Department of Parasitology and Zoology. The seroprevalence of Toxoplasma gondii antibodies in cats from Hungary. By Daniela Nieto
|
|
|
- Janis Norton
- 5 years ago
- Views:
Transcription
1 S S Department of Parasitology and Zoology The seroprevalence of Toxoplasma gondii antibodies in cats from Hungary By Daniela Nieto Supervisor: Professor Róbert Farkas Budapest, Hungary 2015
2 Table of Contents 1. Introduction Literature Review History of Toxoplasma gondii Morphology of Toxoplasma gondii Life cycle of Toxoplasma gondii Life cycle in the definitive host Life cycle in the intermediate hosts Clinical toxoplasmosis in cats Clinical toxoplasmosis in the intermediate hosts Laboratory Diagnosis of toxoplasmosis in cats Laboratory methods Detection of IgM antibody in cats Detection of IgG antibody in cats Studies on Toxoplasma gondii infection in cats Studies on Toxoplasma gondii infection in cats in Hungary Materials and Methods Materials Serological Method Methods Results Discussion Abstract Acknowledgements Bibliography
3 1. Introduction Toxoplasma gondii is an ubiquitous parasite causing infection in a broad spectrum of domestic and wild animal species. It is a zoonotic protozoan which is especially dangerous for pregnant women as it may cause abortion or fetal developmental disorders and in immunocompromised individuals. The felids are the definitive hosts and a wide range of animals serve as the intermediate hosts. Cats play an epidemiological role in the spread of the parasite in the environment because they can shed oocysts with their feces. Cats can obtain the infection by consumption of sporulated oocysts from the environment or by tissue cysts from infected intermediate hosts. The detection of infection is very difficult with finding of oocysts in the feces of cats because the shedding period is 1-3 weeks once in their lifetime. Even though cats can shed up to hundred thousands of oocysts of the parasite, the exact moment of shedding is challenging to determine. On the other hand, the infection caused by T. gondii cannot be diagnosed based on the morphology and size of the unsporulated oocysts, molecular biological studies are needed. The infection of cats can be determined easier by serological methods because antibodies against T. gondii circulate in the blood of the infected animals. These cats have most likely already shed oocysts in their feces and now just harbour the antibodies. The data about the seroprevalence of T. gondii infection in cats is an important parameter for Public Health, because it gives a suggestion about the contamination in the environment and therefore the risk it implies to humans (Gyorke et al., 2011). The aim of my thesis is to study the occurrence of anti-t. gondii antibodies in the sera of 200 cats living in Budapest and the countryside with Immunofluorescence Antibody Test (IFAT). The other aim of this study is to evaluate whether there are relationships between the seroprevalence of the animals living in Budapest and the countryside, their age or gender. 2
4 2. Literature Review 2.1 History of Toxoplasma gondii The parasite was discovered more than 100 years ago by Charles Nicolle and Louis Manceaux in 1908, while they worked at the Pasteur Institute in Tunis, Tunisia. They found the parasite in the tissues of a rodent having the species name Ctenodactylus gundi. Subsequently, they named the parasite Toxoplasma gondii. In Greek, toxo means arc or bow and plasma means anything that is shaped or molded. The name gondii comes from the name of the rodent in which it was discovered. The correct name should have been Toxoplasma gundii, but the scientists identified the rodent incorrectly as Ctenodactylus gondi (Dubey, 2010). T. gondii is regarded as one of the most prevalent parasites in the world (Sykes, 2013). 2.2 Morphology of Toxoplasma gondii Toxoplasma gondii is a protozoan that belongs to the Phylum Apicomplexa. This species has 3 important infectious stages: oocysts, tachyzoites and bradyzoites (Dubey, 2010). The oocysts are approximately micrometers in size; each has two sporocysts, each with 4 sporozoites (Bowman, 2013). The tachyzoites (from Greek: Tachos=speed; Zoon=animal) are the rapidly multiplying stage. They are crescent-shaped with a central nucleus (Dubey, 2010). Bradyzoites (Brados=slow; Zoon=animal) have a more posterior nucleus, they are more slender, contain glycogen and are resistant to digestion (Dubey, 2010; Bowman, 2013). Bradyzoites are the encysted stage found in the tissues of animals and humans. Bradyzoites are also called cystozoites. The size of tissue cysts varies; young cysts are small with only two bradyzoites and older cysts are larger with thousands of bradyzoites. Tissue cysts in the brain are around 70 micrometers and reaching up to 100 micrometers in the muscles (Dubey, 2010). 2.3 Life cycle of Toxoplasma gondii The life cycle of T. gondii was discovered by Frenkel in This protozoan can infect all warm-blooded animals as intermediate hosts (including cats). The only definitive hosts are those species belonging to the family Felidae (wild and domestic cats). Only definitive hosts 3
5 can shed oocysts in their feces. The oocysts undergo sporulation in the environment within 1-5 days. Sporulated oocysts are the infective stage of the parasite. The definitive and intermediate host species become infected by ingesting sporulated oocysts. Once ingested, the wall of the oocysts is digested by the stomach. The sporocysts are released and undergo excystation and then the released sporozoites will penetrate the enterocytes. They develop into tachyzoites which spread to several tissues, the most predilection sites are the neural tissue (e.g. brain and eyes) and muscle tissues (e.g. skeletal and cardiac) and where tissue cysts are formed as early as 3 days post inoculation (Dubey, 2010). It was previously thought that tissue cyst formation was controlled by the host s immune system, but a later study from Dubey and Frenkel (1976) revealed that tissue cysts are an essential part of the life cycle of the parasite and independent from the immune system. These tissue cysts can remain viable and infective for the lifetime of the host (Dubey, 2008) Life cycle in the definitive host The definitive hosts may become infected after ingesting oocysts, tissue cysts or tachyzoites (Dubey, 2010). Cats also serve as intermediate hosts, because the tachyzoites and tissue cysts can form in the extraintestinal tissues (Bowman, 2013). The parasite undergoes an extraintestinal cycle in the host. After ingesting tissue cysts, these are digested in the stomach and small intestines. Bradyzoites are released and penetrate the enterocytes, developing into merozoites and replicate by several cycles of schizogony. Merozoites will transform into micro and macrogametes, which later fertilize and produce a zygote. The cat will shed unsporulated oocysts in their feces. Moreover, after ingesting sporulated oocysts, the sporozoites inside the oocyst will undergo enteroepithelial replication and develop into tachyzoites. Tachyzoites replicate quickly and reach visceral organs via the blood and lymph vessels (Sykes, 2013). Cats will shed oocysts after they ingest any of the three stages of the parasite; tachyzoites, bradyzoites or oocysts. The prepatent period and frequency of the shedding depends on which infectious stage the parasite is ingested (Dubey, 2010). The prepatent period varies between 3-10 days after ingesting tissue cysts and more than 18 days (19-48) after ingesting oocysts (Dubey, 2010; Bowman, 2013). Cats will shed T. gondii oocysts for 1-3 weeks. If reinfection occurs, the shedding is less and for a shorter period. Cats 4
6 that are co-infected with Cystoisospora, can trigger the shedding of T. gondii (Bowman, 2013). Cats can shed millions of oocysts, after ingesting only one bradyzoite and contrarily 100 oocysts may not infect cats (Dubey, 2008). Experimentally infected cats have shown to not shed oocysts again after they were reinfected with T. gondii (De Craeye et al., 2008). Cats that have undergone Toxoplasma infection and have shed oocysts are generally a minor source of infection. These cats are considered safer pets than those that have not contracted the infection yet (Bowman, 2013). Toxoplasma gondii is more efficiently transmitted by carnivorism in felines that ingest tissue cysts from intermediate hosts, whereas in intermediate hosts it is by the ingestion of sporulated oocysts (Dubey, 2010) Life cycle in the intermediate hosts The parasite can cause infection in animals and humans through the following ways: 1. Congenital: the tachyzoites are able to pass through the placenta and reach the fetus (Dubey, 2008). 2. Carnivorism: by the ingestion of tissue cysts in undercooked meat (Dubey, 2008). 3. Fecal-oral route: this explains the widespread infection of vegetarians and herbivores. The cats shed the oocysts in the feces and contaminate the environment (Dubey, 2008). 4. Infection by sexual transmission and transplacental infection has been documented in dogs (Sykes, 2013). 5. Dogs can mechanically transmit T. gondii oocysts after they have ingested feline feces (Sykes, 2013). The parasite has an extraintestinal cycle in the intermediate hosts, when they ingest sporulated oocysts or tissue cysts. After the ingestion of T. gondii oocysts, the sporozoites are released in the intestinal lumen. They penetrate the enterocytes, undergo replication and develop into tachyzoites. The tachyzoites are the rapidly diving stage and can spread to many tissues and further develop into bradyzoites. In case of immunodeficient or pregnant animals, the tachyzoites are what cause the most damage. Most animals can control the infection and the parasite remains enclosed as tissue cysts in the brain, muscles and visceral organs. Intermediate hosts may also become infected by ingesting T. gondii tissue cysts. The tissue 5
7 cysts are digested by the stomach and the resistant bradyzoites are released, these penetrate the enterocytes and develop into tachyzoites and these again into bradyzoites (Dubey, 2010; Bowman, 2013). Some intermediate hosts such as pigs, mice and humans can become infected after ingesting only one oocyst. The ingestion of tissue cysts is less infective in mice than ingestion of oocysts (Dubey, 2008). When rodents are infected with T. gondii, the parasite causes a change in their behaviour, which makes rodents less afraid of cats. This increases the probability that the cat will become infected by eating the rodent and so that the parasite can replicate sexually and complete its life cycle in the definitive host (Sykes, 2013). 2.4 Clinical toxoplasmosis in cats Cats that are immunocompetent, T. gondii usually causes a subclinical infection. On the contrary, the parasite may lead to chronic and clinical toxoplasmosis in immunosuppressed cats, caused either by corticosteroid treatment or viruses (D Amore et al., 1997). Toxoplasma is an opportunistic infection in cats with virus infection (Svobodova et al., 1998). After a primary infection, if there is not a proper immune response; the tachyzoites are responsible for tissue damage. Clinical toxoplasmosis in cats is very rare, during the enteroepithelial stage of the parasite s cycle, only 10-20% of cats will show diarrhea for 1-2 weeks, which is generally self-limiting. Fatal extraintestinal toxoplasmosis has developed in cats that were co-infected with the Feline Leucosis Virus (FeLV), Feline Immunodeficiency Virus (FIV), Feline Infectious Peritonitis Virus (FIP) or received immunosuppressive treatment such as cyclosporine. The tissues most commonly affected are the central nervous system, liver, lungs and pancreas. The most severe signs are seen in kittens that are infected via the placenta or milk from their mothers. They usually die of lung or liver failure and ocular disease is commonly seen. The most common clinical signs of generalized toxoplasmosis in cats are lethargy, anorexia, respiratory signs and uveitis (Sykes, 2013). 6
