Flight feather development: its early specialization during embryogenesis
|
|
|
- Leo Beverly Ward
- 5 years ago
- Views:
Transcription
1 Kondo et al. Zoological Letters (2018) 4:2 DOI /s RESEARCH ARTICLE Flight feather development: its early specialization during embryogenesis Open Access Mao Kondo 1, Tomoe Sekine 1, Taku Miyakoshi 1, Keiichi Kitajima 1, Shiro Egawa 1, Ryohei Seki 1,2, Gembu Abe 1 and Koji Tamura 1* Abstract Background: Flight feathers, a type of feather that is unique to extant/extinct birds and some non-avian dinosaurs, are the most evolutionally advanced type of feather. In general, feather types are formed in the second or later generation of feathers at the first and following molting, and the first molting begins at around two weeks post hatching in chicken. However, it has been stated in some previous reports that the first molting from the natal down feathers to the flight feathers is much earlier than that for other feather types, suggesting that flight feather formation starts as an embryonic event. The aim of this study was to determine the inception of flight feather morphogenesis and to identify embryological processes specific to flight feathers in contrast to those of down feathers. Results: We found that the second generation of feather that shows a flight feather-type arrangement has already started developing by chick embryonic day 18, deep in the skin of the flight feather-forming region. This was confirmed by shh gene expression that shows barb pattern, and the expression pattern revealed that the second generation of feather development in the flight feather-forming region seems to start by embryonic day 14. The first stage at which we detected a specific morphology of the feather bud in the flight feather-forming region was embryonic day 11, when internal invagination of the feather bud starts, while the external morphology of the feather bud is radial down-type. Conclusion: The morphogenesis for the flight feather, the most advanced type of feather, has been drastically modified from the beginning of feather morphogenesis, suggesting that early modification of the embryonic morphogenetic process may have played a crucial role in the morphological evolution of this key innovation. Co-optation of molecular cues for axial morphogenesis in limb skeletal development may be able to modify morphogenesis of the feather bud, giving rise to flight feather-specific morphogenesis of traits. Keywords: Flight feather, Feather development, Chick embryo, Invagination Background Feathers are specialized skin derivatives (integument appendages) that are unique to extant/extinct birds and some non-avian dinosaurs; in living birds, most of the body is covered with some kinds of feathers. Feathers in birds, which serve more than 20 different functions [1], including thermoregulation, physical protection, tactile sensation, various types of visual signaling such as display in courtship, and flight, are functionally refined integument appendages. Bird feathers can be classified * Correspondence: tam@m.tohoku.ac.jp 1 Department of Developmental Biology and Neurosciences, Graduate School of Life Sciences, Tohoku University, Sendai , Japan Full list of author information is available at the end of the article into many types, such as down, bristle, and contour feathers (including flight feathers), and the evolution of various feather types has enabled feathers to exert a variety of functions. The flight feather is the most evolutionarily advanced type of feather [2, 3]. Flight feather evolution was one of the keys for bird evolution because powerful flight of birds is made possible by flight feathers, which have asymmetric shapes of the vanes along the rachis [4] (see also Fig. 1) that aeromechanically enable birds to fly [1]. There are mainly two body regions where flight feathers grow; one region (for remiges) is the posterior margin of the wing (forelimb) and the other (for rectrices) is the lateral margin of the tail. A feather is an exterior structure on The Author(s) Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( applies to the data made available in this article, unless otherwise stated.
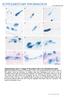
2 Kondo et al. Zoological Letters (2018) 4:2 Page 2 of 11 Fig. 1 Development of remex-type flight feathers after hatching. a A flight feather in the adult chicken. b Natal down feather in the abdominal tract at 1 dph. An uprooted abdominal natal down feather is shown on the right side. c Ventral view of the FFF region at 1 dph. Some rows of feathers in the ventral surface of the wing are trimmed. Staples indicate the FFF region. An magnified photograph is shown on the right side. d f A feather uprooted from the FFF region at 1 dph (d), 5 dph (e), and 7 dph (f). Note that in e, a distal downy part is connected with a proximal bilateral part that starts opening (white arrow). Scale bars: 1 cm the skin in general, but the shaft (quill) of the remextype flight feather is anchored to the bone (ulna and metacarpal/phalanges of digit 2) with extra muscles and neural networks for motor control [5]. These bones have protrusions on the surface (quill knob) that directly attaches to the proximal end of the flight feather calamus (calamus; the short, tubular base of the quill), which functions as mechanics of flight [6, 7]. During embryogenesis and molting, all feathers develop from primordial feather buds with follicles (feather follicles) at the base of it, and repetition of feather development enables cyclic molting. A chick after hatching appears to be covered with fluffy downy feathers (natal down feathers), and diversification of feather types emerges in the second or later feathers that form at the first and following molting. In the chicken, the first molting generally begins at around two weeks post hatching [4], and it is therefore possible that complex features of the flight feather would be provided at molting after hatching. However, it has been stated in some previous reports that the first molting from the natal down feather to the remex-type flight feathers is much earlier than that for other feather types [4, 8, 9] (see also Fig. 1b-f). Moreover, Hosker [8] reported that the feather follicles for the remiges reach the wing bone by the time of hatching, although details remain obscure. In order to clarify the early origin of flight feathers, we speculated that flight feather development may start much earlier as an embryonic event in mid-development in chicken. If this is the case, it is possible that the mechanism for morphogenesis of evolutionally advanced features of the flight feather has not simply added to the mechanism for the morphogenesis of the primitive types of feathers but is instead a modified/peculiar morphogenetic process. Considering the evolutionary and functional peculiarities of the flight feather, when and how flight feathers develop are important issues for feather biology in general. The aim of this study was to determine the incipient morphogenesis of the flight feather, focusing on when morphological traits of the remex-type feather start developing and how special morphologies of remiges (e.g., attachment between the flight feather calamus and the bone) are established. There are already many descriptions of the morphology, morphogenesis and molecular characters of the flight feather. Using such information, we present here morphological, histological, and molecular marker observations of the flight feather bud, and describe its developmental sequence. Integrating such descriptive information will also serve as the basis of feather diversification. Our results suggest that flight feather morphogenesis in the chicken is an embryonic event starting from the middle of chick development and that the first down feathers in the flight featherforming region, the follicles of which have already reached the bone, are special feathers that are different from other natal down feathers. Methods Animals Chicken eggs (Gallus gallus) were purchased from a local chicken ranch (Iwaya poultry farm) and incubated at 38 C, and embryos were staged according to Hamburger and Hamilton (1951) [10] (HHstage). White Leghorn chicks and adult chickens after hatching were supplied by the National BioResource Project (NBRP) Chicken/Quail of the MEXT, Japan, and the age of the chicks was ascertained by the NBRP supplier. Histology Hematoxylin and eosin (HE) staining Forelimb buds and a square area of abdominal skin, including feather follicles, from staged chicken embryos were used for histology. Samples for HE staining were fixed with Bouin s fixative (9% formaldehyde, 5% acetic acid and 75% saturated picric acid) at room temperature (RT) overnight with shaking. The samples were washed with 70% ethanol (with saturated lithium carbonate) for
3 Kondo et al. Zoological Letters (2018) 4:2 Page 3 of days followed by dehydrating with 80%, 90% and 100% ethanol (twice for 100%). Then the samples were permeated with xylene at RT once, 1:1 xylene/paraffin (paraplast, Leica) at 45 C once, and paraffin at 60 C three times and embedded in paraffin at RT. The paraffin blocks were sectioned at 10 μm in thickness on slide glasses (MAS-coated, MATSUNAMI). The sectioned samples were immersed in xylene for 20 min and then quickly immersed in 100% (twice), 90% and 70% ethanol and then rinsed in water. The sections were stained with hematoxylin solution (Muto Pure Chemicals) at RT for 1 min and washed with water followed by eosin staining for 30 s and water washing. The samples were dehydrated with a series of ethanol (70%, 90% and 100% ethanol) and xylene and were mounted with Eukitt (ASONE). Elastica van Gieson (EVG) and Alcian blue (AB) staining The method for EVG + AB staining was essentially the same as that for HE staining except for the thickness of a section (15 μm). See Hayashi et al. [11] for details of EVG + AB staining. We made some modifications for staining: coloring times for Weigert s hematoxylin solution, alcian blue solution and van Gieson s solution were for 3 min, 10 min and 3 min, respectively, in this study. In situ hybridization Forelimb buds and a square of abdominal skin with hypodermal tissues were excised from staged chicken embryos and processed for whole-mount in situ hybridization as described previously [12] using antisense RNA probes for chick shh (see [12, 13] for detailed information of shh RNA probes). In the later stages of feather morphogenesis, shh is expressed in the marginal plate epithelium that will undergo cell death, providing spaces between the barbs [14 16]. In situ hybridization of frozen sections was performed essentially by the same method as that described by Yoshida et al. [17] using antisense RNA probes for chick shh. Results Flight feather morphology and development after hatching In all experiments described below, we used flight feathers in the zeugopod for specimens of flight feathers (Fig. 1a) and their primordia, and compared them with abdominal feathers (Fig. 1b). In this study, we defined the place where remex-type flight feathers (remiges) are formed as the flight feather-forming region (FFF region, see Fig. 1c), which is the posterior margin of the zeugopod and autopod in the wing. In the FFF region, the first generation of feathers is down-type and the second generation is remex-type. An old textbook (Figs in [4]) showed bar charts representing the history of feathers on several body tracts of the chicken, in which the first molting in the FFF region was shown to be very early in the chick, but no definite data were shown in that report. An old report by Hosker [8] showed a feather from the wing of a one-day-old chick (Fig. 27 in [8]) that has clear two parts arranged linearly, but there is no mention about whether that specimen is a flight feather in the FFF region in that report. Therefore, we decided to repeat these observations and confirm how early the first molting occurs in the FFF region in the chicken. In contrast to the morphology of natal down feathers on the abdominal surface at one day post hatching (dph) (Fig. 1b), feathers in the FFF region are composed of two different parts: a distal part resembling down feathers and a proximal part of a shaft without barbs visible at 1 dph (Fig. 1c, d) as described by Hosker [8]. Developing barbs here seem to be still inside the sheath of this proximal part of the developing feather and are therefore not visible yet as described by Alibardi [18]. The root of the proximal part is deeply embedded in the wing. At 5 dph, the distal portion of the proximal part begins to open, and barbs with a bilateral shape along the rachis can be seen (Fig. 1e, showing that the down feather is still on the distal end of the opening bilateral barbs). The remex-type asymmetric barbs further open at 7 dph, and the distal down part has dropped from this specimen (Fig. 1f). A series of observations (Fig. 1) confirmed that the distal part at 1 dph is the first down feather that connects with the second remex-type feather, although the remiges have not been opened yet at that time (Fig. 1d), suggesting that the first molting starts at around the hatching stage. It seems that the remex-type feather has already started developing at embryonic stages before hatching. Flight feather morphology and development before hatching In the FFF region at embryonic day 18 (E18), we observed a row of epidermal sheath for the elongated feather bud, and there was no visible connection of the two feather primordia as judged by externals (Fig. 2a). In contrast, at E19, the epidermal sheath in the FFF region showed a clear joint (white arrowheads in Fig. 2b) that resembles a connection of columns between the second mature remex and third immature remex at the second molting in the adult chicken [4], suggesting that the proximal portion to the joint is the second generation of feather (remex-type). The second feather may thus have already developed before E19. We expected that there may be a primordial remex-type feather under the skin at E18, and histological observations of sections (indicated by a dashed line in Fig. 2a) were attempted. At a
