A Placebo-Controlled Trial of Antibiotics for Smaller Skin Abscesses
|
|
|
- Shanna Casey
- 6 years ago
- Views:
Transcription
1 Original Article A Placebo-Controlled Trial of Antibiotics for Smaller Skin Abscesses Robert S. Daum, M.D., C.M., Loren G. Miller, M.D., M.P.H., Lilly Immergluck, M.D., Stephanie Fritz, M.D., M.S.C.I., C. Buddy Creech, M.D., M.P.H., David Young, M.D., Neha Kumar, M.D., Michele Downing, R.N., M.S.N., Stephanie Pettibone, B.S., Rebecca Hoagland, M.S., Samantha J. Eells, M.P.H., Mary G. Boyle, R.N., M.S.N., Trisha Chan Parker, M.P.H., and Henry F. Chambers, M.D., for the DMID Team* ABSTRACT BACKGROUND Uncomplicated skin abscesses are common, yet the appropriate management of the condition in the era of community-associated methicillin-resistant Staphylococcus aureus (MRSA) is unclear. METHODS We conducted a multicenter, prospective, double-blind trial involving outpatient adults and children. Patients were stratified according to the presence of a surgically drainable abscess, abscess size, the number of sites of skin infection, and the presence of nonpurulent cellulitis. Participants with a skin abscess 5 cm or smaller in diameter were enrolled. After abscess incision and drainage, participants were randomly assigned to receive clindamycin, trimethoprim sulfamethoxazole (TMP-SMX), or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after the end of treatment. RESULTS We enrolled 786 participants: 505 (64.2%) were adults and 281 (35.8%) were children. A total of 448 (57.0%) of the participants were male. S. aureus was isolated from 527 participants (67.0%), and MRSA was isolated from 388 (49.4%). Ten days after therapy in the intention-to-treat population, the cure rate among participants in the clindamycin group was similar to that in the TMP-SMX group (221 of 266 participants [83.1%] and 215 of 263 participants [81.7%], respectively; P = 0.73), and the cure rate in each activetreatment group was higher than that in the placebo group (177 of 257 participants [68.9%], P<0.001 for both comparisons). The results in the population of patients who could be evaluated were similar. This beneficial effect was restricted to participants with S. aureus infection. Among the participants who were initially cured, new infections at 1 month of follow-up were less common in the clindamycin group (15 of 221, 6.8%) than in the TMP-SMX group (29 of 215 [13.5%], P = 0.03) or the placebo group (22 of 177 [12.4%], P = 0.06). Adverse events were more frequent with clindamycin (58 of 265 [21.9%]) than with TMP-SMX (29 of 261 [11.1%]) or placebo (32 of 255 [12.5%]); all adverse events resolved without sequelae. One participant who received TMP-SMX had a hypersensitivity reaction. CONCLUSIONS As compared with incision and drainage alone, clindamycin or TMP-SMX in conjunction with incision and drainage improves short-term outcomes in patients who have a simple abscess. This benefit must be weighed against the known side-effect profile of these antimicrobials. (Funded by the National Institutes of Health; ClinicalTrials.gov number, NCT ) From the University of Chicago Hospitals, Chicago (R.S.D., N.K.); Harbor UCLA Medical Center and Los Angeles BioMedical Research Institute at Harbor UCLA Medical Center, Los Angeles (L.G.M., S.J.E.), and University of California, San Francisco San Francisco General Hospital, San Francisco (D.Y., M.D., H.F.C.); Morehouse School of Medicine and Emory University Grady Memorial Hospital and Children s Healthcare of Atlanta, Atlanta (L.I., T.C.P.); Washington University School of Medicine Barnes-Jewish Hospital and St. Louis Children s Hospital, St. Louis (S.F., M.G.B.); Vanderbilt University School of Medicine and Vanderbilt University Medical Center, Nashville (C.B.C.); EMMES Corporation, Rockville, MD (S.P.); and Cota Enterprises, Meriden, KS (R.H.). Address reprint requests to Dr. Daum at the Dept. of Medicine, University of Maryland School of Medicine, 655 W. Baltimore St., Baltimore, MD 21201, or at rsdaum@gmail. com. * A list of additional investigators in the Division of Microbiology and Infectious Diseases (DMID) Team is provided in the Supplementary Appendix, available at NEJM.org. N Engl J Med 2017;376: DOI: /NEJMoa Copyright 2017 Massachusetts Medical Society. n engl j med 376;26 nejm.org June 29,
2 More than 4 in 100 people seek treatment for skin infections annually in the United States. 1 Abscesses are the most common of these infections, and the majority of patients are treated as outpatients. 1 Serious complications, such as bacteremia, occur in rare cases. 1,2 Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA) strains, causes most skin infections, 3,4 but the appropriate strategy for the treatment of these infections has not been defined. Clindamycin and trimethoprim sulfamethoxazole (TMP-SMX) are recommended for outpatient treatment of abscesses because of their low cost and in vitro activity against community-associated MRSA and methicillin-susceptible strains, 5 but data on their safety and efficacy are limited. One randomized trial showed that outpatients with skin abscesses treated with incision and drainage and TMP-SMX had a slightly higher cure rate than those treated with incision and drainage and placebo, which supports a role for antibiotic therapy. 6 We previously evaluated clindamycin and TMP-SMX in a randomized trial involving outpatient adults and children with large (>5 cm) or multiple skin abscesses and cellulitis (either purulent or nonpurulent). 7 Participants underwent incision and drainage, as appropriate. The cure rates associated with clindamycin were similar to those associated with TMP-SMX. However, the trial did not include the evaluation of a placebo group. Here we report the results of a double-blind, placebo-controlled trial involving children and adults with a single, small abscess. Participants were randomly assigned to receive clindamycin, TMP-SMX, or placebo after incision and drainage. Methods Trial Design and Population We conducted a multicenter, prospective, randomized, double-blind, placebo-controlled clinical trial involving outpatients. Participants were stratified according to the presence of a surgically drainable abscess, abscess size, the number of sites of skin infection, and the presence of nonpurulent cellulitis. Participants with a single skin abscess 5 cm in diameter or smaller were randomly assigned to receive oral clindamycin, TMP-SMX, or placebo in addition to incision and drainage. From May 2009 through January 2015, participants were recruited from urgent care clinics, emergency departments, and affiliated clinics at six sites: the University of Chicago Medical Center, Chicago; San Francisco General Hospital, San Francisco; Harbor University of California, Los Angeles, Medical Center, Torrance; Vanderbilt University Medical Center, Nashville (added in 2011); Washington University, St. Louis (added in 2012); and Morehouse School of Medicine Emory University, Atlanta (added in 2012). All participants or their parents or guardians provided written informed consent and assent, when age-appropriate. The protocol was approved by the institutional review board at each institution. The authors vouch for the accuracy and completeness of the data and the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org Participants were eligible for participation if they had a single abscess (defined as a circumscribed, drainable collection of pus) with a greatest diameter of 5.0 cm or less ( 3 cm for participants 6 to 11 months of age and 4 cm for participants 1 to 8 years of age), evidenced by two or more of the following signs or symptoms for at least 24 hours: erythema, swelling or induration, local warmth, purulent drainage, and tenderness to pain or palpation. Abscess size was evaluated manually by measuring the abscess cavity length in three dimensions (width, length, and depth). A standardized incision-and-drainage procedure was implemented by the treating physician, with packing of the abscess as needed. 8 Exclusion criteria included superficial skin infections (e.g., impetigo), infection at a body site requiring specialized management (e.g., perirectal, genital, or hand infection), human or animal bite, oral temperature higher than 38.5 C (or >38.0 C for children 6 to 11 months of age), presence of systemic inflammatory response syndrome criteria, immunosuppressive therapy or an immunocompromising condition (e.g., diabetes or chronic renal failure), a body-mass index (the weight in kilograms divided by the square of the height in meters) higher than 40, surgical site or prosthetic device infection, or systemic antistaphylococcal antibacterial therapy in the previous 14 days. Participants were ineligible for participation if they required hospitalization, lived in a long-term care facility, had had cancer or an inflammatory disorder treated 2546 n engl j med 376;26 nejm.org June 29, 2017
3 Trial of Antibiotics for Smaller Skin Abscesses in the previous 12 months, or had had major surgery in the previous 12 months. The full list of inclusion and exclusion criteria is provided in Table S3 in the Supplementary Appendix, available at NEJM.org. Randomization and Study Agents After incision and drainage of the abscess and determination of the size of the abscess, participants were randomly assigned in a 1:1:1 ratio to receive placebo, clindamycin, or TMP-SMX. Variable-block randomization was performed by an independent statistics and data-coordinating center (EMMES Corporation). Clindamycin was given as two 150-mg tablets three times daily. TMP-SMX was given as two tablets (each containing 80 mg of trimethoprim and 400 mg of sulfamethoxazole) twice daily. Participants who were randomly assigned to receive TMP-SMX were given a placebo pill for the midday dose. Pills were over-encapsulated to prevent identification by study staff and participants. Clindamycin, TMP-SMX, and placebo capsules were identical in appearance. Pediatric doses were adjusted according to weight (Table S4 in the Supplementary Appendix), and liquid suspensions were available for pediatric dosing. The suspensions did not differ in appearance or taste and were provided in identical medicine bottles. The study agents were administered for 10 days. Adherence was assessed by self-report and drug accountability for those participants who returned blister packs or suspension bottles. Participants and all study staff were unaware of the study-group assignments, with the exception of the research pharmacists, who determined the correct dosing. The study medication was purchased by the study sponsor, the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. Microbiologic and Demographic Data Abscess fluid was submitted for culture, species identification of isolates, and susceptibility testing in accordance with Clinical and Laboratory Standards Institute approved methods 9 by the clinical microbiology laboratory at each participating institution. The investigators were unaware of the microbiologic results, although the results could be obtained on request by an independent data and safety monitor in the case of a treatment failure. Participants