Investigating Enteric Coccidiosis in the Black-footed (Mustela nigripes) and Domestic Ferret (Mustela putorius furo)
|
|
|
- Laura Golden
- 5 years ago
- Views:
Transcription
1 Investigating Enteric Coccidiosis in the Black-footed (Mustela nigripes) and Domestic Ferret (Mustela putorius furo) by Adriana R. Pastor A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Veterinary Science in Zoological Medicine and Pathology Guelph, Ontario, Canada Adriana R. Pastor, November, 2017
2 ABSTRACT INVESTIGATING ENTERIC COCCIDIOSIS IN THE BLACK-FOOTED (MUSTELA NIGRIPES) AND DOMESTIC FERRET (MUSTELA PUTORIUS FURO) Adriana R. Pastor University of Guelph, 2017 Advisors: Dr. D. A. Smith Dr. J. R. Barta Enteric coccidiosis is a major cause of death in both juvenile and adult black-footed ferrets (BFF, Mustela nigripes) in captive breeding programs that reduces the availability of animals for release to their former North American range. Coccidiosis is poorly understood in BFF but in vivo experimental infection in this endangered host is untenable. The goal of this research was to better characterize the etiologic agents and natural history of enteric coccidiosis in BFF and to evaluate the domestic ferret (DF, Mustela putorius furo) as a model for experimental infection. Morphometric and molecular characterization of coccidia from BFF and DF was undertaken. Only Eimeria ictidea was identified in juvenile and adult BFF from at the Toronto Zoo and from BFF at the Louisville Zoo in Eimeria furonis and Isospora (=Cystoisospora) laidlawi were identified in DF fecal and necropsy samples from Canadian and European diagnostic laboratories during Molecular characterization of these parasites included generation of complete mitochondrial genomes and nuclear 18S rdna sequences for Eimeria ictidea and Eimeria furonis from BFF and DF, respectively. Partial sequences were obtained from the same genetic targets from I. (=C.) laidlawi from DF. DNA isolation from formalin fixed paraffin embedded tissues and PCR amplicon sequencing permitted identification of coccidia in BFF and DF tissues dating from 1999 to present. Retrospective and prospective analyses of medical and pathology records supplemented with parasitological evaluation of repeated fecal samples was performed to determine the natural history of
3 coccidiosis in captive BFF. Clinical signs and histopathologic changes associated with infection in BFF were as described previously in the published literature. Average yearly coccidia associated mortality rates were 0.53% in adults and 1.95% in juveniles. Domestic ferrets were confirmed as experimental hosts of E. ictidea isolated from BFF. Seven of 10 juvenile DF inoculated with oocysts from a BFF developed patent infections and mild clinical disease was observed in six of these seven. Infection was confirmed via morphometric, molecular and histologic examination of samples from infected DF. While much is still unknown about enteric coccidiosis in BFF, domestic ferrets provide a promising model for further investigation of this disease.
4 DEDICATION For my mother, Anna Pastor. iv
5 ACKNOWLEDGEMENTS It s hard to believe that my residency and thesis have been completed, and I have a lot of people to thank for that: Dale and Graham, the two people I wanted to be when I grew up, thank you for your mentorship for many years, even before this program. I know that you weren t convinced that this project was DVSc worthy when I first proposed it, but I m hoping that the results have changed your mind. I am sincerely grateful to all the members of my advisory committee: Dale Smith, John Barta and Simon Hollamby, for their insight, support and interest in this project. Dale, you have been an exceptional advisor; I don t know that I will ever get to your level, but thank you for showing me that being a great clinical zoo vet and pathologist are not mutually exclusive. John thank you for spontaneously agreeing to be my advisor when I came to you with this project proposal in my first semester, for your energy and enthusiasm and for supporting my widening interest in parasitology research. My heartfelt appreciation for the Toronto Zoo WHC veterinarians, past and present: Chris Dutton, Pauline Delnatte, Simon Hollamby and Graham Crawshaw. I have learned so much from all of you that I will take forward into my future endeavours. I appreciate the extra time you put in, including comps study sessions, after-hours tête-à-têtes, and the fact that your doors were open when I needed it. For the Toronto Zoo vet techs extraordinaire: Michelle Lovering, Cassia Devison, Dawn Mihailovic and Tasha Long you have been indispensable during this program and there are not enough words to express my gratitude. I would especially like to thank all the Wildlife Health Center staff ( ): Mark Bongelli, Charles Guthrie, Christine McKenzie, Brian Telford, Rick Vos, Gerri Mintha, Margaret Kolakowski, Andrew Lentini, Rebecca Clark, Lydia Attard, Nigel Parr, Paula Roberts, Andrea Dada, Mindy Waisglass and Julie Digiandomenico, for three very memorable years. It is all of you that make the WHC such an amazing place to be. I m not sure I have laughed so hard or so often as I did in that lunchroom, and I hope our paths will cross again. v
