Veterinary Parasitology
|
|
|
- Elizabeth Parrish
- 5 years ago
- Views:
Transcription
1 Veterinary Parasitology 193 (2013) Contents lists available at SciVerse ScienceDirect Veterinary Parasitology jo u rn al hom epa ge : Toxocara spp. infections in paratenic hosts Christina Strube, Lea Heuer, Elisabeth Janecek Institute for Parasitology, University of Veterinary Medicine Hannover, Buenteweg 17, Hannover, Germany a r t i c l e i n f o Article history: In memoriam Thomas Schnieder. Keywords: Toxocara cati Toxocara canis Somatic migration Visceral larva migrans Ocular larva migrans Paratenic host Zoonosis a b s t r a c t The zoonotic roundworms Toxocara canis and T. cati are not only present worldwide in their definitive hosts; they also frequently occur in other animal species, including humans. In those so-called paratenic hosts, the larvae do not develop into the adult stage, but rather migrate throughout the somatic tissue and persist as infectious L3 stage for extensive periods. Those arrested larvae may lead to severe inflammatory reactions and consequently to a wide range of pathological and clinical manifestations. However, the infected paratenic hosts also constitute a potential source of infection for the definitive hosts or humans who may also function as paratenic hosts. In the present review, current knowledge of larval migration in a variety of possible paratenic hosts is summarized including variations of migration routes and susceptibilities. Furthermore, information about the clinical and pathological changes for the presented species and possible consequences of the somatic migration of larvae, i.e. the resulting tissue damage as well as adverse host reactions to arrested larvae are reviewed. There are still many questions unanswered regarding larval behaviour in hosts other than their definitive host. Therefore, it is of great importance to continue further elaboration on the biology of Toxocara spp. to prevent further spreading of larvae in both the paratenic and the definitive host Elsevier B.V. Open access under CC BY license. 1. Introduction Toxocara canis (Werner, 1782) and T. cati (Schrank 1788) are usually gastrointestinal helminths in canids and felids, respectively. Up to 200,000 eggs may be produced by the female Toxocara spp. worm which are shed into the environment and raise the potential infection risk (Glickman and Schantz, 1981). Even though dogs and cats are the definitive host, larvae may also persist or even cause severe disease in a variety of paratenic host species. Hatching larvae behave similarly in the paratenic host compared to the definitive host, however, development into the adult stage does not occur and infectious third-stage larvae (Brunaská et al., 1995) persist in tissues as a developmentally arrested stage. Their importance as zoonotic agents should not be underestimated since toxocarosis is one of the most Corresponding author. Tel.: ; fax: address: christina.strube@tiho-hannover.de (C. Strube). reported zoonotic helminth infections worldwide and has therefore led to an increasing interest in the biology and epidemiology of the genus Toxocara (Magnaval et al., 2001). Prevalence of Toxocara spp. in either host depends on the environmental as well as the hygienic conditions in the surroundings. When shed into the environment by the definitive host, eggs are not infective and take about 3 6 weeks to embryonate and become infectious (Overgaauw, 1997b). Infection risk increases with geophagia or soil eating as well as poor personal hygiene. Eating raw garden vegetables may also lead to infection if contamination through the definitive host has occurred (Magnaval et al., 2001). Human seroprevalence in European countries, namely France, the Czech Republic and Austria, ranges from 2% to 44% with higher values in rural areas (Magnaval et al., 1994a; Uhlíková and Hübner, 1998; Deutz et al., 2005). In tropical countries, seroprevalence ranges from 63% in Bali, Indonesia, (Chomel et al., 1993) to almost 93% in La Réunion, France, (Magnaval et al., 1994b) highlighting the need for prevention of transmission. Parks and playgrounds Elsevier B.V. Open access under CC BY license.
2 376 C. Strube et al. / Veterinary Parasitology 193 (2013) are a potential infection risk for transmission of eggs due to contamination by people routinely walking their pets in those areas. Additionally, stray cats and dogs may also contribute to contamination as well as fox populations in urban and rural areas (Castillo et al., 2000; Reperant et al., 2007). Toxocara canis eggs are mostly found in public parks, whereas T. cati eggs are commonly found in sand boxes (Jansen et al., 1993). To date, it is not clear whether T. cati exhibits the same zoonotic risk as T. canis, since most infections found in humans are considered to be caused by T. canis. However, antigenic fractions are shared and experimentally infected animal models show that both species behave in a similar way after hatching. Therefore, it can be assumed that the zoonotic risk and the behaviour in paratenic hosts are comparable (Fisher, 2003; Cardillo et al., 2009). The risk of environmental contamination increases with infected definitive hosts shedding eggs into the environment. Infection rates in Europe range from 3.5% to 34% T. canis-infected dogs from different environments (e.g. pets, dogs from animal shelters or stray dogs) and from 8% to 76% T. cati-infected cats (Parsons, 1987; Overgaauw, 1997c; Fok et al., 2001; Lee et al., 2010). Prevalences, especially in stray animals, highlight the need to understand the biology of this parasite in hosts other than the definitive one. Since not only humans may take up eggs from the environment, but a number of other animals as well, infection risk rises. Even though paratenic hosts do not shed eggs into the environment, they may be preyed upon by the definitive hosts in which the arrested larvae are reactivated and continue development into egg laying adults. In this review, current literature regarding different paratenic hosts, larval distribution and the pathological changes as well as the impact on the host by T. canis in contrast to T. cati will be discussed in detail. 2. Larval migration 2.1. Larval migration in the definitive host In general, T. canis or T. cati larvae hatch 2 4 h after ingestion of embryonated eggs in the duodenum, where they penetrate the intestinal walls to enter the circulatory system. The liver is reached about 24 h post infection via portal circulation through venous capillaries (Webster, 1958b). About 12 h later, larvae continue migration to the heart where the lung is reached via the pulmonary artery. From there on the migration route strongly depends on several factors such as the age and the immune status of the host as well as the infectious dose ingested. Larvae may penetrate the alveoli wall leading to migration to the pharynx through bronchioles and trachea. After subsequent swallowing, larvae start developing into adult worms in the intestine, which they reach about 7 15 days after tracheal migration (Sprent, 1958). Another possibility of migration from the lung is entering the circulatory system again after penetration of the alveoli walls, in which case larvae are passively distributed to the somatic tissue (Sprent, 1956; Webster, 1958a,b). Larvae, which have been taken up with an infected paratenic host, are also able to develop directly, probably due to a higher degree of maturity (Overgaauw, 1997b). They may therefore exhibit aberrations in their migration patterns, i.e. a lack of tracheal migration. Nevertheless, normal migration routes have been described after consumption of an infected paratenic host (Warren, 1969) Larval migration in the paratenic host In general, the migration pattern in the paratenic host is comparable to the one in the definitive host. However, larval distribution largely depends on the infected species. Commonly, larvae hatch after consumption, penetrate the intestinal wall and during the so-called hepato-pulmonary phase migrate via circulatory system to the liver and then to the lungs. From there, they continue migration into the systemic circulation. During the visceral phase they are distributed throughout the entire body according to species-dependent predilection sites (Abo-Shehada and Herbert, 1984a; Magnaval et al., 2001). Afterwards they persist in the tissue as the infectious stage for extended periods of time (Sprent, 1952). As encapsulated larvae, they remain viable for up to 10 years (Fig. 1). This way they may continue their life cycle at prolonged periods after infection if the paratenic host is consumed by the definitive host (Beaver, 1969; Maruyama et al., 1994; Oryan et al., 2010; Taira et al., 2011). Migration routes as well as predilection sites depend on the host species, however nearly all organs may be affected with varying degrees of larval burdens. In the paratenic host, T. canis seems to exhibit an affinity to the CNS whereas the brain and the eyes are commonly infected at later time point post infection. Toxocara cati shows similar migration behaviour towards the CNS but seems to migrate more slowly (Burren, 1972; Glickman and Summers, 1983; Alba- Hurtado et al., 2000; Akao et al., 2003a). It has been suggested that the degree of blood supply of the different organs plays a role in larval distribution, since larvae are entering the circulatory system and are distributed into the liver that way. Most larvae remain there, but some actively leave the liver. There have been attempts to explain the greater neuroaffinity of T. canisas opposed to T. cati larvae by their size difference. As T. Fig. 1. Toxocara cati larva recovered from the muscle of a mouse after artificial digestion (Institute for Parasitology, archive).
3 C. Strube et al. / Veterinary Parasitology 193 (2013) cati larvae are smaller, they may therefore be able to leave arteries more easily, while T. canis larvae are less likely to leave the circulatory system and finally remain arrested in the brain (Bisseru, 1969; Dunsmore et al., 1983). Possibly, clinical consequences of this neuroaffinity, such as behaviour alterations resulting in an increased probability of being consumed by canid or felid species, may serve as an evolutionary explanation for the parasite s migration route (Olson and Rose, 1966; Cox and Holland, 1998; Hamilton et al., 2006; Chieffi et al., 2010). 3. Paratenic hosts The influence of migrating Toxocara spp. larvae, mainly T. canis, in paratenic hosts has been intensively studied in a variety of animal models. These models mainly serve as a potential comparison to human toxocarosis and give valuable hints to host-parasite interactions. However, the possibility of severe differences between the animal model and human infection biology should not be underestimated (Boes and Helwigh, 2000). Some animal models are more suitable than others, depending on their susceptibility. Organ affection by migrating larvae, effects at time points post infection, as well as the relative distribution and survival, are host species dependent. The most important animal models are described in detail below Rodents Several rodents are used as animal models for toxocarosis, the mouse being the most common. However, rats and gerbils have also been shown to be very useful in the study of larval migration, behavioural changes, as well as the pathology of migrating larvae Mouse strains Different mouse strains exhibit a wide range of susceptibility with some strains being resistant and some retaining high larval counts in somatic tissues. Several studies have been conducted to investigate larval distribution and strain dependent differences. There are several differences between inbred and outbred mouse strains. Furthermore, larval counts are highly dependent on inoculation dose and the day post infection (Kayes et al., 1985; Havasiová-Reiterová et al., 1995; Cox and Holland, 1998; Ollero et al., 2008). In general, the migratory behaviour consists of two phases, namely the hepato-pulmonary phase and the myotropic-neurotropic phase. The first one occurs during the first week post infection when larvae reach liver and lungs, the latter when larvae migrate through the body and accumulate in the carcass and the brain (Abo-Shehada and Herbert, 1984a). After distribution of the larvae, they arrest in the tissue and persist as the infectious stage for at least one year (Sprent, 1952; Burren, 1968). Havasiová-Reiterová et al. (1995) directly compared both roundworm species in C57Bl6/J mice and observed that low infection doses (5 500 eggs) resulted in similar distribution of both Toxocara species. None of the T. canisinfected mice with those low doses presented larvae in the liver. With the high infection dose (1000 eggs), only one larva could be found in the liver and most were found in the brain and in the muscle at day 70 p.i. T. cati, in comparison, was mostly found in the muscle and did not seem to accumulate in the brain, indicating a different migration route (Havasiová-Reiterová et al., 1995). The same phenomenon could be observed by other authors (Bisseru, 1969; Piergili Fioretti et al., 1989; Camparoto et al., 2008). Direct comparison of both species showed that T. cati did not penetrate the brain, leading to the conclusion that T. canis is the causal agent of human neurotoxocarosis. However, larval numbers of T. canis also decreased over time post infection (Burren, 1971). In white mice, larval distribution was determined for T. canis and T. cati leading to the conclusion that the hatching of T. cati seems more rapid than the hatching of T. canis. This is based on the observation that 48 h after infection with T. canis, most larvae were found in the liver, after 4 days in the lung and heart and after 14 days in the brain. Larvae were found in muscle from day 4 on. For T. cati, larvae reached the liver after day 1, the lung on day 2, and the leg muscle on day 3. Larvae could be detected in the brain on days 2 28 p.i. (Prokopic and Figallová, 1982). Migration routes in Clarke s OS1 mice were investigated using an infectious dose of 1000 eggs of either T. canis or T. cati showing that brains and eyes of some T. canis-infected mice contained larvae. Larvae could be found in each part of the brain, however, most were found in the cerebellum. Before day 7 of infection, the number increased with time and stayed constant between day 7 and 44 p.i. Between days p.i., the number of larvae decreased. Some larvae could be found in spinal cords of a couple of individuals. For T. cati, no larvae could be observed in the eye and only single larvae could be detected in the brain (Burren, 1971). The low detection rate of T. cati larvae found in CNS tissue of mice supports an earlier study by Dubey (1968) who found the larvae to be present in the brain for at least 8 weeks, however in very small numbers. BALB/c mice seem exceptionally susceptible to T. canis infection as larval counts are much higher compared to other strains with a maximum number of larvae in the brain on day 8 21 p.i. (Bardón et al., 1994; Epe et al., 1994). Epe et al. (1994) determined differences in several mouse strains (BALB, C3H, C57Bl, DBA and NMRI) and found that even though the larval numbers were highest in BALB/c mice, they usually did not show any clinical symptoms, whereas many individuals of the other strains deceased between weeks p.i. after showing central nervous symptoms. The highest larval burden resulted in the lowest morbidity and mortality. Due to their distinct susceptibility, BALB/c mice were found the most suitable model for toxocarosis in several studies (Pinelli et al., 2001; Hamilton et al., 2006; Ollero et al., 2008). This susceptibility could also been shown for T. cati infection in BALB/c mice in which migration until day 28 p.i. was reported. Observed degenerative muscle fibres in the heart might indicate active migration through the myocardium (Cardillo et al., 2009). Regardless of the mouse strain, the migration route of T. canis and T. cati was comparable in all experiments, as for both larval counts were highest in liver and lungs (Fig. 2) at the beginning of the experiment, with later accumulation in the brain (Fig. 3) (Skerrett and Holland, 1997).