were seen at the end of treatment (day 12), at the test-of-cure visit (7 to 10 days after the prescribed 10-day course of therapy), and at the 1-month follow-up (day 40). Information about clinical response and possible side effects of treatment or placebo were obtained with the use of standardized forms. Statistical Analysis The primary trial outcome was clinical cure by the time of the test-of-cure visit, stratified according to study group. Two primary efficacy analyses were performed: one in the intentionto-treat population (all participants who underwent randomization) and the other in the population that could be evaluated (participants who received treatment or placebo and completed the end-of-treatment and test-of-cure visits) (Fig. 1). A lack of clinical cure was assessed by the research nurse at each site and was defined as lack of resolution of signs or symptoms of the infection, an inability to continue taking the study agent because of adverse effects within the first 48 hours, or any one of the following: recurrence at the original site of infection or occurrence of a skin infection at a new body site, unplanned surgical treatment of the skin infection, or hospitalization related to the infection. The primary null hypothesis was that clindamycin, TMP-SMX, and placebo would have equivalent rates of cure at the test-of-cure visit. The trial was designed as a superiority trial with 80% power to detect a 10-percentage-point absolute difference in cure rates (e.g., 85% vs. 95%) among the three study groups in the population that could be evaluated, with an alpha of Under the assumption of a 20% attrition rate, 786 participants were required (262 per group). The prespecified exploratory secondary outcomes were the cure rates at the end-of-treatment and 1-month follow-up visits; the cure rates in adults and children; the cure rates for patients infected with methicillin-susceptible S. aureus, MRSA, or other strains; and adverse-event rates. Comparisons among the groups were performed with the use of Pearson s chi-square test, Fisher s exact test, an analysis of variance test, or a logistic regression, as appropriate. The statistical analysis plan is available at NEJM.org. Interim analyses for safety and efficacy were performed by an independent data and safety monitoring committee with the use of an O Brien Fleming monitor- n engl j med 376;26 nejm.org June 29,
4 786 Patients were included in intention-to-treat population 266 Were assigned to receive clindamycin 265 Received clindamycin 1 Did not receive clindamycin 263 Were assigned to receive TMP-SMX 261 Received TMP-SMX 2 Did not receive TMP-SMX 257 Were assigned to receive placebo 255 Received placebo 2 Did not receive placebo 22 Discontinued study regimen early 6 Had an adverse reaction to previous study-agent administration 3 Had other illness or injury 3 Withdrew 3 Were withdrawn by investigator 1 Missed a dose 5 Were lost to follow up 1 Had other reasons 24 Discontinued study regimen early 3 Had an adverse reaction to previous study-agent administration 7 Had other illness or injury 1 Withdrew 4 Were withdrawn by investigator 6 Were lost to follow up 3 Had other reasons 41 Discontinued study regimen early 4 Had an adverse reaction to previous study-agent administration 12 Had other illness or injury 4 Withdrew 3 Were withdrawn by investigator 12 Were lost to follow up 6 Had other reasons 238 Were included in population that primary efficacy analysis at TOC 232 Were included in population that primary efficacy analysis at TOC 220 Were included in population that primary efficacy analysis at TOC 28 Were excluded from primary efficacy analysis at TOC 9 Missed a visit 2 Received nonstudy antibiotics 17 Were terminated from 13 Were lost to follow-up 1 Underwent randomization and treatment twice 2 Withdrew 1 Was withdrawn by investigator 31 Were excluded from primary efficacy analysis at TOC 16 Missed a visit 2 Received nonstudy antibiotics 13 Were terminated from 7 Were lost to follow-up 1 Was nonadherent 2 Underwent randomization but were not treated 3 Withdrew 37 Were excluded from primary efficacy analysis at TOC 18 Missed a visit 19 Were terminated from 10 Were lost to follow-up 2 Underwent randomization but were not treated 1 had a serious adverse event 6 Withdrew 234 Were included in population that secondary efficacy analysis at 1-month follow-up 226 Were included in population that secondary efficacy analysis at 1-month follow-up 218 Were included in population that secondary efficacy analysis at 1-month follow-up 32 Were excluded from secondary efficacy analysis at 1-month follow-up 4 Missed a visit 5 Received nonstudy antibiotics 23 Were terminated from 18 Were lost to follow-up 1 Underwent randomization and treatment twice 2 Withdrew 1 Was withdrawn by investigator 37 Were excluded from secondary efficacy analysis at 1-month follow-up 10 Missed a visit 4 Received nonstudy antibiotics 23 Were terminated from 15 Were lost to follow-up 1 Was nonadherent 2 Underwent randomization but were not treated 5 Withdrew 39 Were excluded from secondary efficacy analysis at 1-month follow-up 9 Missed a visit 3 Received nonstudy antibiotics 27 Were terminated from 16 Were lost to follow-up 2 Underwent randomization but were not treated 1 Had a serious adverse event 8 Withdrew 2548 n engl j med 376;26 nejm.org June 29, 2017
5 Trial of Antibiotics for Smaller Skin Abscesses Table 1. Demographic and Clinical Characteristics of the Study Population.* Characteristic Clindamycin (N = 266) TMP-SMX (N = 263) Placebo (N = 257) All Groups (N = 786) Sex no. (%) Male 140 (52.6) 152 (57.8) 156 (60.7) 448 (57.0) Female 126 (47.4) 111 (42.2) 101 (39.3) 338 (43.0) Hispanic ethnic background no. (%) Non-Hispanic and non-latino 216 (81.2) 208 (79.1) 202 (78.6) 626 (79.6) Hispanic or Latino 49 (18.4) 55 (20.9) 55 (21.4) 159 (20.2) Unknown 1 (0.4) (0.1) Race or ethnic group no. (%) Native American or Alaskan Native 0 2 (0.8) 1 (0.4) 3 (0.4) Asian 8 (3.0) 4 (1.5) 2 (0.8) 14 (1.8) Hawaiian or Pacific Islander 2 (0.8) 4 (1.5) 2 (0.8) 8 (1.0) Black or African American 165 (62.0) 152 (57.8) 167 (65.0) 484 (61.6) White 80 (30.1) 87 (33.1) 73 (28.4) 240 (30.5) Multiracial 5 (1.9) 11 (4.2) 8 (3.1) 24 (3.1) Other or unknown 6 (2.3) 3 (1.1) 4 (1.6) 13 (1.7) Age no. (%) <1 yr 6 (2.3) 9 (3.4) 2 (0.8) 17 (2.2) 1 to 8 yr 56 (21.1) 51 (19.4) 59 (23.0) 166 (21.1) 9 to 17 yr 39 (14.7) 31 (11.8) 28 (10.9) 98 (12.5) 18 yr 165 (62.0) 172 (65.4) 168 (65.4) 505 (64.2) Body temperature C 36.67± ± ± ±0.47 Area of wound cm ± ± ± ±4.30 Area of surrounding erythema cm ± ± ± ±86.82 * Percentages may not total to 100 because of rounding. TMP-SMX denotes trimethoprim sulfamethoxazole. Race and ethnic background were reported by the participants. Area was calculated with the use of the formula for an ellipse: (length width π)/4. Figure 1 ( facing page). Enrollment, Randomization, and Follow-up. Five participants underwent randomization but were not treated; 2 of these 5 underwent randomization but study agent was not dispensed, 1 recalled having taken a nonstudy drug before enrollment, and 1 received the incorrect study agent. The population that could be evaluated and was included in the secondary efficacy analysis at the 1-month follow-up included participants who missed the test-of-cure visit (TOC) but completed the 1-month follow-up visit. Patients could have been excluded from the efficacy analyses for more than one reason. TMP-SMX denotes trimethoprim sulfamethoxazole. ing boundary. In the final analysis, P values of 0.04 or lower were considered to indicate statistical significance. Findings from the trial are described in accordance with CONSORT guidelines. 10 Results Participants We enrolled 786 participants: 505 (64.2%) were adults, and 281 (35.8%) were children. The mean age at enrollment was 25.5 years. A total of 448 participants (57.0%) were male (Table 1). Clindamy- n engl j med 376;26 nejm.org June 29,
6 Table 2. Results of Abscess Culture.* Result Patients with Result (N = 786) no. (%) Culture obtained 781 (99.4) Culture not obtained 5 (0.6) Positive culture results 718 (91.3) Culture obtained but no growth 32 (4.1) Culture obtained but results not available 31 (3.9) Staphylococcus aureus isolated 527 (67.0) MRSA 388 (49.4) MSSA 140 (17.8) Coagulase-negative staphylococcus isolated 104 (13.2) Streptococcus species isolated 54 (6.9) Group A streptococcus 7 (0.9) Group B streptococcus 4 (0.5) S. anginosus 1 (0.1) S. agalactiae 1 (0.1) Beta-hemolytic group C streptococcus 2 (0.3) Beta-hemolytic group F streptococcus 3 (0.4) Beta-hemolytic group G streptococcus 1 (0.1) Non group A and B beta-hemolytic streptococcus 1 (0.1) Viridans group streptococcus 36 (4.6) Other species isolated 118 (15.0) Acinetobacter species 4 (0.5) Actinomyces species 3 (0.4) Citrobacter freundii 1 (0.1) Corynebacterium species 31 (3.9) Diphtheroid bacilli 16 (2.0) Eikenella corrodens 3 (0.4) Enterobacter species 2 (0.3) Enterococcus species 7 (0.9) Escherichia coli 2 (0.3) Fusobacterium species 1 (0.1) Haemophilus species 3 (0.4) Klebsiella species 3 (0.4) Lactobacillus species 1 (0.1) Peptostreptococcus species 2 (0.3) Prevotella species 2 (0.3) Proteus mirabilis 9 (1.1) Bacterial growth not otherwise specified 5 (0.6) Other 42 (5.3) * Participants whose lesions grew multiple organisms are counted once for each species identified. The culture from one participant grew a methicillin-resistant S. aureus (MRSA) isolate and a methicillin-susceptible S. aureus (MSSA) isolate. cin was assigned to 266 participants, TMP-SMX to 263 participants, and placebo to 257 participants (Fig. 1). Five participants underwent randomization but were not treated; the studygroup assignments and reasons for withdrawal from the trial are summarized in Figure 1. A total of 343 participants were fully adherent to the study regimen (Table S1 in the Supplementary Appendix). The mean abscess depth was 1.64 cm, and the mean abscess area was 3.89 cm 2 (Table 1). An abscess of 2.0 cm or smaller in diameter was present in 44.6% of participants (Table S2 in the Supplementary Appendix). The results of abscess culture were available for 781 participants (99.4%) (Table 2): S. aureus was isolated in 527 participants (67.0%), MRSA in 388 (49.4%), coagulase-negative staphylococci in 104 (13.2%), streptococcus species in 54 (6.9%), and other organisms in 118 (15.0%). Clinical Cure at the Test-of-Cure Visit The rates of clinical cure at the test-of-cure visit in the intention-to-treat population were 83.1% (221 of 266) in the clindamycin group, 81.7% (215 of 263) in the TMP-SMX group, and 68.9% (177 of 257) in the placebo group (Table 3). The cure rate in the placebo group was significantly lower than that in the clindamycin group (rate difference, 14.2 percentage points; 95% confidence interval [CI], 22.0 to 6.4; P<0.001) and that in the TMP-SMX group (rate difference, 12.9 percentage points; 95% CI, 20.8 to 5.0; P<0.001). The difference between the cure rate in the TMP-SMX group and that in the clindamycin group was not significant ( 1.3 percentage points; 95% CI, 8.4 to 5.7; P = 0.73). The results were similar for the population that could be evaluated (Table 3), with significantly different cure rates for placebo versus either antibiotic but no significant difference between clindamycin and TMP-SMX. A new lesion at a different body site or the use of a rescue medication were more common causes of treatment failure in the placebo group than in the active-treatment groups (Table S8 in the Supplementary Appendix). Treatment failure was rarely due to worsening of the original lesion. Logistic-regression analysis was performed to determine whether cure rates differed according to age and study group (Table 4). In the population that could be evaluated, children had a significantly higher cure rate with clindamycin 2550 n engl j med 376;26 nejm.org June 29, 2017