8 2.5 Clinical toxoplasmosis in the intermediate hosts Tachyzoites are the stage that cause tissue damage and the clinical signs depend on the number of tachyzoites in the host, the immune status of the host and the organs involved. Toxoplasmosis is usually a subclinical infection in immunocompetent animals. In immunosuppressed animals, the infection may cause acute systemic toxoplasmosis. Complications that may arise are interstitial pneumonia, myocarditis, hepatic necrosis, meningoencephalitis, chorioretinitis, lymphadenopathy and myositis. Common clinical signs are fever, diarrhea, coughing, dyspnea, icterus, seizures and death. Toxoplasma can cause abortion in small ruminants and pigs. Toxoplasmosis can cause liver disease in immunocompromised or young dogs (Khan and Line, 2010). Cattle and horses are resistant to clinical toxoplasmosis and clinical cases have not been reported (Dubey, 2008). Marine mammals have also been identified with clinical toxoplasmosis, indicating that the waters are also infected with T. gondii oocysts (Dubey, 2008). Even if animals are treated and recover from toxoplasmosis, they should be considered to be chronically infected with the parasite (Khan and Line, 2010). Humans that have an intact immune system and are infected with T. gondii, can have mild clinical signs such as fever, muscle pain, lymphadenopathy, anorexia and sore throat. Toxoplasmosis is much worse in humans with immunodeficiency such as in fetuses, newborns and old adults. Transplacental transmission can result in abortion, malformations or mental retardation of the fetus. Pregnant women should avoid contact with cat feces and undercooked meat. The most important source of infection for humans is pork meat (Bowman, 2013). The data about the seroprevalence of T. gondii infection in cats is an important parameter for Public Health, because it gives a suggestion about the contamination in the environment and therefore the risk it implies to humans (Gyorke et al., 2011). 7
9 2.6 Laboratory diagnosis of toxoplasmosis in cats There are no pathognomonic signs for toxoplasmosis. For this reason specific laboratory methods must be used for the diagnosis (Sykes, 2013) Laboratory methods 1. Fecal flotation method: Toxoplasma gondii oocysts are morphologically similar to the oocysts of Hammondia, Besnoitia and Neospora genus (Sykes, 2013; Deksne et al., 2013). Therefore their distinction is difficult. This method is not very indicative of clinical toxoplasmosis in cats, because most clinical cases occur after the shedding period. Cats shed the oocysts usually once in their lifetime for 1-3 weeks, so it is difficult to find oocysts at the precise moment of shedding (Sykes, 2013). In spite of cats excreting up to millions of oocysts, they are rarely found in fecal samples (Lopes et al., 2008). 2. Serology diagnosis in cats: this method is the most frequently used (Sykes, 2013). Serological tests cannot exactly determine when a cat got infected or has shed oocysts (Lappin, 1996). The antibodies produced against T. gondii are detected in the serum (Sykes, 2013). Different techniques are available such as ELISA, Immunofluorescence Antibody Test (IFAT), Modified Agglutination Test (MAT), Latex Agglutination Assay (LA), Direct Agglutination (DA), Indirect Hemagglutination Assay (IHA) and Sabin-Feldman dye test. The agglutination assays can be used for cats and can detect all types of antibodies but they very poorly detect antibodies when only IgM type is present (Sykes, 2013). The Direct Agglutination Assay is the main method used in humans and animals (Michalski et al., 2010), although there is no international standard when it comes to the detection of toxoplasmosis in cats, even when using the same method (Hornok et al., 2007). Various articles show different opinions about the serological methods used. According to Da Craeye et al. (2008), the Sabin Feldman dye test and IFAT have higher sensitivity and specificity than ELISA or Agglutination tests. Lopes et al. (2008) considered MAT the most sensitive and specific method. On the contrary, Salant and Spira (2004) used ELISA method as it was 8
10 considered to have higher specificity and sensitivity than the Latex Agglutination and Indirect Hemagglutination Assays. Other serological assays can detect T. gondii immune complexes, but these are not available commercially (Sykes, 2013). Circulating Antigens: give more evidence about the presence of an infection and can be detected in the acute stage of an infection (Wang et al., 2012). The detection of T. gondii DNA with PCR in biological samples is also a good diagnosis for toxoplasmosis, because of the high sensitivity and specificity (Wang et al., 2012) Detection of IgM antibody in cats IgM antibodies were detected within 2-4 weeks after infection of experimentally infected cats with ELISA, but these animals became negative within 16 weeks. IgM titers after 16 weeks have been detected in cats co-infected with FIV and with ocular toxoplasmosis. Some percentages of cats do not have detectable IgM titers. The IgM antibodies cannot be used to predict the exact moment when a cat is shedding oocysts. One study of cats with clinical toxoplasmosis, IgM titers were detected in 93% of cats and IgG in 60% of cats. Thus, IgM antibodies have a higher predictive value for clinical toxoplasmosis than IgG. In the case of cats with chronic toxoplasmosis, they are firstly IgM positive and later negative, but they can become positive again after reinfection due to immunosuppression by FIV or glucocorticoid treatment. In these cats with chronic toxoplasmosis, they do not show clinical signs of the infection, therefore the detection of IgM antibodies will not always indicate clinical toxoplasmosis (Sykes, 2013). The IgM antibodies are difficult to detect in feline sera and results using the Latex Agglutination and Indirect Hemagglutination methods have shown not to be accurate (Lappin and Powell, 1991). 9
11 2.6.3 Detection of IgG antibody in cats With ELISA method, IgG antibodies can be detected in the serum of cats within 3-4 weeks after infection. Usually by the time these antibodies are detected, the shedding period of the oocysts has finished, thus IgG seropositive cats pose a lower threat to the Public Health (Sykes, 2013). In experimentally inoculated cats, the IgG can be detected for at least 6 years in the serum. The parasite and the antibodies produced against it can persist lifelong in the host (Sykes, 2013). Also confirmed by Salant and Spira (2004), IgG antibodies are known to remain high in cats for many years after infection. The low titers indicate a past infection and high titers indicate a more recent or current infection (Michalski et al., 2010), but detection of IgG antibodies do not give information when the infection occurred (Wang et al., 2012). It has been shown that healthy cats can have titers above 1:10,000, thus high titers of IgG do not accurately indicate a recent or active infection. Cats with low number of IgG titers can be considered seronegative based on the cut-off titer and serological method used (even though T. gondii persist in the tissues). Increasing IgG titers indicate a recent or active infection. The time from the first detectable antibody to the highest titer is seen in 2-3 weeks. Increasing IgG titers are seen in healthy and sick animals, so the presence of the antibody alone does not indicate clinical toxoplasmosis. In case of chronic toxoplasmosis, if reinfection occurs after immunosuppression, IgG titers seldom increase (Sykes, 2013). 2.7 Studies on Toxoplasma gondii infection in cats Due to an estimation that 30-40% of cats are infected with T. gondii worldwide (Waap et al., 2012), the serology diagnosis makes it possible to determine the prevalence rates in the different countries. Cats that are seropositive are already likely to have shed T. gondii oocysts and the detection of antibodies in their serum is useful to indicate the degree of contamination of the parasite in the environment and the risk it poses for humans (Lopes et al., 2008). The prevalence of T. gondii antibodies in cats, gives an indirect indication of the presence of T. gondii contamination of the environment (Wang et al., 2012). The prevalence rates of T. gondii infection in cats, varies between countries, within a country, even within the same city (Sukhumavasi et al., 2012). One of the highest prevalence rates was detected in Majorca Spain
12 (84.7%), this indicates that feral cats have highly contaminated the environment and therefore humans are at risk of possible infection (Millan et al., 2009). A study carried out in Romania used IFAT and ELISA, in which higher prevalence was found in older cats, with mixed diet, with outdoor access and from rural area. The sex and breed of the cats did not influence the prevalence rate (Gyorke et al., 2011). From Arad County, Romania, 42 feline fecal samples were collected, all showed negative results for T. gondii oocysts (Hotea et al., 2009). In Czech Republic, sera samples from 390 cats were collected and screened for IgG and IgM antibodies using IFAT (cut off titer 1:10). The overall prevalence was 61.3%. Only one cat was positive for IgM antibody. The flotation method was used to detect T. gondii oocysts and were detected in one fecal sample (Svobodova et al., 1998). In Poland, the sera samples from 135 domestic cats were sampled and IgG was detected using Indirect Agglutination Assay. Eighty-seven (64.4%) cats were seropositive (Michalski et al., 2010). In Scandinavian and Baltic countries, the prevalence ranged from 42% in Sweden and up to 60.8% in Estonia (Uggla et al., 1990; Must et al., 2015). In Latvia, 80 feline fecal samples were examined for T. gondii oocysts. Toxoplasma-like oocysts were found in two samples (Deksne et al., 2013). In Belgium the sera of 567 domestic cats were tested for IgG and IgM antibodies using IFAT. The cut off titer was 1:40. The seroprevalence was 25%. Only one cat was IgM positive and IgG negative, respectively. Older cats showed higher IgG titers (De Craeye et al., 2008). In the Netherlands, the sera samples from 450 cats were collected. The overall seroprevalence was estimated to be 18.2% when the presence of IgG antibodies were detected with ELISA. The determined risk factors were: age, hunting behavior, former stray cat and feeding of raw meat (Opsteegh et al., 2012).
13 In Germany, a total of 18,259 cats fecal samples were analyzed for the detection of T. gondii oocysts with PCR. In total, 68 (0.25%) samples were positive (Herrmann et al., 2010). In Spain, the sera samples from 585 cats were tested for IgG antibodies using IFAT. The cut off titer was 1:80. The prevalence was 32.3%. Higher seroprevalence was detected in stray and farm cats (Miro et al., 2004). In Turkey, the sera samples of 1121 stray cats were collected and tested for IgG antibodies using IFAT and ELISA. The cut off titer was 1:16. The overall prevalence was 33.5% with both assays (Can et al., 2014). In Japan, a total of 1,447 cats were examined, T. gondii antibodies were detected using LA. Seroprevalence was 5.4% (78/1,447). Higher prevalence was seen in outdoor and older cats (Maruyama et al., 2003). During a study in the United States, the sera samples were collected from 12,628 clinically sick cats for detecting IgG and IgM antibodies with ELISA. The overall seroprevalence was 31.6%. The age, gender and breed of cats influenced the seroprevalence (Vollaire et al., 2005). In Mexico, 37 sera samples of 169 domestic cats had IgG antibodies. The seroprevalence was 21.8% determined with ELISA. Risk factors for infection rate were increasing age, female gender and feeding raw meat (Besne-Merida et al., 2008). In Brazil, Bresciani et al. (2006) reported that 25% of 400 cats had T. gondii infection when they used IFAT (cut off titer 1:64).
14 2.8 Studies on Toxoplasma gondii infection in cats in Hungary The first study to be carried on T. gondii in cats was in The unsporulated oocysts were searched in the fecal samples of 60 cats using the flotation method. No cats were found to be actively shedding oocysts at the time of examination (Hegedus, 1979). A second survey was performed in 2004, the sera samples were collected from 264 cats and 112 dogs for studying T. gondii antibodies using the Complement Fixation Test. Seventy-three cats had antibodies and the seroprevalence was 28%. The study showed higher prevalence in outdoor and younger cats (between 1,5-5 years) from the countryside (47%), compared to those living in Budapest (17%). No correlation was found between the seroprevalence and the breed or gender of the cats (Chikan, 2004). A few years ago Hornok et al. (2007) studied the presence of IgG antibodies developed against T. gondii in 330 cats using IFAT. The seroprevalence was 47.6% (157/330). The sera were diluted at 1:20 to 1:10,240. High prevalence 80.3% (126/157) was detected at high titers between 1:640 to 1:5120. The prevalence was 22.4% in urban cats, 50% in suburban cats and 61.3% in rural cats. Females (53.3%) had a higher prevalence than males (39.3%). This study determined that higher prevalence of T. gondii infection occurred in rural, female and older cats.