4 378 C. Strube et al. / Veterinary Parasitology 193 (2013) Fig. 2. Toxocara spp. larva in the lung of an infected mouse (Institute for Parasitology, archive). Additionally, accumulation of T. cati in the muscle tissues was observed (Cardillo et al., 2009). Outbred strains seem to be less susceptible than inbred mouse strains, even though migration patterns were comparable (Canberra vs. C57Bl/6J). It was hypothesized that the outbred strain can trap larvae in the liver and thereby prevent systemic circulation thereby (Dunsmore et al., 1983). Larval persistence in Balb/c mice was determined for up to one year whereas brain and musculature were mainly colonized. After one year larval numbers were found to be diminishing (Bardón et al., 1994). Additionally, invasion of the spinal cord in mice infected with T. canis was observed and data showed that larvae invade the spinal cord early and persist there for at least 4 months, possibly causing the observed motor problems (Olson and Petteway, 1972). Behavioural studies following T. canis infections were mainly conducted in LACA mice which were infected with doses ranging from eggs. It could be shown that there are strong correlations between numbers of larvae recovered from brains of T. canis-infected mice and social behaviour as well as anxiety. High numbers lead to abnormal social behaviour in terms of reduced aggressiveness whilst low larval numbers lead to a decreased amount of risk assessment. Both factors result in greater susceptibility to potential predators in the environment (Cox and Holland, 1998, 2001; Hamilton et al., 2006). Additionally, Fig. 3. Toxocara canis larva in the brain of an infected mouse (Institute for Parasitology, archive). Fig. 4. Toxocara canis larva in the cerebellum (Institute for Parasitology, archive). a more detailed study revealed that high larval burdens in the brain start accumulating day p.i. and mice show behavioural alterations accordingly (Burren, 1971; Skerrett and Holland, 1997). Immobility was increased and a decrease of exploratory behaviour was shown. Mice were less active, less aggressive, spent more time in open areas and showed impairment of learning and memory (Holland and Cox, 2001) Rats Rats are considered a potential paratenic host as well, but are considerably neglected as opposed to mice concerning research. Comparison of larval distribution of T. canis and T. cati in rats revealed a similar migration pattern of larvae as seen in mice. Larval recovery of T. canis in liver, lungs, carcass, and brain indicates a hepato-pulmonary phase during the first week of infection as high numbers of larvae were counted in the liver between day 3 and 5 p.i. of experimentally infected rats. High larval counts could be observed in the lungs between day 5 and 8 p.i., and in the carcass and in the brain from day 5 p.i. on (Lescano et al., 2004). Analysis of larval migration with focus on the CNS revealed that larvae started invading the brain from day 6 p.i. on and could be found until day 28 p.i., the last study day. Notably, as in mice most larvae were detected in the cerebellum (Fig. 4). Only very few individual rats retained larvae in their spinal cords and none retained larvae in their eyes (Burren, 1971, 1972). The T. canis larval counts in the brain are connected to a prolonged learning behaviour as massive impairment of the ability to solve complex maze problems was exhibited by the infected groups (Olson and Rose, 1966). The start of larval invasion of the brain could be observed between day 3 and 7. In addition, behavioural changes after T. canis infection have been observed in terms of mobility. Infected rats lose their natural caution in an open field apparatus by exhibiting higher levels of mobility and exploratory behaviour. Furthermore, muscle strength impairment was measured 30 days p.i. These factors contribute to the assumption that rats as paratenic hosts are influenced by somatic larvae turning them into easier prey for the definitive host in nature (Chieffi et al., 2009, 2010). In contrast, larval distribution of T. cati in rats revealed that larval migration to the brain was a lot less frequent and
5 C. Strube et al. / Veterinary Parasitology 193 (2013) mainly observed on day 15 and 30 p.i. Overall, larval distribution was comparable to T. canis larvae for liver and lungs but larval numbers were lower. The bulk of larvae were found in the carcass, almost rounding up to 100% in relation to the infection dose. Eyes had been examined and larvae could be found on some occasions (Santos et al., 2009). From the migration pattern described above, Santos et al. (2009) concluded that there are significant differences between T. cati and T. canis migration routes and their neural affinity. Santos et al. (2009) investigated distribution of larvae until day 60 p.i. and showed that T. cati larvae migrate to the brain at a slower rate than T. canis. In addition, Zibaei et al. (2010) demonstrated that the T. cati larval burden in the brain did not increase until day p.i Gerbils Gerbils are frequently proposed as a suitable model for human ocular toxocarosis due to their high susceptibility to the ocular form of the parasitosis as well as in terms of induced inflammatory responses, immune responses and host protective mechanisms (Alba-Hurtado et al., 2009). Toxocara canis migration dynamics determined by Alba- Hurtado et al. (2009) revealed that from 12 to 24 h p.i., larvae could be found in the lung and liver but the majority was still located in the intestine. Between day 3 and 5 p.i., larvae were mainly recovered from the lungs, and from day 10 to 60 p.i. from the carcass. Larvae were present in the eyes from day 5 p.i. until the end of the experiment (day 60 p.i.) and most larvae were recovered from the brain on day p.i. Relative distribution was calculated based on the organ s weight and in conclusion neuroaffinity turned out to be much higher than affinity to the muscle. Overall, the migration route could be divided into two phases, namely the visceral phase and the myotropic-neurotropic phase. In contrast to different paratenic hosts like mice, most larvae were recovered from the lung instead of the liver. The second phase lasted up to days p.i. (Alba- Hurtado et al., 2009). The same affinity to the CNS was found by Burren (1972) who investigated brains of gerbils and recovered high larval numbers in the cerebellum. Few larvae continued to migrate into the spinal cord. Comparison of T. cati and T. canis in gerbils revealed a similar severe effect of the somatically migrating larvae. Over half of the gerbils in both groups showed neurological abnormalities which progressed in gerbils infected with T. canis 50 days p.i., whereas gerbils infected with T. cati did not show abnormalities until 120 days p.i. Most larvae were repeatedly found in the cerebellum and pathological changes were identical for both Toxocara species with larvae and lesions apparently existing separately (Akao et al., 2003b). On day 7 p.i., considerably more T. canis larvae than T. cati larvae were detected in the brain (Akao et al., 2000). These findings are supported by other authors who found that just one day after initial infection, 50% of T. canis infected gerbils contained larvae in their eyes whereas ocular T. cati larvae were detected at day 5 p.i. Additionally, it was shown that after T. cati infection, larvae could be recovered from the muscle on day 70 p.i. whereas on day 92 p.i. they were recovered in large numbers from the brain (Zibaei et al., 2010). It was observed that gerbils infected with either T. cati or T. canis, exhibited lack of coordination and immobility (Alba-Hurtado et al., 2000, 2009). The optic nerve is thought to be an important part of the migration route for larvae in OLM in gerbils infected with T. canis. Investigations on gerbil eyes on several days between 0.5 and 60 days p.i. revealed that 90% of the eyes contained larvae (Alba-Hurtado et al., 2000). Larvae are thought to remain in the brain until the host s physiological status changes and then the larvae migrate to the eye where they can elicit severe ophthalmic changes (Takayanagi et al., 1999; Akao et al., 2000; Alba-Hurtado et al., 2000) Other mammals Pigs Experimental infections of pigs with T. canis have been conducted quite frequently in order to propose a model for human toxocarosis since the pig s immunological response is comparable to the human s as well as its many physiological and biochemical similarities (Miller and Ullrey, 1987; Helwigh et al., 1999). No experimental infections of pigs with T. cati could be found in the literature; therefore the migratory route of this species in this particular host remains to be determined. As in the above described paratenic hosts, larvae accumulated in the liver. However, larval recovery was also possible in lungs, heart, brain, and muscles on days p.i. Lymph nodes of the small intestine seemed to be the starting point of larval migration to the liver indicated by high larval counts in lymph nodes around small and large intestines at the beginning of infections. No larvae were detected in the eye on any occasion (Burren, 1968; Helwigh et al., 1999; Sommerfelt et al., 2004). Viable persistence in the pigs tissue was demonstrated by successfully infecting mice with somatic larvae recovered from pigs (Sasmal et al., 2008). However, larvae do not seem to persist in the pigs tissues for prolonged periods of time since a significant decrease in larval numbers had been observed during time points 7, 14, and 21 days p.i. (Helwigh et al., 1999). After 126 days p.i., no larvae were recovered at all, indicating self-limiting factors (Sommerfelt et al., 2004) Avian species Beside mammals, birds were subjected to experimental infections to extend knowledge of somatic migration of Toxocara spp. larvae in paratenic hosts. Overall, the adaptability of Toxocara spp. larvae to the mammalian host seems to be greater than to the avian host (Pahari and Sasmal, 1990) Poultry Comparable to pigs, paratenic chicken hosts serve as a potential source of infection for humans and other mammals. Therefore, chickens have been experimentally infected with either Toxocara species to determine larval distribution, larval persistence and viability in order to assess the infection risk for humans, as accumulating evidence links toxocarosis in humans to the consumption of undercooked chicken (Ito et al., 1986; Nagakura et al., 1989). Since larvae in chicken meat have been shown