7 Trial of Antibiotics for Smaller Skin Abscesses Table 3. Cure Rate at Test-of-Cure Visit in the Overall Population and Relevant Subgroups.* Group Clindamycin TMP-SMX Placebo No. with Cure/ Total No. % (95% CI) No. with Cure/ Total No. % (95% CI) No. with Cure/ Total No. % (95% CI) All participants Intention-to-treat population 221/ ( ) 215/ ( ) 177/ ( ) Population that could be evaluated 221/ ( ) 215/ ( ) 177/ ( ) Children Intention-to-treat population 90/ ( ) 75/ ( ) 61/ ( ) Population that could be evaluated 90/ ( ) 75/ ( ) 61/ ( ) Adults Intention-to-treat population 131/ ( ) 140/ ( ) 116/ ( ) Population that could be evaluated 131/ ( ) 140/ ( ) 116/ ( ) S. aureus isolated Intention-to-treat population 157/ ( ) 149/ ( ) 102/ ( ) Population that could be evaluated 157/ ( ) 149/ ( ) 102/ ( ) MRSA isolated Intention-to-treat population 116/ ( ) 110/ ( ) 73/ ( ) Population that could be evaluated 116/ ( ) 110/ ( ) 73/ ( ) MSSA isolated Intention-to-treat population 41/ ( ) 39/ ( ) 29/ ( ) Population that could be evaluated 41/ ( ) 39/ ( ) 29/ ( ) No S. aureus isolated Intention-to-treat population 57/ ( ) 59/ ( ) 69/ ( ) Population that could be evaluated 57/ ( ) 59/ ( ) 69/ ( ) * The actual confidence interval was 95.6% after adjustment for the interim analysis. The intention-to-treat population includes all participants who underwent randomization, and the population that could be evaluated includes participants who received treatment or placebo and completed the required study visits. n engl j med 376;26 nejm.org June 29,
8 Table 4. Logistic-Regression Model of Cure Rates among Children and Adults. Variable and Population P Value in the Logistic-Regression Model* Study group TMP-SMX vs. Clindamycin Placebo vs. Clindamycin Placebo vs. TMP-SMX Intention-to-treat population 0.37 < Population that could be evaluated 0.17 <0.001 <0.001 Age group Intention-to-treat population Population that could be evaluated Interaction Intention-to-treat population Population that could be evaluated * The P values refer to the results of a logistic-regression model incorporating study group (clindamycin vs. TMP-SMX) and age group (children vs. adults). After controlling for the effect of age, the differences between placebo and clindamycin and between placebo and TMP-SMX were significant in both the intention-to-treat population and the population that could be evaluated (P<0.001). The cure rates for clindamycin in the population that could be evaluated were significantly higher among children than among adults (among children, P = 0.04 for TMP-SMX vs. clindamycin and P = 0.03 for placebo vs. clindamycin). None of the interaction terms for the logistic-regression models were significant, indicating that the differences in cure rates between children and adults were not significant in each respective study-group comparison (P>0.05 for the intention-to-treat population and the population that could be evaluated). than with TMP-SMX or placebo, and this treatment advantage with clindamycin was significantly greater than that seen among adults (for the age-group differences in cure rates, P = 0.04 for clindamycin vs. TMP-SMX and P = 0.03 for clindamycin vs. placebo); there was no significant difference in cure rates between adults and children in the comparison between the TMP- SMX group and the placebo group (P = 0.87). In the intention-to-treat population, there were no significant differences between children and adults in any study-group comparisons (Table 4). The cure rates among participants in the intention-to-treat population who were culture-positive for S. aureus were 83.5% in the clindamycin group and 83.2% in the TMP-SMX group (P = 0.99) (Table 3). These rates were significantly higher than the cure rate of 63.8% in the placebo group (P<0.001 for both comparisons). The results were similar for the population that could be evaluated. Among MRSA-infected participants in the intention-to-treat population, 81.7% of those treated with clindamycin had been cured by the time of the test-of-cure visit, as compared with 84.6% of those treated with TMP-SMX and 62.9% of those who received placebo (Table 3). The cure rates in the clindamycin and TMP-SMX groups did not differ significantly (P = 0.63), whereas the cure rate in the placebo group was significantly lower than that in either the TMP- SMX group (P = 0.001) or the clindamycin group (P<0.001). The results were similar for the population that could be evaluated. Among the participants who were infected with methicillin-susceptible S. aureus in the intention-to-treat population, 89.1% of the participants in the clindamycin group were cured, as compared with 79.6% of participants in the TMP-SMX group and 65.9% of participants in the placebo group (Table 3). The cure rate in the placebo group was significantly lower than that in the clindamycin group (P = 0.01) but not significantly lower than that in the TMP-SMX group (P = 0.16). The difference between the cure rate in the clindamycin group and that in the TMP-SMX group was not significant (P = 0.26). Similar results were observed for the population that could be evaluated. The cure rates among participants with an abscess that did not grow S. aureus in culture were similar for all treatment groups in the intention-to-treat population and the population that could be evaluated (P = 0.99 for all comparisons) (Table 3). There were 13 participants with S. aureus isolates that were resistant to clindamycin n engl j med 376;26 nejm.org June 29, 2017
9 Trial of Antibiotics for Smaller Skin Abscesses isolates found to be resistant by single-agent testing and 1 found to be resistant by disk-diffusion (D-zone) testing. The cure rate among clindamycin recipients with clindamycin-resistant isolates was significantly lower than that among participants with clindamycin-susceptible S. aureus isolates (7 of 13 [53.8%] vs. 145 of 170 [85.3%], P = 0.01). Clinical Cure at the 1-Month Follow-up Visit At the 1-month follow-up visit in the intentionto-treat population, 78.6% (209 of 266) of the clindamycin-treated participants, 73.0% (192 of 263) of the TMP-SMX treated participants, and 62.6% (161 of 257) of the placebo-treated participants remained cured. The difference in cure rates between the TMP-SMX group and the clindamycin group was not significant ( 5.6 percentage points; 95% CI, 13.2 to 2.1; P = 0.16). The differences in cure rates between the placebo group and the clindamycin group ( 15.9 percentage points; 95% CI, 24.0 to 7.8; P<0.001) and between the placebo group and the TMP-SMX group ( 10.4 percentage points; 95% CI, 18.7 to 2.0; P = 0.01) were significant. The results were similar in the population that could be evaluated. At the 1-month follow-up visit among participants who had been found to be cured by the time of the test-of-cure visit, new infections at a different body site or a recurrent infection at the original body site had occurred in 6.8% (15 of 221) of clindamycin recipients, 13.5% (29 of 215) of TMP-SMX recipients, and 12.4% (22 of 177) of placebo recipients. The difference in the rates of interval or recurrent infections between the TMP-SMX and clindamycin groups was significant (6.7 percentage points; 95% CI, 0.6 to 12.8; P = 0.03), but the difference between the placebo and clindamycin groups (5.6 percentage points; 95% CI, 0.8 to 12.0; P = 0.06) and the difference between the placebo and TMP-SMX groups ( 1.1 percentage points; 95% CI, 8.2 to 6.1; P = 0.88) were not significant. A new lesion at a new location or worsening of the original lesion were among the reasons for failure at the 1-month follow-up visit, although the latter reason was infrequent. There was also a nonsignificant trend toward higher rates of interval or recurrent infections among children who were treated with TMP-SMX; the rate of interval or recurrent infections in this group was 13.3% (10 of 75), as compared with 4.4% (4 of 90) in the clindamycin group, a difference of 8.9 percentage points (95% CI, 1.1 to 18.9; P = 0.05). Adverse Events The rate of treatment-associated adverse events was higher in the clindamycin group (21.9%) than in the TMP-SMX group (11.1%) or the placebo group (12.5%) (Table S5 in the Supplementary Appendix). The most common adverse events were diarrhea (16.2%, 5.4%, and 6.7% respectively) and nausea (2.3%, 4.2%, and 2.4% respectively). Most adverse events were mild or moderate and resolved without sequelae (Table S6 in the Supplementary Appendix). There were no episodes of Clostridium difficile associated diarrhea. There were nine serious adverse events reported in 8 participants (Table S7 in the Supplementary Appendix). Eight of the events resolved without sequelae and were judged by the investigator not to be related to the study agent; these included a motor-vehicle accident, a case of status asthmaticus, a case of pneumonia, three episodes of worsening cellulitis or abscess, a new perirectal abscess, and a case of emesis. Only one episode, a hypersensitivity reaction with fever, rash, thrombocytopenia, and hepatitis, was thought by the investigator to be related to the study drug (TMP-SMX), and the reaction resolved without sequelae. Discussion The cure rates for simple abscesses treated with incision and drainage plus clindamycin or incision and drainage plus TMP-SMX were similar, and both cure rates were significantly higher (by 12 to 13 percentage points) than that among participants who were treated with incision and drainage plus placebo. Our findings show a clinical benefit of antibiotic therapy in addition to incision and drainage that seems limited to patients with S. aureus infection. The results complement findings from the trial conducted by Talan et al., 6 who found higher cure rates among TMP-SMX treated participants than among placebo-treated participants in conjunction with abscess drainage (however, this trial did not include children 12 years of age or younger). Our trial yielded other new findings. First, clindamycin was as effective as TMP-SMX, and the cure rates associated with either agent were n engl j med 376;26 nejm.org June 29,