15 3. Materials and methods 3.1 Materials Altogether sera samples were collected from 100 cats kept in Budapest and 100 cats from the countryside. There were 99 male and 101 female cats, some of them were castrated. The majority of cats (168) were non-purebred. Based on the age the cats belonged to the following groups: Group 1 (11 cats) < 1 year, Group 2 (53 cats) from 1 to 5 years of age, Group 3 (123 cats) between 6 and 15 years of age and Group 4 (13 cats) > 15 years. The sera were stored at minus 20º C until examined at the Department of Parasitology and Zoology, Faculty of Veterinary Science, Budapest. 3.2 Serological Method The IgG antibody of cats developed against Toxoplasma gondii were detected with an Indirect Immunofluorescence Antibody Test using MegaFLUO TOXOPLASMA g. (Diagnostik Megacor Laboratory, Austria). The test kit contained: - 10 slides with 10 wells coated with toxoplasma tachyzoites - 1 dropper bottle with 0,5 ml Positive control - 1 dropper bottle with 0,5 ml Negative control - 1 dropper bottle with 3,0 ml of anti cat-fitc Conjugate - 1 dropper bottle with 3,0 Mounting fluid Laboratory materials used: - Phosphate buffered saline solution (PBS, ph 7,4) - Micropipettes - Mixing wells for sera and PBS dilutions - Coverslips for the slides - Humid chamber for incubation of slides at 37 degrees Celsius - Fluorescent microscope (Carl Zeiss Jena, Germany) at 400x magnification
16 3.3 Methods Firstly, the slides were removed from the foil pouch and placed on a plastic container. The slides contained T. gondii tachyzoites. Each slide contained 10 wells; the first and the second well of each slide were used for positive and negative control, respectively. The sera of cats were diluted in PBS at the standard titer of 1:50. This is the cut-off titer written in the manual. If the serum was positive it was furthered screened at every twofold serial dilutions from 1:50 until it was seronegative. After incubating the slides at 37ºC for 30 minutes they were washed in PBS solution for removing the excess of unbound serum proteins. After this, the fluorescein isothiocyanate (FITC) labelled anti-cat IgG antibodies, as the conjugate, was placed in each well of the slides. The slides were incubated again for 30 minutes at 37ºC and after that washed to remove the unreacted conjugate. Finally, the results were detected under the fluorescent microscope in a dark room. The positive reaction shows bright green fluorescent colour forming a complete ring around the membrane of the T. gondii tachyzoites (Figure 1). The negative reaction is red fluorescent colour on the membrane of the T. gondii tachyzoites due to Evan s Blue counterstain or just one pole of the membrane of the tachyzoite is stained. Figure 1: IgG antibody positive result (The bright green fluorescent rings indicate positive IgG antibody reaction)
17 () Results The results are summarized in Table 1 and Figures 2-5. Seropositivity of cats From 200 cat sera samples 136 were found to be positive using the cut-off titer (1:50) (Table 1) and 64 were negative. The overall seroprevalence was 68.0% (Figure 2), with 69 positive cats from Budapest and 67 cats from the countryside (Table 1). Table 1: Number of cats from Budapest and countryside having T. gondii IgG antibody D*+,-*./.: ;<=>?@AB?@?BB?@CBB?@EBB?@FBB?@?GBB?@HCBB?@GEBB?@?CFBB?@CAGBB IJ GK AH EE HG?F?C L H? B M GL AB EC HL CL CB L H C B TNOQR UVW UXV YW ZV ([ V\ U( W V X BP: Budapest C: Countryside Prevalence 100% 80% 60% 40% 20% 0! 68% P"#$%$&' 32% Negative Figure 2: Prevalence of seropositive and seronegative cats
18 l f kj f g h f i e { }~ ƒ }~ ˆ} Š }~ ƒ }~ ˆ Œ Š } Ž ƒ ƒ ~ ~ ~ } dilution of sera when more than half of the cats were still seropositive was 1:400 among the cats from Budapest as well as from countryside (Table 1, Figure 3). 100% 80% 60% 40% 20% _` abcd 1:100 1:200 1:400 1:800 1:1600 1:3200 1:6400 1: :25600 mnopqrst uvnwtxyszor Dilutions Figure 3: Seropositive samples at different dilutions The seroprevalence of the tested cats from Budapest was almost the same as the seroprevalence of cats tested from the countryside in the first three dilutions (1:50, 1:100 and 1:200). In the next dilution (1:400) high percentage of cats from Budapest still remained positive comparing with the other group. In the next two dilutions (1:800 and 1:1600) the seroprevalence was higher among the cats from the countryside. From Budapest and countryside, equal positive values were seen at the dilutions of 1:3200 and 1:6400. The number of seropositive animals decreased with the dilution and no infected animals were found at the highest dilution (1:25600) (Table 1, Figure 3). ]^
19 ª«««±² ±³ ±² µ± ª Among the cats kept in Budapest, 33 out of 52 (63.5%) males had IgG antibody against T. gondii. The seroprevalence of females was higher (75%, 36/48) comparing with the results of males (Figure 4). Higher percentage of males (72.3%, 34/47) than females (62.3%, 33/53) were found to be seropositive which lived in the countryside (Figure 4). Prevalence 100% 80% 60% 40% 20% 63.5% 75.0% 72.3% 62.3% š œ ž Females Ÿ ž œ Figure 4: Seroprevalence according to gender The seroprevalence of cats increased with age, in both groups, seen the highest in cats above 15 years of age (Figure 5). Among the cats which lived in the countryside, more than 60% of animals were seropositive above 1-year-old. This level of seropositivity was also found in cats kept in Budapest. 100% 80% Prevalence 60% 40% 20% 33.3% 40% 76.5% 71.4% 65.0% 44.4% 87.5% 80% ¹ 1 to 5 6 to 15 >15 º»¼½¾ ÀÁ ÂûÄÁÅÆÀǼ Age in years Figure 5: Seroprevalence of cats according to different age groups
20 5. Discussion Toxoplasma gondii is a zoonotic parasitic species found worldwide, which has great importance in humans, especially pregnant women and immunocompromised patients (Dubey, 2010; Sykes, 2013; Bowman, 2013). It is also important in veterinary medicine because of causing abortion, mild or severe diseases in domestic animals (Khan and Line, 2010). Domestic and wild felids play a key role in the epidemiology of T. gondii, because they are the only definitive hosts of the parasite. The oocysts of T. gondii shed with the feces of the cats and contaminate the environment from where humans and all warm blooded animals as the intermediate hosts of the parasite can become infected (Dubey, 2010). Humans as well as carnivorous domestic animals can also become infected by eating the meat of infected animals, which contain tissue cysts (Dubey, 2008). From epidemiological point of view it is important to know whether the domestic cats living around humans and domestic animals are infected with T. gondii or not. It is difficult to find the oocysts at the time of shedding because the infected cats shed oocysts for 1-3 weeks usually once in their lifetime (Sykes, 2013). According to the investigations done earlier, only a few carried out fecal examinations. In these studies, they found negative results (Hegedus, 1979; Hotea et al., 2009) or very few infected cats with shedding oocysts. In Germany, a large sample size (18,259 cats) was used and the rate of oocyst shedding was 0.25% (Herrmann et al., 2010). According to these reports, it is difficult to determine positive cats at the precise moment of shedding and it varies among the sample number examined. The likelihood of more positive cats should be found in larger sample numbers and in spite of cats excreting up to millions of oocysts, they are rarely found in fecal samples (Lopes et al., 2008). Further difficulty arises with microscopical examination, since the oocysts of T. gondii cannot be distinguished from those of Hammondia, Besnoitia and Neospora spp. (Sykes et al., 2013; Deksne et al., 2013). Therefore, PCR detection of the T. gondii DNA has proven to be more accurate in detecting positive feline fecal samples (Wang et al., 2012). However, nowadays the most frequent method used for detecting T. gondii infection in cats is by serological methods (Sykes, 2013). The advantages of serological methods is that it gives an indirect ÈÉ
21 evaluation about the presence of T. gondii in the environment, thus providing the risks it poses for humans and warm blooded animals (Lopes et al., 2008; Gyorke et al., 2011; Wang et al., 2012). With serological methods, the antibodies produced against T. gondii by the definitive hosts can be detected in their sera. Usually IgG and IgM antibody types are detected. The presence of IgG antibodies are found in a latent infection, as for IgM antibodies appear in more recent infections (Sykes, 2013). The importance of the different dilutions of the sera is that it may indicate an acute or chronic infection. As mentioned by Michalski et al. (2010), the low titers indicate a past infection and high titers indicate a more recent infection. There are some difficulties that arise when detecting IgM antibody type, because it is poorly detected with the Agglutination Tests (Lappin and Powell, 1991; Sykes, 2013) and some cats do not have detectable IgM titers (Sykes, 2013). For this reason, only IgG antibodies were detected in this study. It is important to mention that cats which are IgG seropositive, are considered safer pets and pose a lower risk to humans, because they most likely have shed T. gondii oocysts (Sykes, 2013; Bowman, 2013). The estimated prevalence of infected cats with T. gondii is thought to be around 30-40% worldwide (Waap et al., 2012). However the number of Toxoplasma infected cats ranges from a few percentages up to 80-90%. It was reported from Mexico that the seroprevalence of the tested cats was 21.8% (Besne-Merida et al., 2008), 25% in Brazil (Bresciani et al., 2006) and 31.6% in the USA (Vollaire et al., 2005). In Far East Asia, the prevalence rates were much lower, only 5.4% in Japan (Maruyama et al., 2003). In Europe the percentage of the infected cats varied from 18.2% reported in the Netherlands (Opsteegh et al., 2012) up to 84.7% reported in Majorca, Spain (Millan et al., 2009). The results obtained in this study are similar to those reported from Czech Republic (61.3%) and Poland (64.4%) where the sera of 390 and 135 cats were studied using IFAT and Indirect Agglutination Assay, respectively (Svobodova et al., 1998; Michalski et al., 2010). There are several different serological kits on the market. The disadvantage of this diagnostic technique is that all serological methods have shown different sensitivity and specificity as ÊË
22 discussed by many authors (Salant and Spira, 2004; Da Craeye et al., 2008; Lopes et al., 2008). For this reason and the sample size used, the seroprevalence rates vary regarding the data reported from other countries (Sukhumavasi et al., 2012). For instance, the scientists who worked with IFAT for detecting anti-t. gondii IgG used different cut-off titers. The cut-off titer was 1:10 in Czech Republic (Svobodova et al., 1998), 1:16 in Turkey (Can et al., 2014), 1:40 in Belgium (Da Craeye et al., 2008), 1:64 in Brazil (Bresciani et al., 2006) and 1:80 in Spain (Miro et al., 2004). Therefore it is almost impossible to compare the results with other published data. On the other hand, cats with low titers can be considered seronegative based on the cut-off titer used as discussed by Sykes (2013). As mentioned by several authors, there is no international standard for diagnosing T. gondii infection and results vary between countries, within a country and even within the same city (Hornok et al., 2007; Sukhumavasi et al., 2012). The Spanish scientists concluded that the results also varied depending on the origin of the animals, whether they were stray or household cats (Miro et al., 2004). Opsteegh et al. (2012) confirmed the conclusion of Miro et al. (2004). Other scientists concluded that the prevalence was not influenced by the origin of cats (Deksne et al., 2013). Generally it can be stated that amongst stray cats, the seroprevalence of T. gondii infection may be higher because of outdoor access, therefore the cats may become infected by eating rodents and birds as the intermediate hosts of the parasite. Mostly, outdoor access was proven to be a risk factor for T. gondii infection in cats as reported by some authors (Maruyama et al., 2003; Gyorke et al., 2011). From Hungary, the author Chikan (2004) also concluded the same. Ospteegh et al. (2012) determined hunting behavior was also a risk factor. In previous serological studies of cats carried out in Hungary, high seroprevalence rate was also found among rural cats (Chikan, 2004; Hornok et al., 2007). Fourty-seven percent of rural cats out of 264 were found seropositive by Chikan (2004) who used the Complement Fixation Test. Three years later when Hornok et al. (2007) checked Toxoplasma infection in 330 cats with IFAT, they found that 61.3% of rural cats were seropositive. In our study the prevalence (67%) in rural cats was higher than those compared to the previous Hungarian studies. The difference in results may be due to the different sample size and serological method used. All ÌÍ