6 380 C. Strube et al. / Veterinary Parasitology 193 (2013) to be extremely infective even after an extended period of time or prolonged periods of low temperatures, Toxocara spp. infected chickens pose a potential health risk (Sprent, 1953; Taira et al., 2011, 2012). Chickens kept in free-ranging systems are likely to ingest embryonated eggs or infected paratenic hosts like earthworms (Pahari and Sasmal, 1991). Investigations of the possible hepato-pulmonary migration of T. canis in chickens revealed the highest larval burdens in the liver and the lungs, while only few larvae were recovered from other tissues. It is assumed that T. canis larvae leave the intestine after hatching around 24 h p.i. and start migration to the liver, which they in turn leave and migrate to the lungs around day 3 p.i. Re-migration to the liver occurs around day 6 p.i., most likely via the circulatory system. A small number of larvae was recovered from the breast muscle, the brain, the heart and duodenum 3 days p.i., possibly indicating alternative routes of migration (Taira et al., 2003a). The liver as predilection site may harbour persisting T. canis larvae for 3 month to 3.5 years (Beaver, 1956; Galvin, 1964; Tsvetaeva et al., 1979; Maruyama et al., 1994). Contrarily, assessment of the migration route of T. cati revealed highest larval counts in the liver and the brain 14 days p.i., however, the total number of recovered larvae was rather low (Azizi et al., 2007). In a follow-up study, chronic infections were observed until day 240 p.i. However, larvae were detected solely in the brain and only in low numbers (Oryan et al., 2010) Other avian species In pigeons experimentally infected with T. canis, a total recovery rate of 4 27% was achieved with the majority of larvae persisting in the liver and lungs as demonstrated in chickens previously. Some degree of tracheal migration is suspected in pigeons, because large numbers of larvae could be observed in the lungs at the beginning of infection and relatively few in somatic tissue later on (Galvin, 1964). Furthermore, a remarkable amount of larvae persists in the liver for up to 3 months (Beaver, 1956). Japanese quails showed similar distribution patterns to chickens with the highest number of recovered larvae in the liver at day 10, 20 and 30 p.i., whereas only few larvae were found in muscle, brain and heart tissue. Larvae recovered from these animals revealed infectious potential as they reappeared in mice tissues after experimental infection (Pahari and Sasmal, 1990) Humans and primates Many cases of toxocarosis in humans have been observed caused by ingested embyronated eggs from soil or infective larvae from undercooked meat (Glickman and Schantz, 1981; Ito et al., 1986; Stürchler et al., 1990; Aragane et al., 1999; Magnaval et al., 2001). Larval distribution has only been observed for T. canis in very few studies including primates: Crab-eating macaques (Macaca fascicularis) infected with embryonated eggs of T. canis suffered from acute and chronic phases of the infection. However, as tissues were not investigated for presence of larvae, the migration route remains unclear, but CNS lesions indicate larval migration to the brain, supporting the assumed neuroaffinity of T. canis (Glickman and Summers, 1983). Neuroaffinity in primates was also shown by Tomimura et al. (1976), who observed larvae in the cerebrum of infected Macaca spp. besides liver and lung tissue. Occasionally larvae were detected in the spinal cord. Severe neurological signs could be observed on day 8 16 p.i., leading to the assumption that larvae start migrating to the brain around that time. Persistence and survival of larvae was noted up to 15 months in the eyes of monkeys and up to 10 years in other tissues (Beaver, 1969). It was suggested that larvae retain the ability to actively migrate through ocular tissue and exit via the optical nerve (Watzke et al., 1984). However, definite migration routes have yet to be determined. 4. Continuance of somatic larvae Somatic larvae can complete their lifecycle after ingestion of the paratenic host by the definitive host (cf. Section 2.1). Another possibility of larvae continuance is transmission to the offspring, which, at least in the definitive host, provides again the opportunity to reach adulthood and thus the completion of the life cycle Transmission to the offspring in the definitive host Transmission of somatic larvae to the definitive hosts offspring plays an important role in the lifecycle of Toxocara species. This form of transmission usually occurs at a very early point of development resulting in a very high prevalence among puppies and kittens. With increasing age of the host, the development of infective larvae to adult worms decreases, which is thought to be connected to acquired immunity (Greve, 1971; Oshima, 1976; Barriga, 1988). Canids, as the definitive host for Toxocara canis, have a high chance of getting infected as soon as they reach the foetal stage (Koutz et al., 1966). Infection of the foetus may either be induced by oral ingestion of embryonated eggs by the bitch during pregnancy, or reactivation of somatic tissue larvae which are suggested to reach the foetuses by transplacental transmission (Shillinger and Cram, 1923; Yutuc, 1949; Webster, 1958a; Scothorn et al., 1965; Schnieder et al., 2011). Transplacental transmission is the common route of infection whereas lactogenic transmission is of minor importance in dogs (Burke and Roberson, 1985a,b). In contrast, the primary transmission route of T. cati in cats is defined as lactogenic (Sprent, 1956; Swerczek et al., 1971; Coati et al., 2004). If infection occurs during the pregnancy of the queen, it is assumed that the larvae migrate directly to the mammary glands, ensuring survival and further development of the larvae by vertical transmission (Swerczek et al., 1971). Reactivation of T. cati larvae arrested in the somatic tissue of the cat and migration to
7 C. Strube et al. / Veterinary Parasitology 193 (2013) the mammary glands seem to be less frequent (Coati et al., 2004) Transmission to the offspring in the paratenic host To date, no final conclusions can be drawn on whether or not prenatal or lactogenic infections play a major role in paratenic hosts. There are several studies dealing with this mode of transmission in the paratenic host concerning T. canis infection, however very few dealing with T. cati. For T. canis, prenatal transmission only seems to occur under special conditions and only to a small extent. A possible explanation may be the recurrent somatic migration in the paratenic host as opposed to the very frequent haematogenic migration in the definitive host. Therefore, the placenta may not be affected. Indeed, several authors could not show any transplacental transmission in mice (Oshima, 1961; Taylor et al., 1996; Jin et al., 2008). However, transplacental migration of T. canis larvae in mice was demonstrated when mice were infected during pregnancy. First signs of larval migration to the uterus and the placenta were detected from day 9 of pregnancy while foetal infection started on day 11 of pregnancy (Lee et al., 1976). Further evidence for transplacental transmission was provided in very few cases (Oteifa et al., 1996; Reiterová et al., 2003). By contrast to the definitive host, lactogenic T. canis transmission seems to occur quite frequently in paratenic hosts. In this context it was shown for mice that larval counts in offspring as well as milk were high when mothers were infected during pregnancy (Baumm and Stoye, 1981). Oshima (1961) demonstrated strong effects of gestation and lactation on T. canis distribution in mice. The author suggests that the hormonal status during pregnancy plays an important role in reactivation of larvae. After injection of prolactin, a decrease in the number of T. canis larvae in the mice tissue was observed, leading to the conclusion that the hormone may stimulate arrested larvae to continue migration. This study was supported by results by Jin et al. (2008), who also investigated the trans-lactational maternal-neonatal transmission of T. canis larvae in mice taking the effect of prolactin into account. Vertical transmission of T. cati in the paratenic host was described in studies which provided similar data to vertical transmission of T. canis. Intrauterine transmission was observed on occasion in mice if they had been infected during gravidity (Prokopic and Figallová, 1982; Schön and Stoye, 1986). Schön and Stoye (1986) demonstrated that lactogenic transmission occurred frequently when the mother was infected around the time of confinement. The lactogenic infection rate of the offspring decreased as the time span between infection and lactation increased. Furthermore, lactogenically infected offspring revealed the ability to pass on larvae to their progeny by lactogenic transmission as well. Notably, lactogenically infected offspring show partial protection against reinfection, marked by a significantly reduced amount of larvae compared to an orally infected control group (Reiterová et al., 2006). In addition to larval transfer to the offspring, abortion of foetuses is another important consequence of intrauterine transmission. A significant decrease in mouse litter size compared to the control group as well as up to 54% abortions could be observed if mothers were infected at an early gestational stage. Mothers larval numbers decreased continuously while larva in the offspring could first be detected from day 5 after birth onwards with gradually increasing numbers during the rest of the study. The litter size of mothers infected at late gestational stage showed significant differences from that of uninfected mice, but only 21% suffered abortion (Reiterová et al., 2003). These findings concerning the influence of the infection on litter size support an earlier study demonstrating a decrease in litter size after infection (Akao et al., 1990). 5. Clinical and pathological changes 5.1. Human toxocarosis The clinical picture of toxocarosis in humans has been systematically classified in four groups: Visceral larva migrans syndrome (VLM), neurological toxocarosis (NT), ocular larva migrans syndrome (OLM; Fig. 5) and the more recently described covert toxocarosis (Magnaval et al., 2001). The broad spectrum of clinical and pathological consequences of toxocarosis as well as the frequency of unapparent infections may cause some difficulty in identifying and classifying clinical cases. The severity and range of symptoms depends on the tissue invaded, the number of migrating larvae, and the age of the host. The immediate hypersensitivity response to the death of larvae is thought to be the main cause for symptoms of VLM (Despommier, 2003). The first VLM report described a multi-systemic disease with hypereosinophilia and hepatomegaly in three children (Beaver et al., 1952). Generally young children (<5 years) are most often affected and usually present with fever, abdominal pain, probably due to hepato- and splenomegaly, as well as lower respiratory symptoms such as coughing, bronchospasms and asthma caused by parasitic pneumonia or bronchitis (Kayes and Oaks, 1978; Magnaval et al., 2001; Despommier, 2003). Laboratory Fig. 5. Ocular larva migrans (Institute for Parasitology, archive).