10 higher than that associated with placebo. Table S8 in the Supplementary Appendix shows that new infections developed more frequently in participants who received placebo than among participants in either antibiotic group. Second, TMP-SMX was effective at half the dose used by Talan et al., although TMP-SMX was administered for 10 days instead of the 7 days used in that trial. 6 Third, children and adults both benefited from active therapy, although clindamycin may have performed slightly better than TMP- SMX among children. Fourth, clindamycin may be more effective than TMP-SMX in preventing recurrences or new infections after completion of therapy, particularly in children; perhaps a higher dose of TMP-SMX would have been more effective in preventing recurrent or new infections. 6 Finally, these data underscore the potential clinical relevance of in vitro resistance to clindamycin. Participants infected with a clindamycin-resistant S. aureus isolate who were treated with clindamycin had cure rates similar to those who were given placebo. The contribution of resistance to TMP-SMX to treatment failure could not be assessed because there were no resistant isolates in vitro. The cumulative data from our investigation and that of Talan et al. 6 call into question the perception largely based on expert opinion or smaller, underpowered, and lower-quality noninferiority trials that cure rates do not improve with the addition of systemic antibiotic treatment after incision and drainage. 15 These two larger trials show that adjunctive antibiotic therapy improves cure rates for skin abscesses and decreases the recurrence rate. Antibiotic-related side effects, particularly if frequent or serious, should be taken into account when deciding whether to treat a drained abscess with an antibiotic. In this trial, TMP- SMX was associated with a hypersensitivity reaction, and clindamycin was associated with more adverse events than TMP-SMX. Although no cases of C. difficile associated diarrhea or severe allergic reactions were observed, these and other known side effects must be considered. Our findings suggest that there is a trade-off between more adverse effects and a lower likelihood of infection recurrence when one uses clindamycin rather than TMP-SMX. Such information and the local prevalence of resistance should be used by treating physicians and policy makers when choosing an antibiotic for adjunctive therapy of cutaneous abscesses. Our trial has limitations. Two antibiotics that are commonly used and recommended for the treatment of uncomplicated skin infections were studied, but there are others that may be just as effective. For example, doxycycline (which is contraindicated for children younger than 8 years of age and was not studied in this trial) is active against MRSA strains. 16 However, given the higher cure rates that we found, the marginal benefit, if any, would probably be small at best. We followed participants for 1 month; a longer follow-up period may have captured more recurrences. 17 The potential for participants to have treatment failure but be cured of infection subsequently, such as at the 1-month follow-up visit, was not assessed (i.e., if a patient had a treatment failure at the test-of-cure visit, the patient was not evaluated at the 1-month follow-up visit). The exploratory secondary analyses we have discussed provide a potential direction for future analyses. In conclusion, our results show that short-term outcomes among patients with uncomplicated cutaneous abscesses, particularly those caused by S. aureus, are improved by antibiotic treatment with either clindamycin or TMP-SMX in addition to abscess incision and drainage. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. Supported by a contract from the National Institute of Allergy and Infectious Diseases (NIAID) (HHSN C to Dr. Chambers) and the National Center for Research Resources (UL1RR033176, now at the National Center for Advancing Translational Sciences, UL1TR000124). Dr. Daum reports receiving fees for serving on an advisory board from Pfizer and Dynavax and grant support from Theravance and Merck; Dr. Miller, receiving grant support from Gilead Sciences, Achaogen, Merck, Abbott, and Cepheid and consulting fees from Tetraphase; Dr. Creech, receiving grant support from Pfizer, grant support and consulting fees from GlaxoSmith- Kline, and consulting fees from Theravance; and Dr. Chambers, previously holding stock in Merck and receiving grant support and fees for serving on an advisory board from Genentech and fees for serving on an advisory board from AstraZeneca, Pfizer, Cubist (now Merck), Theravance, Allergan, and Cempra. No other potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. We thank Christine Chiou, M.D., Maureen Mehigan, R.N., B.S.N., and Hyung Koo, R.N., B.S.N. (all at the Division of Microbiology and Infectious Diseases, NIAID) for assistance and advice n engl j med 376;26 nejm.org June 29, 2017
11 Trial of Antibiotics for Smaller Skin Abscesses References 1. Miller LG, Eisenberg DF, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, BMC Infect Dis 2015; 15: Lipsky BA, Kollef MH, Miller LG, Sun X, Johannes RS, Tabak YP. Predicting bacteremia among patients hospitalized for skin and skin-structure infections: derivation and validation of a risk score. Infect Control Hosp Epidemiol 2010; 31: Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006; 355: David MZ, Daum RS. Communityassociated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010; 23: Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52: Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med 2016; 374: Miller LG, Daum RS, Creech CB, et al. Clindamycin versus trimethoprim sulfamethoxazole for uncomplicated skin infections. N Engl J Med 2015; 372: Fitch MT, Manthey DE, McGinnis HD, Nicks BA, Pariyadath M. Abscess incision and drainage. N Engl J Med 2007; 357(19): e Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria which grow aerobically: approved standard. 10th ed. CLSI document M07-A10. Wayne, PA: CLSI, January Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010; 152: Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J 2004; 23: Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med 2010; 56: Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebocontrolled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother 2007; 51: Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med 2010; 55: Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59(2): e10-e Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3): e18- e Holmes L, Ma C, Qiao H, et al. Trimethoprim-sulfamethoxazole therapy reduces failure and recurrence in methicillinresistant Staphylococcus aureus skin abscesses after surgical drainage. J Pediatr 2016; 169: e1. Copyright 2017 Massachusetts Medical Society. n engl j med 376;26 nejm.org June 29,
HHS Public Access Author manuscript N Engl J Med. Author manuscript; available in PMC 2015 September 19.
 Clindamycin versus Trimethoprim Sulfamethoxazole for Uncomplicated Skin Infections Loren G. Miller, M.D., M.P.H., Robert S. Daum, M.D., C.M., C. Buddy Creech, M.D., M.P.H., David Young, M.D., Michele D.
Clindamycin versus Trimethoprim Sulfamethoxazole for Uncomplicated Skin Infections Loren G. Miller, M.D., M.P.H., Robert S. Daum, M.D., C.M., C. Buddy Creech, M.D., M.P.H., David Young, M.D., Michele D.
abstract n engl j med 372;12 nejm.org March 19,
 The new england journal of medicine established in 1812 March 19, 2015 vol. 372 no. 12 Clindamycin versus Trimethoprim Sulfamethoxazole for Uncomplicated Skin Infections Loren G. Miller, M.D., M.P.H.,
The new england journal of medicine established in 1812 March 19, 2015 vol. 372 no. 12 Clindamycin versus Trimethoprim Sulfamethoxazole for Uncomplicated Skin Infections Loren G. Miller, M.D., M.P.H.,
Trimethoprim Sulfamethoxazole versus Placebo for Uncomplicated Skin Abscess
 The new england journal of medicine Original Article Trimethoprim Sulfamethoxazole versus Placebo for Uncomplicated Skin Abscess David A. Talan, M.D., William R. Mower, M.D., Ph.D., Anusha Krishnadasan,
The new england journal of medicine Original Article Trimethoprim Sulfamethoxazole versus Placebo for Uncomplicated Skin Abscess David A. Talan, M.D., William R. Mower, M.D., Ph.D., Anusha Krishnadasan,
A Prospective Investigation of Nasal Mupirocin, Hexachlorophene Body Wash, and Systemic
 AAC Accepts, published online ahead of print on 14 November 2011 Antimicrob. Agents Chemother. doi:10.1128/aac.01608-10 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions.
AAC Accepts, published online ahead of print on 14 November 2011 Antimicrob. Agents Chemother. doi:10.1128/aac.01608-10 Copyright 2011, American Society for Microbiology and/or the Listed Authors/Institutions.
Randomized, Controlled Trial of Antibiotics in the Management of Community-Acquired Skin Abscesses in the Pediatric Patient
 PEDIATRICS/ORIGINAL RESEARCH Randomized, Controlled Trial of Antibiotics in the Management of Community-Acquired Skin Abscesses in the Pediatric Patient Myto Duong, MD, MS Stephen Markwell, MA John Peter,
PEDIATRICS/ORIGINAL RESEARCH Randomized, Controlled Trial of Antibiotics in the Management of Community-Acquired Skin Abscesses in the Pediatric Patient Myto Duong, MD, MS Stephen Markwell, MA John Peter,
Optimizing Antibiotic Treatment of Skin and Soft Tissue Infections
 Optimizing Antibiotic Treatment of Skin and Soft Tissue Infections 15th Annual Rocky Mountain Hospital Medicine Symposium November 6, 2017 Tim Jenkins, MD Director, Antibiotic Stewardship Program Denver
Optimizing Antibiotic Treatment of Skin and Soft Tissue Infections 15th Annual Rocky Mountain Hospital Medicine Symposium November 6, 2017 Tim Jenkins, MD Director, Antibiotic Stewardship Program Denver
Scottish Medicines Consortium
 Scottish Medicines Consortium tigecycline 50mg vial of powder for intravenous infusion (Tygacil ) (277/06) Wyeth 9 June 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Scottish Medicines Consortium tigecycline 50mg vial of powder for intravenous infusion (Tygacil ) (277/06) Wyeth 9 June 2006 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Skin and Soft Tissue Infections Emerging Therapies and 5 things to know
 2011 MFMER slide-1 Skin and Soft Tissue Infections Emerging Therapies and 5 things to know Aaron Tande, MD Assistant Professor of Medicine October 27, 2017 Division of INFECTIOUS DISEASES 2011 MFMER slide-2
2011 MFMER slide-1 Skin and Soft Tissue Infections Emerging Therapies and 5 things to know Aaron Tande, MD Assistant Professor of Medicine October 27, 2017 Division of INFECTIOUS DISEASES 2011 MFMER slide-2
INFECTIOUS DISEASE/ORIGINAL RESEARCH
 INFECTIOUS DISEASE/ORIGINAL RESEARCH Randomized Controlled Trial of Trimethoprim-Sulfamethoxazole for Uncomplicated Skin Abscesses in Patients at Risk for Community-Associated Methicillin-Resistant Staphylococcus
INFECTIOUS DISEASE/ORIGINAL RESEARCH Randomized Controlled Trial of Trimethoprim-Sulfamethoxazole for Uncomplicated Skin Abscesses in Patients at Risk for Community-Associated Methicillin-Resistant Staphylococcus
Management of Skin and Soft-Tissue Infection
 Clinical Decisions Interactive at www.nejm.org Management of Skin and Soft-Tissue Infection This interactive feature addresses the diagnosis or management of a clinical case. A case vignette is followed