23 the cats collected in this study were domestic household pets. Concerning our findings, the seroprevalence of cats from Budapest and the countryside was 69% and 67%, respectively. This difference was not considered to be statistically significant. Based on these results it could be assumed that the infection was homogenously spread among the cat population in Hungary. However, this conclusion may not be true because only a limited number of cats were screened in this study, due to the difficulties of sampling and the financial situation. According to the gender, this study showed higher seroprevalence (75%) in females than males (63.5%) amongst cats living in Budapest. On the contrary, male cats showed higher seroprevalence (72.3%) than females (62.3%) kept in the countryside. Some earlier studies reported that the rate of the seroprevalence did not differ between the female and male cats (Chikan, 2004; Gyorke et al., 2011), while other authors found a difference between the infection rate of females and males (Vollaire et al., 2005; Besne-Merida et al., 2008). Interestingly, females showed higher prevalence rates in two studies (Hornok et al., 2007; Besne-Merida et al., 2008). Due to these conflicting results, it is difficult to regard the gender as a strong risk factor for T. gondii infection in cats. Further investigation would be needed to figure out whether T. gondii infection is more prevalent in male or female cats and what is/are its reason(s). Taking into account the age of the cats, this studied showed that the seroprevalence of cats increased with advancing of age. The results showed the highest (> 80%) seropositivity above 15 year old cats, originating both from Budapest and the countryside. The majority of cats were infected with T. gondii above one year of age. This indicates that a high percentage of cats are infected with T. gondii from an early age in both Budapest and the countryside. The results are in connection with those from previous studies in Hungary. The seroprevalence was also found to increase with age reported by Hornok et al. (2007) and Chikan (2004). The relationship between seroprevalence rate of T. gondii infection and increasing age of cats, is in accordance with the majority of the results from other countries, such as in Belgium (De Craeye et al., 2008), Romania (Gyorke et al., 2011), Netherlands (Opsteegh et al., 2012), Japan (Maruyama et al., 2003), USA (Vollaire et al., 2005) and Mexico (Besne-Merida et al., 2008). ÎÎ
24 Finally, it can be concluded that the sample size used in this study was small, but the data confirmed that T. gondii infection is prevalent among local cats of Hungary, like reported all over the world. This accounts for the high amount of oocyst shedding in the environment and many animals and humans are at risk of infection. Further investigation would be needed on how to minimize the infection of T. gondii in cats and ultimately to the intermediate hosts and humans. ÏÐ
25 6. Abstract The aim of this study was to detect the anti-t. gondii IgG antibodies in the sera collected from 100 cats living in Budapest and 100 kept in the countryside. The serological method used was a marketed Immunofluorescence Antibody Test (MegaFLUO TOXOPLASMA g). We also investigated the relationship between the seroprevalence rate of T. gondii infected cats with factors such as the origin of the cats, their gender and age. A total of 136 (68%) cats were found to be seropositive, with 69 and 67 positive cats from Budapest and from the countryside, respectively. The cut off titer was 1:50. The number of seropositive cats decreased at higher dilutions, the highest dilution titer of positive sera was at 1: The seroprevalence varied only slightly according to the gender of the cats from both Budapest and the countryside. More than 60% of the cats above 1 year old were seropositive, from both Budapest and countryside. Higher seroprevalence was found in older cats above 15 years of age. Overall the results showed a high seroprevalence rate of Toxoplasma gondii infection among cats from Hungary and the most important associated factor determined was the increasing of age. ÑÒ
26 7. Acknowledgements Firstly, I would like to thank the Budaors Diagnostic Laboratory for supplying many of the feline sera samples. I would like to thank the Department of Parasitology and Zoology, Faculty of Veterinary Sciences, Budapest for letting me take part in their research. Especially a warm thanks to the Head of the Department, Professor Róbert Farkas as the supervisor of my thesis, for his guidance and patience. I thank Mónika Gyurkovsky, the laboratory assistant, for her help and support during the laboratory work. Finally, I would also like to thank my family and friends who have helped me accomplish my thesis. ÓÔ
27 8. Bibliography BESNE-MERIDA, A., FIGUEROA-CASTILLO, J., MARTINEZ-MAYA, J., LUNA- PASTEN, H., CALDERON-SEGURA, E., CORREA, D. (2008). Prevalence of antibodies against Toxoplasma gondii in domestic cats from Mexico City. Veterinary Parasitology, 157(3-4), p BOWMAN, D. (2013). Georgis' Parasitology for Veterinarians.10th ed. St.Louis, Missouri: Saunders, p BRESCIANI, K., GENNARI, S., SERRANO, A., RODRIGUES, A., UENO, T., FRANCO, L., PERRI, S., AMARANTE, A. (2006). Antibodies to Neospora caninum and Toxoplasma gondii in domestic cats from Brazil. Parasitology Research, 100(2), p CAN, H., DOSKAYA, M., AJZENBERG, D., OZDEMIR, H., CANER, A., IZ, S., DOSKAYA, A., ATALAY, E., CETINKAYA, C., URGEN, S., KARACALI, S., UN, C., DARDE, M., GURUZ, Y. (2014). Genetic characterization of Toxoplasma gondii isolates and toxoplasmosis seroprevalence in stray cats of Izmir, Turkey. PLOS ONE, 9(8), p.e ØÙÚÛÜÝ Þß àáââãäß Macskak es kutyak Toxoplasma gondii okozta fertozottsegenek åæçèéêéëìíì îìæågalata. Szakdolgozat. D'AMORE, E., FALCONE, E., BUSANI, L., TOLLIS, M. (1997). A serological survey of feline immunodeficiency virus and Toxoplasma gondii in stray cats. Veterinary Research Communications, 21(5), p DE CRAEYE, S., FRANCART, A., CHABAUTY, J., DE VRIENDT, V., VAN GUCHT, S., LEROUX, I., JONGERT, E. (2008). Prevalence of Toxoplasma gondii infection in Belgian house cats. Veterinary Parasitology, 157(1-2), p DEKSNE, G., PETRUSEVICA, A., KIRJUSINA, M. (2013). Seroprevalence and factors associated with toxoplasma gondii infection in domestic cats from urban areas in Latvia. Journal of Parasitology, 99(1), p ÕÖ
28 DUBEY, J. (2008). The history of Toxoplasma gondii-the first 100 years. Journal of Eukaryotic Microbiology, 55(6), p DUBEY, J. (2010). Toxoplasmosis of animals and humans.2 nd ed. Boca Raton, Florida: CRC Press. p GYORKE, A., OPSTEEGH, M., MIRCEAN, V., IOVU, A., COZMA, V. (2011). Toxoplasma gondii in Romanian household cats: Evaluation of serological tests, epidemiology and risk factors. Preventive Veterinary Medicine, 102(4), p HEGEDUS, B. (1979). Adatok a macska toxoplasmosis-terjesztesenek szerepehez. Szakdolgozat. HERRMANN, D., PANTCHEV, N., VRHOVEC, M., BARUTZKI, D., WILKING, H., FROHLICH, A., LUDER, C., CONRATHS, F., SCHARES, G. (2010). Atypical Toxoplasma gondii genotypes identified in oocysts shed by cats in Germany. International Journal for Parasitology, 40(3), p HORNOK, S., EDELHOFER, R., JOACHIM, A., FARKAS, R., BERTA, K., REPASI, A., LAKATOS, B. (2007). Seroprevalence of Toxoplasma gondii and Neospora caninum infection of cats in Hungary. Acta Veterinaria Hungarica, 56(1), p HOTEA, I., DARABUS, G., ILIE, M., IMRE, K., CIOCAN, R., BALINT, A., INDRE, D., MANDITA, D. (2009). The prevalence of Toxoplasma gondii infection in cats from Hunedoara county. Scientific Works, 52(11), p KHAN, C., LINE, S. (2010). The Merck Veterinary Manual. 10th ed. Whitehouse Station, New Jersey: Merck & Co, p. 420,620. LAPPIN, M. (1996). Feline toxoplasmosis: Interpretation of diagnostic test results. Seminars in Veterinary Medicine and Surgery: Small Animal, 11(3), p LAPPIN, M., POWELL, C. (1991). Comparison of Latex Agglutination, Indirect Hemagglutination, and ELISA techniques for the detection of Toxoplasma gondii-specific antibodies in the serum of cats. Journal of Veterinary Internal Medicine, 5(5), p ïð
29 LOPES, A., CARDOSO, L., RODRIGUES, M. (2008). Serological survey of Toxoplasma gondii infection in domestic cats from northeastern Portugal. Veterinary Parasitology, 155(3-4), p MARUYAMA, S., KABEYA, H., NAKAO, R., TANAKA, S., SAKAI, T., XUAN, X., KATSUBE, Y., MIKAMI, T. (2003). Seroprevalence of Bartonella henselae, Toxoplasma gondii, FIV and FeLV infections in domestic cats in Japan. Microbiology and Immunology, 47(2), p MICHALSKI, M., PLATT-SAMORAJ, A., MIKULSKA-SKUPIEN, E. (2010). Toxoplasma gondii antibodies in domestic cats in Olsztyn urban area, Poland. Wiadomosci Parazytologiczne, 53(3), p MILLAN, J., CABEZON, O., PABON, M., DUBEY, J., ALMERIA, S. (2009). Seroprevalence of Toxoplasma gondii and Neospora caninum in feral cats (Felis silvestris catus) in Majorca, Balearic Islands, Spain. Veterinary Parasitology, 165(3-4), p MIRO, G., MONTOYA, A., JIMENEZ, S., FRISUELOS, C., MATEO, M., FUENTES, I. (2004). Prevalence of antibodies to Toxoplasma gondii and intestinal parasites in stray, farm and household cats in Spain. Veterinary Parasitology, 126(3), p MUST, K., LASSEN, B., JOKELAINEN, P. (2015). Seroprevalence of and risk factors for Toxoplasma gondii infection in cats in Estonia. Vector-Borne and Zoonotic Diseases, 15(10), p OPSTEEGH, M., HAVEMAN, R., SWART, A., MENSINK-BEEREPOOT, M., HOFHUIS, A., LANGELAAR, M., VAN DER GIESSEN, J. (2012). Seroprevalence and risk factors for Toxoplasma gondii infection in domestic cats in The Netherlands. Preventive Veterinary Medicine, 104(3-4), p SALANT, H., SPIRA, D. (2004). A cross-sectional survey of anti-toxoplasma gondii antibodies in Jerusalem cats. Veterinary Parasitology, 124(3-4), p ñò
30 SUKHUMAVASI, W., BELLOSA, M., LUCIO-FORSTER, A., LIOTTA, J., LEE, A., PORNMINGMAS, P., CHUNGPIVAT, S., MOHAMMED, H., LORENTZEN, L., DUBEY, J., BOWMAN, D. (2012). Serological survey of Toxoplasma gondii, Dirofilaria immitis, Feline Immunodeficiency Virus (FIV) and Feline Leukemia Virus (FeLV) infections in pet cats in Bangkok and vicinities, Thailand. Veterinary Parasitology, 188(1-2), pp SVOBODOVA, V., KNOTEK, Z., SVOBODA, M. (1998). Prevalence of IgG and IgM antibodies specific to Toxoplasma gondii in cats. Veterinary Parasitology, 80(2), p SYKES, J. (2013). Canine and feline infectious diseases. St.Louis, Missouri: Saunders, p UGGLA, A., MATTSON, S., JUNTTI, N. (1990). Prevalence of antibodies to Toxoplasma gondii in cats, dogs and horses in Sweden. Acta Veterinaria Scandinavia, 31(2), p VOLLAIRE, M., RADECKI, S., LAPPIN, M. (2005). Seroprevalence of Toxoplasma gondii antibodies in clinically ill cats in the United States. American Journal of Veterinary Research, 66(5), p WAAP, H., CARDOSO, R., LEITAO, A., NUNES, T., VILARES, A., GARGATE, M., MEIRELES, J., CORTES, H., ANGELO, H. (2012). In vitro isolation and seroprevalence of Toxoplasma gondii in stray cats and pigeons in Lisbon, Portugal. Veterinary Parasitology, 187(3-4), p WANG, Q., JIANG, W., CHEN, Y., LIU, C., SHI, J., LI, X. (2012). Prevalence of Toxoplasma gondii antibodies, circulating antigens and DNA in stray cats in Shanghai, China. Parasites & Vectors, 5(1), p.190. óô
JOURNAL OF INTERNATIONAL ACADEMIC RESEARCH FOR MULTIDISCIPLINARY Impact Factor 2.417, ISSN: , Volume 4, Issue 2, March 2016
 EPIDEMIOLOGY OF TOXOPLASMA GONDII INFECTION OF CATS IN SOUTHWEST OF ALBANIA SHEMSHO LAMAJ 1 GERTA DHAMO 2 ILIR DOVA 2 1 Regional Agricultural Directory of Gjirokastra 2 Faculty of Veterinary Medicine,
EPIDEMIOLOGY OF TOXOPLASMA GONDII INFECTION OF CATS IN SOUTHWEST OF ALBANIA SHEMSHO LAMAJ 1 GERTA DHAMO 2 ILIR DOVA 2 1 Regional Agricultural Directory of Gjirokastra 2 Faculty of Veterinary Medicine,
Systemic Apicomplexans. Toxoplasma
 Systemic Apicomplexans Toxoplasma Protozoan Groups Historically, protozoa have been grouped by mode of motility. Flagellates Hemoflagellates Trypanosoma cruzi Leishmania infantum Mucoflagellates Tritrichomonas
Systemic Apicomplexans Toxoplasma Protozoan Groups Historically, protozoa have been grouped by mode of motility. Flagellates Hemoflagellates Trypanosoma cruzi Leishmania infantum Mucoflagellates Tritrichomonas
Above: life cycle of toxoplasma gondii. Below: transmission of this infection.