8 382 C. Strube et al. / Veterinary Parasitology 193 (2013) diagnosis in these patients commonly reveals leucocytosis, persistent eosinophilia as well as hypergammaglobulinaemia and an elevated GT-level (Cypess, 1978; Schantz, 1989). Other organ involvement such as myocarditis, myalgia with eosinophilic polymyositis, arthritis, and nephritis may also occur (Kayes and Oaks, 1978; Dromer et al., 1993; Shetty and Aviles, 1999; Prunier et al., 2001). VLM has additionally been associated with dermatological changes such as rash, pruritus, excema, panniculitis, urticara and vasculitis (Holland and Smith, 2006). Although generally most T. canis infections remain unapparent, long-term effects such as development of asthma and promotion of pulmonary fibrosis are suspected to occur (Aderele and Oduwole, 1982; Grove, 1982; Richards et al., 1983; de Sylva et al., 1990; Feldman and Parker, 1992; Kayes, 1997). The number of reported cases of neurotoxocarosis is scarce and varies between 29 (Moreira-Silva et al., 2004) and 50 cases (Finsterer and Auer, 2007) listed in literature reviews of case reports from the early 1950s until to date. In contrast, various animal experiments have indicated frequent CNS involvement in paratenic hosts as described below. Migration of Toxocara spp. larvae to the human brain is most often not associated with clinical central nervous signs, but may in rare cases result in eosinophilic meningitis, encephalitis, myelitis or combined pathological presentations (Engel et al., 1971; Mikhael et al., 1974; Wang et al., 1983; Gould et al., 1985; Villano et al., 1992; Ota et al., 1994; Sommer et al., 1994; Barra et al., 1996; Duprez et al., 1996; Goffette et al., 2000; Vidal et al., 2003; Moreira-Silva et al., 2004; Mrissa et al., 2005; Caldera et al., 2012). Cerebral vasculitis, which might even develop under anthelmintic therapy (Dousset et al., 2003; Oujamaa et al., 2003; Xinou et al., 2003), optic neuritis (Komiyama et al., 1995) and other cranial nerve involvement (Moreira-Silva et al., 2004) as well as a case of meningo-radiculitis (Robinson et al., 2002) have also been described. Cerebral lesions are predominantly located in the white matter and additional occlusion of cerebral arterial vessels has been reported (Sommer et al., 1994; Xinou et al., 2003). Clinical patients present with a large variety of symptoms according to their individual pathology ranging from headache, fever, photophobia, weakness, dorsalgia, confusion, tiredness, visual impairment to epileptic seizures, neuropsychological disturbances, dementia and depression (Marmor et al., 1987; Fortenberry et al., 1991; Sommer et al., 1994; Richartz and Buchkremer, 2002; Bachli et al., 2004; Finsterer and Auer, 2007). Furthermore, motor impairment such as ataxia, rigor, para- or tetraparesis and dysaesthesia as well as urinary retention and faecal incontinence occurred in human cases of toxocarosis (Wang et al., 1983; Russegger and Schmutzhard, 1989; Fortenberry et al., 1991; Villano et al., 1992; Sommer et al., 1994; Goffette et al., 2000; Moreira-Silva et al., 2004). Ocular larva migrans syndrome (OLM) is characterized by an eosinophilic immune response to larval migration into the eye. After formation of an eosinophilic abscess, a granulomatous inflammatory reaction surrounds the larvae (Shields, 1984; Taylor, 2006). Histopathological examinations revealed multiple retinal and vitreous haemorrhages, eosinophilic abscesses and granulomatous lesions with or without larvae. The lack of larvae in some lesions was attributed to destruction of the causative organism and its mobility (Wilder, 1950; Lyness et al., 1987; Taylor, 2006). OLM occurs predominantly unilaterally. Bilateral ocular involvement has been described but can be considered uncommon (Taylor, 2006). Clinical findings predominantly comprise visual impairment, strabismus, leukocoria, solid retinal mass predominantly at the posterior pole, vitreous mass or haze, vitritis, retinal detachment, cataract, endophthalmitis, papillitis and uveitis. Possible clinical consequences of OLM are blindness and secondary glaucoma (Brown, 1970; Gillespie et al., 1993; Despommier, 2003). The term covert toxocarosis was first introduced by Taylor et al. (1987) describing a non-specific clinical syndrome in children caused by Toxocara spp. infection which could not be subsumed under VLM, OLM or NT. Unspecific symptoms such as fever, anorexia, nausea, headache, abdominal pain, vomiting, sleep and behaviour disorders, pharyngitis, pneumonia, cough, wheeze, limb pains, cervical lymphadenitis could be observed (Taylor et al., 1987; Magnaval et al., 2001) Toxocarosis in animal models The haematological and serological changes in experimental T. canis infection in primates such as macaques (M. fascicularis) are similar to the ones observed in children suffering from VLM (Glickman and Summers, 1983). Furthermore, histopathological findings like granulomatous hepatitis and encephalomyelitis are also consistent with the disease induced by Toxocara spp. in humans and the other paratenic hosts described below. Throughout the experiments carried out by Glickman and Summers (1983) and Tomimura et al. (1976), some of the monkeys developed neurological symptoms such as ataxia, nystagmus, or paralysis. Additionally swelling of the tracheobronchial lymph nodes, granulomatous lesions in lungs and kidneys as described in other paratenic hosts were reported. But unlike in humans, OLM does not occur in primates after oral infection. A clinical picture similar to human OLM has only been reported after artificial intravitreal injection of larvae (Watzke et al., 1984). Mice have frequently been used in studies investigating the biology of Toxocara spp. infection. It has been noted that different in- and outbred strains of mice show varying larval distribution patterns and brain inflammatory immune response (Koizumi and Hayakawa, 1984; Epe et al., 1994; Cox and Holland, 1998; Pinelli et al., 2008; Cardillo et al., 2009). Clinically, Toxocara canis-infected mice of various strains present with behaviour alterations as described in Section and, furthermore, develop central nervous symptoms such as dullness, somnolence, kyphosis, paresis, incoordination and tremor (Epe et al., 1994; Cox and Holland, 2001). Epe et al. (1994) showed that a high larval CNS burden does not correlate with the severity of the symptoms, indicating that not mechanical damage by migrating larvae but underlying adverse immune reactions are causing the pathology. Pathohistological examinations of infected mouse brains revealed demyelization, focal malacia and mixed cell infiltration (Epe et al., 1994; Kayes,
9 C. Strube et al. / Veterinary Parasitology 193 (2013) Fig. 8. Toxocara spp. larva near the renal cortex (Institute for Parasitology, archive). Fig. 6. Haemorrhagic lesions in the brain of a C57Bl/6J mouse infected with T. cati on day 7 p.i. (Institute for Parasitology, archive). 1997). Similar neuropathological changes predominantly in the heavily myelated tracts of the brain such as the corpus callosum, internal and external capsules, cerebellar peduncles and cerebellar medulla have been described by Summers et al. (1983) and Dolinsky et al. (1985). These findings lead to the conclusion of larval affinity to the white matter of the brain (Holland and Hamilton, 2006). Cardillo et al. (2009) reported macroscopic lesions such as spotted haemorrhagic foci on the cortex surface of mouse brains during the first week of infection with T. canis (Fig. 6). Histologically, areas of haemorrhage, congestion and neuronal necrosis without reaction of the microglia could be detected. Furthermore, haemorrhagic foci in lungs (Fig. 7) and kidneys (Fig. 8) of infected animals as well as parenchymatose congestion in the kidneys and areas of red hepatisation and haemorrhagic lung consolidation were noted by the authors. Larvae in the skeletal muscle and Fig. 7. Haemorrhagic lesions in the lung of a C57Bl/6J mouse infected with T. canis on day 2 p.i. (Institute for Parasitology, archive). other organs of paratenic hosts are enclosed in granulomas with exception of the CNS (Burren, 1968; Kayes and Oaks, 1978; Parsons, 1987). Despite the lack of inflammatory cell infiltrates, increased blood-brain barrier permeability and enhanced expression of brain injury-associated biomarkers such as TGF-beta1, S100B, GFAP, NF-L, ttg, AbetaPP as well as Tau occur in the course of Toxocara spp. brain infection (Liao et al., 2008a,b). Although the utility of the mouse model is proven for the investigation of a broad spectrum of aspects regarding Toxocara spp. infections, mice show a rather low incidence of OLM after oral infection with T. canis in some studies (Burren, 1968; Ghafoor et al., 1984). This and the timeconsuming investigation of mice eyeballs led Akao (2006) to question the suitability of the mouse model for the investigation of OLM. Nevertheless, a study carried out by Stangogiannis et al. (2007) reported some degree of ocular damage in one or both eyes of the experimentally infected mice although segments of larvae could only be recovered from two eyes with no directly associated inflammatory reaction. In consistence with the findings in humans, larvae were mainly found at the posterior pole. However, granulomatous lesions have rarely been reported in mice or gerbils, a novel animal model for OLM (Akao, 2006; Ghafoor et al., 1984). These differences to the clinical picture in humans may be partly due to the relatively early stage of infection in laboratory animals as compared to humans, which develop the condition after several years (Taylor, 2006). Ocular lesions in mice most commonly comprise vitreal and retinal haemorrhages, uveitis, iridocyclitis, retinal vasculitis, microinfarcts, lymphocytic and haemorrhagic vitreal infiltrates (Ghafoor et al., 1984; Taylor, 2006). Mongolian gerbils (Meriones unguiculatus) are very susceptible to OLM by T. canis and T. cati (Takayanagi et al., 1999; Akao et al., 2000). Compared to mice, the incidence of OLM in gerbils after oral infection has been stated as high as 90% (Alba-Hurtado et al., 2000). The types of ocular pathomorphology recorded are similar to the ones observed in mice, including vitreous and retinal haemorrhages, exudative lesions and vasculitis (Akao, 2006). Additionally, a study carried out by Alba-Hurtado et al. (2000) revealed granulomatous lesions towards the end of the observation period (60 days p.i.) similar to the picture described
10 384 C. Strube et al. / Veterinary Parasitology 193 (2013) in humans. Mongolian gerbils frequently develop neurological symptoms such as gait difficulty, progressive ataxia, paraplegia, urinary incontinence and coma. Histopathological changes are mostly limited to the cerebellum area and present as a loss of Purkinje cells, glial fibres and nerve sheaths. These changes also occurred independently of the presence of larvae and were generally less severe in T. catithan in T. canis-infected gerbils (Akao et al., 2003b; Akao, 2006). Only a few investigations regarding Toxocara spp. infection have been carried out using a rat model, although it is considered to be of similar scientific value as the mouse model (Olson and Rose, 1966; Burren, 1968; Schaffer et al., 1992; Okada et al., 1996; Akao, 2006; Chieffi et al., 2010). However, for investigations on OLM the rat model cannot be recommended, since OLM in rats is even less common than in mice (Burren, 1972). The pig has been considered a useful animal model for human covert toxocarosis and VLM since T. canis larvae exhibit an extensive migration pattern and induce a strong immune response (Helwigh et al., 1999; Taira et al., 2004). Taira et al. (2003b, 2004) demonstrated a lack of clinical symptoms in infected pigs despite extended larval migration and the presence of pathological changes in different tissues. The organs most severely affected by pathological changes due to larval migration were liver, kidneys, lungs and lymph nodes (Helwigh et al., 1999; Sommerfelt et al., 2004). On the surface of liver and kidneys numerous white spots, similar to those commonly observed during Ascaris suum infection, were detected. Livers appeared white and fibrotic, whereas lungs showed numerous granulomas and consolidation (Helwigh et al., 1999; Sommerfelt et al., 2004). Another study additionally revealed severe pathological aberrations in the brain such as congestion, oedema, shrinkage of nerve cells, vacuolization, gliosis, satellitosis, neurophagia and liquefactive necrosis (Sasmal et al., 2008). The disease in the pig shows a clear capacity of self-limitation since there is a significant decrease in larval numbers over time and the lesions show a tendency to heal from 28 days p.i. on (Sommerfelt et al., 2004). In chickens no clinical symptoms have been described after experimental infection with T. cati or T. canis (Galvin, 1964; Taira et al., 2003a, 2011). Pathological findings during T. cati infection in chickens mainly include haemorrhagic areas and foci of necrosis in the liver and to a lesser extent in the lungs and kidneys 3 14 days p.i. (Azizi et al., 2007). Oryan et al. (2010) reported lymphocytic peribronichiolitis with BALT hyperplasia and mild haemorrhages, lymphocytic and eosinophilic infiltration of the meninges covering the cerebellum as well as perivascular cuffing in some areas of the cerebellum as late as 240 days after T. cati infection. 