Clinical Decisions Interactive at www.nejm.org Management of Skin and Soft-Tissue Infection This interactive feature addresses the diagnosis or management of a clinical case. A case vignette is followed
Scottish Medicines Consortium
 Scottish Medicines Consortium daptomycin 350mg powder for concentrate for solution for infusion (Cubicin ) Chiron Corporation Limited No. (248/06) 10 March 2006 The Scottish Medicines Consortium (SMC)
Scottish Medicines Consortium daptomycin 350mg powder for concentrate for solution for infusion (Cubicin ) Chiron Corporation Limited No. (248/06) 10 March 2006 The Scottish Medicines Consortium (SMC)
S aureus infections: outpatient treatment. Dirk Vogelaers Dept of Infectious Diseases University Hospital Gent Belgium
 S aureus infections: outpatient treatment Dirk Vogelaers Dept of Infectious Diseases University Hospital Gent Belgium Intern Med J. 2005 Feb;36(2):142-3 Intern Med J. 2005 Feb;36(2):142-3 Treatment of
S aureus infections: outpatient treatment Dirk Vogelaers Dept of Infectious Diseases University Hospital Gent Belgium Intern Med J. 2005 Feb;36(2):142-3 Intern Med J. 2005 Feb;36(2):142-3 Treatment of
11/10/2016. Skin and Soft Tissue Infections. Disclosures. Educational Need/Practice Gap. Objectives. Case #1
 Disclosures Selecting Antimicrobials for Common Infections in Children FMR-Contemporary Pediatrics 11/2016 Sean McTigue, MD Assistant Professor of Pediatrics, Pediatric Infectious Diseases Medical Director
Disclosures Selecting Antimicrobials for Common Infections in Children FMR-Contemporary Pediatrics 11/2016 Sean McTigue, MD Assistant Professor of Pediatrics, Pediatric Infectious Diseases Medical Director
Mrsa abscess and cellulitis
 Search Mrsa abscess and cellulitis An abscess is a collection of pus that has built up within the tissue of the body. Signs and symptoms of abscesses include redness, pain, warmth, and swelling. The. Staph
Search Mrsa abscess and cellulitis An abscess is a collection of pus that has built up within the tissue of the body. Signs and symptoms of abscesses include redness, pain, warmth, and swelling. The. Staph
Period of study: 12 Nov 2002 to 08 Apr 2004 (first subject s first visit to last subject s last visit)
 Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency of Bayer's
Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency of Bayer's
Impact of a Standardized Protocol to Address Outbreak of Methicillin-resistant
 Impact of a Standardized Protocol to Address Outbreak of Methicillin-resistant Staphylococcus Aureus Skin Infections at a large, urban County Jail System Earl J. Goldstein, MD* Gladys Hradecky, RN* Gary
Impact of a Standardized Protocol to Address Outbreak of Methicillin-resistant Staphylococcus Aureus Skin Infections at a large, urban County Jail System Earl J. Goldstein, MD* Gladys Hradecky, RN* Gary
Bacterial skin and soft tissues infections (SSTI) are one of the most common 1. infections among different age groups
 Bacterial skin and soft tissues infections (SSTI) are one of the most common 1 infections among different age groups Gram-positive bacteria are the most frequently isolated pathogens from SSTI, with a
Bacterial skin and soft tissues infections (SSTI) are one of the most common 1 infections among different age groups Gram-positive bacteria are the most frequently isolated pathogens from SSTI, with a
Clinical Policy: Linezolid (Zyvox) Reference Number: CP.PMN.27 Effective Date: Last Review Date: Line of Business: HIM*, Medicaid
 Clinical Policy: (Zyvox) Reference Number: CP.PMN.27 Effective Date: 09.01.06 Last Review Date: 02.19 Line of Business: HIM*, Medicaid Coding Implications Revision Log See Important Reminder at the end
Clinical Policy: (Zyvox) Reference Number: CP.PMN.27 Effective Date: 09.01.06 Last Review Date: 02.19 Line of Business: HIM*, Medicaid Coding Implications Revision Log See Important Reminder at the end
Clinical Policy: Linezolid (Zyvox) Reference Number: CP.PMN.27 Effective Date: Last Review Date: Line of Business: Oregon Health Plan
 Clinical Policy: (Zyvox) Reference Number: CP.PMN.27 Effective Date: 07.01.18 Last Review Date: 05.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Zyvox) Reference Number: CP.PMN.27 Effective Date: 07.01.18 Last Review Date: 05.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end of this policy
Cellulitis. Assoc Prof Mark Thomas. Conference for General Practice Auckland Saturday 28 July 2018
 Cellulitis Assoc Prof Mark Thomas Conference for General Practice Auckland Saturday 28 July 2018 Summary Cellulitis Usual treatment flucloxacillin for 5 days Frequent recurrences consider penicillin 250mg
Cellulitis Assoc Prof Mark Thomas Conference for General Practice Auckland Saturday 28 July 2018 Summary Cellulitis Usual treatment flucloxacillin for 5 days Frequent recurrences consider penicillin 250mg
Bacterial skin infection
 D i v i s i o n o f P e d i a t r i c E m e r g e n c y M e d i c i n e P a g e 1 Bacterial skin infection Cellulitis w/o abscess Abscess Deep tissue involvement Multiple abscesses Perirectal Anterior
D i v i s i o n o f P e d i a t r i c E m e r g e n c y M e d i c i n e P a g e 1 Bacterial skin infection Cellulitis w/o abscess Abscess Deep tissue involvement Multiple abscesses Perirectal Anterior
Development of Drugs for Skin Infections
 EFPIA - Skin Infection comments 1 Development of Drugs for Skin Infections John H Rex, MD EFPIA - Skin Infection comments 2 Skin Infections Significant recent debate: Acceptable forms: A focus on fever
EFPIA - Skin Infection comments 1 Development of Drugs for Skin Infections John H Rex, MD EFPIA - Skin Infection comments 2 Skin Infections Significant recent debate: Acceptable forms: A focus on fever
Antibiotic Updates: Part II
 Antibiotic Updates: Part II Fredrick M. Abrahamian, DO, FACEP, FIDSA Health Sciences Clinical Professor of Emergency Medicine David Geffen School of Medicine at UCLA Los Angeles, California Financial Disclosures
Antibiotic Updates: Part II Fredrick M. Abrahamian, DO, FACEP, FIDSA Health Sciences Clinical Professor of Emergency Medicine David Geffen School of Medicine at UCLA Los Angeles, California Financial Disclosures
Antibiotic Stewardship Program (ASP) CHRISTUS SETX
 Antibiotic Stewardship Program (ASP) CHRISTUS SETX Program Goals I. Judicious use of antibiotics Decrease use of broad spectrum antibiotics and deescalate use based on clinical symptoms Therapeutic duplication:
Antibiotic Stewardship Program (ASP) CHRISTUS SETX Program Goals I. Judicious use of antibiotics Decrease use of broad spectrum antibiotics and deescalate use based on clinical symptoms Therapeutic duplication:
Impact of Systemic Antibiotics on Staphylococcus aureus Colonization and Recurrent Skin Infection
 Clinical Infectious Diseases MAJOR ARTICLE Impact of Systemic Antibiotics on Staphylococcus aureus Colonization and Recurrent Skin Infection Patrick G. Hogan, 1 Marcela Rodriguez, 2 Allison M. Spenner,
Clinical Infectious Diseases MAJOR ARTICLE Impact of Systemic Antibiotics on Staphylococcus aureus Colonization and Recurrent Skin Infection Patrick G. Hogan, 1 Marcela Rodriguez, 2 Allison M. Spenner,
Antibiotic Abyss. Discussion Points. MRSA Treatment Guidelines
 Antibiotic Abyss Fredrick M. Abrahamian, D.O., FACEP, FIDSA Professor of Medicine UCLA School of Medicine Director of Education Department of Emergency Medicine Olive View-UCLA Medical Center Sylmar, California
Antibiotic Abyss Fredrick M. Abrahamian, D.O., FACEP, FIDSA Professor of Medicine UCLA School of Medicine Director of Education Department of Emergency Medicine Olive View-UCLA Medical Center Sylmar, California
New Antibiotics for MRSA
 New Antibiotics for MRSA Faculty Warren S. Joseph, DPM, FIDSA Consultant, Lower Extremity Infectious Diseases Roxborough Memorial Hospital Philadelphia, Pennsylvania Faculty Disclosure Dr. Joseph: Speaker
New Antibiotics for MRSA Faculty Warren S. Joseph, DPM, FIDSA Consultant, Lower Extremity Infectious Diseases Roxborough Memorial Hospital Philadelphia, Pennsylvania Faculty Disclosure Dr. Joseph: Speaker
Epidemiology of early-onset bloodstream infection and implications for treatment
 Epidemiology of early-onset bloodstream infection and implications for treatment Richard S. Johannes, MD, MS Marlborough, Massachusetts Health care-associated infections: For over 35 years, infections
Epidemiology of early-onset bloodstream infection and implications for treatment Richard S. Johannes, MD, MS Marlborough, Massachusetts Health care-associated infections: For over 35 years, infections
Simplicef is Used to Treat Animals with Skin Infections
 Simplicef is Used to Treat Animals with Skin Infections PRODUCT INFO Simplicef tablets are a semi-synthetic cephalosporin antibiotic cefpodoxime proxetil used to cure infections caused by the susceptible
Simplicef is Used to Treat Animals with Skin Infections PRODUCT INFO Simplicef tablets are a semi-synthetic cephalosporin antibiotic cefpodoxime proxetil used to cure infections caused by the susceptible
Reduce the risk of recurrence Clear bacterial infections fast and thoroughly
 Reduce the risk of recurrence Clear bacterial infections fast and thoroughly Clearly advanced 140916_Print-Detailer_Englisch_V2_BAH-05-01-14-003_RZ.indd 1 23.09.14 16:59 In bacterial infections, bacteriological
Reduce the risk of recurrence Clear bacterial infections fast and thoroughly Clearly advanced 140916_Print-Detailer_Englisch_V2_BAH-05-01-14-003_RZ.indd 1 23.09.14 16:59 In bacterial infections, bacteriological
A Randomized, Double-Blinded Study for the Prevention of Exit Site Infections in Pediatric Peritoneal Dialysis Patients
 A Randomized, Double-Blinded Study for the Prevention of Exit Site Infections in Pediatric Peritoneal Dialysis Patients Joshua Zaritsky, MD PhD, Barbara Gales, RN, Georgina Ramos, and Isidro B. Salusky,
A Randomized, Double-Blinded Study for the Prevention of Exit Site Infections in Pediatric Peritoneal Dialysis Patients Joshua Zaritsky, MD PhD, Barbara Gales, RN, Georgina Ramos, and Isidro B. Salusky,
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES
 DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
DAYTON CHILDREN S HOSPITAL CLINICAL PRACTICE GUIDELINES DISCLAIMER: This Clinical Practice Guideline (CPG) generally describes a recommended course of treatment for patients with the identified health
CA-MRSA lesions: What works, what doesn t
 For mass reproduction, content licensing and permissions contact Dowden Health Media. FAMILY David McBride, MD University Student Health Services and the Department of Family Medicine, Boston University
For mass reproduction, content licensing and permissions contact Dowden Health Media. FAMILY David McBride, MD University Student Health Services and the Department of Family Medicine, Boston University
Standing Orders for the Treatment of Outpatient Peritonitis
 Standing Orders for the Treatment of Outpatient Peritonitis 1. Definition of Peritonitis: a. Cloudy effluent. b. WBC > 100 cells/mm3 with >50% polymorphonuclear (PMN) cells with minimum 2 hour dwell. c.
Standing Orders for the Treatment of Outpatient Peritonitis 1. Definition of Peritonitis: a. Cloudy effluent. b. WBC > 100 cells/mm3 with >50% polymorphonuclear (PMN) cells with minimum 2 hour dwell. c.