4 Kondo et al. Zoological Letters (2018) 4:2 Page 4 of 11 Second generation of feather development in the FFF region Fig. 2 Development of remex-type flight feathers before hatching. a, b Ventral view of the FFF region at E18 (a) and E19 (b). Scale bars: 1 mm. Arrowheads indicate joints between distal and proximal parts of the elongated feather bud in the FFF region. c e, Sections of the feather bud under the skin at E 18; (c), a shallow level, (d), an intermediate level and (e), a deep level. Scale bars: 100 μm. Asterisk in (e) indicates the position of the prospective rachis that exhibits an irregular pattern of barb ridges. f Rows of feather buds in the posterior wing, including the FFF region, at E18. Distal to the right and dorsal to the bottom. U: ulna. Asterisks indicate the position of prospective rachis, showing that the position is biased to the dorsal. Scale bar: 100 μm shallow level (Fig. 2c), radially symmetric barb primordia on the circumference could be seen in the feather follicle, and this configuration resembles that of a primordial natal down feather in which all barb ridges are parallel to the long axis [4, 19]. At the middle level (Fig. 2d), there was a layer where no barb primordia were visible. At a deeper level (Fig. 2e) of the same feather follicle as that shown in Fig. 2c and d, there was a bilateral barb arrangement on the circumference, and this configuration indicates a typical flight feather primordium [20]. These observations revealed that the second generation of feather that shows a remex-type feather arrangement has already started developing by E18 deeply in the skin of the FFF region. In addition, the position of the rachis primordium in each feather bud (indicated by asterisks in Fig. 2f ) was biased to the dorsal side of the wing. To further investigate when the remex-type feather development starts, we analyzed the expression pattern of a gene, sonic hedgehog (shh). Expression of shh starts early in the feather placode and then remains in the epidermal marginal plate (presumptive inter-barb region) of the feather bud [14 16], and we therefore thought that we could estimate feather types by the shh expression pattern. At E10, many feather buds protuberated on the abdominal surface of the body trunk (Fig. 3a), and shh was expressed in the epidermis of the protrusion, with the pattern of expression becoming obvious at E11 (Fig. 3b) and E12 (Fig. 3c). In the abdominal feather buds, shh expression showed a radially symmetric pattern that corresponds to the barb arrangement of a natal down feather. In the FFF region, a line of feather buds (arrowheads in Fig. 3d 3f, 3j, 3k) were much larger than those in the surrounding area of the wing skin surface. Expression of shh in feather buds in the FFF region showed a radially symmetric pattern similar to that in abdominal feather buds. We never observed a bilateral pattern of shh expression (indicating the pattern for contour feathers) by wholemount in situ hybridization on the embryonic days we conducted observations. Thus, it appears that the elongating feather bud in the FFF region gives rise to down Fig. 3 Whole-mount observations of shh expression in the abdominal and FFF regions. a c, g i shh expression in the abdominal tract by whole-mount in situ hybridization from E10 (HHstage 36) to E16 (HHstage 42). d f, j l shh expression in the FFF region. Arrowheads in (d f), (j), (k) indicate feather buds in the FFF region: note that in j and k, feather buds in the FFF region have little shh expression
5 Kondo et al. Zoological Letters (2018) 4:2 feather during this developmental period of E10-E12. The expression of shh in the abdominal feather buds continues to E13 15 (Fig. 3g, h and not shown), and signals for shh expression could not be detected at E16 (Fig. 3i). On the other hand, shh expression in the FFF region had disappeared by E13 (black arrowheads in Fig. 3j), although in surrounding feather buds shh continued to be expressed (Fig. 3j and k) and the shh expression disappeared by E16 (Fig. 3l), indicating that the first generation of feather development in the FFF region ceases much earlier than that in other feather buds. Thus, feather development of the first generation in the FFF region may proceed more rapidly. We found such heterochronic modification of shh expression, but did not detect a second generation of remex-type feather formation by whole-mount observation of the shh expression pattern. As can be seen in histological images shown in Fig. 2, it remained possible that the second generation of feathers was developing in a deep area under the skin, and we thus examined shh expression by in situ hybridization for sections of feather buds in the FFF region (Fig. 4). At E13, when a natal feather pattern could Page 5 of 11 not be detected by whole-mount observation (Fig. 3j), clear expression of shh was detected by section in situ hybridization at a relatively shallow level under the skin (Fig. 4a, see Fig. 4j, an illustrated image showing positional relation of sections along depth direction). The expression of shh was circumferentially spotted with a radially symmetric pattern, typical for a natal down feather [21]. The feather development generally proceeds in a proximal-to-distal direction, and the more distal region of a feather bud develops to be more mature. This could account for the continued expression of shh in the proximal region of the feather bud retains (Fig. 4a), while shh expression ceases in the distal external region (Fig. 3j). Faint expression of shh at a shallow level was detected until E15 (Fig. 4b and c). At a middle level, clear shh expression was not detected in the period we examined (E13-E15, Fig. 4d, e and f ). Interestingly, at a deep level of the feather bud in the FFF region (slightly upper from the bottom), we detected a clear signal of shh at E14 and later (compare Fig. 4g and Fig. 4h, i). The expression pattern of shh at this depth became not radially symmetric but bilateral (Fig. 4i, a prospective rachis is indicated by an asterisk), resembling the barb arrangement in the feather follicle of the molting flight feather in the adult [20]. We found that the second generation of feather development in the FFF region, which gives rise to remextype feathers, appears to start by E14 at latest, a week before hatching. A unique morphogenetic process for first generation of feathers in the FFF region Fig. 4 Shh expression detected by section in situ hybridization in the FFF region. Cross sections around the bottom region of feather buds in the FFF region at E13 (a, d and g), E14 (b, e and h) and E15 (c, f and i) at shallow (a c), middle (d f) and deep (g i) levels under the skin. Asterisk in i indicates an irregular pattern of shh expression that represents the position of the prospective rachis. j. an illustrated image showing positional relation of sections (a i) along depth direction. Scale bars: 100 μm In the above-described observations of section in situ hybridization, we realized that feather buds in the FFF region extend the base of the calamus in a deep layer under the skin of the wing. This surprised us, as feather buds are generally external structures on the skin surface (Fig. 5d) (see also panels with histological sections of developing chick skin in [16, 22]) and drove us to further observe histologically the process of feather bud formation in the FFF region. Feather buds in the abdominal region develop on the skin surface with underlying mesenchyme (Fig. 5a). The buds grow toward the outside and make a sheath-like structure (Fig. 5b and c). The sheath-like elongated feather bud was a surface structure (Fig. 5d). Although the base of the epidermal sheet made a slight invagination to create a cavity-like bend (follicular cavity, see [3]) and the surrounding dermal layer became thicker, embedding the follicle in the dermis, the feather follicles did not enter the hypodermal layer but remained external to it. On the other hand, in the FFF region, the feather bud showed a peculiar process of development different from that of the abdominal feather bud. At E10 (Fig. 5e), the feather bud in the FFF region looks similar to the abdominal feather bud. External
6 Kondo et al. Zoological Letters (2018) 4:2 Page 6 of 11 Fig. 5 Deep invagination of the feather bud toward the bone primordium in the FFF region. a h Sagittal sections of abdominal skin (a d) and coronal sections of the FFF region (e h) at E10 (a, e), E11 (b, f), E12 (c, g) and E13 (d, h). Arrowheads in (f) indicate the follicular cavity that starts invagination. i l. Transverse sections of the FFF region showing the invagination process of the feather bud in the FFF region at E10 (i), E11 (j), E12 (k) and E13 (l). Scale bars: 100 μm. The blue core is the cartilaginous rudiment of the ulna, and the red layer surrounding the ulna cartilage is the ossifying bone collar morphology of the feather bud in the FFF region still looks similar at E11 (Fig. 5f, compare to 5b and see also Fig. 4b and e), but there was a slit-like bend of the epithelium surrounding the base of the protruding feather bud (arrowheads in Fig. 5f and j). At E12, the epithelial bend became deeper, and the feather bud created cylindrical invagination into the hypodermal layer (Fig. 5g and k). The proximal end of the feather bud sank into the deep limb mesenchyme and reached the bone (ulna) by E13 (Fig. 5h and l). Observation of a series of transverse sections of the developing wing with bone (in red) and cartilage (in blue) (Fig. 5i 5l) revealed that the base of the feather bud reaches the ulna before ossification of the bone collar is completely finished (Fig. 5k and l, showing the ossifying bone collar in red). We never observed such invagination of the feather bud in the abdominal region (Fig. 5a 5d). These observations strongly suggest that the initial feather bud in the FFF region has a unique morphogenetic process for flight featherspecific traits, deep invagination and direct attachment to the bone, although the first generation of feathers in the FFF region shows an external down feather-like morphology, as seen in Fig. 3. Discussion In this study, we traced the morphogenesis of the flight feather back until embryonic stages and revealed some specialties of its process. In Fig. 6, the morphogenesis from initiation of feather bud formation to later embryogenesis is shown. The first stage at which we detected a specific morphology of the feather bud in the FFF region was E11, when a slit-like bend forms around the bud. At this stage, the feather bud starts to change its relative position to the skin surface and seems to make invagination into the skin. It is possible that both invagination of the bud and swelling of the surrounding skin occur simultaneously. The invagination continues, and the feather follicle reaches the bone at E13. At this time point, the second wave of shh expression (for remex-type) has not yet been initiated, while shh is expressed in the external region of the feather bud with a radially symmetric pattern. The radial shh expression results in the natal down feather morphology with a radial barb pattern. The second wave of shh expression, which has a bilateral pattern, can be detected around the bottom of the feather bud deeply in the wing at E14. Therefore, the feather bud in the FFF
7 Kondo et al. Zoological Letters (2018) 4:2 Page 7 of 11 Fig. 6 Schematic representation of the developmental process of feather formation in the FFF region. Illustrated images in the lower column indicate posterior halves of transverse sections of the developing wing buds with a feather bud in the FFF region and ulna (ellipse). See the text for details region at this stage can be divided into several parts from proximal to distal: shh-positive (bilateral), shh-negative (as a joint), and shh-positive (radial). The proximal bilateral pattern of shh expression results in the remex-type feather pattern that is visible externally at around five days post hatching. The remex-type feather with a joint to the first generation of the down-type feather can be seen internally at E18 and externally at E19. Invagination of the feather bud in the FFF region and formation of the quill knob Invagination is a morphogenetic process of epithelial tissue that changes a flat epithelial sheet to complex structures such as tubes, furrows, and capsules. The skin epithelium creates many kinds of structures, such as secretory glands, nails/claws/hoofs, and scales/feathers/ hairs, by interacting with the underlying mesenchyme [2, 9, 14, 23, 24]. Morphogenesis of skin derivatives is preceded by epithelial invagination. In the developing feather bud, invagination gives rise to a collar-like bend, the bottom of which serves as a feather follicle, the cellular source of the feather. The feather follicle reaches the dermal tissue, but the invagination is generally not so deep, retaining the follicle in the hypodermal layers of the skin. In contrast, the feather follicle in the FFF region deeply sinks to the posterior-dorsal surface of the bone. Although the mechanism of epithelial invagination of a feather bud in the FFF region is largely unknown, an interesting report by Lin et al. [13] suggested that tubular invagination is achieved through loosening of the bud mesenchyme. Epithelial cell proliferation and elongation in the bud may lead to the formation of a sprout that invades the surrounding loosened mesenchyme, and epithelial-mesenchymal interaction may contribute to the epithelium organization as seen in the morphogenesis of other types of epithelial tissues such as mammary glands [25]. Rows of feather buds more dorsal to the FFF region, which give rise to wing coverts, also make deep invagination into the wing bud mesenchyme (Fig. 5l). Since the feather buds at the ventral side of the wing do not show such deep invagination (Fig. 5k, and not shown), the dorsal property of the limb that is provided by dorsal determinants such as lmx1 might participate in the deep invagination. It could be hypothesized that this contact results in the formation of a bump on the bone surface. The quill knob is a trait associated with the flight feather and plays important roles in flight because the attachment point between the knob and the calamus functions as a mechanical link that transfers aerodynamic forces generated by flight feathers to the wing skeleton [5]. The characteristics of the flight feather start developing in the initial process of feather bud formation, earlier than the remex-type feather morphogenesis. The remex-type morphology is thought to be the most evolutionarily and developmentally advanced phenotype of the feather [2, 3]