6. Diagnosis and treatment of toxocarosis in the paratenic host 6.1. Diagnosis In humans, lesions caused by Toxocara spp. larvae can be detected with various medical imaging techniques, such as ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) (Dupas et al., 1986; Ruttinger and Hadidi, 1991; Baldisserotto et al., 1999). Additionally, biopsy and subsequent histopathological examination using a special staining technique leads to a definite diagnosis (Parsons et al., 1986). However, the most commonly used diagnostic methods are serological techniques such as the enzyme-linked immunosorbent assay (ELISA) with Toxocara spp. excretory-secretory (TES) antigen or Western blot (de Savigny et al., 1979; Magnaval et al., 1991). Positive ELISA test results have to be interpreted cautiously since cross reactions due to other parasitic infections may occur. Furthermore, TES-antigen contains parasitederived human A and B blood group-like substances, which could interfere with anti-a or -B isohaemagglutinins in human sera (van Knapen et al., 1983; Smith et al., 1984; Overgaauw, 1997a). To overcome these difficulties, an IgE- ELISA has been suggested for differential diagnosis since the above mentioned interfering antibodies are mainly of the IgG class (Girdwood, 1986; Magnaval et al., 1992). In experimentally infected animals, seroconversion was detected between 4 days and 4 weeks p.i. and persisted for months to years (Cypess et al., 1977; van Knapen et al., 1982; Glickman and Summers, 1983; Overgaauw, 1997a). Seroconversion in mice was induced by merely five infective eggs (Kayes et al., 1985; Girdwood, 1986). For interpretation of serological results in humans, it has been proposed that a seropositivity alone is of little clinical significance, only the combination of seropositivity and blood eosinophilia is indicative of active toxocarosis (Magnaval et al., 2001). Furthermore, patients may suffer from OLM whilst serologically negative. This is probably due to a lower larval burden combined with a prolonged interval between infection and testing (Overgaauw, 1997a). In general, only patients with clinical signs of a Toxocara spp. infection are eligible for therapy (Magnaval et al., 2001) Treatment Most anthelmintic drug efficiency studies have been carried out on experimentally infected mice (Magnaval and Glickman, 2006). A large number of substances has been tested and revealed larvicidal or anti-migratory effects (Dafalla, 1972; Abdel-Hameed, 1984; Abo-Shehada and Herbert, 1984b; Bardón et al., 1995; Fok and Kassai, 1998; Reis et al., 2010). Fok and Kassai (1998) assessed the larval reduction in mice experimentally infected with T. canis following treatment with albendazole, fenbendazole, flubendazole, oxibendazole or ivermectin. They recorded high larvicidal activities of these substances with reductions of mean group larval counts of 81.1% or 88.2% for a 20 day course of oxibendazole or flubendazole and 98.8% or 100% for a 30 day course of fenbendazole or albendazole. Similar larvicidal effects have been described for diethylcarbamazinen (DEC) administered intraperitoneally on three consecutive days (Dafalla, 1972). By contrast, ivermectin and mebendazole exhibited only moderate larvicidal activities (Bardón et al., 1995; Fok and Kassai, 1998). An inhibition of larval migration combined with minor larvicidal potential has been reported for thiabendazole (Abdel-Hameed, 1984). Similar antimigratory effects with enhancement of liver-entrapment
B. Good, C.V. Holland* and P. Stafford. Department of Zoology, Trinity College, Dublin 2, Ireland
 Journal of Helminthology (2001) 75, 175±181 DOI: 10.1079/JOH200178 The influence of inoculum size and time post-infection on the number and position of Toxocara canis larvae recovered from the brains of
Journal of Helminthology (2001) 75, 175±181 DOI: 10.1079/JOH200178 The influence of inoculum size and time post-infection on the number and position of Toxocara canis larvae recovered from the brains of
Fighting feline worms: Toxocara in cats and its role in human toxocarosis
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Fighting feline worms: Toxocara in cats and its role in human toxocarosis Author : Ian Wright Categories : Companion animal,
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Fighting feline worms: Toxocara in cats and its role in human toxocarosis Author : Ian Wright Categories : Companion animal,
Neurotoxocarosis: marked preference of Toxocara canis for the cerebrum and T. cati for the cerebellum in the paratenic model host mouse
 Janecek et al. Parasites & Vectors 2014, 7:194 RESEARCH Open Access Neurotoxocarosis: marked preference of Toxocara canis for the cerebrum and T. cati for the cerebellum in the paratenic model host mouse
Janecek et al. Parasites & Vectors 2014, 7:194 RESEARCH Open Access Neurotoxocarosis: marked preference of Toxocara canis for the cerebrum and T. cati for the cerebellum in the paratenic model host mouse
HUSK, LUNGWORMS AND CATTLE
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk HUSK, LUNGWORMS AND CATTLE Author : Alastair Hayton Categories : Vets Date : July 20, 2009 Alastair Hayton discusses how best
Vet Times The website for the veterinary profession https://www.vettimes.co.uk HUSK, LUNGWORMS AND CATTLE Author : Alastair Hayton Categories : Vets Date : July 20, 2009 Alastair Hayton discusses how best
Canine Distemper Virus
 Photo: LE Carmichael, MJ Appel Photo: LE Carmichael, MJ Appel Photo: LE Carmichael, MJ Appel Canine Distemper Virus Canine Distemper (CD) is a highly contagious infectious disease of dogs worldwide caused
Photo: LE Carmichael, MJ Appel Photo: LE Carmichael, MJ Appel Photo: LE Carmichael, MJ Appel Canine Distemper Virus Canine Distemper (CD) is a highly contagious infectious disease of dogs worldwide caused
Toxocara canis larvae reinfecting BALB/c mice exhibit accelerated speed of migration to the host CNS
 Parasitol Res (2011) 109:1267 1278 DOI 10.1007/s00436-011-2371-y ORIGINAL PAPER Toxocara canis larvae reinfecting BALB/c mice exhibit accelerated speed of migration to the host CNS Petra Kolbeková & David
Parasitol Res (2011) 109:1267 1278 DOI 10.1007/s00436-011-2371-y ORIGINAL PAPER Toxocara canis larvae reinfecting BALB/c mice exhibit accelerated speed of migration to the host CNS Petra Kolbeková & David
Helminthic food-borne infection in Japan
 Helminthic food-borne infection in Japan Raw meat consumption as a risk factor for zoonotic roundworm infections Ayako Yoshida Laboratory of Veterinary Parasitic Diseases, Department of Veterinary Sciences,
Helminthic food-borne infection in Japan Raw meat consumption as a risk factor for zoonotic roundworm infections Ayako Yoshida Laboratory of Veterinary Parasitic Diseases, Department of Veterinary Sciences,
Nematodes 2. Lecture topics. Ascarid life cycle. Main features of the Ascarids. Adults L 5 L 1 L 4 L 2 L 3. Groups that you need to know about
 Lecture topics Nematodes 2 BVM&S Parasitology T.W.Jones The Ascarids Migratory & non-migratory species Hypobiosis Paratenic hosts The Strongyles Tissue feeders Migratory & non-migratory species The Hookworms
Lecture topics Nematodes 2 BVM&S Parasitology T.W.Jones The Ascarids Migratory & non-migratory species Hypobiosis Paratenic hosts The Strongyles Tissue feeders Migratory & non-migratory species The Hookworms
Nematodes 2. BVM&S Parasitology T.W.Jones
 Nematodes 2 BVM&S Parasitology T.W.Jones Lecture topics The Ascarids Migratory & non-migratory species Hypobiosis Paratenic hosts The Strongyles Tissue feeders Migratory & non-migratory species The Hookworms
Nematodes 2 BVM&S Parasitology T.W.Jones Lecture topics The Ascarids Migratory & non-migratory species Hypobiosis Paratenic hosts The Strongyles Tissue feeders Migratory & non-migratory species The Hookworms
Antihelminthic Trematodes (flukes): Cestodes (tapeworms): Nematodes (roundworms, pinworm, whipworms and hookworms):
 Antihelminthic Drugs used to treat parasitic worm infections: helminthic infections Unlike protozoa, helminthes are large and have complex cellular structures It is very important to identify the causative
Antihelminthic Drugs used to treat parasitic worm infections: helminthic infections Unlike protozoa, helminthes are large and have complex cellular structures It is very important to identify the causative
Guard against intestinal worms with Palatable All-wormer
 Guard against intestinal worms with Palatable All-wormer WHIPWORMS HOOKWORMS TAPEWORMS ROUNDWORMS Palatable All-wormer, for superior, flexible protection of dogs and cats. GENTLE ON PETS, TOUGH ON WORMS.
Guard against intestinal worms with Palatable All-wormer WHIPWORMS HOOKWORMS TAPEWORMS ROUNDWORMS Palatable All-wormer, for superior, flexible protection of dogs and cats. GENTLE ON PETS, TOUGH ON WORMS.
TREATMENT OF EXPERIMENTAL TOXOCARA CATI INFECTION IN MICE WITH IVERMECTIN AND MOXIDECTIN
 Bull Vet Inst Pulawy 51, 371-377, 2007 TREATMENT OF EXPERIMENTAL TOXOCARA CATI INFECTION IN MICE WITH IVERMECTIN AND MOXIDECTIN MELTEM ULUTAS ESATGIL Department of Parasitology, Faculty of Veterinary Medicine,
Bull Vet Inst Pulawy 51, 371-377, 2007 TREATMENT OF EXPERIMENTAL TOXOCARA CATI INFECTION IN MICE WITH IVERMECTIN AND MOXIDECTIN MELTEM ULUTAS ESATGIL Department of Parasitology, Faculty of Veterinary Medicine,
Feline zoonoses. Institutional Animal Care and Use Committee 12/09
 Feline zoonoses Institutional Animal Care and Use Committee 12/09 Cat scratch disease Bacterial infection caused by Bartonella henselae Associated with a cat bite or scratch Infection at point of injury,
Feline zoonoses Institutional Animal Care and Use Committee 12/09 Cat scratch disease Bacterial infection caused by Bartonella henselae Associated with a cat bite or scratch Infection at point of injury,
Data were analysed by SPSS, version 10 and the chi-squared test was used to assess statistical differences. P < 0.05 was considered significant.
 Toxocara canis is one of the commonest nematodes of the dog and most often this nematode is the cause of toxocariasis (visceral larva migrans) [1]. People become infected by ingestion of eggs from soil,
Toxocara canis is one of the commonest nematodes of the dog and most often this nematode is the cause of toxocariasis (visceral larva migrans) [1]. People become infected by ingestion of eggs from soil,
Diagnosing intestinal parasites. Clinical reference guide for Fecal Dx antigen testing
 Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Canine and Feline Distemper. Description. The following chart indicates the animals which are susceptible to infection by canine and feline distemp
 Canine and Feline Distemper Description Canine and feline distemper are diseases affecting many wild and domestic carnivo The following chart indicates the animals which are susceptible to infection by
Canine and Feline Distemper Description Canine and feline distemper are diseases affecting many wild and domestic carnivo The following chart indicates the animals which are susceptible to infection by
Diagnosing intestinal parasites. Clinical reference guide for Fecal Dx antigen testing
 Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Lecture 4: Dr. Jabar Etaby
 Lecture 4: Dr. Jabar Etaby 1 Introduction : Cutaneous larva migrans(clm),frequently termed creeping eruption,is a parasitic skin infection that is caused by the filariform larvae of various animal hookworm
Lecture 4: Dr. Jabar Etaby 1 Introduction : Cutaneous larva migrans(clm),frequently termed creeping eruption,is a parasitic skin infection that is caused by the filariform larvae of various animal hookworm
04/02/2013. Parasites and breeding dogs: These parasites we don t hear so much about. Main internal parasites found in breeding kennels
 Parasites and breeding dogs: These parasites we don t hear so much about Main internal parasites found in breeding kennels Isospora sp. Giardia sp. Toxocara canis Something else? Breeders burden I m kind
Parasites and breeding dogs: These parasites we don t hear so much about Main internal parasites found in breeding kennels Isospora sp. Giardia sp. Toxocara canis Something else? Breeders burden I m kind
Above: life cycle of toxoplasma gondii. Below: transmission of this infection.
 Toxoplasmosis PDF This article is based on a paid for research paper dated 1972 of similar title and authored by J.K.Frenkel and J.P. Dubey. It was published by The Journal of Infectious Diseases Vol.
Toxoplasmosis PDF This article is based on a paid for research paper dated 1972 of similar title and authored by J.K.Frenkel and J.P. Dubey. It was published by The Journal of Infectious Diseases Vol.