Skin Infections and Antibiotic Stewardship: Analysis of Emergency Department Prescribing Practices,
 Original Research Skin Infections and Antibiotic Stewardship: Analysis of Emergency Department Prescribing Practices, 2007-2010 Daniel J. Pallin, MD, MPH Carlos A. Camargo Jr, MD, DrPH Jeremiah D. Schuur,
Original Research Skin Infections and Antibiotic Stewardship: Analysis of Emergency Department Prescribing Practices, 2007-2010 Daniel J. Pallin, MD, MPH Carlos A. Camargo Jr, MD, DrPH Jeremiah D. Schuur,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Nuzyra) Reference Number: CP.PMN.## Effective Date: 11.20.18 Last Review Date: 02.19 Line of Business: Commercial, TBD HIM*, Medicaid Coding Implications Revision Log See Important Reminder
Clinical Policy: (Nuzyra) Reference Number: CP.PMN.## Effective Date: 11.20.18 Last Review Date: 02.19 Line of Business: Commercial, TBD HIM*, Medicaid Coding Implications Revision Log See Important Reminder
moxifloxacin intravenous, 400mg/250mL, solution for infusion (Avelox ) SMC No. (650/10) Bayer Schering
 moxifloxacin intravenous, 400mg/250mL, solution for infusion (Avelox ) SMC No. (650/10) Bayer Schering 05 November 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
moxifloxacin intravenous, 400mg/250mL, solution for infusion (Avelox ) SMC No. (650/10) Bayer Schering 05 November 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above
FM - Male, 38YO. MRSA nasal swab (+) Due to positive MRSA nasal swab test, patient will be continued on Vancomycin 1500mg IV q12 for MRSA treatment...
 Jillian O Keefe Doctor of Pharmacy Candidate 2016 September 15, 2015 FM - Male, 38YO HPI: Previously healthy male presents to ED febrile (102F) and in moderate distress ~2 weeks after getting a tattoo
Jillian O Keefe Doctor of Pharmacy Candidate 2016 September 15, 2015 FM - Male, 38YO HPI: Previously healthy male presents to ED febrile (102F) and in moderate distress ~2 weeks after getting a tattoo
Clinical Management of Skin and Soft Tissue Infections in the U.S. Emergency Departments
 Original Research Clinical Management of Skin and Soft Tissue Infections in the U.S. Emergency Departments Rakesh D. Mistry, MD, MS* Daniel J. Shapiro, BA Monika K. Goyal, MD Theoklis E. Zaoutis Jeffrey
Original Research Clinical Management of Skin and Soft Tissue Infections in the U.S. Emergency Departments Rakesh D. Mistry, MD, MS* Daniel J. Shapiro, BA Monika K. Goyal, MD Theoklis E. Zaoutis Jeffrey
11/22/2016. Antimicrobial Stewardship Update Disclosures. Outline. No conflicts of interest to disclose
 Antimicrobial Stewardship Update 2016 APIC-CI Conference November 17 th, 2016 Jay R. McDonald, MD Chief, ID Section VA St. Louis Health Care System Assistant Professor of medicine Washington University
Antimicrobial Stewardship Update 2016 APIC-CI Conference November 17 th, 2016 Jay R. McDonald, MD Chief, ID Section VA St. Louis Health Care System Assistant Professor of medicine Washington University
HEALTH SERVICES POLICY & PROCEDURE MANUAL
 PAGE 1 of 3 PURPOSE To assure that DOP inmates with Soft Tissue Infections are receiving high quality Primary Care for their infections and that the risk of infecting other inmates or staff is minimized.
PAGE 1 of 3 PURPOSE To assure that DOP inmates with Soft Tissue Infections are receiving high quality Primary Care for their infections and that the risk of infecting other inmates or staff is minimized.
Preventing and Responding to Antibiotic Resistant Infections in New Hampshire
 Preventing and Responding to Antibiotic Resistant Infections in New Hampshire Benjamin P. Chan, MD, MPH NH Dept. of Health & Human Services Division of Public Health Services May 23, 2017 To bring a greater
Preventing and Responding to Antibiotic Resistant Infections in New Hampshire Benjamin P. Chan, MD, MPH NH Dept. of Health & Human Services Division of Public Health Services May 23, 2017 To bring a greater
Source: Portland State University Population Research Center (
 Methicillin Resistant Staphylococcus aureus (MRSA) Surveillance Report 2010 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Health Authority Updated:
Methicillin Resistant Staphylococcus aureus (MRSA) Surveillance Report 2010 Oregon Active Bacterial Core Surveillance (ABCs) Office of Disease Prevention & Epidemiology Oregon Health Authority Updated:
Barriers to Intravenous Penicillin Use for Treatment of Nonmeningitis
 JCM Accepts, published online ahead of print on 7 July 2010 J. Clin. Microbiol. doi:10.1128/jcm.01012-10 Copyright 2010, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
JCM Accepts, published online ahead of print on 7 July 2010 J. Clin. Microbiol. doi:10.1128/jcm.01012-10 Copyright 2010, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
Staph Cases. Case #1
 Staph Cases Lisa Winston University of California, San Francisco San Francisco General Hospital Case #1 A 60 y.o. man with well controlled HIV and DM presents to clinic with ten days of redness and swelling
Staph Cases Lisa Winston University of California, San Francisco San Francisco General Hospital Case #1 A 60 y.o. man with well controlled HIV and DM presents to clinic with ten days of redness and swelling
Treatment Duration for Uncomplicated Community-Acquired Pneumonia: The Evidence in Support of 5 Days
 Treatment Duration for Uncomplicated Community-Acquired Pneumonia: The Evidence in Support of 5 Days Executive Summary National consensus guidelines created jointly by the Infectious Diseases Society of
Treatment Duration for Uncomplicated Community-Acquired Pneumonia: The Evidence in Support of 5 Days Executive Summary National consensus guidelines created jointly by the Infectious Diseases Society of
Appropriate Management of Common Pediatric Infections. Blaise L. Congeni M.D. Akron Children s Hospital Division of Pediatric Infectious Diseases
 Appropriate Management of Common Pediatric Infections Blaise L. Congeni M.D. Akron Children s Hospital Division of Pediatric Infectious Diseases It s all about the microorganism The common pathogens Viruses
Appropriate Management of Common Pediatric Infections Blaise L. Congeni M.D. Akron Children s Hospital Division of Pediatric Infectious Diseases It s all about the microorganism The common pathogens Viruses
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Appropriate antimicrobial therapy in HAP: What does this mean?
 Appropriate antimicrobial therapy in HAP: What does this mean? Jaehee Lee, M.D. Kyungpook National University Hospital, Korea KNUH since 1907 Presentation outline Empiric antimicrobial choice: right spectrum,
Appropriate antimicrobial therapy in HAP: What does this mean? Jaehee Lee, M.D. Kyungpook National University Hospital, Korea KNUH since 1907 Presentation outline Empiric antimicrobial choice: right spectrum,
Treating Rosacea in the Era of Bacterial Resistance. This presentation is sponsored by Galderma Laboratories, L.P.
 Treating Rosacea in the Era of Bacterial Resistance This presentation is sponsored by Galderma Laboratories, L.P. Lecture Discuss rosacea as an inflammatory condition Assess the psychosocial impact of
Treating Rosacea in the Era of Bacterial Resistance This presentation is sponsored by Galderma Laboratories, L.P. Lecture Discuss rosacea as an inflammatory condition Assess the psychosocial impact of
Suitability of Antibiotic Treatment for CAP (CAPTIME) The duration of antibiotic treatment in community acquired pneumonia (CAP)
 STUDY PROTOCOL Suitability of Antibiotic Treatment for CAP (CAPTIME) Purpose The duration of antibiotic treatment in community acquired pneumonia (CAP) lasts about 9 10 days, and is determined empirically.
STUDY PROTOCOL Suitability of Antibiotic Treatment for CAP (CAPTIME) Purpose The duration of antibiotic treatment in community acquired pneumonia (CAP) lasts about 9 10 days, and is determined empirically.
4/3/2017 CLINICAL PEARLS: UPDATES IN THE MANAGEMENT OF NOSOCOMIAL PNEUMONIA DISCLOSURE LEARNING OBJECTIVES
 CLINICAL PEARLS: UPDATES IN THE MANAGEMENT OF NOSOCOMIAL PNEUMONIA BILLIE BARTEL, PHARMD, BCCCP APRIL 7 TH, 2017 DISCLOSURE I have had no financial relationship over the past 12 months with any commercial
CLINICAL PEARLS: UPDATES IN THE MANAGEMENT OF NOSOCOMIAL PNEUMONIA BILLIE BARTEL, PHARMD, BCCCP APRIL 7 TH, 2017 DISCLOSURE I have had no financial relationship over the past 12 months with any commercial
Author - Dr. Josie Traub-Dargatz
 Author - Dr. Josie Traub-Dargatz Dr. Josie Traub-Dargatz is a professor of equine medicine at Colorado State University (CSU) College of Veterinary Medicine and Biomedical Sciences. She began her veterinary
Author - Dr. Josie Traub-Dargatz Dr. Josie Traub-Dargatz is a professor of equine medicine at Colorado State University (CSU) College of Veterinary Medicine and Biomedical Sciences. She began her veterinary
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Bennett-Guerrero E, Pappas TN, Koltun WA, et al. Gentamicin
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Bennett-Guerrero E, Pappas TN, Koltun WA, et al. Gentamicin
New Antibiotics & New Insights into Old Antibiotics
 New Antibiotics & New Insights into Old Antibiotics Louisiana Chapter of the American Academy of Pediatrics August 18, 2018 Baton Rouge, Louisiana John Bradley MD Rady Children s Hospital San Diego University
New Antibiotics & New Insights into Old Antibiotics Louisiana Chapter of the American Academy of Pediatrics August 18, 2018 Baton Rouge, Louisiana John Bradley MD Rady Children s Hospital San Diego University
Duration of antibiotic therapy:
 Duration of antibiotic therapy: How low can you go? Thomas Holland, MD Hilton Head, SC July 2017 Disclosures Consulting: The Medicines Company, Basilea Pharmaceutica Adjudication committee: Achaogen Grant
Duration of antibiotic therapy: How low can you go? Thomas Holland, MD Hilton Head, SC July 2017 Disclosures Consulting: The Medicines Company, Basilea Pharmaceutica Adjudication committee: Achaogen Grant
Antimicrobial Stewardship Strategy: Antibiograms
 Antimicrobial Stewardship Strategy: Antibiograms A summary of the cumulative susceptibility of bacterial isolates to formulary antibiotics in a given institution or region. Its main functions are to guide
Antimicrobial Stewardship Strategy: Antibiograms A summary of the cumulative susceptibility of bacterial isolates to formulary antibiotics in a given institution or region. Its main functions are to guide
Antibacterial Resistance: Research Efforts. Henry F. Chambers, MD Professor of Medicine University of California San Francisco
 Antibacterial Resistance: Research Efforts Henry F. Chambers, MD Professor of Medicine University of California San Francisco Resistance Resistance Dose-Response Curve Antibiotic Exposure Anti-Resistance
Antibacterial Resistance: Research Efforts Henry F. Chambers, MD Professor of Medicine University of California San Francisco Resistance Resistance Dose-Response Curve Antibiotic Exposure Anti-Resistance
Inappropriate Use of Antibiotics and Clostridium difficile Infection. Jocelyn Srigley, MD, FRCPC November 1, 2012
 Inappropriate Use of Antibiotics and Clostridium difficile Infection Jocelyn Srigley, MD, FRCPC November 1, 2012 Financial Disclosures } No conflicts of interest } The study was supported by a Hamilton
Inappropriate Use of Antibiotics and Clostridium difficile Infection Jocelyn Srigley, MD, FRCPC November 1, 2012 Financial Disclosures } No conflicts of interest } The study was supported by a Hamilton
Le infezioni di cute e tessuti molli
 Le infezioni di cute e tessuti molli SCELTE e STRATEGIE TERAPEUTICHE Pierluigi Viale Clinica di Malattie Infettive Policlinico S. Orsola Malpighi Treatment of complicated skin and skin structure infections
Le infezioni di cute e tessuti molli SCELTE e STRATEGIE TERAPEUTICHE Pierluigi Viale Clinica di Malattie Infettive Policlinico S. Orsola Malpighi Treatment of complicated skin and skin structure infections
Newsflash: Hospital Medicine JOHN C. CHRISTENSEN, MD FACP AMERICAN COLLEGE OF PHYSICIANS, UTAH CHAPTER SCIENTIFIC MEETING FEBRUARY 10, 2017
 Newsflash: Hospital Medicine JOHN C. CHRISTENSEN, MD FACP AMERICAN COLLEGE OF PHYSICIANS, UTAH CHAPTER SCIENTIFIC MEETING FEBRUARY 10, 2017 Newsflash: Fluoroquinolones Newsflash: Fluoroquinolones Don t
Newsflash: Hospital Medicine JOHN C. CHRISTENSEN, MD FACP AMERICAN COLLEGE OF PHYSICIANS, UTAH CHAPTER SCIENTIFIC MEETING FEBRUARY 10, 2017 Newsflash: Fluoroquinolones Newsflash: Fluoroquinolones Don t
B. PACKAGE LEAFLET 1
 B. PACKAGE LEAFLET 1 PACKAGE LEAFLET NICILAN 400 mg/100 mg tablets for dogs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
B. PACKAGE LEAFLET 1 PACKAGE LEAFLET NICILAN 400 mg/100 mg tablets for dogs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH
Who should read this document? 2. Key practice points 2. Background/ Scope/ Definitions 2. What is new in this version? 3
 Neurosurgical infections (adult only) Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): as above Authors Division: DCSS & Tertiary
Neurosurgical infections (adult only) Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): as above Authors Division: DCSS & Tertiary
Standing Orders for the Treatment of Outpatient Peritonitis
 Standing Orders for the Treatment of Outpatient Peritonitis 1. Definition of Peritonitis: a. Cloudy effluent. b. WBC > 100 cells/mm3 with >50% polymorphonuclear (PMN) cells with minimum 2 hour dwell. c.