 Toxoplasmosis PDF This article is based on a paid for research paper dated 1972 of similar title and authored by J.K.Frenkel and J.P. Dubey. It was published by The Journal of Infectious Diseases Vol.
Toxoplasmosis PDF This article is based on a paid for research paper dated 1972 of similar title and authored by J.K.Frenkel and J.P. Dubey. It was published by The Journal of Infectious Diseases Vol.
Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51
 Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual reproduction Intestinal
Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual reproduction Intestinal
For Vets General Information Prevalence of Tox Prevalence of opl Tox asm opl asm Humans Hum Animals Zoonotic Risk & Other Ris Zoonotic Risk & Ot
 For Vets General Information Toxoplasma gondii is a protozoal parasite capable of infecting any warm-blooded animal, including humans. Wild and domestic cats are the only known definitive hosts of Toxoplasma;
For Vets General Information Toxoplasma gondii is a protozoal parasite capable of infecting any warm-blooded animal, including humans. Wild and domestic cats are the only known definitive hosts of Toxoplasma;
For Public Health Personnel
 For Public Health Personnel General Information Toxoplasma gondii is a protozoal parasite capable of infecting any warm-blooded animal, including humans. Wild and domestic cats are the only known definitive
For Public Health Personnel General Information Toxoplasma gondii is a protozoal parasite capable of infecting any warm-blooded animal, including humans. Wild and domestic cats are the only known definitive
Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49
 Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual
Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual
Outline 1/13/15. Range is mostly surrounding Puerto Rico Important for Tourism and ecological balance
 1/13/15 Prevalence of Toxoplasma gondii in Antillean manatees (Trichechus manatus manatus) and investigating transmission from feral cat feces in Puerto Rico Heidi Wyrosdick M.S. Candidate University of
1/13/15 Prevalence of Toxoplasma gondii in Antillean manatees (Trichechus manatus manatus) and investigating transmission from feral cat feces in Puerto Rico Heidi Wyrosdick M.S. Candidate University of
Archives of Razi Institute, Vol. 69, No. 2, December (2014) Razi Vaccine & Serum Research Institute
 Archives of Razi Institute, Vol. 69, No. 2, December (2014) 165-170 Copyright 2014 by Razi Vaccine & Serum Research Institute Full Article Evaluation of Humoral Immune Response of Cats to the Experimental
Archives of Razi Institute, Vol. 69, No. 2, December (2014) 165-170 Copyright 2014 by Razi Vaccine & Serum Research Institute Full Article Evaluation of Humoral Immune Response of Cats to the Experimental
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign
 A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
Doctor B s BARF & Toxoplasmosis
 Doctor B s BARF & Toxoplasmosis Copyright Ian Billinghurst Introduction Ignorance is bliss so they say! Sometimes the less we know, the happier we are. Ignorance can most definitely be a source of bliss
Doctor B s BARF & Toxoplasmosis Copyright Ian Billinghurst Introduction Ignorance is bliss so they say! Sometimes the less we know, the happier we are. Ignorance can most definitely be a source of bliss
Seroprevalence and risk factors of infections with Neospora caninum and Toxoplasma gondii in hunting dogs from Campania region, southern Italy
 Institute of Parasitology, Biology Centre CAS doi: http://folia.paru.cas.cz Research Article Seroprevalence and risk factors of infections with Neospora caninum and Toxoplasma gondii in hunting dogs from
Institute of Parasitology, Biology Centre CAS doi: http://folia.paru.cas.cz Research Article Seroprevalence and risk factors of infections with Neospora caninum and Toxoplasma gondii in hunting dogs from
Eukaryotic Organisms
 Eukaryotic Organisms A Pictoral Guide of Supportive Illustrations to accompany Select Topics on Eukaryotic Oranisms Bacteria (Not Shown) Agent of Disease Reservoir Vector By Noel Ways Favorable Environmental
Eukaryotic Organisms A Pictoral Guide of Supportive Illustrations to accompany Select Topics on Eukaryotic Oranisms Bacteria (Not Shown) Agent of Disease Reservoir Vector By Noel Ways Favorable Environmental
Feline zoonoses. Institutional Animal Care and Use Committee 12/09
 Feline zoonoses Institutional Animal Care and Use Committee 12/09 Cat scratch disease Bacterial infection caused by Bartonella henselae Associated with a cat bite or scratch Infection at point of injury,
Feline zoonoses Institutional Animal Care and Use Committee 12/09 Cat scratch disease Bacterial infection caused by Bartonella henselae Associated with a cat bite or scratch Infection at point of injury,
Phylum:Apicomplexa Class:Sporozoa
 Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Coccidia. Nimit Morakote, Ph.D.
 Coccidia Nimit Morakote, Ph.D. 1 Learning objectives After class, students will be able to: Describe morphology, life cycle, signs and symptoms, prevention and control, laboratory diagnosis and treatment
Coccidia Nimit Morakote, Ph.D. 1 Learning objectives After class, students will be able to: Describe morphology, life cycle, signs and symptoms, prevention and control, laboratory diagnosis and treatment
Surveillance of animal brucellosis
 Surveillance of animal brucellosis Assoc.Prof.Dr. Theera Rukkwamsuk Department of large Animal and Wildlife Clinical Science Faculty of Veterinary Medicine Kasetsart University Review of the epidemiology
Surveillance of animal brucellosis Assoc.Prof.Dr. Theera Rukkwamsuk Department of large Animal and Wildlife Clinical Science Faculty of Veterinary Medicine Kasetsart University Review of the epidemiology
PARASITOLOGICAL EXAMINATIONS CATALOGUE OF SERVICES AND PRICE LIST
 INSTITUTE OF PARASITOLOGY Biomedical Research Center Seltersberg Justus Liebig University Giessen Schubertstrasse 81 35392 Giessen Germany Office: +49 (0) 641 99 38461 Fax: +49 (0) 641 99 38469 Coprological
INSTITUTE OF PARASITOLOGY Biomedical Research Center Seltersberg Justus Liebig University Giessen Schubertstrasse 81 35392 Giessen Germany Office: +49 (0) 641 99 38461 Fax: +49 (0) 641 99 38469 Coprological
SEROPREVALENCE OF BRUCELLA SPP, LEPSTOSPIRA SPP AND TOXOPLASMA GONDII IN WILD BOARD (SUS SCROFA) FROM SOUTHERN BRAZIL
 SEROPREVALENCE OF BRUCELLA SPP, LEPSTOSPIRA SPP AND TOXOPLASMA GONDII IN WILD BOARD (SUS SCROFA) FROM SOUTHERN BRAZIL Iara Maria Trevisol 1, Beatris Kramer 1, Arlei Coldebella¹, Virginia Santiago Silva
SEROPREVALENCE OF BRUCELLA SPP, LEPSTOSPIRA SPP AND TOXOPLASMA GONDII IN WILD BOARD (SUS SCROFA) FROM SOUTHERN BRAZIL Iara Maria Trevisol 1, Beatris Kramer 1, Arlei Coldebella¹, Virginia Santiago Silva
Seroprevalence of IgG and IgM antibodies and associated risk factors for toxoplasmosis in cats and dogs from subtropical arid parts of Pakistan
 Tropical Biomedicine 31(4): 777 784 (2014) Seroprevalence of IgG and IgM antibodies and associated risk factors for toxoplasmosis in cats and dogs from subtropical arid parts of Pakistan Ahmad, N. 1*,
Tropical Biomedicine 31(4): 777 784 (2014) Seroprevalence of IgG and IgM antibodies and associated risk factors for toxoplasmosis in cats and dogs from subtropical arid parts of Pakistan Ahmad, N. 1*,
Canine Distemper Virus
 Photo: LE Carmichael, MJ Appel Photo: LE Carmichael, MJ Appel Photo: LE Carmichael, MJ Appel Canine Distemper Virus Canine Distemper (CD) is a highly contagious infectious disease of dogs worldwide caused
Photo: LE Carmichael, MJ Appel Photo: LE Carmichael, MJ Appel Photo: LE Carmichael, MJ Appel Canine Distemper Virus Canine Distemper (CD) is a highly contagious infectious disease of dogs worldwide caused
PCR detection of Leptospira in. stray cat and
 PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
Sero-diagnosis of toxoplasmosis by using lateral flow chromatographic assay
 International Journal of Natural and Social Sciences, 2018, 5(1): 25-29 ISSN: 2313-4461 Sero-diagnosis of toxoplasmosis by using lateral flow chromatographic assay Md. Billal Hossain 1 *, Md. Younus Ali
International Journal of Natural and Social Sciences, 2018, 5(1): 25-29 ISSN: 2313-4461 Sero-diagnosis of toxoplasmosis by using lateral flow chromatographic assay Md. Billal Hossain 1 *, Md. Younus Ali
Feline Vaccines: Benefits and Risks
 Feline Vaccines: Benefits and Risks Deciding which vaccines your cat should receive requires that you have a complete understanding of the benefits and risks of the procedure. For this reason, it is extremely
Feline Vaccines: Benefits and Risks Deciding which vaccines your cat should receive requires that you have a complete understanding of the benefits and risks of the procedure. For this reason, it is extremely
Rapid Diagnostic Test for pet
 In vitro Diagnostic Rapid Diagnostic Test for pet Canine / Feline Rapid Test offers highly sensitive and specificity for the detection of antigen and antibody from various kinds of easily obtainable specimen.
In vitro Diagnostic Rapid Diagnostic Test for pet Canine / Feline Rapid Test offers highly sensitive and specificity for the detection of antigen and antibody from various kinds of easily obtainable specimen.