8 Kondo et al. Zoological Letters (2018) 4:2 Page 8 of 11 with secondary modification of the developmental process of the feather pattern, but it does not necessarily mean that the modification was added subsequently to morphogenesis of primitive type. Our results suggest that at least some characteristics of the flight feather are provided by modification of processes in its early development. Remex-type feather formation Our observation of shh expression in the feather bud of the FFF region revealed that its expression in the FFF region has a non-radial but bilateral pattern at E14 and E15. This suggests that there is the second generation of feather formation, the morphology of which is different from that of natal down in the first generation, already at the embryonic stage before hatching in the FFF region. The molecular mechanism for transition of shh expression from radial to bilateral remains unknown. Gradient signaling such as Wnt3a [26] and retinoic acid (RA) [27] are thought to provide the bilateral and asymmetric shape of the feather with a rachis since modification of Wnt3a and RA gradients in the feather bud transforms feather morphology. In these experiments, young adult chickens at the molting stage were focused on, but also in the embryonic stage, change in these signaling pathways from a homogenous pattern to a localized pattern in the feather bud might occur before establishment of bilateral shh expression at E14. Vanes in remiges are bilaterally asymmetric to the rachis, and this asymmetry is more distinguishable in the distal wing (primary remiges). Feo and Prum [28] examined the developmental process of the asymmetric barb shape of rectrices in the tail of adult parrots, and they found that the developing feather germ of rectrices has asymmetry of barb ridges to the rachis in a cross section. We did not detect clear asymmetry of the feather bud in the FFF region, but the shh-expressing region shown in Fig. 4i seems to be asymmetric to the hinge of expression (a prospective rachis, indicated by an asterisk), corresponding to the discussion of the origin of asymmetric feathers [18, 29]. It would be interesting to investigate the relationship between asymmetry of the feather pattern and axis formation of the limb. The feather bud in the FFF region and its invagination are biased to the dorsal side of the limb, and the position of the future rachis (asterisk in Fig. 4i) is also biased to the dorsal side of the limb (Fig. 2f). The axis formation of the limb regarding dorsal-ventral, proximal-distal and anteriorposterior may play a role in the axis formation of the feather. We would like to discuss this point further in the next section. Hypotheses on specification of the FFF region The FFF region can be defined as a region satisfying four criteria of topological identity in the bird body. The first criterion is the forelimb. The second one is the zeugopod and autopod. The third one is the posterior margin. The last criterion is the dorsal side. The position where the above four criteria meet, the dorsal side of the posterior margin in the zeugopod and autopod of the forelimb, is the position for the FFF region. In the process of limb development, each of the four criteria can be specified by specific gene expression patterns and functions, and it is possible that these molecular networks for specifying the four criteria in the limb bud may also be applied to specifying the FFF region and providing peculiar morphogenesis of the feather follicle in the FFF region. We hypothesize that feather formation in the FFF region is special from the beginning, and this enables us to discuss relationships between molecular mechanisms for establishing the above four criteria and feather formation in the FFF region. Criterion 1 for the FFF region: forelimb The remex-type flight feather in the extant bird is forelimb-specific, although some modern birds such as some species of raptors bear feathers in the hindlimb zeugopod that seem to be important in flight when catching and carrying prey [30]. A reliable marker for the tetrapod forelimb bud is tbx5, which has essential functions in initiation of development and morphology of the forelimb [31, 32]. tbx5 deficiency results in a limbless phenotype, and evidence for the direct function of tbx5 in flight feather morphology is therefore sparse. Nevertheless, recent findings on avian genetics and embryology have revealed crucial functions of tbx5 in flight feather formation. There are some mutants (strains) of domestic chickens and pigeons that have flight featherlike asymmetrical feathers in the hindlimb. Domyan et al. [33] found that this intriguing phenotype is associated with cis-regulatory changes in the tbx5 locus that give rise to ectopic expression of tbx5 in the hindlimb bud, suggesting a role of this gene in flight feather formation. Criterion 2 for the FFF region: zeugopod and autopod Mayerson and Fallon [34] suggested that feathers arise sequentially and independently within different feather tracts (reviewed in [14]). Johansson and Headon [35] suggested that positional information mediated by transcription factors, including Hox genes, can code regionalization of a skin appendage. Indeed, some homeobox genes show different expression patterns in skin tracts in the chick embryonic body [36 38]. Hox genes are also involved in regionalization of the limb along the proximo-distal axis (from stylopod through zeugopod to autopod); hoxa11 is a key molecule for zeugopod specification, and hoxa13 is a key molecule for the autopod, and both of molecules have essential functions in morphogenesis of the regionalization [39 41]
9 Kondo et al. Zoological Letters (2018) 4:2 Page 9 of 11 (reviewed in [42, 43]), although the direct functions of these Hox genes in flight feather formation remain undetermined. Functional studies should be carried out to determine their role in the establishment of regional differences of feather type. Li et al. [27] reported that retinoic acid signaling mediates a continuum of asymmetric vanes of flight feathers along the proximo-distal axis of the wing, and they discussed the possibility that the limb RA gradient that is used for the proximo-distal limb patterning is somehow imprinted within remex pulp cells and that the limb RA gradient is also used to establish asymmetry levels in remiges along the wing. It is possible that information of the proximo-distal axis in the wing mediated by retinoic acid signaling is embedded into the developing feather bud at the zeugopod and autopod through different expression patterns of Hox genes. Criterion 3 for the FFF region: posterior margin Flight feather buds appear at the posterior margin of the forelimb bud. The posterior margin is the region where the feather primordia develop earliest in the chick forelimb bud [34]. The posterior margin of the limb bud includes a special mesenchymal region, so-called zone of polarizing activity (ZPA). Anterior implantation of ZPA tissue induces a set of digits with mirror-image duplication, in which large feather buds develop at the anterior margin (with newly-acquired posterior identity) of the duplicated limb [44], suggesting that the zone contributes to the FFF region. Shh, a secretory molecule responsible for polarizing activity of the zone, also induces posterior feather buds when applied to the anterior margin [44]. Furthermore, ectopic flight feathers in pigeon and chicken mutants are biased to the posterior foot, and ectopic expression of tbx5 in the hindlimb in these mutants is also posterior-biased [33, 45]. Taken together with the fact that flight feathers in the raptor hindlimbs are also posterior-biased [30], it is thought that molecular networks for forelimb identity and posterior identity of the limb morphology coordinate formation of the FFF region. Since shh expression in the forelimb ZPA disappears at around HHstage 29 [46] before the feather budinitiating stage, shh-experienced posterior mesenchyme (descendants of mesenchymal cells that had expressed shh gene in the early stages) might contribute to the induction of the flight feather bud in the FFF region. Criterion 4 for the FFF region: dorsal side Quill knobs are formed on the dorsal surface of the bone [7]. This would be because invagination of the FFF feather bud proceeds to the dorsal side (see Fig. 5l), and the proximal end of the feather bud reaches to the dorsal surface of the bone. It is known that dorsal-ventral reorientation of developing chick limb ectoderm makes a bi-dorsal feather pattern, including a double row of flight feather buds [47]. It is also noteworthy that limbless mutants of the chicken, which have no dorsal-ventral boundary due to the complete dorsalization of the early limb field, resulting in no outgrowth of the limb bud, do not form wing (thus no flight feathers) [48]. Interestingly, when the mutant phenotype was partially rescued by application of FGF, double lines of feathers that looked like flight feathers in the sheath appeared at the posterior margin of the developed forelimb [48]. These results strongly suggest a correlation between the dorsal-ventral organization of the limb and flight feather formation, although the molecular mechanism remains unknown. lmx1, a determinant of limb dorsal morphology, is expressed in the dorsal part of the developing wing bud [46], and feather placodes in the FFF region are included in the lmx1-positive dorsal territory (unpublished observations by TS, TM, KK and KT). However, miss-expressed lmx1 does not change the ventral feather pattern when it is ectopically expressed in the ventral side of limb bud [46], suggesting that the molecule is not sufficient to provide the FFF region. The FFF region that satisfies the above four criteria may develop special traits of the feather bud from the beginning of feather development, and one of the first visible specialties in feather development in the FFF region is the formation of a slit-like bend surrounding the feather bud at E11, followed by deep invagination. We recently reported that the gene Sim1 is expressed in the posterior margin of the zeugopod and autopod in the developing wing of the avian embryo, nicely corresponding to the FFF region [45]. Sim1 expression that is specific to the region where the above four criteria meet starts at around E7 and ceases at around E14 ([45] and our unpublished observations). Although investigation of the functions of Sim1 and the other molecules described above in the feather formation are necessary, we suggest that Sim1 expression in the FFF region is not necessarily too early to contribute to flight feather formation. Conclusion Our results indicated that the second generation for the remex-type flight feather has already started developing before hatching as an embryonic event. The morphogenesis in the FFF region is modified from the beginning of feather morphogenesis as the first generation of the feather also has a unique morphogenetic process. The flight feather is thought to be the most evolutionarily and developmentally advanced type of feather, and our results presented here suggest that the morphogenesis for the advanced type has been drastically modified from the beginning of feather morphogenesis. At the beginning of feather bud formation in the FFF region, molecular cues for axial morphogenesis of the wing along the 3D axes may be co-opted to modify morphogenesis
10 Kondo et al. Zoological Letters (2018) 4:2 Page 10 of 11 of the feather bud, giving rise to flight feather-specific morphogenesis of traits. Such early modification would enable the feather bud to make at least some of the flight feather-specific traits, including direct connection with the bonny structure, quill knobs. Flight feathers in the wing connect with specific muscles for coordinating wing/feather movements and changing the wing to an airfoil shape [5], and the connection between the muscle and flight feather calamus, which is an important trait of the flight feather, is probably established during feather morphogenesis in the embryo. Quill knobs are evident not only in extant bird species but also in basal taxa such as Ichthyornis and non-avian theropod dinosaurs such as Velociraptor [7]. Some nonavian theropod dinosaurs and basal taxa of birds are equipped with remex-type flight feathers with asymmetric shapes in their forelimb [45]. Analyses of juvenile specimens referable to the oviraptorosaur have suggested that morphological changes in remiges occurred during feather development [49]. Ancestors of birds might have already experienced the modification of early embryonic morphogenesis in the FFF region. This is a good example of how early modification of the embryonic morphogenetic process may have played a crucial role in the morphological evolution of key innovation. Acknowledgements A series of post-hatching chicks were kindly provided by NBRP Chicken/Quail of the MEXT, Japan. Funding This research was supported by the following grants; JSPS KAKENHI Grant number 15H04374, Naito, Novartis foundations to KT. RS and SE are JSPS Research Fellows (JSPS KAKENHI Grant Numbers JP14J07050 for RS and JP15J06859 for SE). Availability of data and materials The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Authors contributions MK conceived of the study, and MK and KT designed the experiments. MK carried out morphological, histological and gene expression analysis. TS, TM, KK, SE helped the experiments and participated in section in situ hybridization and histology. KT prepared the draft manuscript, and SE, RS and GA revised it. All authors read and approved the final manuscript. Ethics approval All animal experiments were properly performed in accordance with guidelines provided by Tohoku University. Consent for publication Not applicable. Competing interests The authors declare that they have no competing interests. Publisher s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Author details 1 Department of Developmental Biology and Neurosciences, Graduate School of Life Sciences, Tohoku University, Sendai , Japan. 2 Mammalian Genetics Laboratory, Genetic Strains Research Center, National Institute of Genetics, 1111 Yata, Mishima, Shizuoka , Japan. Received: 4 September 2017 Accepted: 29 December 2017 References 1. Stettenheim PR. The integumentary morphology of modern birds - an overview. Am Zool. 2000;40: Chuong CM, Chodankar R, Widelitz RB, Jiang TX. Evo-devo of feathers and scales: building complex epithelial appendages - commentary. Curr Opin Genet Dev. 2000;10: Prum RO. Development and evolutionary origin of feathers. J Exp Zool. 1999;285: Lucas A, Stettenheim P. Avian anatomy: integument (agricultural handbook 362) Washington, DC. US Department of Agriculture Hieronymus TL. Flight feather attachment in rock pigeons (Columba Livia): covert feathers and smooth muscle coordinate a morphing wing. J Anat. 2016;229: Hieronymus TL. Qualitative skeletal correlates of wing shape in extant birds (Aves: Neoaves). BMC Evol Biol. 2015;15: Turner AH, Makovicky PJ, Norell MA. Feather quill knobs in the dinosaur velociraptor. Science. 2007; 317: Hosker A. Studies on the epidermal structures of birds. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1936;226:143 U Yu MK, Yue ZC, Wu P, Wu DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong CM. The developmental biology of feather follicles. Int J Dev Biol. 2004;48: Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88: Hayashi S, Kobayashi T, Yano T, Kamiyama N, Egawa S, Seki R, Takizawa K, Okabe M, Yokoyama H, Tamura K. Evidence for an amphibian sixth digit. Zoological Lett. 2015;1: Yonei S, Tamura K, Ohsugi K, Ide H. Mrc-5 cells induce the Aer prior to the duplicated pattern-formation in Chick limb bud. Dev Biol. 1995;170: Matsubara H, Saito D, Abe G, Yokoyama H, Suzuki T, Tamura K. Upstream regulation for initiation of restricted Shh expression in the chick limb bud. Dev Dyn. 2017;246: Lin CM, Jiang TX, Widelitz RB, Chuong CM. Molecular signaling in feather morphogenesis. Curr Opin Cell Biol. 2006;18: Nohno T, Kawakami Y, Ohuchi H, Fujiwara A, Yoshioka H, Noji S. Involvement of the sonic hedgehog gene in chick feather formation. Biochem Biophys Res Commun. 1995;206: TingBerreth SA, Chuong CM. Sonic hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev Dyn. 1996;207: Yoshida N, Urase K, Takahashi J, Ishii Y, Yasugi S. Mucus-associated antigen in epithelial cells of the chicken digestive tract: developmental change in expression and implications for morphogenesis-function relationships. Develop Growth Differ. 1996;38: Alibardi L. Review: cornification, morphogenesis and evolution of feathers. Protoplasma. 2017;254: Rawles ME. Tissue Interactions in Scale and Feather Development as Studied in Dermal-Epidermal Recombinations. J Embryol Exp Morphol. 1963;11: Yu MK, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420: Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. 2002;294: Widelitz RB, Jiang TX, Lu JF, Chuong CM. Beta-catenin in epithelial morphogenesis: conversion of part of avian foot scales into feather buds with a mutated beta-catenin. Dev Biol. 2000;219: Dhouailly D. A new scenario for the evolutionary origin of hair, feather, and avian scales. J Anat. 2009;214: Dhouailly D, Godefroit P, Martin T, Nonchev S, Caraguel F, Oftedal O. Getting to the root of scales, feather and hair: as deep as odontodes? Exp Dermatol. 2017;00:1 6.