Standing Orders for the Treatment of Outpatient Peritonitis 1. Definition of Peritonitis: a. Cloudy effluent. b. WBC > 100 cells/mm3 with >50% polymorphonuclear (PMN) cells with minimum 2 hour dwell. c.
Replaces:04/14/16. Formulated: 1997 SKIN AND SOFT TISSUE INFECTION
 Effective Date: 04/13/17 Replaces:04/14/16 Page 1 of 7 POLICY To standardize the clinical management and housing of offenders with skin and soft tissue infections, thereby reducing the transmission and
Effective Date: 04/13/17 Replaces:04/14/16 Page 1 of 7 POLICY To standardize the clinical management and housing of offenders with skin and soft tissue infections, thereby reducing the transmission and
An Approach to Appropriate Antibiotic Prescribing in Outpatient and LTC Settings?
 An Approach to Appropriate Antibiotic Prescribing in Outpatient and LTC Settings? Dr. Andrew Morris Antimicrobial Stewardship ProgramMt. Sinai Hospital University Health Network amorris@mtsinai.on.ca andrew.morris@uhn.ca
An Approach to Appropriate Antibiotic Prescribing in Outpatient and LTC Settings? Dr. Andrew Morris Antimicrobial Stewardship ProgramMt. Sinai Hospital University Health Network amorris@mtsinai.on.ca andrew.morris@uhn.ca
against Clinical Isolates of Gram-Positive Bacteria
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 993, p. 366-370 Vol. 37, No. 0066-0/93/00366-05$0.00/0 Copyright 993, American Society for Microbiology In Vitro Activity of CP-99,9, a New Fluoroquinolone,
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Feb. 993, p. 366-370 Vol. 37, No. 0066-0/93/00366-05$0.00/0 Copyright 993, American Society for Microbiology In Vitro Activity of CP-99,9, a New Fluoroquinolone,
Synopsis. Takeda Pharmaceutical Company Limited Name of the finished product UNISIA Combination Tablets LD, UNISIA Combination Tablets
 Synopsis Name of the sponsor Takeda Pharmaceutical Company Limited Name of the finished product UNISIA Combination Tablets LD, UNISIA Combination Tablets Name of active ingredient Title of the study Study
Synopsis Name of the sponsor Takeda Pharmaceutical Company Limited Name of the finished product UNISIA Combination Tablets LD, UNISIA Combination Tablets Name of active ingredient Title of the study Study
The CARI Guidelines Caring for Australians with Renal Impairment. 10. Treatment of peritoneal dialysis associated fungal peritonitis
 10. Treatment of peritoneal dialysis associated fungal peritonitis Date written: February 2003 Final submission: July 2004 Guidelines (Include recommendations based on level I or II evidence) The use of
10. Treatment of peritoneal dialysis associated fungal peritonitis Date written: February 2003 Final submission: July 2004 Guidelines (Include recommendations based on level I or II evidence) The use of
Adequacy of Early Empiric Antibiotic Treatment and Survival in Severe Sepsis: Experience from the MONARCS Trial
 BRIEF REPORT Adequacy of Early Empiric Antibiotic Treatment and Survival in Severe Sepsis: Experience from the MONARCS Trial Rodger D. MacArthur, 1 Mark Miller, 2 Timothy Albertson, 3 Edward Panacek, 3
BRIEF REPORT Adequacy of Early Empiric Antibiotic Treatment and Survival in Severe Sepsis: Experience from the MONARCS Trial Rodger D. MacArthur, 1 Mark Miller, 2 Timothy Albertson, 3 Edward Panacek, 3
UPDATE ON ANTIMICROBIAL STEWARDSHIP REGULATIONS AND IMPLEMENTATION OF AN AMS PROGRAM
 UPDATE ON ANTIMICROBIAL STEWARDSHIP REGULATIONS AND IMPLEMENTATION OF AN AMS PROGRAM Diane Rhee, Pharm.D. Associate Professor of Pharmacy Practice Roseman University of Health Sciences Chair, Valley Health
UPDATE ON ANTIMICROBIAL STEWARDSHIP REGULATIONS AND IMPLEMENTATION OF AN AMS PROGRAM Diane Rhee, Pharm.D. Associate Professor of Pharmacy Practice Roseman University of Health Sciences Chair, Valley Health
CARBAPENEM RESISTANT ENTEROBACTERIACEAE (KPC CRE)
 CARBAPENEM RESISTANT ENTEROBACTERIACEAE (KPC CRE) Bartsch SM et al. Potential economic burden of carbapenem-resistent Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect 2017;23(1):48e9-e16.
CARBAPENEM RESISTANT ENTEROBACTERIACEAE (KPC CRE) Bartsch SM et al. Potential economic burden of carbapenem-resistent Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect 2017;23(1):48e9-e16.
SIVEXTRO (tedizolid phosphate) oral tablet ZYVOX (linezolid) oral suspension and tablet
 ZYVOX (linezolid) oral suspension and tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
ZYVOX (linezolid) oral suspension and tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
Pharmacist Coordinated Antimicrobial Therapy: OPAT and Transitions of Care
 Pharmacist Coordinated Antimicrobial Therapy: OPAT and Transitions of Care Jennifer McCann, PharmD, BCCCP State Director of Clinical Pharmacy Services St. Vincent Health Indiana Conflicts of Interest No
Pharmacist Coordinated Antimicrobial Therapy: OPAT and Transitions of Care Jennifer McCann, PharmD, BCCCP State Director of Clinical Pharmacy Services St. Vincent Health Indiana Conflicts of Interest No
SKIN AND SOFT TISSUE INFECTIONS OCTOBER 3-4, 2015
 SKIN AND SOFT TISSUE INFECTIONS OCTOBER 3-4, 2015 Disclosures I have no financial conflicts of interest to disclose or report. Steven Tran, PharmD NEFSHP Fall Meeting 2015 Objectives for Pharmacists Review
SKIN AND SOFT TISSUE INFECTIONS OCTOBER 3-4, 2015 Disclosures I have no financial conflicts of interest to disclose or report. Steven Tran, PharmD NEFSHP Fall Meeting 2015 Objectives for Pharmacists Review
SUMMARY OF PRODUCT CHARACTERISTICS. Cephacare flavour 50 mg tablets for cats and dogs. Excipients: For a full list of excipients, see section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Cephacare flavour 50 mg tablets for cats and dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Cephacare flavour 50 mg tablets for cats and dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active
Understanding the Hospital Antibiogram
 Understanding the Hospital Antibiogram Sharon Erdman, PharmD Clinical Professor Purdue University College of Pharmacy Infectious Diseases Clinical Pharmacist Eskenazi Health 5 Understanding the Hospital
Understanding the Hospital Antibiogram Sharon Erdman, PharmD Clinical Professor Purdue University College of Pharmacy Infectious Diseases Clinical Pharmacist Eskenazi Health 5 Understanding the Hospital
Delayed Prescribing for Minor Infections Resource Pack for Prescribers
 Delayed Prescribing for Minor Infections Resource Pack for Prescribers Background: Antibiotic resistance is an alarming threat to modern healthcare, and infectious illness remains a major global threat
Delayed Prescribing for Minor Infections Resource Pack for Prescribers Background: Antibiotic resistance is an alarming threat to modern healthcare, and infectious illness remains a major global threat
Concise Antibiogram Toolkit Background
 Background This toolkit is designed to guide nursing homes in creating their own antibiograms, an important tool for guiding empiric antimicrobial therapy. Information about antibiograms and instructions
Background This toolkit is designed to guide nursing homes in creating their own antibiograms, an important tool for guiding empiric antimicrobial therapy. Information about antibiograms and instructions
Srirupa Das, Associate Director, Medical Affairs, Tushar Fegade, Manager, Clinical Research Abbott Healthcare Private Limited, Mumbai.