Feline Immunodeficiency Virus (FIV)
 Virus (FeLV) FIV and FeLV are both viruses within the same family of retroviruses, but they are in different groups within that family: FIV is in one group called lentiviruses these cause lifelong infections
Virus (FeLV) FIV and FeLV are both viruses within the same family of retroviruses, but they are in different groups within that family: FIV is in one group called lentiviruses these cause lifelong infections
9 Parasitology 9 EXERCISE EQA. Objectives EXERCISE
 0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
Evidence of Feline Immunodeficiency Virus, Feline Leukemia Virus, and Toxoplasma gondii in Feral Cats on Mauna Kea, Hawaii
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2007 Evidence of Feline Immunodeficiency Virus, Feline Leukemia Virus,
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2007 Evidence of Feline Immunodeficiency Virus, Feline Leukemia Virus,
04/02/2013. Parasites and breeding dogs: These parasites we don t hear so much about. Main internal parasites found in breeding kennels
 Parasites and breeding dogs: These parasites we don t hear so much about Main internal parasites found in breeding kennels Isospora sp. Giardia sp. Toxocara canis Something else? Breeders burden I m kind
Parasites and breeding dogs: These parasites we don t hear so much about Main internal parasites found in breeding kennels Isospora sp. Giardia sp. Toxocara canis Something else? Breeders burden I m kind
Canine Anaplasmosis Anaplasma phagocytophilum Anaplasma platys
 Canine Anaplasmosis Anaplasma phagocytophilum Anaplasma platys It takes just hours for an infected tick to transmit Anaplasma organisms to a dog. What is canine anaplasmosis? Canine anaplasmosis is a disease
Canine Anaplasmosis Anaplasma phagocytophilum Anaplasma platys It takes just hours for an infected tick to transmit Anaplasma organisms to a dog. What is canine anaplasmosis? Canine anaplasmosis is a disease
Data were analysed by SPSS, version 10 and the chi-squared test was used to assess statistical differences. P < 0.05 was considered significant.
 Toxocara canis is one of the commonest nematodes of the dog and most often this nematode is the cause of toxocariasis (visceral larva migrans) [1]. People become infected by ingestion of eggs from soil,
Toxocara canis is one of the commonest nematodes of the dog and most often this nematode is the cause of toxocariasis (visceral larva migrans) [1]. People become infected by ingestion of eggs from soil,
Control of Intestinal Protozoa in Dogs and Cats
 6 Control of Intestinal Protozoa in Dogs and Cats ESCCAP Guideline 06 Second Edition February 2018 1 ESCCAP Malvern Hills Science Park, Geraldine Road, Malvern, Worcestershire, WR14 3SZ, United Kingdom
6 Control of Intestinal Protozoa in Dogs and Cats ESCCAP Guideline 06 Second Edition February 2018 1 ESCCAP Malvern Hills Science Park, Geraldine Road, Malvern, Worcestershire, WR14 3SZ, United Kingdom
////////////////////////////////////////// Shelter Medicine
 ////////////////////////////////////////// Shelter Medicine To Test or Not to Test Confronting feline leukemia and feline immunodeficiency virus By Lila Miller, D.V.M. Just because a cat tests positive
////////////////////////////////////////// Shelter Medicine To Test or Not to Test Confronting feline leukemia and feline immunodeficiency virus By Lila Miller, D.V.M. Just because a cat tests positive
Control of Intestinal Protozoa in Dogs and Cats
 6 Control of Intestinal Protozoa in Dogs and Cats ESCCAP Guideline 06 Second Edition February 2018 1 TABLE OF CONTENTS INTRODUCTION 4 1: CONSIDERATION OF PET HEALTH AND LIFESTYLE FACTORS 5 2: LIFELONG
6 Control of Intestinal Protozoa in Dogs and Cats ESCCAP Guideline 06 Second Edition February 2018 1 TABLE OF CONTENTS INTRODUCTION 4 1: CONSIDERATION OF PET HEALTH AND LIFESTYLE FACTORS 5 2: LIFELONG
LABORATORY. The Protozoa. At the Bench
 LABORATORY Laboratory 8, Page 1 8 The Protozoa Introduction: The protozoa are unicellular animals that are classified on the basis of the organelles used for locomotion (flagella, pseudopodia, cilia or
LABORATORY Laboratory 8, Page 1 8 The Protozoa Introduction: The protozoa are unicellular animals that are classified on the basis of the organelles used for locomotion (flagella, pseudopodia, cilia or
Diagnosing intestinal parasites. Clinical reference guide for Fecal Dx antigen testing
 Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Diagnosing intestinal parasites. Clinical reference guide for Fecal Dx antigen testing
 Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
TOXOPLASMOSIS - AN OVERVIEW
 TOXOPLASMOSIS - AN OVERVIEW I JP Dubey Zoonotic Diseases Laboratory, Livestock and Poultry Sciences Institute, Agricultural Research Service, US Department of Agriculture, Beltsville, Maryland 20705-2530,
TOXOPLASMOSIS - AN OVERVIEW I JP Dubey Zoonotic Diseases Laboratory, Livestock and Poultry Sciences Institute, Agricultural Research Service, US Department of Agriculture, Beltsville, Maryland 20705-2530,
Feline Immunodefficiency Virus
 Feline Immunodefficiency Virus by Skye Patterson - Revised 1-Jun-15 Cats who are infected with feline immunodeficiency virus (FIV) may not show symptoms until years after the initial infection occurred.
Feline Immunodefficiency Virus by Skye Patterson - Revised 1-Jun-15 Cats who are infected with feline immunodeficiency virus (FIV) may not show symptoms until years after the initial infection occurred.
Hydatid Cyst Dr. Nora L. El-Tantawy
 Hydatid Cyst Dr. Nora L. El-Tantawy Ass. Prof. of Parasitology Faculty of Medicine, Mansoura university, Egypt Echinococcus granulosus Geographical Distribution: cosmopolitan especially in sheep raising
Hydatid Cyst Dr. Nora L. El-Tantawy Ass. Prof. of Parasitology Faculty of Medicine, Mansoura university, Egypt Echinococcus granulosus Geographical Distribution: cosmopolitan especially in sheep raising
EVALUATION OF THE SENSITIVITY AND SPECIFICITY OF THE EHRLICHIA CANIS DIAGNOSTIC TEST: Anigen Rapid E.canis Ab Test Kit
 EVALUATION OF THE SENSITIVITY AND SPECIFICITY OF THE EHRLICHIA CANIS DIAGNOSTIC TEST: Anigen Rapid E.canis Ab Test Kit FINAL REPORT Research contract (art. 83 of the L.O.U) between the Ehrlichiosis Diagnostic
EVALUATION OF THE SENSITIVITY AND SPECIFICITY OF THE EHRLICHIA CANIS DIAGNOSTIC TEST: Anigen Rapid E.canis Ab Test Kit FINAL REPORT Research contract (art. 83 of the L.O.U) between the Ehrlichiosis Diagnostic
PLASMODIUM MODULE 39.1 INTRODUCTION OBJECTIVES 39.2 MALARIAL PARASITE. Notes
 Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Antibody Test Kit for Feline Calici, Herpes and Panleukopenia Viruses (2011)
 Sensitivity-specificity and accuracy of the ImmunoComb Feline VacciCheck Antibody Test Kit for Feline Calici, Herpes and Panleukopenia Viruses (2011) Mazar S 1, DiGangi B 2, Levy J 2 and Dubovi E 3 1 Biogal,
Sensitivity-specificity and accuracy of the ImmunoComb Feline VacciCheck Antibody Test Kit for Feline Calici, Herpes and Panleukopenia Viruses (2011) Mazar S 1, DiGangi B 2, Levy J 2 and Dubovi E 3 1 Biogal,
Hurricane Animal Hospital 2120 Mount Vernon Road Hurricane, WV or
 Hurricane Animal Hospital 2120 Mount Vernon Road Hurricane, WV 25526 304-757-5937 or 304-757-2287 www.hurricaneanimalhospital.com Feline Leukemia Virus (FELV) This information handout is designed as a
Hurricane Animal Hospital 2120 Mount Vernon Road Hurricane, WV 25526 304-757-5937 or 304-757-2287 www.hurricaneanimalhospital.com Feline Leukemia Virus (FELV) This information handout is designed as a
FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT
![FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT](/thumbs/93/113164093.jpg) FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 22 APR 2018 Biogal Galed Laboratories Acs Ltd. tel: 972-4-9898605. fax: 972-4-9898690 e-mail:info@biogal.co.il
FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 22 APR 2018 Biogal Galed Laboratories Acs Ltd. tel: 972-4-9898605. fax: 972-4-9898690 e-mail:info@biogal.co.il
Diagnosis, treatment and control: dealing with coccidiosis in cattle
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Diagnosis, treatment and control: dealing with coccidiosis in cattle Author : Adam Martin Categories : Vets Date : January
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Diagnosis, treatment and control: dealing with coccidiosis in cattle Author : Adam Martin Categories : Vets Date : January
Feline Leukemia Holly Nash, DVM, MS
 1 of 7 2/5/2008 4:36 PM Feline Leukemia Holly Nash, DVM, MS Veterinary Services Department, Drs. Foster & Smith, Inc. What is feline leukemia? Feline leukemia is a cancerous disease caused by feline leukemia
1 of 7 2/5/2008 4:36 PM Feline Leukemia Holly Nash, DVM, MS Veterinary Services Department, Drs. Foster & Smith, Inc. What is feline leukemia? Feline leukemia is a cancerous disease caused by feline leukemia
Research Article Seroprevalence of Toxoplasma gondii in Dairy Cattle with Reproductive Problems in Sudan
 ISRN Veterinary Science Volume 2013, Article ID 895165, 4 pages http://dx.doi.org/10.1155/2013/895165 Research Article Seroprevalence of Toxoplasma gondii in Dairy Cattle with Reproductive Problems in
ISRN Veterinary Science Volume 2013, Article ID 895165, 4 pages http://dx.doi.org/10.1155/2013/895165 Research Article Seroprevalence of Toxoplasma gondii in Dairy Cattle with Reproductive Problems in
Scientific background concerning Echinococcus multilocularis. Muza Kirjušina, Daugavpils University, Latvia
 Scientific background concerning Echinococcus multilocularis Muza Kirjušina, Daugavpils University, Latvia Echinococcus multilocularis Infection with the larval form causes alveolar echinococcosis (AE).
Scientific background concerning Echinococcus multilocularis Muza Kirjušina, Daugavpils University, Latvia Echinococcus multilocularis Infection with the larval form causes alveolar echinococcosis (AE).