11 Kondo et al. Zoological Letters (2018) 4:2 Page 11 of Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development. 2015;142: Yue ZC, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc Natl Acad Sci U S A. 2006;103: Li A, Figueroa S, Jiang TX, Wu P, Widelitz R, Nie Q, Chuong CM. Diverse feather shape evolution enabled by coupling anisotropic signalling modules with self-organizing branching programme. Nat Commun. 2017;8:ncomms Feo TJ, Prum RO. Theoretical morphology and development of flight feather vane asymmetry with experimental tests in parrots. J Exp Zool B Mol Dev Evol. 2014;322: Alibardi L. Follicular patterns during feather morphogenesis in relation to the formation of asymmetric feathers, filoplumes and bristles. Italian Journal of Zoology. 2009;76: Chatterjee S, Templin RJ. Biplane wing planform and flight performance of the feathered dinosaur microraptor gui. Proc Natl Acad Sci U S A. 2007;104: Rodriguez-Esteban C, Tsukui T, Yonei S, Magallon J, Tamura K, Belmonte JCI. The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature. 1999;398: Takeuchi JK, Koshiba-Takeuchi K,MatsumotoK,Vogel-HopkerA, Naitoh-Matsuo M, Ogura K, Takahashi N, Yasuda K, Ogura T. Tbx5 and Tbx4 genes determine the wing/leg identity of limb buds. Nature. 1999;398: Domyan ET, Kronenberg Z, Infante CR, Vickrey AI, Stringham SA, Bruders R, Guernsey MW, Park S, Payne J, Beckstead RB, et al. Molecular shifts in limb identity underlie development of feathered feet in two domestic avian species. elife. 2016;5:e Mayerson PL, Fallon JF. The spatial pattern and temporal sequence in which feather germs Arise in the white leghorn Chick-embryo. Dev Biol. 1985;109: Johansson JA, Headon DJ. Regionalisation of the skin. Semin Cell Dev Biol. 2014;25: Chuong CM, Oliver G, Ting SA, Jegalian BG, Chen HM, De Robertis EM. Gradients of homeoproteins in developing feather buds. Development. 1990;110: Hughes MW, Wu P, Jiang TX, Lin SJ, Dong CY, Li A, Hsieh FJ, Widelitz RB, Chuong CM. In search of the golden fleece: unraveling principles of morphogenesis by studying the integrative biology of skin appendages. Integr Biol. 2011;3: Reid AI, Gaunt SJ. Colinearity and non-colinearity in the expression of Hox genes in developing chick skin. Int J Dev Biol. 2002;46: Nelson CE, Morgan BA, Burke AC, Laufer E, DiMambro E, Murtaugh LC, Gonzales E, Tessarollo L, Parada LF, Tabin C. Analysis of Hox gene expression in the chick limb bud. Development. 1996;122: Sato K, Koizumi Y, Takahashi M, Kuroiwa A, Tamura K. Specification of cell fate along the proximal-distal axis in the developing chick limb bud. Development. 2007;134: Yokouchi Y, Nakazato S, Yamamoto M, Goto Y, Kameda T, Iba H, Kuroiwa A. Misexpression of Hoxa-13 induces cartilage homeotic transformation and changes cell adhesiveness in Chick limb buds. Genes Dev. 1995;9: Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007;21: Tamura K, Yonei-Tamura S, Yano T, Yokoyama H, Ide H. The autopod: its formation during limb development. Develop Growth Differ. 2008;50:S Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic-hedgehog mediates the polarizing activity of the Zpa. Cell. 1993;75: Seki R, Li C, Fang Q, Hayashi S, Egawa S, Hu J, Xu L, Pan H, Kondo M, Sato T, et al. Functional roles of Aves class-specific cis-regulatory elements on macroevolution of bird-specific features. Nat Commun. 2017;8: Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C. Induction of the Lim Homeobox gene Lmx1 by Wnt7a establishes Dorsoventral pattern in the vertebrate limb. Cell. 1995;83: Geduspan JS, MacCabe JA. The ectodermal control of mesodermal patterns of differentiation in the developing chick wing. Dev Biol. 1987;124: Grieshammer U, Minowada G, Pisenti JM, Abbott UK, Martin GR. The chick limbless mutation causes abnormalities in limb bud dorsal-ventral patterning: implications for the mechanism of apical ridge formation. Development. 1996;122: Xu X, Zheng XT, You HL. Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature. 2010;464: Submit your next manuscript to BioMed Central and we will help you at every step: We accept pre-submission inquiries Our selector tool helps you to find the most relevant journal We provide round the clock customer support Convenient online submission Thorough peer review Inclusion in PubMed and all major indexing services Maximum visibility for your research Submit your manuscript at
Biology 340 Comparative Embryology Lecture 12 Dr. Stuart Sumida. Evo-Devo Revisited. Development of the Tetrapod Limb
 Biology 340 Comparative Embryology Lecture 12 Dr. Stuart Sumida Evo-Devo Revisited Development of the Tetrapod Limb Limbs whether fins or arms/legs for only in particular regions or LIMB FIELDS. Primitively
Biology 340 Comparative Embryology Lecture 12 Dr. Stuart Sumida Evo-Devo Revisited Development of the Tetrapod Limb Limbs whether fins or arms/legs for only in particular regions or LIMB FIELDS. Primitively
Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling
 Developmental Cell, Volume 29 Supplemental Information Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling Danyi Li, Rui
Developmental Cell, Volume 29 Supplemental Information Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling Danyi Li, Rui
Accepted Manuscript. News & Views. Primary feather vane asymmetry should not be used to predict the flight capabilities of feathered fossils
 Accepted Manuscript News & Views Primary feather vane asymmetry should not be used to predict the flight capabilities of feathered fossils Xia Wang, Robert L. Nudds, Colin Palmer, Gareth J. Dyke PII: S2095-9273(17)30453-X
Accepted Manuscript News & Views Primary feather vane asymmetry should not be used to predict the flight capabilities of feathered fossils Xia Wang, Robert L. Nudds, Colin Palmer, Gareth J. Dyke PII: S2095-9273(17)30453-X
1/9/2013. Divisions of the Skeleton: Topic 8: Appendicular Skeleton. Appendicular Components. Appendicular Components
 /9/203 Topic 8: Appendicular Skeleton Divisions of the Skeleton: Cranial Postcranial What makes up the appendicular skeleton? What is the pattern of serial homology of the limbs? Tetrapod front limb morphology
/9/203 Topic 8: Appendicular Skeleton Divisions of the Skeleton: Cranial Postcranial What makes up the appendicular skeleton? What is the pattern of serial homology of the limbs? Tetrapod front limb morphology
Supplementary Figure 1 Cartilaginous stages in non-avian amniotes. (a) Drawing of early ankle development of Alligator mississippiensis, as reported
 Supplementary Figure 1 Cartilaginous stages in non-avian amniotes. (a) Drawing of early ankle development of Alligator mississippiensis, as reported by a previous study 1. The intermedium is formed at
Supplementary Figure 1 Cartilaginous stages in non-avian amniotes. (a) Drawing of early ankle development of Alligator mississippiensis, as reported by a previous study 1. The intermedium is formed at
the Feather or the Bird?