 Indian Medical Gazette JUNE 2015 225 Comparative A Randomized, Open Label, Prospective, Comparative Evaluating the Efficacy and Safety of Fixed Dose Combination of Cefpodoxime 200 Mg + Clavulanic Acid
Indian Medical Gazette JUNE 2015 225 Comparative A Randomized, Open Label, Prospective, Comparative Evaluating the Efficacy and Safety of Fixed Dose Combination of Cefpodoxime 200 Mg + Clavulanic Acid
Aberdeen Hospital. Antibiotic Susceptibility Patterns For Commonly Isolated Organisms For 2015
 Aberdeen Hospital Antibiotic Susceptibility Patterns For Commonly Isolated s For 2015 Services Laboratory Microbiology Department Aberdeen Hospital Nova Scotia Health Authority 835 East River Road New
Aberdeen Hospital Antibiotic Susceptibility Patterns For Commonly Isolated s For 2015 Services Laboratory Microbiology Department Aberdeen Hospital Nova Scotia Health Authority 835 East River Road New
Short Course Antibiotic Therapy of Streptococcal Pharyngitis: Comparison of Clarithromycin with Amoxicillin/ Clavulanate and Cefuroxime Axetil
 Short Course Antibiotic Therapy of Streptococcal Pharyngitis: Comparison of Clarithromycin with Amoxicillin/ Clavulanate and Cefuroxime Axetil D. Adam, MD PhD, Munich, Germany H. Scholz, MD PhD, Berlin,
Short Course Antibiotic Therapy of Streptococcal Pharyngitis: Comparison of Clarithromycin with Amoxicillin/ Clavulanate and Cefuroxime Axetil D. Adam, MD PhD, Munich, Germany H. Scholz, MD PhD, Berlin,
Randomized Controlled Trial on Adjunctive Lavage for Severe Peritoneal Dialysis- Related Peritonitis
 Randomized Controlled Trial on Adjunctive Lavage for Severe Peritoneal Dialysis- Related Peritonitis Steve SM Wong Alice Ho Miu Ling Nethersole Hospital Background PD peritonitis is a major cause of PD
Randomized Controlled Trial on Adjunctive Lavage for Severe Peritoneal Dialysis- Related Peritonitis Steve SM Wong Alice Ho Miu Ling Nethersole Hospital Background PD peritonitis is a major cause of PD
The CARI Guidelines Caring for Australians with Renal Impairment. 8. Prophylactic antibiotics for insertion of peritoneal dialysis catheter
 8. Prophylactic antibiotics for insertion of peritoneal dialysis catheter Date written: February 2003 Final submission: May 2004 Guidelines (Include recommendations based on level I or II evidence) Antibiotic
8. Prophylactic antibiotics for insertion of peritoneal dialysis catheter Date written: February 2003 Final submission: May 2004 Guidelines (Include recommendations based on level I or II evidence) Antibiotic
Summary Report Relating to a Pilot Program to Require Reporting of Methicillin-resistant Staphylococcus aureus
 Summary Report Relating to a Pilot Program to Require Reporting of Methicillin-resistant Staphylococcus aureus Prepared by the Texas Department of State Health Services as required by House Bill 1082,
Summary Report Relating to a Pilot Program to Require Reporting of Methicillin-resistant Staphylococcus aureus Prepared by the Texas Department of State Health Services as required by House Bill 1082,
Does Screening for MRSA Colonization Have A Role In Healthcare-Associated Infection Prevention Programs?
 Does Screening for MRSA Colonization Have A Role In Healthcare-Associated Infection Prevention Programs? John A. Jernigan, MD, MS Division of Healthcare Quality Promotion Centers for Disease Control and
Does Screening for MRSA Colonization Have A Role In Healthcare-Associated Infection Prevention Programs? John A. Jernigan, MD, MS Division of Healthcare Quality Promotion Centers for Disease Control and
Perichondritis: Source: UpToDate Ciprofloxacin 10 mg/kg/dose PO (max 500 mg/dose) BID Inpatient: Ceftazidime 50 mg/kg/dose q8 hours IV
 Empiric Antibiotics for Pediatric Infections Seen in ED NOTE: Choice of empiric antibiotic therapy must take into account local pathogen frequency and resistance patterns, individual patient characteristics,
Empiric Antibiotics for Pediatric Infections Seen in ED NOTE: Choice of empiric antibiotic therapy must take into account local pathogen frequency and resistance patterns, individual patient characteristics,
The Nuts and Bolts of Antibiograms in Long-Term Care Facilities
 The Nuts and Bolts of Antibiograms in Long-Term Care Facilities J. Kristie Johnson, Ph.D., D(ABMM) Professor, Department of Pathology University of Maryland School of Medicine Director, Microbiology Laboratories
The Nuts and Bolts of Antibiograms in Long-Term Care Facilities J. Kristie Johnson, Ph.D., D(ABMM) Professor, Department of Pathology University of Maryland School of Medicine Director, Microbiology Laboratories
Critical Appraisal Topic. Antibiotic Duration in Acute Otitis Media in Children. Carissa Schatz, BSN, RN, FNP-s. University of Mary
 Running head: ANTIBIOTIC DURATION IN AOM 1 Critical Appraisal Topic Antibiotic Duration in Acute Otitis Media in Children Carissa Schatz, BSN, RN, FNP-s University of Mary 2 Evidence-Based Practice: Critical
Running head: ANTIBIOTIC DURATION IN AOM 1 Critical Appraisal Topic Antibiotic Duration in Acute Otitis Media in Children Carissa Schatz, BSN, RN, FNP-s University of Mary 2 Evidence-Based Practice: Critical
ANTIMICROBIAL STEWARDSHIP: ADVANCING PATIENT CARE BY IMPROVING MEDICATION USE
 ANTIMICROBIAL STEWARDSHIP: ADVANCING PATIENT CARE BY IMPROVING MEDICATION USE ANTHONY M. CASAPAO, PHARM.D. ASSISTANT PROFESSOR OF PHARMACY PRACTICE HUSSON UNIVERSITY SCHOOL OF PHARMACY CLINICAL INFECTIOUS
ANTIMICROBIAL STEWARDSHIP: ADVANCING PATIENT CARE BY IMPROVING MEDICATION USE ANTHONY M. CASAPAO, PHARM.D. ASSISTANT PROFESSOR OF PHARMACY PRACTICE HUSSON UNIVERSITY SCHOOL OF PHARMACY CLINICAL INFECTIOUS
Antibiotic Prophylaxis in Spinal Surgery Antibiotic Guidelines. Contents
 Antibiotic Prophylaxis in Spinal Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): Authors Division: DCSS & Tertiary Medicine Unique
Antibiotic Prophylaxis in Spinal Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Antibiotic Steering Committee Additional author(s): Authors Division: DCSS & Tertiary Medicine Unique
Cefazolin vs. Antistaphyloccal Penicillins: The Great Debate
 Cefazolin vs. Antistaphyloccal Penicillins: The Great Debate Annie Heble, PharmD PGY2 Pediatric Pharmacy Resident Children s Hospital Colorado Microbiology Rounds March 22, 2017 Image Source: Buck cartoons
Cefazolin vs. Antistaphyloccal Penicillins: The Great Debate Annie Heble, PharmD PGY2 Pediatric Pharmacy Resident Children s Hospital Colorado Microbiology Rounds March 22, 2017 Image Source: Buck cartoons
Methicillin Resistant Staphylococcus Aureus (MRSA) The drug resistant `Superbug that won t die
 Methicillin Resistant Staphylococcus Aureus (MRSA) The drug resistant `Superbug that won t die Michael A. Miller, MD Assistant Professor of Pediatrics -Jacksonville OBJECTIVES 1. Understand the basic microbiology
Methicillin Resistant Staphylococcus Aureus (MRSA) The drug resistant `Superbug that won t die Michael A. Miller, MD Assistant Professor of Pediatrics -Jacksonville OBJECTIVES 1. Understand the basic microbiology
BACTERIAL SUSCEPTIBILITY REPORT: 2016 (January 2016 December 2016)
 BACTERIAL SUSCEPTIBILITY REPORT: 2016 (January 2016 December 2016) VA Palo Alto Health Care System April 14, 2017 Trisha Nakasone, PharmD, Pharmacy Service Russell Ryono, PharmD, Public Health Surveillance
BACTERIAL SUSCEPTIBILITY REPORT: 2016 (January 2016 December 2016) VA Palo Alto Health Care System April 14, 2017 Trisha Nakasone, PharmD, Pharmacy Service Russell Ryono, PharmD, Public Health Surveillance
Active Bacterial Core Surveillance Site and Epidemiologic Classification, United States, 2005a. Copyright restrictions may apply.
 Impact of routine surgical ward and intensive care unit admission surveillance cultures on hospital-wide nosocomial methicillin-resistant Staphylococcus aureus infections in a university hospital: an interrupted
Impact of routine surgical ward and intensive care unit admission surveillance cultures on hospital-wide nosocomial methicillin-resistant Staphylococcus aureus infections in a university hospital: an interrupted
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Cellulitis and Abscess: ED Phase v 1.1
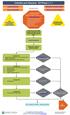 Cellulitis and Abscess: ED Phase v 1.1 Executive Summary Test Your Knowledge PHASE I (E.D.) Explanation of Evidence Ratings Summary of Version Changes! Labs if systemic illness or necrotizing fasciitis
Cellulitis and Abscess: ED Phase v 1.1 Executive Summary Test Your Knowledge PHASE I (E.D.) Explanation of Evidence Ratings Summary of Version Changes! Labs if systemic illness or necrotizing fasciitis
Antibiotic Susceptibility Patterns of Community-Acquired Urinary Tract Infection Isolates from Female Patients on the US (Texas)- Mexico Border
 Antibiotic Susceptibility Patterns of Community-Acquired Urinary Tract Infection Isolates from Female Patients on the US (Texas)- Mexico Border Yvonne Vasquez, MPH W. Lee Hand, MD Department of Research
Antibiotic Susceptibility Patterns of Community-Acquired Urinary Tract Infection Isolates from Female Patients on the US (Texas)- Mexico Border Yvonne Vasquez, MPH W. Lee Hand, MD Department of Research
Objectives 4/26/2017. Co-Investigators Sadie Giuliani, PharmD, BCPS Claude Tonnerre, MD Jayme Hartzell, PharmD, MS, BCPS
 IMPLEMENTATION AND ASSESSMENT OF A GUIDELINE-BASED TREATMENT ALGORITHM FOR COMMUNITY-ACQUIRED PNEUMONIA (CAP) Lucas Schonsberg, PharmD PGY-1 Pharmacy Practice Resident Providence St. Patrick Hospital Missoula,
IMPLEMENTATION AND ASSESSMENT OF A GUIDELINE-BASED TREATMENT ALGORITHM FOR COMMUNITY-ACQUIRED PNEUMONIA (CAP) Lucas Schonsberg, PharmD PGY-1 Pharmacy Practice Resident Providence St. Patrick Hospital Missoula,
4 th and 5 th generation cephalosporins. Naderi HR Associate professor of Infectious Diseases
 4 th and 5 th generation cephalosporins Naderi HR Associate professor of Infectious Diseases Classification Forth generation: Cefclidine, cefepime (Maxipime),cefluprenam, cefoselis,cefozopran, cefpirome
4 th and 5 th generation cephalosporins Naderi HR Associate professor of Infectious Diseases Classification Forth generation: Cefclidine, cefepime (Maxipime),cefluprenam, cefoselis,cefozopran, cefpirome