Apicomplexans Apicomplexa Intro
 Apicomplexans Apicomplexa Intro Cryptosporidium Apicomplexan Select Characteristics Gliding motility Apical Complex organelle for invasion of host cell Life cycle alternates b/w sexual and asexual phases
Apicomplexans Apicomplexa Intro Cryptosporidium Apicomplexan Select Characteristics Gliding motility Apical Complex organelle for invasion of host cell Life cycle alternates b/w sexual and asexual phases
Szent István University, Faculty of Veterinary Sciences. Department of Parasitology and Zoology
 Szent István University, Faculty of Veterinary Sciences Department of Parasitology and Zoology Serosurvey of Toxoplasma gondii infection in Hungarian sheep and goat flocks By Larissa Windmeier Supervisor:
Szent István University, Faculty of Veterinary Sciences Department of Parasitology and Zoology Serosurvey of Toxoplasma gondii infection in Hungarian sheep and goat flocks By Larissa Windmeier Supervisor:
OIE Collaborating Centres Reports Activities
 OIE Collaborating Centres Reports Activities Activities in 2016 This report has been submitted : 2017-03-25 00:33:18 Title of collaborating centre: Food-Borne Zoonotic Parasites Address of Collaborating
OIE Collaborating Centres Reports Activities Activities in 2016 This report has been submitted : 2017-03-25 00:33:18 Title of collaborating centre: Food-Borne Zoonotic Parasites Address of Collaborating
Epidemiology and Molecular Prevalence of Toxoplasma gondii in Cattle Slaughtered in Zahedan and Zabol Districts, South East of Iran
 Iran J Parasitol: Vol. 13, No. 1, Jan-Mar 2018, pp.114-119 Iran J Parasitol Tehran University of Medical Sciences Publication http://tums.ac.ir Open access Journal at http://ijpa.tums.ac.ir Iranian Society
Iran J Parasitol: Vol. 13, No. 1, Jan-Mar 2018, pp.114-119 Iran J Parasitol Tehran University of Medical Sciences Publication http://tums.ac.ir Open access Journal at http://ijpa.tums.ac.ir Iranian Society
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress PETS AS RESERVOIRS OF FOR ZOONOTIC DISEASE WHAT SHOULD WE ADVISE OUR CLINETS? Gad Baneth, DVM. Ph.D., Dipl. ECVCP
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress PETS AS RESERVOIRS OF FOR ZOONOTIC DISEASE WHAT SHOULD WE ADVISE OUR CLINETS? Gad Baneth, DVM. Ph.D., Dipl. ECVCP
Feline and Canine Internal Parasites
 Feline and Canine Internal Parasites Internal parasites are a very common problem among dogs. Almost all puppies are already infected with roundworm when still in the uterus, or get the infection immediately
Feline and Canine Internal Parasites Internal parasites are a very common problem among dogs. Almost all puppies are already infected with roundworm when still in the uterus, or get the infection immediately
Vaccines for Cats. 2. Feline viral rhinotracheitis, FVR caused by FVR virus, also known as herpes virus type 1, FHV-1
 Vaccines for Cats Recent advances in veterinary medical science have resulted in an increase in the number and type of vaccines that are available for use in cats, and improvements are continuously being
Vaccines for Cats Recent advances in veterinary medical science have resulted in an increase in the number and type of vaccines that are available for use in cats, and improvements are continuously being
Coccidia in a Shelter Setting Video Transcript July 2013
 Coccidia in a Shelter Setting Video Transcript July 2013 This transcript has been automatically generated and may not be 100% accurate. This text may not be in its final form and may be updated or revised
Coccidia in a Shelter Setting Video Transcript July 2013 This transcript has been automatically generated and may not be 100% accurate. This text may not be in its final form and may be updated or revised
Seroprevalence of Encephalitozoon cuniculi and Toxoplasma gondii in domestic rabbits (Oryctolagus cuniculus) in China
 ISSN (Print) 0023-4001 ISSN (Online) 1738-0006 BRIEF COMMUNICATION Korean J Parasitol Vol. 53, No. 6: 759-763, December 2015 http://dx.doi.org/10.3347/kjp.2015.53.6.759 Seroprevalence of Encephalitozoon
ISSN (Print) 0023-4001 ISSN (Online) 1738-0006 BRIEF COMMUNICATION Korean J Parasitol Vol. 53, No. 6: 759-763, December 2015 http://dx.doi.org/10.3347/kjp.2015.53.6.759 Seroprevalence of Encephalitozoon
INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER
 Canine VacciCheck INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER IgG ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 13 JUL 2015 Biogal Galed Laboratories Acs. Ltd., tel: 972-4-9898605.
Canine VacciCheck INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER IgG ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 13 JUL 2015 Biogal Galed Laboratories Acs. Ltd., tel: 972-4-9898605.
Seroprevalence of Toxoplasma gondii in Sheep, Cattle and Horses in Urmia North-West of Iran
 Tehran University of Medical Sciences Publication http:// tums.ac.ir Short Communication Iranian J Parasitol Open access Journal at http:// ijpa.tums.ac.ir Iranian Society of Parasitology http:// isp.tums.ac.ir
Tehran University of Medical Sciences Publication http:// tums.ac.ir Short Communication Iranian J Parasitol Open access Journal at http:// ijpa.tums.ac.ir Iranian Society of Parasitology http:// isp.tums.ac.ir
Salmonella Dublin: Clinical Challenges and Control
 Salmonella Dublin: Clinical Challenges and Control Simon Peek BVSc, MRCVS PhD, DACVIM, University of Wisconsin-Madison School of Veterinary Medicine Advancing animal and human health with science and compassion
Salmonella Dublin: Clinical Challenges and Control Simon Peek BVSc, MRCVS PhD, DACVIM, University of Wisconsin-Madison School of Veterinary Medicine Advancing animal and human health with science and compassion
FIV/FeLV testing FLOW CHARTS
 FIV/FeLV testing FLOW CHARTS The following FIV and FeLV test result flow charts should be used as guidance for the management of cats in CP care and interpretation of test results. There may be situations
FIV/FeLV testing FLOW CHARTS The following FIV and FeLV test result flow charts should be used as guidance for the management of cats in CP care and interpretation of test results. There may be situations
Classificatie: intern
 Classificatie: intern Animal Health Service Deventer Jet Mars part 1: Paratuberculosis ParaTB approach In the NL: control program, not an eradication program Quality of dairy products as starting point
Classificatie: intern Animal Health Service Deventer Jet Mars part 1: Paratuberculosis ParaTB approach In the NL: control program, not an eradication program Quality of dairy products as starting point
Short Communication INTRODUCTION
 EcoHealth DOI: 10.1007/s10393-014-0975-2 Ó 2014 International Association for Ecology and Health Short Communication Seroprevalence of Toxoplasma gondii in White-Tailed Deer (Odocoileus virginianus) and
EcoHealth DOI: 10.1007/s10393-014-0975-2 Ó 2014 International Association for Ecology and Health Short Communication Seroprevalence of Toxoplasma gondii in White-Tailed Deer (Odocoileus virginianus) and
General introduction
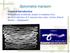 Spirometra mansoni General introduction Distributed worldwide, mainly in southeast Asia. Larval infection of S. mansoni may cause serious clinical disease ---Sparganosis Morphology Adult worm measures
Spirometra mansoni General introduction Distributed worldwide, mainly in southeast Asia. Larval infection of S. mansoni may cause serious clinical disease ---Sparganosis Morphology Adult worm measures
New Mexico Department of Agriculture
 Veterinary Diagnostic Services New Mexico Department of Agriculture The New Mexico Organic Farming Conference 2018 New Mexico Scientific Laboratories New Mexico Department of Agriculture Veterinary Diagnostic
Veterinary Diagnostic Services New Mexico Department of Agriculture The New Mexico Organic Farming Conference 2018 New Mexico Scientific Laboratories New Mexico Department of Agriculture Veterinary Diagnostic
Biology of toxoplasmosis
 1 Biology of toxoplasmosis E. Petersen 1 and J. P. Dubey 2 1 Statens Seruminstitut, Copenhagen, Denmark 2 U.S. Department of Agriculture, Beltsville, USA History Toxoplasma gondii is a coccidium, with
1 Biology of toxoplasmosis E. Petersen 1 and J. P. Dubey 2 1 Statens Seruminstitut, Copenhagen, Denmark 2 U.S. Department of Agriculture, Beltsville, USA History Toxoplasma gondii is a coccidium, with
Serological assays and PCR for detection of Toxoplasma gondii infection in an ostrich farm at Ismailia Provine, Egypt
 IOSR Journal of Agriculture and Veterinary Science (IOSR-JAVS) e-issn: 2319-2380, p-issn: 2319-2372. Volume 2, Issue 3 (Jan. - Feb. 2013), PP 56-60 Serological assays and PCR for detection of Toxoplasma
IOSR Journal of Agriculture and Veterinary Science (IOSR-JAVS) e-issn: 2319-2380, p-issn: 2319-2372. Volume 2, Issue 3 (Jan. - Feb. 2013), PP 56-60 Serological assays and PCR for detection of Toxoplasma
Eukaryotic Parasites. An Illustrated Guide to Parsitic Life Cycles to Accompany Lecture. By Noel Ways
 Eukaryotic Parasites An Illustrated Guide to Parsitic Life Cycles to Accompany Lecture By Noel Ways Giardia lamblia Life Cycle Reservoir: Beavers strongly implicated. Also, many other wild animals as well
Eukaryotic Parasites An Illustrated Guide to Parsitic Life Cycles to Accompany Lecture By Noel Ways Giardia lamblia Life Cycle Reservoir: Beavers strongly implicated. Also, many other wild animals as well
SensPERT TM Giardia Test Kit
 SensPERT TM Giardia Test Kit Giardia Test Kit Summary : Detection of specific antigens of Giardia within 10 minutes Principle : One-step immunochromatographic assay Detection Target : Giardia Lamblia antigen
SensPERT TM Giardia Test Kit Giardia Test Kit Summary : Detection of specific antigens of Giardia within 10 minutes Principle : One-step immunochromatographic assay Detection Target : Giardia Lamblia antigen
Epidemiology of Opisthorchis felineus in the European Union
 Epidemiology of Opisthorchis felineus in the European Union Edoardo Pozio European Union Reference Laboratory for Parasites Istituto Superiore di Sanità Rome, Italy World distribution and human prevalence
Epidemiology of Opisthorchis felineus in the European Union Edoardo Pozio European Union Reference Laboratory for Parasites Istituto Superiore di Sanità Rome, Italy World distribution and human prevalence
Protozoan Parasites of Veterinary importance 2017
 Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
ECHINOCOCCOSIS. By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine).
 ECHINOCOCCOSIS By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine). INTRODUCTION Species under genus Echinococcus are small tapeworms of carnivores with larval stages known as hydatids proliferating
ECHINOCOCCOSIS By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine). INTRODUCTION Species under genus Echinococcus are small tapeworms of carnivores with larval stages known as hydatids proliferating
Prevalence of Toxoplasma gondii in cats from Colombia, South America and genetic characterization of T. gondii isolates
 Veterinary Parasitology 141 (2006) 42 47 www.elsevier.com/locate/vetpar Prevalence of Toxoplasma gondii in cats from Colombia, South America and genetic characterization of T. gondii isolates J.P. Dubey
Veterinary Parasitology 141 (2006) 42 47 www.elsevier.com/locate/vetpar Prevalence of Toxoplasma gondii in cats from Colombia, South America and genetic characterization of T. gondii isolates J.P. Dubey
Hydatid Disease. Overview
 Hydatid Disease Overview Hydatid disease in man is caused principally by infection with the larval stage of the dog tapeworm Echinococcus granulosus. It is an important pathogenic zoonotic parasitic infection
Hydatid Disease Overview Hydatid disease in man is caused principally by infection with the larval stage of the dog tapeworm Echinococcus granulosus. It is an important pathogenic zoonotic parasitic infection
Seroprevalence of antibodies to Schmallenberg virus in livestock
 Seroprevalence of antibodies to Schmallenberg virus in livestock Armin R.W. Elbers Dept. Epidemiology, Crisis organisation and Diagnostics Central Veterinary Institute (CVI) part of Wageningen UR armin.elbers@wur.nl
Seroprevalence of antibodies to Schmallenberg virus in livestock Armin R.W. Elbers Dept. Epidemiology, Crisis organisation and Diagnostics Central Veterinary Institute (CVI) part of Wageningen UR armin.elbers@wur.nl
Coccidia and Giardia Diagnosis, Prevention and Treatment
 Coccidia and Giardia Diagnosis, Prevention and Treatment Coccidia and Giardia are both intestinal protozoan parasites that are common in young puppies and kittens and older or debilitated adults. Their
Coccidia and Giardia Diagnosis, Prevention and Treatment Coccidia and Giardia are both intestinal protozoan parasites that are common in young puppies and kittens and older or debilitated adults. Their
Prevalence of antibodies against Neospora caninum in dogs from urban areas in Central Poland
 Parasitol Res (2011) 108:991 996 DOI 10.1007/s00436-010-2143-0 ORIGINAL PAPER Prevalence of antibodies against Neospora caninum in dogs from urban areas in Central Poland Katarzyna Goździk & Robert Wrzesień
Parasitol Res (2011) 108:991 996 DOI 10.1007/s00436-010-2143-0 ORIGINAL PAPER Prevalence of antibodies against Neospora caninum in dogs from urban areas in Central Poland Katarzyna Goździk & Robert Wrzesień
What causes heartworm disease?