 Which Came First, the Feather or the Bird? FEATHERS EVOLVED in carnivorous, bipedal dinosaurs before the origin of birds. The creatures depicted here are reconstructions of fossils found recently in northern
Which Came First, the Feather or the Bird? FEATHERS EVOLVED in carnivorous, bipedal dinosaurs before the origin of birds. The creatures depicted here are reconstructions of fossils found recently in northern
Adaptation to the Sky: Defining The Feather With Integument Fossils From Mesozoic China and Experimental Evidence From Molecular Laboratories
 JOURNAL OF EXPERIMENTAL ZOOLOGY (MOL DEV EVOL) 298B:42 56 (2003) Adaptation to the Sky: Defining The Feather With Integument Fossils From Mesozoic China and Experimental Evidence From Molecular Laboratories
JOURNAL OF EXPERIMENTAL ZOOLOGY (MOL DEV EVOL) 298B:42 56 (2003) Adaptation to the Sky: Defining The Feather With Integument Fossils From Mesozoic China and Experimental Evidence From Molecular Laboratories
DEVELOPMENT OF THE HEAD AND NECK PLACODES
 DEVELOPMENT OF THE HEAD AND NECK Placodes and the development of organs of special sense L. Moss-Salentijn PLACODES Localized thickened areas of specialized ectoderm, lateral to the neural crest, at the
DEVELOPMENT OF THE HEAD AND NECK Placodes and the development of organs of special sense L. Moss-Salentijn PLACODES Localized thickened areas of specialized ectoderm, lateral to the neural crest, at the
Morphoregulation of Avian Beaks: Comparative Mapping of Growth Zone Activities and Morphological Evolution
 DEVELOPMENTAL DYNAMICS 235:1400 1412, 2006 RESEARCH ARTICLE Morphoregulation of Avian Beaks: Comparative Mapping of Growth Zone Activities and Morphological Evolution Ping Wu, 1 Ting-Xin Jiang, 1 Jen-Yee
DEVELOPMENTAL DYNAMICS 235:1400 1412, 2006 RESEARCH ARTICLE Morphoregulation of Avian Beaks: Comparative Mapping of Growth Zone Activities and Morphological Evolution Ping Wu, 1 Ting-Xin Jiang, 1 Jen-Yee
COMBINATIONS BETWEEN CHICK EMBRYOS OF DIFFERENT
 446 ZOOLOG Y: WILLIER AND RA WLES PROC. N. A. S. FEA THER CHARA CTERIZA TION AS STUDIED IN HOST-GRA FT COMBINATIONS BETWEEN CHICK EMBRYOS OF DIFFERENT BREEDS By B. H. WILLIER AND MARY E. RAWLES DEPARTMENT
446 ZOOLOG Y: WILLIER AND RA WLES PROC. N. A. S. FEA THER CHARA CTERIZA TION AS STUDIED IN HOST-GRA FT COMBINATIONS BETWEEN CHICK EMBRYOS OF DIFFERENT BREEDS By B. H. WILLIER AND MARY E. RAWLES DEPARTMENT
Lesson 7. References: Chapter 6: Chapter 12: Reading for Next Lesson: Chapter 6:
 Lesson 7 Lesson Outline: Embryonic Origins of the Dermis Specializations of the Dermis o Scales in Fish o Dermal Armour in Tetrapods Epidermal/Dermal Interactions o Feathers o Hair o Teeth Objectives:
Lesson 7 Lesson Outline: Embryonic Origins of the Dermis Specializations of the Dermis o Scales in Fish o Dermal Armour in Tetrapods Epidermal/Dermal Interactions o Feathers o Hair o Teeth Objectives:
(1) the behavior of pigmented skin grafts on non-pigmented hosts
 542 ZOOLOGY: WILLIER, RA WLES AND HADORN PROC. N. A. S. 3. Fagus-Araucaria zones-eogene. 4. Lower Miocene flora-part equivalent of Santa Cruz. However lacking in detail or in completeness, this sequence
542 ZOOLOGY: WILLIER, RA WLES AND HADORN PROC. N. A. S. 3. Fagus-Araucaria zones-eogene. 4. Lower Miocene flora-part equivalent of Santa Cruz. However lacking in detail or in completeness, this sequence
Theoretical Morphology and Development of Flight Feather Vane Asymmetry with Experimental Tests in Parrots
 RESEARCH ARTICLE Theoretical Morphology and Development of Flight Feather Vane Asymmetry with Experimental Tests in Parrots TERESA J. FEO 1,2 * AND RICHARD O. PRUM 1,2 1 Department of Ecology and Evolutionary
RESEARCH ARTICLE Theoretical Morphology and Development of Flight Feather Vane Asymmetry with Experimental Tests in Parrots TERESA J. FEO 1,2 * AND RICHARD O. PRUM 1,2 1 Department of Ecology and Evolutionary
Evolution as Fact. The figure below shows transitional fossils in the whale lineage.
 Evolution as Fact Evolution is a fact. Organisms descend from others with modification. Phylogeny, the lineage of ancestors and descendants, is the scientific term to Darwin's phrase "descent with modification."
Evolution as Fact Evolution is a fact. Organisms descend from others with modification. Phylogeny, the lineage of ancestors and descendants, is the scientific term to Darwin's phrase "descent with modification."
Developmental basis of limblessness and axial patterning in snakes
 are expanded along the body axis in python embryos, and that this can account for both the absence of forelimbs and the expansion of thoracic identity in the axial skeleton. Hindlimb buds are initiated,
are expanded along the body axis in python embryos, and that this can account for both the absence of forelimbs and the expansion of thoracic identity in the axial skeleton. Hindlimb buds are initiated,
2 nd Term Final. Revision Sheet. Students Name: Grade: 11 A/B. Subject: Biology. Teacher Signature. Page 1 of 11
 2 nd Term Final Revision Sheet Students Name: Grade: 11 A/B Subject: Biology Teacher Signature Page 1 of 11 Nour Al Maref International School Riyadh, Saudi Arabia Biology Worksheet (2 nd Term) Chapter-26
2 nd Term Final Revision Sheet Students Name: Grade: 11 A/B Subject: Biology Teacher Signature Page 1 of 11 Nour Al Maref International School Riyadh, Saudi Arabia Biology Worksheet (2 nd Term) Chapter-26
BEAK AND FEATHER DYSTROPHY IN WILD SULPHUR-CRESTED COCKATOOS (CACATUA GALERITA)
 BEAK AND FEATHER DYSTROPHY IN WILD SULPHUR-CRESTED COCKATOOS (CACATUA GALERITA) Author(s): Steven McOrist, Douglas G. Black, David A. Pass, Peter C. Scott, and John Marshall Source: Journal of Wildlife
BEAK AND FEATHER DYSTROPHY IN WILD SULPHUR-CRESTED COCKATOOS (CACATUA GALERITA) Author(s): Steven McOrist, Douglas G. Black, David A. Pass, Peter C. Scott, and John Marshall Source: Journal of Wildlife
VARIATION IN MONIEZIA EXPANSA RUDOLPHI
 VARIATION IN MONIEZIA EXPANSA RUDOLPHI STEPHEN R. WILLIAMS, Miami University, Oxford, Ohio In making a number of preparations of proglottids for class study at the stage when sex organs are mature and
VARIATION IN MONIEZIA EXPANSA RUDOLPHI STEPHEN R. WILLIAMS, Miami University, Oxford, Ohio In making a number of preparations of proglottids for class study at the stage when sex organs are mature and
Biology 1B Evolution Lecture 11 (March 19, 2010), Insights from the Fossil Record and Evo-Devo
 Biology 1B Evolution Lecture 11 (March 19, 2010), Insights from the Fossil Record and Evo-Devo Extinction Important points on extinction rates: Background rate of extinctions per million species per year:
Biology 1B Evolution Lecture 11 (March 19, 2010), Insights from the Fossil Record and Evo-Devo Extinction Important points on extinction rates: Background rate of extinctions per million species per year:
Evo-Devo of amniote integuments and appendages
 Int. J. Dev. Biol. 48: 249-270 (2004) Evo-Devo of amniote integuments and appendages PING WU 1, LIANHAI HOU 2, MAKSIM PLIKUS 1, MICHAEL HUGHES 1, JEFFREY SCEHNET 1, SANONG SUKSAWEANG 1, RANDALL B. WIDELITZ
Int. J. Dev. Biol. 48: 249-270 (2004) Evo-Devo of amniote integuments and appendages PING WU 1, LIANHAI HOU 2, MAKSIM PLIKUS 1, MICHAEL HUGHES 1, JEFFREY SCEHNET 1, SANONG SUKSAWEANG 1, RANDALL B. WIDELITZ
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii. Yates, Lauren A.
 A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
Video Assignments. Microraptor PBS The Four-winged Dinosaur Mark Davis SUNY Cortland Library Online
 Video Assignments Microraptor PBS The Four-winged Dinosaur Mark Davis SUNY Cortland Library Online Radiolab Apocalyptical http://www.youtube.com/watch?v=k52vd4wbdlw&feature=youtu.be Minute 13 through minute
Video Assignments Microraptor PBS The Four-winged Dinosaur Mark Davis SUNY Cortland Library Online Radiolab Apocalyptical http://www.youtube.com/watch?v=k52vd4wbdlw&feature=youtu.be Minute 13 through minute
A new scenario for the evolutionary origin of hair, feather, and avian scales.
 REVIEW A new scenario for the evolutionary origin of hair, feather, and avian scales. Danielle Dhouailly 1 Equipe Ontogenèse et Cellules Souches du Tégument, Centre de Recherche INSERM UJF U823, Institut
REVIEW A new scenario for the evolutionary origin of hair, feather, and avian scales. Danielle Dhouailly 1 Equipe Ontogenèse et Cellules Souches du Tégument, Centre de Recherche INSERM UJF U823, Institut
Molecular embryology of the foregut.
 Author manuscript, published in "Journal of Pediatric Gastroenterology & Nutrition 2011;52 Suppl 1:S2-3" DOI : 10.1097/MPG.0b013e3182105a1a Molecular embryology of the foregut. Sandrine Faure, Ph.D. and
Author manuscript, published in "Journal of Pediatric Gastroenterology & Nutrition 2011;52 Suppl 1:S2-3" DOI : 10.1097/MPG.0b013e3182105a1a Molecular embryology of the foregut. Sandrine Faure, Ph.D. and
Diapsida. BIO2135 Animal Form and Function. Page 1. Diapsida (Reptilia, Sauropsida) Amniote eggs. Amniote egg. Temporal fenestra.
 Diapsida (Reptilia, Sauropsida) Vertebrate phylogeny Mixini Chondrichthyes Sarcopterygii Mammalia Pteromyzontida Actinopterygii Amphibia Reptilia! 1! Amniota (autapomorphies) Costal ventilation Amniote
Diapsida (Reptilia, Sauropsida) Vertebrate phylogeny Mixini Chondrichthyes Sarcopterygii Mammalia Pteromyzontida Actinopterygii Amphibia Reptilia! 1! Amniota (autapomorphies) Costal ventilation Amniote
Diapsida. BIO2135 Animal Form and Function. Page 1. Diapsida (Reptilia, Sauropsida) Amniote egg. Membranes. Vertebrate phylogeny
 Diapsida (Reptilia, Sauropsida) 1 Vertebrate phylogeny Mixini Chondrichthyes Sarcopterygii Mammalia Pteromyzontida Actinopterygii Amphibia Reptilia!! Amniota (autapomorphies) Costal ventilation Amniote
Diapsida (Reptilia, Sauropsida) 1 Vertebrate phylogeny Mixini Chondrichthyes Sarcopterygii Mammalia Pteromyzontida Actinopterygii Amphibia Reptilia!! Amniota (autapomorphies) Costal ventilation Amniote
What is evolution? Transitional fossils: evidence for evolution. In its broadest sense, evolution is simply the change in life through time.
 Transitional fossils: evidence for evolution http://domain- of- darwin.deviantart.com/art/no- Transitional- Fossils- 52231284 Western MA Atheists and Secular Humanists 28 May 2016 What is evolution? In
Transitional fossils: evidence for evolution http://domain- of- darwin.deviantart.com/art/no- Transitional- Fossils- 52231284 Western MA Atheists and Secular Humanists 28 May 2016 What is evolution? In
Modern taxonomy. Building family trees 10/10/2011. Knowing a lot about lots of creatures. Tom Hartman. Systematics includes: 1.