Drug therapy of Filariasis. Dr. Shareef sm Asst. professor pharmacology
 Drug therapy of Filariasis Dr. Shareef sm Asst. professor pharmacology Signs and symptoms Lymphatic filariasis Fever Inguinal or axillary lymphadenopathy Testicular and/or inguinal pain Skin exfoliation
Drug therapy of Filariasis Dr. Shareef sm Asst. professor pharmacology Signs and symptoms Lymphatic filariasis Fever Inguinal or axillary lymphadenopathy Testicular and/or inguinal pain Skin exfoliation
Contains most of the medically important tapeworms Scolex has 4 suckers and compact vitelline gland are characteristic Range from mm to >10m
 Cyclophyllidae Contains most of the medically important tapeworms Scolex has 4 suckers and compact vitelline gland are characteristic Range from mm to >10m Family Taeniidae Taenia saginata: beef tapeworm
Cyclophyllidae Contains most of the medically important tapeworms Scolex has 4 suckers and compact vitelline gland are characteristic Range from mm to >10m Family Taeniidae Taenia saginata: beef tapeworm
Feline and Canine Internal Parasites
 Feline and Canine Internal Parasites Internal parasites are a very common problem among dogs. Almost all puppies are already infected with roundworm when still in the uterus, or get the infection immediately
Feline and Canine Internal Parasites Internal parasites are a very common problem among dogs. Almost all puppies are already infected with roundworm when still in the uterus, or get the infection immediately
General introduction
 Spirometra mansoni General introduction Distributed worldwide, mainly in southeast Asia. Larval infection of S. mansoni may cause serious clinical disease ---Sparganosis Morphology Adult worm measures
Spirometra mansoni General introduction Distributed worldwide, mainly in southeast Asia. Larval infection of S. mansoni may cause serious clinical disease ---Sparganosis Morphology Adult worm measures
Presentation of Quiz #85
 Presentation of Quiz #85 ***Reminder: Slides are copyrighted and cannot be copied for publication. A 36 year old male from Columbia was admitted to the hospital with seizures. This patient had previously
Presentation of Quiz #85 ***Reminder: Slides are copyrighted and cannot be copied for publication. A 36 year old male from Columbia was admitted to the hospital with seizures. This patient had previously
HEARTWORM DISEASE AND THE DAMAGE DONE
 HEARTWORM DISEASE AND THE DAMAGE DONE Stephen Jones, DVM There are now more months of the year where environmental conditions favor mosquito survival and reproduction. Warmer temperatures Indoor environments
HEARTWORM DISEASE AND THE DAMAGE DONE Stephen Jones, DVM There are now more months of the year where environmental conditions favor mosquito survival and reproduction. Warmer temperatures Indoor environments
Lecture # 24: Order Oxyurida & Order Ascaridida
 Lecture # 24: Order Oxyurida & Order Ascaridida Objectives: 1. Describe the unique egg laying habits of Oxyuris equi and the pathological consequences. 2. What is characteristic about the lips at the anterior
Lecture # 24: Order Oxyurida & Order Ascaridida Objectives: 1. Describe the unique egg laying habits of Oxyuris equi and the pathological consequences. 2. What is characteristic about the lips at the anterior
FACULTY OF MEDICINE INSTITUTE OF BIOLOGY SYSTEMS AND GENETIC RESEARCH HELENE TONNER
 FACULTY OF MEDICINE INSTITUTE OF BIOLOGY SYSTEMS AND GENETIC RESEARCH HELENE TONNER THE MOST LIKELY INFECTION PATHWAYS OF TOXOCARA SPP. AND COMPARISON OF INFECTION RISK AMONG STUDENTS OF THE LITHUANIAN
FACULTY OF MEDICINE INSTITUTE OF BIOLOGY SYSTEMS AND GENETIC RESEARCH HELENE TONNER THE MOST LIKELY INFECTION PATHWAYS OF TOXOCARA SPP. AND COMPARISON OF INFECTION RISK AMONG STUDENTS OF THE LITHUANIAN
Order Strongylida. Superfamilies: Trichostrongyloidea Strongyloidea Metastrongyloidea Ancylostomatoidea (hookworms)
 Order Strongylida Superfamilies: Trichostrongyloidea Strongyloidea Metastrongyloidea Ancylostomatoidea (hookworms) ORDER STRONGYLIDA - Bursate worms Superfamily - Ancylostomatoidea HOOKWORMS *dorsally
Order Strongylida Superfamilies: Trichostrongyloidea Strongyloidea Metastrongyloidea Ancylostomatoidea (hookworms) ORDER STRONGYLIDA - Bursate worms Superfamily - Ancylostomatoidea HOOKWORMS *dorsally
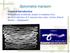
Hepatic Toxocariasis with Atypical CT and MR Imaging Findings: a Case Report
 pissn 2384-1095 eissn 2384-1109 imri 2018;22:113-118 https://doi.org/10.13104/imri.2018.22.2.113 Hepatic Toxocariasis with Atypical CT and MR Imaging Findings: a Case Report Hye Soo Shin, Kyung Sook Shin,
pissn 2384-1095 eissn 2384-1109 imri 2018;22:113-118 https://doi.org/10.13104/imri.2018.22.2.113 Hepatic Toxocariasis with Atypical CT and MR Imaging Findings: a Case Report Hye Soo Shin, Kyung Sook Shin,
SEMESTER ONE 2007 INFECTION and IMMUNITY GRADUATE ENTRY PROGRAMME PARASITOLOGY PRACTICAL 9 Dr TW Jones NEMATODES
 SEMESTER ONE 2007 INFECTION and IMMUNITY GRADUATE ENTRY PROGRAMME PARASITOLOGY PRACTICAL 9 Dr TW Jones NEMATODES Objectives After this class I expect you to be able to: 1. Describe and recognise the range
SEMESTER ONE 2007 INFECTION and IMMUNITY GRADUATE ENTRY PROGRAMME PARASITOLOGY PRACTICAL 9 Dr TW Jones NEMATODES Objectives After this class I expect you to be able to: 1. Describe and recognise the range
Aspects of Toxocara epidemiology in the Netherlands
 Aspects of Toxocara epidemiology in the Netherlands Epidemiologische aspecten van Toxocara in Nederland (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan
Aspects of Toxocara epidemiology in the Netherlands Epidemiologische aspecten van Toxocara in Nederland (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan
Systemic Apicomplexans. Toxoplasma
 Systemic Apicomplexans Toxoplasma Protozoan Groups Historically, protozoa have been grouped by mode of motility. Flagellates Hemoflagellates Trypanosoma cruzi Leishmania infantum Mucoflagellates Tritrichomonas
Systemic Apicomplexans Toxoplasma Protozoan Groups Historically, protozoa have been grouped by mode of motility. Flagellates Hemoflagellates Trypanosoma cruzi Leishmania infantum Mucoflagellates Tritrichomonas
Canine Anaplasmosis Anaplasma phagocytophilum Anaplasma platys
 Canine Anaplasmosis Anaplasma phagocytophilum Anaplasma platys It takes just hours for an infected tick to transmit Anaplasma organisms to a dog. What is canine anaplasmosis? Canine anaplasmosis is a disease
Canine Anaplasmosis Anaplasma phagocytophilum Anaplasma platys It takes just hours for an infected tick to transmit Anaplasma organisms to a dog. What is canine anaplasmosis? Canine anaplasmosis is a disease
Scientific background concerning Echinococcus multilocularis. Muza Kirjušina, Daugavpils University, Latvia
 Scientific background concerning Echinococcus multilocularis Muza Kirjušina, Daugavpils University, Latvia Echinococcus multilocularis Infection with the larval form causes alveolar echinococcosis (AE).
Scientific background concerning Echinococcus multilocularis Muza Kirjušina, Daugavpils University, Latvia Echinococcus multilocularis Infection with the larval form causes alveolar echinococcosis (AE).
Hydatid Disease. Overview
 Hydatid Disease Overview Hydatid disease in man is caused principally by infection with the larval stage of the dog tapeworm Echinococcus granulosus. It is an important pathogenic zoonotic parasitic infection
Hydatid Disease Overview Hydatid disease in man is caused principally by infection with the larval stage of the dog tapeworm Echinococcus granulosus. It is an important pathogenic zoonotic parasitic infection
Questions and answers on serious non-fatal adverse events and reporting rules
 12 April 2017 EMA/CVMP/PhVWP/303762/2012-Rev.1 Committee for Medicinal Products for Veterinary Use Questions and answers on serious non-fatal adverse events and reporting rules This questions and answers
12 April 2017 EMA/CVMP/PhVWP/303762/2012-Rev.1 Committee for Medicinal Products for Veterinary Use Questions and answers on serious non-fatal adverse events and reporting rules This questions and answers
Efficacies of fenbendazole and albendazole in the treatment of commercial turkeys artificially infected with Ascaridia dissimilis
 Efficacies of fenbendazole and albendazole in the treatment of commercial turkeys artificially infected with Ascaridia dissimilis Jessica Perkins, Thomas Yazwinski, Chris Tucker Abstract The goal of this
Efficacies of fenbendazole and albendazole in the treatment of commercial turkeys artificially infected with Ascaridia dissimilis Jessica Perkins, Thomas Yazwinski, Chris Tucker Abstract The goal of this
Hydatid Cyst Dr. Nora L. El-Tantawy
 Hydatid Cyst Dr. Nora L. El-Tantawy Ass. Prof. of Parasitology Faculty of Medicine, Mansoura university, Egypt Echinococcus granulosus Geographical Distribution: cosmopolitan especially in sheep raising
Hydatid Cyst Dr. Nora L. El-Tantawy Ass. Prof. of Parasitology Faculty of Medicine, Mansoura university, Egypt Echinococcus granulosus Geographical Distribution: cosmopolitan especially in sheep raising
Ocular Larva Migrans: A Severe Manifestation of an Unseen Epidemic
 Curr Trop Med Rep (2014) 1:69 73 DOI 10.1007/s40475-013-0004-5 PARASITOLOGY TROPICAL MEDICINE (C FRANCO-PAREDES, SECTION EDITOR) Ocular Larva Migrans: A Severe Manifestation of an Unseen Epidemic Anna
Curr Trop Med Rep (2014) 1:69 73 DOI 10.1007/s40475-013-0004-5 PARASITOLOGY TROPICAL MEDICINE (C FRANCO-PAREDES, SECTION EDITOR) Ocular Larva Migrans: A Severe Manifestation of an Unseen Epidemic Anna
HOOKWORM FAQ SHEET (rev ) Adapted from the CDC Fact Sheet
 HOOKWORM FAQ SHEET (rev 3-1-10) Adapted from the CDC Fact Sheet Hookworm Infection FAQ Sheet Contents What is hookworm? Where are hookworms commonly found? How do I get a hookworm infection? Who is at
HOOKWORM FAQ SHEET (rev 3-1-10) Adapted from the CDC Fact Sheet Hookworm Infection FAQ Sheet Contents What is hookworm? Where are hookworms commonly found? How do I get a hookworm infection? Who is at
Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51
 Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual reproduction Intestinal
Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual reproduction Intestinal
2018 General Health Survey
 2018 General Health Survey Standard Manchester Terrier Summary From February 1 March 31, 2018, the Canadian and American Manchester Terrier Clubs administered a comprehensive online health survey of Manchester
2018 General Health Survey Standard Manchester Terrier Summary From February 1 March 31, 2018, the Canadian and American Manchester Terrier Clubs administered a comprehensive online health survey of Manchester
REVIEW NEUROTOXOCAROSIS
 Rev. Inst. Med. trop. S. Paulo 49(5):279-287, September-October, 2007 REVIEW NEUROTOXOCAROSIS Josef FINSTERER(1) & Herbert AUER(2) SUMMARY Infection of humans with embryonated eggs of Toxocara canis (larva
Rev. Inst. Med. trop. S. Paulo 49(5):279-287, September-October, 2007 REVIEW NEUROTOXOCAROSIS Josef FINSTERER(1) & Herbert AUER(2) SUMMARY Infection of humans with embryonated eggs of Toxocara canis (larva
Eukaryotic Organisms
 Eukaryotic Organisms A Pictoral Guide of Supportive Illustrations to accompany Select Topics on Eukaryotic Oranisms Bacteria (Not Shown) Agent of Disease Reservoir Vector By Noel Ways Favorable Environmental
Eukaryotic Organisms A Pictoral Guide of Supportive Illustrations to accompany Select Topics on Eukaryotic Oranisms Bacteria (Not Shown) Agent of Disease Reservoir Vector By Noel Ways Favorable Environmental
Parasites in Sheep Flocks
 Parasites in Sheep Flocks 1 WHAT IS NEW IN PARASITE CONTROL FOR SHEEP FLOCKS? Drew E. Hunnisett, DVM Honeywood and Warder Veterinary Services 132 Commerce Park Drive, Unit N Barrie, Ontario L4N 8W8 705
Parasites in Sheep Flocks 1 WHAT IS NEW IN PARASITE CONTROL FOR SHEEP FLOCKS? Drew E. Hunnisett, DVM Honeywood and Warder Veterinary Services 132 Commerce Park Drive, Unit N Barrie, Ontario L4N 8W8 705
A Thesis Presented to. The Faculty of Alfred University. Addressing Toxocara in humans and companion animals as a public health issue
 1 A Thesis Presented to The Faculty of Alfred University Addressing Toxocara in humans and companion animals as a public health issue Allison Naclerio In Partial Fulfillment of the Requirements for The
1 A Thesis Presented to The Faculty of Alfred University Addressing Toxocara in humans and companion animals as a public health issue Allison Naclerio In Partial Fulfillment of the Requirements for The
Chapter 8. Effect of a government education campaign in the Netherlands on awareness of Toxocara and toxocarosis. P.A.M. Overgaauw
 Chapter 8 Effect of a government education campaign in the Netherlands on awareness of Toxocara and toxocarosis. P.A.M. Overgaauw Virbac Nederland B.V, P.O. Box 313, 3770 AH Barneveld, The Netherlands
Chapter 8 Effect of a government education campaign in the Netherlands on awareness of Toxocara and toxocarosis. P.A.M. Overgaauw Virbac Nederland B.V, P.O. Box 313, 3770 AH Barneveld, The Netherlands
What s Hiding in your Pet?