 Heartworm Disease: What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs and cats. It is caused by a blood-borne parasite called Dirofilaria
Heartworm Disease: What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs and cats. It is caused by a blood-borne parasite called Dirofilaria
EFSA Scientific Opinion on canine leishmaniosis
 EFSA Scientific Opinion on canine leishmaniosis Andrea Gervelmeyer Animal Health and Welfare Team Animal and Plant Health Unit AHAC meeting 19 June 2015 PRESENTATION OUTLINE Outline Background ToR Approach
EFSA Scientific Opinion on canine leishmaniosis Andrea Gervelmeyer Animal Health and Welfare Team Animal and Plant Health Unit AHAC meeting 19 June 2015 PRESENTATION OUTLINE Outline Background ToR Approach
Prevalence of Toxoplasma gondii antibodies, circulating antigens and DNA in stray cats in Shanghai, China
 Wang et al. Parasites & Vectors 2012, 5:190 RESEARCH Open Access Prevalence of Toxoplasma gondii antibodies, circulating antigens and DNA in stray cats in Shanghai, China Quan Wang *, Wei Jiang, Yong-Jun
Wang et al. Parasites & Vectors 2012, 5:190 RESEARCH Open Access Prevalence of Toxoplasma gondii antibodies, circulating antigens and DNA in stray cats in Shanghai, China Quan Wang *, Wei Jiang, Yong-Jun
CMED 526/EPI 526 B.J. Weigler Spring 2009
 CMED 526/EPI 526 B.J. Weigler Spring 2009 Toxoplasmosis Agent: Taxonomy: Toxoplasma gondii Phylum Apicomplexa (= ~5000 spp.) Sporozoon coccidia Distribution: Definitive Host: Intermed. Hosts: Invertebrates:
CMED 526/EPI 526 B.J. Weigler Spring 2009 Toxoplasmosis Agent: Taxonomy: Toxoplasma gondii Phylum Apicomplexa (= ~5000 spp.) Sporozoon coccidia Distribution: Definitive Host: Intermed. Hosts: Invertebrates:
Diurnal variation in microfilaremia in cats experimentally infected with larvae of
 Hayasaki et al., Page 1 Short Communication Diurnal variation in microfilaremia in cats experimentally infected with larvae of Dirofilaria immitis M. Hayasaki a,*, J. Okajima b, K.H. Song a, K. Shiramizu
Hayasaki et al., Page 1 Short Communication Diurnal variation in microfilaremia in cats experimentally infected with larvae of Dirofilaria immitis M. Hayasaki a,*, J. Okajima b, K.H. Song a, K. Shiramizu
Standard Operating Procedure for Rabies. November Key facts
 Standard Operating Procedure for Rabies November 2011 Key facts Rabies occurs in more than 150 countries and territories. Dogs are the source of 99% of human rabies deaths. Worldwide, more than 55 000
Standard Operating Procedure for Rabies November 2011 Key facts Rabies occurs in more than 150 countries and territories. Dogs are the source of 99% of human rabies deaths. Worldwide, more than 55 000
Asociación Mexicana de Médicos Veterinarios Especialistas en Pequeñas Especies
 Asociación Mexicana de Médicos Veterinarios Especialistas en Pequeñas Especies XXXI CONGRESO NACIONAL DE LA ASOCIACIÓN MEXICANA DE MÉDICOS VETERINARIOS ESPECIALISTAS EN PEQUEÑAS ESPECIES, A.C. DRA. IRENE
Asociación Mexicana de Médicos Veterinarios Especialistas en Pequeñas Especies XXXI CONGRESO NACIONAL DE LA ASOCIACIÓN MEXICANA DE MÉDICOS VETERINARIOS ESPECIALISTAS EN PEQUEÑAS ESPECIES, A.C. DRA. IRENE
CANINE HEARTWORM DISEASE
 ! CANINE HEARTWORM DISEASE What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs. It is caused by a blood-borne parasite called Dirofilaria
! CANINE HEARTWORM DISEASE What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs. It is caused by a blood-borne parasite called Dirofilaria
Research Article Prevalence and Risk Factors of Intestinal Parasites in Cats from China
 Hindawi Publishing Corporation BioMed Research International Volume 2015, Article ID 967238, 5 pages http://dx.doi.org/10.1155/2015/967238 Research Article Prevalence and Risk Factors of Intestinal Parasites
Hindawi Publishing Corporation BioMed Research International Volume 2015, Article ID 967238, 5 pages http://dx.doi.org/10.1155/2015/967238 Research Article Prevalence and Risk Factors of Intestinal Parasites
Zoonotic Diseases. Risks of working with wildlife. Maria Baron Palamar, Wildlife Veterinarian
 Zoonotic Diseases Risks of working with wildlife www.cdc.gov Definition Zoonoses: infectious diseases of vertebrate animals that can be naturally transmitted to humans Health vs. Disease Transmission -
Zoonotic Diseases Risks of working with wildlife www.cdc.gov Definition Zoonoses: infectious diseases of vertebrate animals that can be naturally transmitted to humans Health vs. Disease Transmission -
Humane Society of West Michigan
 Humane Society of West Michigan Health Concerns & Medical Treatment Feline Upper Respiratory Infections Your cat may have a cold when you get him home. Cats are subject to airborne virus disease that is
Humane Society of West Michigan Health Concerns & Medical Treatment Feline Upper Respiratory Infections Your cat may have a cold when you get him home. Cats are subject to airborne virus disease that is
Sero-Prevalence of Toxoplasma Gondii in Different Horses Groups from Khartoum State, Sudan
 Research Article 152 Sero-Prevalence of Toxoplasma Gondii in Different Horses Groups from Khartoum State, Sudan Abdalla Mohamed Ibrahim 1* ; Osman Mukhtar Osman 2 ; Rabab Haroun Mohamed Ali 1 ; Ahmed Ali
Research Article 152 Sero-Prevalence of Toxoplasma Gondii in Different Horses Groups from Khartoum State, Sudan Abdalla Mohamed Ibrahim 1* ; Osman Mukhtar Osman 2 ; Rabab Haroun Mohamed Ali 1 ; Ahmed Ali
Parvovirus Type 2c An Emerging Pathogen in Dogs. Sanjay Kapil, DVM, MS, PhD Professor Center for Veterinary Health Sciences OADDL Stillwater, OK
 Parvovirus Type 2c An Emerging Pathogen in Dogs Sanjay Kapil, DVM, MS, PhD Professor Center for Veterinary Health Sciences OADDL Stillwater, OK Properties of Canine Parvovirus Single-stranded DNA virus
Parvovirus Type 2c An Emerging Pathogen in Dogs Sanjay Kapil, DVM, MS, PhD Professor Center for Veterinary Health Sciences OADDL Stillwater, OK Properties of Canine Parvovirus Single-stranded DNA virus
Prevalence of antibodies against Toxoplasma gondii in pets and their owners in Shandong province, Eastern China
 Cong et al. BMC Infectious Diseases (2018) 18:430 https://doi.org/10.1186/s12879-018-3307-2 RESEARCH ARTICLE Open Access Prevalence of antibodies against Toxoplasma gondii in pets and their owners in Shandong
Cong et al. BMC Infectious Diseases (2018) 18:430 https://doi.org/10.1186/s12879-018-3307-2 RESEARCH ARTICLE Open Access Prevalence of antibodies against Toxoplasma gondii in pets and their owners in Shandong
Suggested vector-borne disease screening guidelines
 Suggested vector-borne disease screening guidelines SNAP Dx Test Screen your dog every year with the SNAP Dx Test to detect exposure to pathogens that cause heartworm disease, ehrlichiosis, Lyme disease
Suggested vector-borne disease screening guidelines SNAP Dx Test Screen your dog every year with the SNAP Dx Test to detect exposure to pathogens that cause heartworm disease, ehrlichiosis, Lyme disease
ELISA assays for parasitic and tick-borne diseases
 ELISA assays for parasitic and tick-borne diseases We are passionate about the health and well-being of humans and animals. Immunodiagnostics from contribute to a global, adequate supply of safe and nutritious
ELISA assays for parasitic and tick-borne diseases We are passionate about the health and well-being of humans and animals. Immunodiagnostics from contribute to a global, adequate supply of safe and nutritious
Toxoplasmosis By Amanda Baugh
 Toxoplasmosis By Amanda Baugh Toxoplasmosis Etiological agent: protozoan parasite Toxoplasma gondii (1). Domain: Eukaryota (unranked): Sar (unranked): Alveolata Phylum: Apicomplexa Class: Conoidasida Order:
Toxoplasmosis By Amanda Baugh Toxoplasmosis Etiological agent: protozoan parasite Toxoplasma gondii (1). Domain: Eukaryota (unranked): Sar (unranked): Alveolata Phylum: Apicomplexa Class: Conoidasida Order:
What s Hiding in your Pet?
 What s Hiding in your Pet? by Erin Quigley, DVM Potentially harmful parasites! A parasite is an organism that lives on (external) or in (internal) an organism of another species (such as dog, cat or human),
What s Hiding in your Pet? by Erin Quigley, DVM Potentially harmful parasites! A parasite is an organism that lives on (external) or in (internal) an organism of another species (such as dog, cat or human),
Providing links to additional websites for more information:
 Over Vaccinating you pets can kill them! There is much information available online concerning new guidelines for vaccinating your pets and we highly encourage you to do some additional research on this
Over Vaccinating you pets can kill them! There is much information available online concerning new guidelines for vaccinating your pets and we highly encourage you to do some additional research on this
The Seroprevalence of Toxoplasma gondii in Cats from the Kars Region, Turkey
 The Seroprevalence of Toxoplasma gondii in Cats from the Kars Region, Turkey Erkılıç, E.E., 1 * Mor, N., 2 Babür, C., 3 Kırmızıgül, A.H. 1 and Beyhan, Y.E. 3 1 Department of Internal Medicine, Faculty
The Seroprevalence of Toxoplasma gondii in Cats from the Kars Region, Turkey Erkılıç, E.E., 1 * Mor, N., 2 Babür, C., 3 Kırmızıgül, A.H. 1 and Beyhan, Y.E. 3 1 Department of Internal Medicine, Faculty
Mexican Wolves and Infectious Diseases
 Mexican Wolves and Infectious Diseases Mexican wolves are susceptible to many of the same diseases that can affect domestic dogs, coyotes, foxes and other wildlife. In general, very little infectious disease
Mexican Wolves and Infectious Diseases Mexican wolves are susceptible to many of the same diseases that can affect domestic dogs, coyotes, foxes and other wildlife. In general, very little infectious disease
SEROLOGIC SURVEY OF TOXOPLASMA GONDII ANTIBODIES IN CATS (FELIS CATUS) SOLD AT LIVE ANIMAL MARKETS IN SOUTHWESTERN NIGERIA
 Bulgarian Journal of Veterinary Medicine, 2017, 20, No 1, 58 64 ISSN 1311-1477; DOI: 10.15547/bjvm.957 Original article SEROLOGIC SURVEY OF TOXOPLASMA GONDII ANTIBODIES IN CATS (FELIS CATUS) SOLD AT LIVE
Bulgarian Journal of Veterinary Medicine, 2017, 20, No 1, 58 64 ISSN 1311-1477; DOI: 10.15547/bjvm.957 Original article SEROLOGIC SURVEY OF TOXOPLASMA GONDII ANTIBODIES IN CATS (FELIS CATUS) SOLD AT LIVE