 Modern taxonomy Building family trees Tom Hartman www.tuatara9.co.uk Classification has moved away from the simple grouping of organisms according to their similarities (phenetics) and has become the study
Modern taxonomy Building family trees Tom Hartman www.tuatara9.co.uk Classification has moved away from the simple grouping of organisms according to their similarities (phenetics) and has become the study
The genetics and development of fused and supernumerary molars in the rice rat
 /. Embryol. exp. Morph. Vol. 26, 1, pp. 99-109, 1971 99 Printed in Great Britain The genetics and development of fused and supernumerary molars in the rice rat By J. A. SOFAER 1 AND J. H. SHAW 2 From the
/. Embryol. exp. Morph. Vol. 26, 1, pp. 99-109, 1971 99 Printed in Great Britain The genetics and development of fused and supernumerary molars in the rice rat By J. A. SOFAER 1 AND J. H. SHAW 2 From the
VERTEBRATE READING. Fishes
 VERTEBRATE READING Fishes The first vertebrates to become a widespread, predominant life form on earth were fishes. Prior to this, only invertebrates, such as mollusks, worms and squid-like animals, would
VERTEBRATE READING Fishes The first vertebrates to become a widespread, predominant life form on earth were fishes. Prior to this, only invertebrates, such as mollusks, worms and squid-like animals, would
Shifts in axial patterning in snake and caecilian embryos
 Chapter 5 Shifts in axial patterning in snake and caecilian embryos Joost M. Woltering, Freek J. Vonk, Hendrik Müller, Antony J. Durston & Michael K. Richardson Abstract The macro evolutionary morphological
Chapter 5 Shifts in axial patterning in snake and caecilian embryos Joost M. Woltering, Freek J. Vonk, Hendrik Müller, Antony J. Durston & Michael K. Richardson Abstract The macro evolutionary morphological
MORPHOLOGICAL DESCRIPTION OF THE DEVELOPING OSTRICH EMBRYO: A TOOL FOR EMBRYONIC AGE ESTIMATION
 ISRAEL JOURNAL OF ZOOLOGY, Vol. 47, 2001, pp. 87 97 MORPHOLOGICAL DESCRIPTION OF THE DEVELOPING OSTRICH EMBRYO: A TOOL FOR EMBRYONIC AGE ESTIMATION ERAN GEFEN* AND AMOS AR Department of Zoology, Tel Aviv
ISRAEL JOURNAL OF ZOOLOGY, Vol. 47, 2001, pp. 87 97 MORPHOLOGICAL DESCRIPTION OF THE DEVELOPING OSTRICH EMBRYO: A TOOL FOR EMBRYONIC AGE ESTIMATION ERAN GEFEN* AND AMOS AR Department of Zoology, Tel Aviv
Hox Expression in the American Alligator and Evolution of Archosaurian Axial Patterning
 RESEARCH ARTICLE Hox Expression in the American Alligator and Evolution of Archosaurian Axial Patterning JENNIFER H. MANSFIELD 1 AND ARHAT ABZHANOV 2 1 Department of Biological Sciences, Barnard College,
RESEARCH ARTICLE Hox Expression in the American Alligator and Evolution of Archosaurian Axial Patterning JENNIFER H. MANSFIELD 1 AND ARHAT ABZHANOV 2 1 Department of Biological Sciences, Barnard College,
Diversity of Animals
 Classifying Animals Diversity of Animals Animals can be classified and grouped based on similarities in their characteristics. Animals make up one of the major biological groups of classification. All
Classifying Animals Diversity of Animals Animals can be classified and grouped based on similarities in their characteristics. Animals make up one of the major biological groups of classification. All
Vertebrates. Vertebrate Characteristics. 444 Chapter 14
 4 Vertebrates Key Concept All vertebrates have a backbone, which supports other specialized body structures and functions. What You Will Learn Vertebrates have an endoskeleton that provides support and
4 Vertebrates Key Concept All vertebrates have a backbone, which supports other specialized body structures and functions. What You Will Learn Vertebrates have an endoskeleton that provides support and
A Scanning Electron Microscopic Study of Eggshell Surface Topography of Leidynema portentosae and L. appendiculatum (Nematoda: Oxyuroidea)
 The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 88, Issue 5 (December, 1988) 1988-12 A Scanning Electron Microscopic
The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 88, Issue 5 (December, 1988) 1988-12 A Scanning Electron Microscopic
HISTOPATHOLOGY. Introduction:
 Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
ECOL330 4/8/2019. Structure of Adult Beak. Where does the beak come from? A developmental perspective. What determines beak shape?
 Beak Evolution in Birds Structure of Adult Beak Evolution of Animal Form & Function Dr Alex Badyaev Office hours: T 11 12, by apt BSW 416 Lecture 15 2019 Instructor: Sarah Britton ECOL330 A developmental
Beak Evolution in Birds Structure of Adult Beak Evolution of Animal Form & Function Dr Alex Badyaev Office hours: T 11 12, by apt BSW 416 Lecture 15 2019 Instructor: Sarah Britton ECOL330 A developmental
Page # Diversity of Arthropoda Crustacea Morphology. Diversity of Arthropoda. Diversity of Arthropoda. Diversity of Arthropoda. Arthropods, from last
 Arthropods, from last time Crustacea are the dominant marine arthropods Crustacea are the dominant marine arthropods any terrestrial crustaceans? Should we call them shellfish? sowbugs 2 3 Crustacea Morphology
Arthropods, from last time Crustacea are the dominant marine arthropods Crustacea are the dominant marine arthropods any terrestrial crustaceans? Should we call them shellfish? sowbugs 2 3 Crustacea Morphology
The Evolution of HoxD-11 Expression in the Bird Wing: Insights from Alligator mississippiensis
 The Evolution of HoxD-11 Expression in the Bird Wing: Insights from Alligator mississippiensis Alexander O. Vargas 1 a *, Tiana Kohlsdorf 1 b, John F. Fallon 2, John VandenBrooks 3 c, Günter P. Wagner
The Evolution of HoxD-11 Expression in the Bird Wing: Insights from Alligator mississippiensis Alexander O. Vargas 1 a *, Tiana Kohlsdorf 1 b, John F. Fallon 2, John VandenBrooks 3 c, Günter P. Wagner
Title: Phylogenetic Methods and Vertebrate Phylogeny
 Title: Phylogenetic Methods and Vertebrate Phylogeny Central Question: How can evolutionary relationships be determined objectively? Sub-questions: 1. What affect does the selection of the outgroup have
Title: Phylogenetic Methods and Vertebrate Phylogeny Central Question: How can evolutionary relationships be determined objectively? Sub-questions: 1. What affect does the selection of the outgroup have
This is a series of skulls and front leg fossils of organisms believed to be ancestors of the modern-day horse.
 Evidence of Evolution Background When Charles Darwin first proposed the idea that all new species descend from an ancestor, he performed an exhaustive amount of research to provide as much evidence as
Evidence of Evolution Background When Charles Darwin first proposed the idea that all new species descend from an ancestor, he performed an exhaustive amount of research to provide as much evidence as
A quantitative study of hair growth using mouse and rat vibrissal follicles
 /. Embryol. exp. Morph. Vol. 72, pp. 209-224, 1982 209 Printed in Great Britain Company of Biologists Limited 1982 A quantitative study of hair growth using mouse and rat vibrissal follicles I. Dermal
/. Embryol. exp. Morph. Vol. 72, pp. 209-224, 1982 209 Printed in Great Britain Company of Biologists Limited 1982 A quantitative study of hair growth using mouse and rat vibrissal follicles I. Dermal
Birds & Mammals. Chapter 15
 Birds & Mammals Chapter 15 What is a Bird? Vertebrate Endothermic Feathered 4 chambered heart Egg laying Fore-limbs adapted for flight Bones nearly hollow (allow for lighter weight) Bird Internal Anatomy
Birds & Mammals Chapter 15 What is a Bird? Vertebrate Endothermic Feathered 4 chambered heart Egg laying Fore-limbs adapted for flight Bones nearly hollow (allow for lighter weight) Bird Internal Anatomy
What is the evidence for evolution?
 What is the evidence for evolution? 1. Geographic Distribution 2. Fossil Evidence & Transitional Species 3. Comparative Anatomy 1. Homologous Structures 2. Analogous Structures 3. Vestigial Structures
What is the evidence for evolution? 1. Geographic Distribution 2. Fossil Evidence & Transitional Species 3. Comparative Anatomy 1. Homologous Structures 2. Analogous Structures 3. Vestigial Structures
17.2 Classification Based on Evolutionary Relationships Organization of all that speciation!
 Organization of all that speciation! Patterns of evolution.. Taxonomy gets an over haul! Using more than morphology! 3 domains, 6 kingdoms KEY CONCEPT Modern classification is based on evolutionary relationships.
Organization of all that speciation! Patterns of evolution.. Taxonomy gets an over haul! Using more than morphology! 3 domains, 6 kingdoms KEY CONCEPT Modern classification is based on evolutionary relationships.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
Name Class Date. After you read this section, you should be able to answer these questions:
 CHAPTER 14 4 Vertebrates SECTION Introduction to Animals BEFORE YOU READ After you read this section, you should be able to answer these questions: How are vertebrates different from invertebrates? How
CHAPTER 14 4 Vertebrates SECTION Introduction to Animals BEFORE YOU READ After you read this section, you should be able to answer these questions: How are vertebrates different from invertebrates? How
Aging by molt patterns of flight feathers of non adult Steller s Sea Eagle
 First Symposium on Steller s and White-tailed Sea Eagles in East Asia pp. 11-16, 2000 UETA, M. & MCGRADY, M.J. (eds) Wild Bird Society of Japan, Tokyo Japan Aging by molt patterns of flight feathers of
First Symposium on Steller s and White-tailed Sea Eagles in East Asia pp. 11-16, 2000 UETA, M. & MCGRADY, M.J. (eds) Wild Bird Society of Japan, Tokyo Japan Aging by molt patterns of flight feathers of
Animal Diversity wrap-up Lecture 9 Winter 2014
 Animal Diversity wrap-up Lecture 9 Winter 2014 1 Animal phylogeny based on morphology & development Fig. 32.10 2 Animal phylogeny based on molecular data Fig. 32.11 New Clades 3 Lophotrochozoa Lophophore:
Animal Diversity wrap-up Lecture 9 Winter 2014 1 Animal phylogeny based on morphology & development Fig. 32.10 2 Animal phylogeny based on molecular data Fig. 32.11 New Clades 3 Lophotrochozoa Lophophore:
I another of a genetically different breed of fowl or species of bird that the
 GENOTYPIC CONTROL OF FEATHER COLOR PATTERN AS DEMONSTRATED BY THE EFFECTS OF A SEX- LINKED GENE UPON THE MELANOPHORES* B. H. WILLIER AND MARY E. RAWLES Department of Biology, The Johns Hopkins University,
GENOTYPIC CONTROL OF FEATHER COLOR PATTERN AS DEMONSTRATED BY THE EFFECTS OF A SEX- LINKED GENE UPON THE MELANOPHORES* B. H. WILLIER AND MARY E. RAWLES Department of Biology, The Johns Hopkins University,
ABSTRACT. aspect is very sparse and in view of its importance. MATERIALS AND METHODS
 MICROMETRICAL STUDIES ON THE SKIN OF MADRAS RED SHEEP (OVIS ARIES) IN DIFEERENT AGE GROUPS Mir Shabir Ahmad 1, O.R. Sathyamoorthy 2, Geetha Ramesh 3 and C. Balachandran 4 Department of Veterinary Anatomy
MICROMETRICAL STUDIES ON THE SKIN OF MADRAS RED SHEEP (OVIS ARIES) IN DIFEERENT AGE GROUPS Mir Shabir Ahmad 1, O.R. Sathyamoorthy 2, Geetha Ramesh 3 and C. Balachandran 4 Department of Veterinary Anatomy
Modern Evolutionary Classification. Lesson Overview. Lesson Overview Modern Evolutionary Classification
 Lesson Overview 18.2 Modern Evolutionary Classification THINK ABOUT IT Darwin s ideas about a tree of life suggested a new way to classify organisms not just based on similarities and differences, but
Lesson Overview 18.2 Modern Evolutionary Classification THINK ABOUT IT Darwin s ideas about a tree of life suggested a new way to classify organisms not just based on similarities and differences, but
Testing Phylogenetic Hypotheses with Molecular Data 1
 Testing Phylogenetic Hypotheses with Molecular Data 1 How does an evolutionary biologist quantify the timing and pathways for diversification (speciation)? If we observe diversification today, the processes
Testing Phylogenetic Hypotheses with Molecular Data 1 How does an evolutionary biologist quantify the timing and pathways for diversification (speciation)? If we observe diversification today, the processes
CLADISTICS Student Packet SUMMARY Phylogeny Phylogenetic trees/cladograms
 CLADISTICS Student Packet SUMMARY PHYLOGENETIC TREES AND CLADOGRAMS ARE MODELS OF EVOLUTIONARY HISTORY THAT CAN BE TESTED Phylogeny is the history of descent of organisms from their common ancestor. Phylogenetic
CLADISTICS Student Packet SUMMARY PHYLOGENETIC TREES AND CLADOGRAMS ARE MODELS OF EVOLUTIONARY HISTORY THAT CAN BE TESTED Phylogeny is the history of descent of organisms from their common ancestor. Phylogenetic
What Makes a Bird a Bird?