 What s Hiding in your Pet? by Erin Quigley, DVM Potentially harmful parasites! A parasite is an organism that lives on (external) or in (internal) an organism of another species (such as dog, cat or human),
What s Hiding in your Pet? by Erin Quigley, DVM Potentially harmful parasites! A parasite is an organism that lives on (external) or in (internal) an organism of another species (such as dog, cat or human),
ELlSA Seropositivity for Toxocara canis Antibodies in Malaysia,
 ELlSA Seropositivity for Toxocara canis Antibodies in Malaysia, 1989.. 1991 S. L. Hakim, MSc ].w. Mak, MRCPath P.L.W. Lam, MSc Institute for Medical Research, Jalan Pahang, 50588 Kuala Lumpur Introduction
ELlSA Seropositivity for Toxocara canis Antibodies in Malaysia, 1989.. 1991 S. L. Hakim, MSc ].w. Mak, MRCPath P.L.W. Lam, MSc Institute for Medical Research, Jalan Pahang, 50588 Kuala Lumpur Introduction
Eukaryotic Parasites. An Illustrated Guide to Parsitic Life Cycles to Accompany Lecture. By Noel Ways
 Eukaryotic Parasites An Illustrated Guide to Parsitic Life Cycles to Accompany Lecture By Noel Ways Giardia lamblia Life Cycle Reservoir: Beavers strongly implicated. Also, many other wild animals as well
Eukaryotic Parasites An Illustrated Guide to Parsitic Life Cycles to Accompany Lecture By Noel Ways Giardia lamblia Life Cycle Reservoir: Beavers strongly implicated. Also, many other wild animals as well
Title: ontamination of the hair of owned dogs with the eggs of Toxocara spp.
 Title: ontamination of the hair of owned dogs with the eggs of Toxocara spp. Authors: Jason Devoy Keegan, Celia V. Holland PII: S0304-4017(10)00343-2 DOI: doi:10.1016/j.vetpar.2010.06.010 Reference: VETPAR
Title: ontamination of the hair of owned dogs with the eggs of Toxocara spp. Authors: Jason Devoy Keegan, Celia V. Holland PII: S0304-4017(10)00343-2 DOI: doi:10.1016/j.vetpar.2010.06.010 Reference: VETPAR
COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE
 European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/211249/2005-FINAL July 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE DIHYDROSTREPTOMYCIN (Extrapolation to all ruminants)
European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/211249/2005-FINAL July 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE DIHYDROSTREPTOMYCIN (Extrapolation to all ruminants)
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT IVOMEC Injection for Pigs 10 mg/ml 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active Substance: Ivermectin
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT IVOMEC Injection for Pigs 10 mg/ml 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active Substance: Ivermectin
Metacam is an anti-inflammatory medicine used in cattle, pigs, horses, dogs, cats and guinea pigs.
 EMA/CVMP/259397/2006 EMEA/V/C/000033 An overview of Metacam and why it is authorised in the EU What is Metacam and what is it used for? Metacam is an anti-inflammatory medicine used in cattle, pigs, horses,
EMA/CVMP/259397/2006 EMEA/V/C/000033 An overview of Metacam and why it is authorised in the EU What is Metacam and what is it used for? Metacam is an anti-inflammatory medicine used in cattle, pigs, horses,
New Mexico Department of Agriculture
 Veterinary Diagnostic Services New Mexico Department of Agriculture The New Mexico Organic Farming Conference 2018 New Mexico Scientific Laboratories New Mexico Department of Agriculture Veterinary Diagnostic
Veterinary Diagnostic Services New Mexico Department of Agriculture The New Mexico Organic Farming Conference 2018 New Mexico Scientific Laboratories New Mexico Department of Agriculture Veterinary Diagnostic
The Rat Lungworm Lifecycle
 Hawaii Island Rat Lungworm Working Group Daniel K. Inouye College of Pharmacy University of Hawaii, Hilo The Rat Lungworm Lifecycle Rat Lungworm IPM RLWL-3 It is important to understand the lifecycle of
Hawaii Island Rat Lungworm Working Group Daniel K. Inouye College of Pharmacy University of Hawaii, Hilo The Rat Lungworm Lifecycle Rat Lungworm IPM RLWL-3 It is important to understand the lifecycle of
Schistosoma mansoni, S. japonicum, S. haematobium
 Schistosoma mansoni, S. japonicum, S. haematobium The Organisms More than 200 million people are infected worldwide with Schistosoma species. The adult worms are long and slender (males are 6 12 mm in
Schistosoma mansoni, S. japonicum, S. haematobium The Organisms More than 200 million people are infected worldwide with Schistosoma species. The adult worms are long and slender (males are 6 12 mm in
ECHINOCOCCOSIS. By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine).
 ECHINOCOCCOSIS By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine). INTRODUCTION Species under genus Echinococcus are small tapeworms of carnivores with larval stages known as hydatids proliferating
ECHINOCOCCOSIS By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine). INTRODUCTION Species under genus Echinococcus are small tapeworms of carnivores with larval stages known as hydatids proliferating
Surveillance of animal brucellosis
 Surveillance of animal brucellosis Assoc.Prof.Dr. Theera Rukkwamsuk Department of large Animal and Wildlife Clinical Science Faculty of Veterinary Medicine Kasetsart University Review of the epidemiology
Surveillance of animal brucellosis Assoc.Prof.Dr. Theera Rukkwamsuk Department of large Animal and Wildlife Clinical Science Faculty of Veterinary Medicine Kasetsart University Review of the epidemiology
Diurnal variation in microfilaremia in cats experimentally infected with larvae of
 Hayasaki et al., Page 1 Short Communication Diurnal variation in microfilaremia in cats experimentally infected with larvae of Dirofilaria immitis M. Hayasaki a,*, J. Okajima b, K.H. Song a, K. Shiramizu
Hayasaki et al., Page 1 Short Communication Diurnal variation in microfilaremia in cats experimentally infected with larvae of Dirofilaria immitis M. Hayasaki a,*, J. Okajima b, K.H. Song a, K. Shiramizu
EFFICACY OF ANTHELMINTICS: SPECIFIC RECOMMENDATIONS FOR CANINES
 VICH GL19 (ANTHELMINTICS: CANINE) June 2001 For implementation at Step 7 - Draft 1 EFFICACY OF ANTHELMINTICS: SPECIFIC RECOMMENDATIONS FOR CANINES Recommended for Implementation on June 2001 by the VICH
VICH GL19 (ANTHELMINTICS: CANINE) June 2001 For implementation at Step 7 - Draft 1 EFFICACY OF ANTHELMINTICS: SPECIFIC RECOMMENDATIONS FOR CANINES Recommended for Implementation on June 2001 by the VICH
HYDATID CYST DISEASE
 HYDATID CYST DISEASE Hydatid disease, also called hydatidosis or echinococcosis, is a cystforming disease resulting from an infection with the metacestode, or larval form, of parasitic dog tapeworms from
HYDATID CYST DISEASE Hydatid disease, also called hydatidosis or echinococcosis, is a cystforming disease resulting from an infection with the metacestode, or larval form, of parasitic dog tapeworms from
Lactation. Macroscopic Anatomy of the Mammary Gland. Anatomy AS 1124
 Lactation AS 1124 Macroscopic Anatomy of the Mammary Gland Species differences in numbers and locations of glands inguinal - caudal to the abdomen, between the hind legs (cow, mare, ewe) abdominal - along
Lactation AS 1124 Macroscopic Anatomy of the Mammary Gland Species differences in numbers and locations of glands inguinal - caudal to the abdomen, between the hind legs (cow, mare, ewe) abdominal - along
IDEXX PetChek IP A new approach to intestinal parasites in veterinary medicine
 IDEXX PetChek IP A new approach to intestinal parasites in veterinary medicine Making next-generation testing a part of parasite control programmes Introduction Veterinary practices routinely implement
IDEXX PetChek IP A new approach to intestinal parasites in veterinary medicine Making next-generation testing a part of parasite control programmes Introduction Veterinary practices routinely implement
VICH Topic GL19 EFFICACY OF ANTHELMINTICS: SPECIFIC RECOMMENDATIONS FOR CANINES
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology CVMP/VICH/835/99-FINAL London, 30 July 2001 VICH Topic GL19 Step 7 EFFICACY OF ANTHELMINTICS:
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology CVMP/VICH/835/99-FINAL London, 30 July 2001 VICH Topic GL19 Step 7 EFFICACY OF ANTHELMINTICS:
Name Class Date. After you read this section, you should be able to answer these questions:
 CHAPTER 14 4 Vertebrates SECTION Introduction to Animals BEFORE YOU READ After you read this section, you should be able to answer these questions: How are vertebrates different from invertebrates? How
CHAPTER 14 4 Vertebrates SECTION Introduction to Animals BEFORE YOU READ After you read this section, you should be able to answer these questions: How are vertebrates different from invertebrates? How
RABIES CONTROL INTRODUCTION
 RABIES CONTROL INTRODUCTION Throughout human history, few illnesses have provoked as much anxiety as has rabies. Known as a distinct entity since at least 500 B.C., rabies has been the subject of myths
RABIES CONTROL INTRODUCTION Throughout human history, few illnesses have provoked as much anxiety as has rabies. Known as a distinct entity since at least 500 B.C., rabies has been the subject of myths
INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER
 Canine VacciCheck INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER IgG ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 13 JUL 2015 Biogal Galed Laboratories Acs. Ltd., tel: 972-4-9898605.
Canine VacciCheck INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER IgG ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 13 JUL 2015 Biogal Galed Laboratories Acs. Ltd., tel: 972-4-9898605.