 What Makes a Bird a Bird? Overview Students will compare types of feathers by examining structure and function of each. California Science Standards Grade 5: 6.g.-I&E Grade 6: 7.b.-I&E Grade 7: 7.a.-I&E
What Makes a Bird a Bird? Overview Students will compare types of feathers by examining structure and function of each. California Science Standards Grade 5: 6.g.-I&E Grade 6: 7.b.-I&E Grade 7: 7.a.-I&E
Introduction to phylogenetic trees and tree-thinking Copyright 2005, D. A. Baum (Free use for non-commercial educational pruposes)
 Introduction to phylogenetic trees and tree-thinking Copyright 2005, D. A. Baum (Free use for non-commercial educational pruposes) Phylogenetics is the study of the relationships of organisms to each other.
Introduction to phylogenetic trees and tree-thinking Copyright 2005, D. A. Baum (Free use for non-commercial educational pruposes) Phylogenetics is the study of the relationships of organisms to each other.
Origin and Evolution of Birds. Read: Chapters 1-3 in Gill but limited review of systematics
 Origin and Evolution of Birds Read: Chapters 1-3 in Gill but limited review of systematics Review of Taxonomy Kingdom: Animalia Phylum: Chordata Subphylum: Vertebrata Class: Aves Characteristics: wings,
Origin and Evolution of Birds Read: Chapters 1-3 in Gill but limited review of systematics Review of Taxonomy Kingdom: Animalia Phylum: Chordata Subphylum: Vertebrata Class: Aves Characteristics: wings,
Frog Dissection Information Manuel
 Frog Dissection Information Manuel Anatomical Terms: Used to explain directions and orientation of a organism Directions or Positions: Anterior (cranial)- toward the head Posterior (caudal)- towards the
Frog Dissection Information Manuel Anatomical Terms: Used to explain directions and orientation of a organism Directions or Positions: Anterior (cranial)- toward the head Posterior (caudal)- towards the
These small issues are easily addressed by small changes in wording, and should in no way delay publication of this first- rate paper.
 Reviewers' comments: Reviewer #1 (Remarks to the Author): This paper reports on a highly significant discovery and associated analysis that are likely to be of broad interest to the scientific community.
Reviewers' comments: Reviewer #1 (Remarks to the Author): This paper reports on a highly significant discovery and associated analysis that are likely to be of broad interest to the scientific community.
Evidence for Evolution by Natural Selection. Hunting for evolution clues Elementary, my dear, Darwin!
 Evidence for Evolution by Natural Selection Hunting for evolution clues Elementary, my dear, Darwin! 2006-2007 Evidence supporting evolution Fossil record shows change over time Anatomical record comparing
Evidence for Evolution by Natural Selection Hunting for evolution clues Elementary, my dear, Darwin! 2006-2007 Evidence supporting evolution Fossil record shows change over time Anatomical record comparing
Phylogeny Reconstruction
 Phylogeny Reconstruction Trees, Methods and Characters Reading: Gregory, 2008. Understanding Evolutionary Trees (Polly, 2006) Lab tomorrow Meet in Geology GY522 Bring computers if you have them (they will
Phylogeny Reconstruction Trees, Methods and Characters Reading: Gregory, 2008. Understanding Evolutionary Trees (Polly, 2006) Lab tomorrow Meet in Geology GY522 Bring computers if you have them (they will
Echinoderms. Copyright 2011 LessonSnips
 Echinoderms The ocean is home to different creatures from animals that are found on land and the phylum of echinoderms is a prime example. The phylum Echinodermata is a scientific classification of simple
Echinoderms The ocean is home to different creatures from animals that are found on land and the phylum of echinoderms is a prime example. The phylum Echinodermata is a scientific classification of simple
THE EFFECTS OF THE ENVIRONMENTAL CONDITIONS ON CURLY EXPRESSIVITY IN DROSOPHILA MELANOGAST ER. Ken NOZAWA
 THE EFFECTS OF THE ENVIRONMENTAL CONDITIONS ON CURLY EXPRESSIVITY IN DROSOPHILA MELANOGAST ER Ken NOZAWA Department of Animal Breeding, Faculty of Agriculture, Nagoya University, Anjo, Japan Received August
THE EFFECTS OF THE ENVIRONMENTAL CONDITIONS ON CURLY EXPRESSIVITY IN DROSOPHILA MELANOGAST ER Ken NOZAWA Department of Animal Breeding, Faculty of Agriculture, Nagoya University, Anjo, Japan Received August
Selection and Evaluation
 Selection and Evaluation Lesson 2: Selection and Evaluation Selecting high quality poultry is a skill that is important to egg and meat production. By evaluating and selecting the most productive birds,
Selection and Evaluation Lesson 2: Selection and Evaluation Selecting high quality poultry is a skill that is important to egg and meat production. By evaluating and selecting the most productive birds,
The Development of Behavior
 The Development of Behavior 0 people liked this 0 discussions READING ASSIGNMENT Read this assignment. Though you've already read the textbook reading assignment that accompanies this assignment, you may
The Development of Behavior 0 people liked this 0 discussions READING ASSIGNMENT Read this assignment. Though you've already read the textbook reading assignment that accompanies this assignment, you may
ARE CURRENT CRITIQUES OF THE THEROPOD ORIGIN OF BIRDS SCIENCE? REBUTTAL TO FEDUCCIA (2002)
 Commentary The Auk 120(2):550 561, 2003 ARE CURRENT CRITIQUES OF THE THEROPOD ORIGIN OF BIRDS SCIENCE? REBUTTAL TO FEDUCCIA (2002) Department of Ecology and Evolutionary Biology, and Natural History Museum,
Commentary The Auk 120(2):550 561, 2003 ARE CURRENT CRITIQUES OF THE THEROPOD ORIGIN OF BIRDS SCIENCE? REBUTTAL TO FEDUCCIA (2002) Department of Ecology and Evolutionary Biology, and Natural History Museum,
Origin and Evolution of Birds. Read: Chapters 1-3 in Gill but limited review of systematics
 Origin and Evolution of Birds Read: Chapters 1-3 in Gill but limited review of systematics Review of Taxonomy Kingdom: Animalia Phylum: Chordata Subphylum: Vertebrata Class: Aves Characteristics: wings,
Origin and Evolution of Birds Read: Chapters 1-3 in Gill but limited review of systematics Review of Taxonomy Kingdom: Animalia Phylum: Chordata Subphylum: Vertebrata Class: Aves Characteristics: wings,
INHERITANCE OF BODY WEIGHT IN DOMESTIC FOWL. Single Comb White Leghorn breeds of fowl and in their hybrids.
 440 GENETICS: N. F. WATERS PROC. N. A. S. and genetical behavior of this form is not incompatible with the segmental interchange theory of circle formation in Oenothera. Summary.-It is impossible for the
440 GENETICS: N. F. WATERS PROC. N. A. S. and genetical behavior of this form is not incompatible with the segmental interchange theory of circle formation in Oenothera. Summary.-It is impossible for the
Title. CitationJapanese Journal of Veterinary Research, 24(1-2): 37. Issue Date DOI. Doc URL. Type. File Information
 Title DISTRIBUTION OF LYMPHATIC TISSUES IN DUCK CAECA Author(s)KITAMURA, Hirokazu; SUGIMURA, Makoto; HASHIMOTO, Yos CitationJapanese Journal of Veterinary Research, 24(1-2): 37 Issue Date 1976-05 DOI 10.14943/jjvr.24.1-2.37
Title DISTRIBUTION OF LYMPHATIC TISSUES IN DUCK CAECA Author(s)KITAMURA, Hirokazu; SUGIMURA, Makoto; HASHIMOTO, Yos CitationJapanese Journal of Veterinary Research, 24(1-2): 37 Issue Date 1976-05 DOI 10.14943/jjvr.24.1-2.37
2. Using an appropriate illustration and words, describe the physics of flight.
 1. Besides the obvious, like feathers and wings, birds have many special features that allow them to fly. Explain how each of the characteristics are specialized to help birds fly. A. Skeletal System-
1. Besides the obvious, like feathers and wings, birds have many special features that allow them to fly. Explain how each of the characteristics are specialized to help birds fly. A. Skeletal System-
Evolution of Birds. Summary:
 Oregon State Standards OR Science 7.1, 7.2, 7.3, 7.3S.1, 7.3S.2 8.1, 8.2, 8.2L.1, 8.3, 8.3S.1, 8.3S.2 H.1, H.2, H.2L.4, H.2L.5, H.3, H.3S.1, H.3S.2, H.3S.3 Summary: Students create phylogenetic trees to
Oregon State Standards OR Science 7.1, 7.2, 7.3, 7.3S.1, 7.3S.2 8.1, 8.2, 8.2L.1, 8.3, 8.3S.1, 8.3S.2 H.1, H.2, H.2L.4, H.2L.5, H.3, H.3S.1, H.3S.2, H.3S.3 Summary: Students create phylogenetic trees to
d a Name Vertebrate Evolution - Exam 2 1. (12) Fill in the blanks
 Vertebrate Evolution - Exam 2 1. (12) Fill in the blanks 100 points Name f e c d a Identify the structures (for c and e, identify the entire structure, not the individual elements. b a. b. c. d. e. f.
Vertebrate Evolution - Exam 2 1. (12) Fill in the blanks 100 points Name f e c d a Identify the structures (for c and e, identify the entire structure, not the individual elements. b a. b. c. d. e. f.
Vertebrates. Vertebrates are animals that have a backbone and an endoskeleton.
 Vertebrates Vertebrates are animals that have a backbone and an endoskeleton. The backbone replaces the notochord and contains bones called vertebrae. An endoskeleton is an internal skeleton that protects
Vertebrates Vertebrates are animals that have a backbone and an endoskeleton. The backbone replaces the notochord and contains bones called vertebrae. An endoskeleton is an internal skeleton that protects
S06-5 Selection for feather structure. 1 Introduction
 52(Supplement): 131 135, 2006 S06-5 Selection for feather structure Edward H. BURTT Jr. 1, Jann M. ICHIDA 2 1. Dept. of Zoology, Ohio Wesleyan University, Delaware, OH 43015, USA; ehburtt@owu.edu 2. Dept.
52(Supplement): 131 135, 2006 S06-5 Selection for feather structure Edward H. BURTT Jr. 1, Jann M. ICHIDA 2 1. Dept. of Zoology, Ohio Wesleyan University, Delaware, OH 43015, USA; ehburtt@owu.edu 2. Dept.
Developmental Morphology of Limb Reduction in Hemiergis (Squamata: Scincidae): Chondrogenesis, Osteogenesis, and Heterochrony
 JOURNAL OF MORPHOLOGY 254:211 231 (2002) Developmental Morphology of Limb Reduction in Hemiergis (Squamata: Scincidae): Chondrogenesis, Osteogenesis, and Heterochrony Michael D. Shapiro* Department of
JOURNAL OF MORPHOLOGY 254:211 231 (2002) Developmental Morphology of Limb Reduction in Hemiergis (Squamata: Scincidae): Chondrogenesis, Osteogenesis, and Heterochrony Michael D. Shapiro* Department of
Lesson 6. References: Chapter 6: Reading for Next Lesson: Chapter 6:
 Lesson 6 Lesson Outline: General Features of the Integument Embryonic Origins of the Epidermis Specializations of the Epidermis o Glands o Keratin and Stratum Corneum Objectives: At the end of this lesson
Lesson 6 Lesson Outline: General Features of the Integument Embryonic Origins of the Epidermis Specializations of the Epidermis o Glands o Keratin and Stratum Corneum Objectives: At the end of this lesson
From Reptiles to Aves
 First Vertebrates From Reptiles to Aves Evolutions of Fish to Amphibians Evolution of Amphibians to Reptiles Evolution of Reptiles to Dinosaurs to Birds Common Ancestor of Birds and Reptiles: Thecodonts
First Vertebrates From Reptiles to Aves Evolutions of Fish to Amphibians Evolution of Amphibians to Reptiles Evolution of Reptiles to Dinosaurs to Birds Common Ancestor of Birds and Reptiles: Thecodonts
Incubation Conditions and Integrity in Pekin Ducks
 Incubation Conditions and Integrity in Pekin Ducks Ozan Akkus 1, Co-PI; Todd Applegate 2, Co-PI; Serife Agcaoglu 1 1 Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907,
Incubation Conditions and Integrity in Pekin Ducks Ozan Akkus 1, Co-PI; Todd Applegate 2, Co-PI; Serife Agcaoglu 1 1 Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907,
Calendar : Timeframe: 1 st 9 Weeks
 Subject: Advanced Animal Science Calendar : Timeframe: 1 st 9 Weeks Level/Grade: 9-12 Unit A Knowledge of the employability characteristics of a successful worker Unit B Demonstration of principles Unit
Subject: Advanced Animal Science Calendar : Timeframe: 1 st 9 Weeks Level/Grade: 9-12 Unit A Knowledge of the employability characteristics of a successful worker Unit B Demonstration of principles Unit
muscles (enhancing biting strength). Possible states: none, one, or two.
 Reconstructing Evolutionary Relationships S-1 Practice Exercise: Phylogeny of Terrestrial Vertebrates In this example we will construct a phylogenetic hypothesis of the relationships between seven taxa
Reconstructing Evolutionary Relationships S-1 Practice Exercise: Phylogeny of Terrestrial Vertebrates In this example we will construct a phylogenetic hypothesis of the relationships between seven taxa
Fischthal and Kuntz (1964) reported the
 Zoological Studies 41(3): 283-287 (2002) Meristocotyle provitellaria sp. nov. (Digenea: Meristocotylidae) from Varanus salvator in China Wei Liu 1, Qing-Kui Li 2, Hsiu-Hui Shih 3 and Zhao-Zhi Qiu 1, *
Zoological Studies 41(3): 283-287 (2002) Meristocotyle provitellaria sp. nov. (Digenea: Meristocotylidae) from Varanus salvator in China Wei Liu 1, Qing-Kui Li 2, Hsiu-Hui Shih 3 and Zhao-Zhi Qiu 1, *
Shedding Light on the Dinosaur-Bird Connection
 Shedding Light on the Dinosaur-Bird Connection This text is provided courtesy of the American Museum of Natural History. When people think of dinosaurs, two types generally come to mind: the huge herbivores
Shedding Light on the Dinosaur-Bird Connection This text is provided courtesy of the American Museum of Natural History. When people think of dinosaurs, two types generally come to mind: the huge herbivores
May 10, SWBAT analyze and evaluate the scientific evidence provided by the fossil record.
 May 10, 2017 Aims: SWBAT analyze and evaluate the scientific evidence provided by the fossil record. Agenda 1. Do Now 2. Class Notes 3. Guided Practice 4. Independent Practice 5. Practicing our AIMS: E.3-Examining
May 10, 2017 Aims: SWBAT analyze and evaluate the scientific evidence provided by the fossil record. Agenda 1. Do Now 2. Class Notes 3. Guided Practice 4. Independent Practice 5. Practicing our AIMS: E.3-Examining
University of Bristol - Explore Bristol Research. Peer reviewed version. Link to published version (if available): /pala.
 Saitta, E. T., Gelernter, R., & Vinther, J. (2018). Additional information on the primitive contour and wing feathering of paravian dinosaurs. Palaeontology, 61(2), 273-288. DOI: 10.1111/pala.12342 Peer
Saitta, E. T., Gelernter, R., & Vinther, J. (2018). Additional information on the primitive contour and wing feathering of paravian dinosaurs. Palaeontology, 61(2), 273-288. DOI: 10.1111/pala.12342 Peer
Bio homework #5. Biology Homework #5
 Biology Homework #5 Bio homework #5 The information presented during the first five weeks of INS is very important and will be useful to know in the future (next quarter and beyond).the purpose of this
Biology Homework #5 Bio homework #5 The information presented during the first five weeks of INS is very important and will be useful to know in the future (next quarter and beyond).the purpose of this
Avian Reproductive System Female
 extension Avian Reproductive System Female articles.extension.org/pages/65372/avian-reproductive-systemfemale Written by: Dr. Jacquie Jacob, University of Kentucky For anyone interested in raising chickens
extension Avian Reproductive System Female articles.extension.org/pages/65372/avian-reproductive-systemfemale Written by: Dr. Jacquie Jacob, University of Kentucky For anyone interested in raising chickens
Species: Panthera pardus Genus: Panthera Family: Felidae Order: Carnivora Class: Mammalia Phylum: Chordata
 CHAPTER 6: PHYLOGENY AND THE TREE OF LIFE AP Biology 3 PHYLOGENY AND SYSTEMATICS Phylogeny - evolutionary history of a species or group of related species Systematics - analytical approach to understanding
CHAPTER 6: PHYLOGENY AND THE TREE OF LIFE AP Biology 3 PHYLOGENY AND SYSTEMATICS Phylogeny - evolutionary history of a species or group of related species Systematics - analytical approach to understanding
Mammalogy Lecture 8 - Evolution of Ear Ossicles
 Mammalogy Lecture 8 - Evolution of Ear Ossicles I. To begin, let s examine briefly the end point, that is, modern mammalian ears. Inner Ear The cochlea contains sensory cells for hearing and balance. -
Mammalogy Lecture 8 - Evolution of Ear Ossicles I. To begin, let s examine briefly the end point, that is, modern mammalian ears. Inner Ear The cochlea contains sensory cells for hearing and balance. -
A record of a first year dark plumage Augur Buzzard moulting into normal plumage.
 A record of a first year dark plumage Augur Buzzard moulting into normal plumage. Simon Thomsett The Peregrine Fund, 5668 West Flying Hawk Lane, Boise Idaho, 83709, USA Also: Dept. of Ornithology, National
A record of a first year dark plumage Augur Buzzard moulting into normal plumage. Simon Thomsett The Peregrine Fund, 5668 West Flying Hawk Lane, Boise Idaho, 83709, USA Also: Dept. of Ornithology, National
Phylogeny of Animalia (overview)
 The Diversity of Animals 2 Chapter 23 Phylogeny of Animalia (overview) Key features of Chordates Phylum Chordata (the Chordates) includes both invertebrates and vertebrates that share (at some point in
The Diversity of Animals 2 Chapter 23 Phylogeny of Animalia (overview) Key features of Chordates Phylum Chordata (the Chordates) includes both invertebrates and vertebrates that share (at some point in
Developmental expression of synthetic cis-regulatory systems composed of spatial control elements from two different genes
 Proc. Natl. Acad. Sci. USA Vol. 93, pp. 13849 13854, November 1996 Developmental Biology Developmental expression of synthetic cis-regulatory systems composed of spatial control elements from two different
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 13849 13854, November 1996 Developmental Biology Developmental expression of synthetic cis-regulatory systems composed of spatial control elements from two different
Gross and Microscopic Features of the Interdigital Sinus in the Barbados Black Belly Sheep in Trinidad
 Original Research Article International Journal of Current Research in Medical Sciences ISSN: 2454-5716 www.ijcrims.com Volume 2, Issue 7-2016 SOI: http://s-o-i.org/1.15/ijcrms-2016-2-7-4 Gross and Microscopic
Original Research Article International Journal of Current Research in Medical Sciences ISSN: 2454-5716 www.ijcrims.com Volume 2, Issue 7-2016 SOI: http://s-o-i.org/1.15/ijcrms-2016-2-7-4 Gross and Microscopic
Name: Block: Due Date: Starfish Dissection
 Name: Block: Due Date: Starfish Dissection Introduction Echinoderms are radially symmetrical animals that are only found in the sea (there are none on land or in fresh water). Echinoderms mean "spiny skin"
Name: Block: Due Date: Starfish Dissection Introduction Echinoderms are radially symmetrical animals that are only found in the sea (there are none on land or in fresh water). Echinoderms mean "spiny skin"
1/27/10 More complications to Mendel
 1/27/10 More complications to Mendel Required Reading: The Interpretation of Genes Natural History 10/02 pg. 52-58 http://fire.biol.wwu.edu/trent/trent/interpretationofgenes.pdf NOTE: In this and subsequent
1/27/10 More complications to Mendel Required Reading: The Interpretation of Genes Natural History 10/02 pg. 52-58 http://fire.biol.wwu.edu/trent/trent/interpretationofgenes.pdf NOTE: In this and subsequent
SELECTION FOR AN INVARIANT CHARACTER, VIBRISSA NUMBER, IN THE HOUSE MOUSE. IV. PROBIT ANALYSIS
 SELECTION FOR AN INVARIANT CHARACTER, VIBRISSA NUMBER, IN THE HOUSE MOUSE. IV. PROBIT ANALYSIS BERENICE KINDRED Division of Animal Genetics, C.S.I.R.O., University of Sydney, Australia Received November
SELECTION FOR AN INVARIANT CHARACTER, VIBRISSA NUMBER, IN THE HOUSE MOUSE. IV. PROBIT ANALYSIS BERENICE KINDRED Division of Animal Genetics, C.S.I.R.O., University of Sydney, Australia Received November
Exceptional fossil preservation demonstrates a new mode of axial skeleton elongation in early ray-finned fishes
 Supplementary Information Exceptional fossil preservation demonstrates a new mode of axial skeleton elongation in early ray-finned fishes Erin E. Maxwell, Heinz Furrer, Marcelo R. Sánchez-Villagra Supplementary
Supplementary Information Exceptional fossil preservation demonstrates a new mode of axial skeleton elongation in early ray-finned fishes Erin E. Maxwell, Heinz Furrer, Marcelo R. Sánchez-Villagra Supplementary
Flatworms Flatworms Platyhelminthes dorsoventrally free-living planarian parasitic fluke tapeworm label three body layers ectoderm mesoderm
 Flatworms Flatworms are in the phylum Platyhelminthes. Flatworms are flattened dorsoventrally (top to bottom). The group includes the freshwater, free-living planarian and the parasitic fluke and tapeworm.
Flatworms Flatworms are in the phylum Platyhelminthes. Flatworms are flattened dorsoventrally (top to bottom). The group includes the freshwater, free-living planarian and the parasitic fluke and tapeworm.
Phylum Platyhelminthes Flatworms
 Phylum Platyhelminthes Flatworms The Acoelomates The acoelomates are animals that lack a coelom. Acoelomates lack a body cavity, and instead the space between the body wall and the digestive tract is filled
Phylum Platyhelminthes Flatworms The Acoelomates The acoelomates are animals that lack a coelom. Acoelomates lack a body cavity, and instead the space between the body wall and the digestive tract is filled
The Fossil Record of Vertebrate Transitions
 The Fossil Record of Vertebrate Transitions The Fossil Evidence of Evolution 1. Fossils show a pattern of change through geologic time of new species appearing in the fossil record that are similar to
The Fossil Record of Vertebrate Transitions The Fossil Evidence of Evolution 1. Fossils show a pattern of change through geologic time of new species appearing in the fossil record that are similar to