CANINE HEARTWORM DISEASE
 ! CANINE HEARTWORM DISEASE What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs. It is caused by a blood-borne parasite called Dirofilaria
! CANINE HEARTWORM DISEASE What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs. It is caused by a blood-borne parasite called Dirofilaria
Helminth Infections. Pinworms
 Helminth Infections Pinworms Helminths Worm classified as a parasite Contaminate food, water, air, feces, pets, wild animals, toilet seats and door handles Prevention: Frequent hand washing Frequent cleaning
Helminth Infections Pinworms Helminths Worm classified as a parasite Contaminate food, water, air, feces, pets, wild animals, toilet seats and door handles Prevention: Frequent hand washing Frequent cleaning
VICH Topic GL20 EFFICACY OF ANTHELMINTICS: SPECIFIC RECOMMENDATIONS FOR FELINE
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology CVMP/VICH/545/00-FINAL London, 30 July 2001 VICH Topic GL20 Step 7 EFFICACY OF ANTHELMINTICS:
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology CVMP/VICH/545/00-FINAL London, 30 July 2001 VICH Topic GL20 Step 7 EFFICACY OF ANTHELMINTICS:
ophthalmoscopic observation
 Br J Ophthalmol 1999;83:967 972 967 Department of Medical Zoology, Faculty of Medicine, Tokyo Medical and Dental University, Tokyo, Japan T H Takayanagi N Akao R Suzuki M Tomoda S Tsukidate K Fujita Correspondence
Br J Ophthalmol 1999;83:967 972 967 Department of Medical Zoology, Faculty of Medicine, Tokyo Medical and Dental University, Tokyo, Japan T H Takayanagi N Akao R Suzuki M Tomoda S Tsukidate K Fujita Correspondence
Campylobacter species
 ISSUE NO. 1 SEPTEMBER 2011 1. What are Campylobacter spp.? Campylobacter spp. are microaerophilic, Gram-negative, spiral shaped cells with corkscrew-like motility. They are the most common cause of bacterial
ISSUE NO. 1 SEPTEMBER 2011 1. What are Campylobacter spp.? Campylobacter spp. are microaerophilic, Gram-negative, spiral shaped cells with corkscrew-like motility. They are the most common cause of bacterial
COMPARATIVE HISTOLOGY SLIDE SETS
 COMPARATIVE HISTOLOGY SLIDE SETS Cat #: CH-COMP1 - COMPARATIVE EPITHELIUM & CONNECTIVE TISSUE SLIDE SET - 28 slides 1 - Surface of Simple squamous epithelium (silver staining) 2 - Simple squamous epithelium
COMPARATIVE HISTOLOGY SLIDE SETS Cat #: CH-COMP1 - COMPARATIVE EPITHELIUM & CONNECTIVE TISSUE SLIDE SET - 28 slides 1 - Surface of Simple squamous epithelium (silver staining) 2 - Simple squamous epithelium
How to talk to clients about heartworm disease
 Client Communication How to talk to clients about heartworm disease Detecting heartworm infection early generally allows for a faster and more effective response to treatment. Answers to pet owners most
Client Communication How to talk to clients about heartworm disease Detecting heartworm infection early generally allows for a faster and more effective response to treatment. Answers to pet owners most
This is the smallest tapeworm that can affect human being but it s not really proper human tapeworm (the human is not the primary host).
 Echinococcus Granulosus Small Tapeworm (1 cm), Cestode. This is the smallest tapeworm that can affect human being but it s not really proper human tapeworm (the human is not the primary host). The primary
Echinococcus Granulosus Small Tapeworm (1 cm), Cestode. This is the smallest tapeworm that can affect human being but it s not really proper human tapeworm (the human is not the primary host). The primary
Worming: key decision factors and ways to improve compliance
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Worming: key decision factors and ways to improve compliance Author : Emma Gerrard Categories : RVNs Date : February 1, 2013
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Worming: key decision factors and ways to improve compliance Author : Emma Gerrard Categories : RVNs Date : February 1, 2013
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology EMEA/MRL/728/00-FINAL April 2000 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS STREPTOMYCIN AND
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology EMEA/MRL/728/00-FINAL April 2000 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS STREPTOMYCIN AND
Fungal Disease. What is a fungus?
 Fungal Disease What is a fungus? A fungus is a living organism. It goes through a complicated life cycle and is able to spread in the environment by producing large numbers of spores that are easily dispersed
Fungal Disease What is a fungus? A fungus is a living organism. It goes through a complicated life cycle and is able to spread in the environment by producing large numbers of spores that are easily dispersed
Clinical Manifestations and Treatment of Plague Dr. Jacky Chan. Associate Consultant Infectious Disease Centre, PMH
 Clinical Manifestations and Treatment of Plague Dr. Jacky Chan Associate Consultant Infectious Disease Centre, PMH Update of plague outbreak situation in Madagascar A large outbreak since 1 Aug 2017 As
Clinical Manifestations and Treatment of Plague Dr. Jacky Chan Associate Consultant Infectious Disease Centre, PMH Update of plague outbreak situation in Madagascar A large outbreak since 1 Aug 2017 As
Clinical Study Investigation of Anti-Toxocara Antibodies in Epileptic Patients and Comparison of Two Methods: ELISA and Western Blotting
 Epilepsy Research and Treatment Volume 2013, Article ID 156815, 5 pages http://dx.doi.org/10.1155/2013/156815 Clinical Study Investigation of Anti-Toxocara Antibodies in Epileptic Patients and Comparison
Epilepsy Research and Treatment Volume 2013, Article ID 156815, 5 pages http://dx.doi.org/10.1155/2013/156815 Clinical Study Investigation of Anti-Toxocara Antibodies in Epileptic Patients and Comparison
Heartworm Disease in Dogs
 Kingsbrook Animal Hospital 5322 New Design Road, Frederick, MD, 21703 Phone: (301) 631-6900 Website: KingsbrookVet.com What causes heartworm disease? Heartworm Disease in Dogs Heartworm disease or dirofilariasis
Kingsbrook Animal Hospital 5322 New Design Road, Frederick, MD, 21703 Phone: (301) 631-6900 Website: KingsbrookVet.com What causes heartworm disease? Heartworm Disease in Dogs Heartworm disease or dirofilariasis
Hookworms in Dogs & Cats Blood-Sucking Parasites in our Pets
 Hookworms in Dogs & Cats Blood-Sucking Parasites in our Pets Recently I came across a news story of a couple who visited the Dominican Republic. While in the tropical paradise, they became infected with
Hookworms in Dogs & Cats Blood-Sucking Parasites in our Pets Recently I came across a news story of a couple who visited the Dominican Republic. While in the tropical paradise, they became infected with
THE VETERINARIAN'S CHOICE. Compendium clinical Trials. Introducing new MILPRO. from Virbac. Go pro. Go MILPRO..
 THE VETERINARIAN'S CHOICE. Introducing new MILPRO from Virbac. Compendium clinical Trials Go pro. Go MILPRO.. milbemycin/praziquantel Content INTRODUCTION 05 I. EFFICACY STUDIES IN CATS 06 I.I. Efficacy
THE VETERINARIAN'S CHOICE. Introducing new MILPRO from Virbac. Compendium clinical Trials Go pro. Go MILPRO.. milbemycin/praziquantel Content INTRODUCTION 05 I. EFFICACY STUDIES IN CATS 06 I.I. Efficacy
What causes heartworm disease?
 Heartworm Disease: What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs and cats. It is caused by a blood-borne parasite called Dirofilaria
Heartworm Disease: What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs and cats. It is caused by a blood-borne parasite called Dirofilaria
D.M. Cox and C.V. Holland* Department of Zoology, Trinity College, Dublin 2, Ireland
 Journal of Helminthology (2001) 75, 33±41 DOI: 10.1079/JOH200028 Relationship between three intensity levels of Toxocara canis larvae in the brain and effects on exploration, anxiety, learning and memory
Journal of Helminthology (2001) 75, 33±41 DOI: 10.1079/JOH200028 Relationship between three intensity levels of Toxocara canis larvae in the brain and effects on exploration, anxiety, learning and memory
We will need to know your pets weight in order to prescribe the correct dose of medication.
 Care Guide Flea and worm prevention. There are many medications available to treat and protect your pets against parasites. We are always happy to advise you on a specific regime tailored to meet your
Care Guide Flea and worm prevention. There are many medications available to treat and protect your pets against parasites. We are always happy to advise you on a specific regime tailored to meet your
Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49
 Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual
Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual
VACCINATION GUIDELINES
 WHY VACCINATE? VACCINATION GUIDELINES Vaccines help prepare the body's immune system to fight the invasion of disease-causing organisms. Vaccines contain antigens, which look like the disease-causing organism
WHY VACCINATE? VACCINATION GUIDELINES Vaccines help prepare the body's immune system to fight the invasion of disease-causing organisms. Vaccines contain antigens, which look like the disease-causing organism
Federal law (U.S.A.) restricts this drug to use by or on the order of a licensed veterinarian.
 BAYER HEALTHCARE LLC Animal Health Division USA Product Label http://www.vetdepot.com P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390 Customer Service Tel.: 800-633-3796 Customer Service Fax: 800-344-4219
BAYER HEALTHCARE LLC Animal Health Division USA Product Label http://www.vetdepot.com P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390 Customer Service Tel.: 800-633-3796 Customer Service Fax: 800-344-4219
Indication for laser acupuncture, body and ear acupuncture treatment
 108 Indication for laser acupuncture, body and ear acupuncture treatment Orthopedics 1. Back pain 2. Tying up 3. Acute lameness, distortion and contusion 4. Acute and chronic laminitis 5. Acute and chronic
108 Indication for laser acupuncture, body and ear acupuncture treatment Orthopedics 1. Back pain 2. Tying up 3. Acute lameness, distortion and contusion 4. Acute and chronic laminitis 5. Acute and chronic
Metacam 1.5 mg/ml oral suspension for dogs
 Metacam 1.5 mg/ml oral suspension for dogs Species:Dogs Therapeutic indication:pharmaceuticals: Neurological preparations: Analgesics, Other NSAIDs, Locomotor (including navicular and osteoarthritis) Active
Metacam 1.5 mg/ml oral suspension for dogs Species:Dogs Therapeutic indication:pharmaceuticals: Neurological preparations: Analgesics, Other NSAIDs, Locomotor (including navicular and osteoarthritis) Active
Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine
 Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine The Master Degree in Theriogenology & Artificial Insemination /Faculty of Veterinary Medicine
Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine The Master Degree in Theriogenology & Artificial Insemination /Faculty of Veterinary Medicine
Senior Pet Care and Early Disease Detection
 Senior Pet Care and Early Disease Detection Thanks to advances in veterinary medicine, pets are living longer than ever before. However, with this increased lifespan comes an increase in the types of ailments
Senior Pet Care and Early Disease Detection Thanks to advances in veterinary medicine, pets are living longer than ever before. However, with this increased lifespan comes an increase in the types of ailments
Dear Doctor: Our sincerest thanks, Stephen A. Connell, DVM Director, Technical, Academic and Consumer Services Elanco Companion Animal Health
 Dear Doctor: As a trained professional, you understand the loss of a pet is incredibly difficult. Every pet owner responds differently as they grieve. We believe the recent negative media coverage of Trifexis
Dear Doctor: As a trained professional, you understand the loss of a pet is incredibly difficult. Every pet owner responds differently as they grieve. We believe the recent negative media coverage of Trifexis
Oral and intestinal candidiasis. As adjuvant treatment with other local nystatin preparations to prevent reinfection.
 1. NAME OF THE MEDICINAL PRODUCT Nystatin Orifarm, 100 000 IU/ml oral suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains 100 000 IU nystatin. Excipients with known effect: - Methyl parahydroxybenzoate
1. NAME OF THE MEDICINAL PRODUCT Nystatin Orifarm, 100 000 IU/ml oral suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains 100 000 IU nystatin. Excipients with known effect: - Methyl parahydroxybenzoate
Lyme Disease. Lyme disease is a bacterial infection spread by tick bites from infected blacklegged
 Lyme Disease Lyme disease is a bacterial infection spread by tick bites from infected blacklegged ticks. The bacteria that causes the disease is Borrelia burgdorferi, a spirochete. The earliest symptoms
Lyme Disease Lyme disease is a bacterial infection spread by tick bites from infected blacklegged ticks. The bacteria that causes the disease is Borrelia burgdorferi, a spirochete. The earliest symptoms
Feline Immunodeficiency Virus (FIV)
 Virus (FeLV) FIV and FeLV are both viruses within the same family of retroviruses, but they are in different groups within that family: FIV is in one group called lentiviruses these cause lifelong infections
Virus (FeLV) FIV and FeLV are both viruses within the same family of retroviruses, but they are in different groups within that family: FIV is in one group called lentiviruses these cause lifelong infections
Feline Vaccines: Benefits and Risks
 Feline Vaccines: Benefits and Risks Deciding which vaccines your cat should receive requires that you have a complete understanding of the benefits and risks of the procedure. For this reason, it is extremely
Feline Vaccines: Benefits and Risks Deciding which vaccines your cat should receive requires that you have a complete understanding of the benefits and risks of the procedure. For this reason, it is extremely