6 I don t think that I can truly express how thankful I am to Pathobiology laboratory technicians Julie Cobean and Julia Whale. Without your assistance, patient teaching and friendship, I would probably still be screening fecal samples years from now, and scratching my head as to how our lab protocols actually work. It is people like you who make sure graduate students become successful doctorates, and I can t imagine Pathobio without you both in it. I would also like to thank my labmates in the Barta lab Mian Hafeez, Evelyn Rejman, Rachel Imai, Perryn Kruth, Ryan Snyder and Mosun Ogedengbe. A special thank you goes to Alex Leveille, without whom my many adventures in parasitology research, from coccidia to Babesia, would not have been as successful. To all the students who helped with ferret fecal sample processing, data compilation, and necropsies: Nathalie Ferriman, Janessa Price, Thisuri Eagalle, Sarah Brisson thank you so much for your hard work and excitement about my project even when it was very smelly! So many thanks to the amazing staff of Central Animal Facility - Linda Groocock, Vicky Carson, Tony Cengija and Mary Fowler for the daily care and enrichment of my experimental ferrets. Your excitement about working with our ferrets and your assistance with all parts of the process helped made this project a success. To Adriana Nielsen, who was not only my better half but the other fifty percent of my brain for several years. It is your friendship, fortitude and our endless phone conversations that got me through the never-ending Toronto-Guelph commute, and this program. To all the scope room pathology co-residents past and present - thank you for being wonderful friends and colleagues. It is indeed rare to find so many amazing people in one place, and I know this program and my sanity would not have been the same without you. To the anatomic pathology faculty and senior graduate students - thank you for all the time, teaching and guidance you provided during my program. While I can t say that I have become an amazing pathologist, I can say that because of your mentorship I am a better diagnostician and the type of clinician who asks better questions, takes better samples and understands that you can t just make a PCR for that. vi
7 A special thank you to Tony van Dreumel who came out of retirement for a semester to try to teach the Adrianas zoo pathology, screening cases with you was always a pleasure. To all the lovely Histo Ladies, P.M. room staff and the other AHL staff who helped me with Toronto Zoo and HSC pathology cases along the way - I don t think the anatomic path students could survive without you. Thank you for always smiling, assisting and accommodating me, even when I made near-impossible processing requests during my weekly Guelph visits. I would also like to acknowledge and sincerely thank all the individuals who helped with resource and sample acquisition for this project. A special mention for those who went above and beyond because of their interest in this project: Don Duszynski who was instrumental in acquiring, and then providing a translator for many of the original mustelid Eimeria descriptions; and Majda Globokar, Nikola Pantchev, and Donald Martin who supplied my domestic ferret fecal samples and historical data. A shout-out to Julie Swenson, Gary West and the Phoenix Zoo BFF team who fostered my love of this endangered species and helped develop the idea for this project. As always, I continue to go out into the world and pursue my dreams with the knowledge that I have the support of my incredible family, long-time friends, and my partner Keith Morris. I am so lucky that my residency brought me home, and that it afforded us all more time spent together. For my aunt, Veronica Lacey, who has never failed to believe in my potential and always pushed me to become an academic you ll never get that PhD from me but I think this is pretty close! Finally, for my mother, Anna Pastor, who never lived to see my greatest achievements but had absolute faith that I could reach any goal I worked towards. this is for you. Finally, none of this would have been possible without the generous support of the Toronto Zoo Residency program and funding through the Barta Laboratory, University of Guelph. Adriana Pastor Toronto, August 2017 vii
8 DECLARATION OF WORK PERFORMED I declare that all the work reported in this thesis was performed by myself, with the following exceptions: Fecal samples were collected by personnel at the Toronto Zoo, Louisville Zoo and participating diagnostic laboratories. Fecal oocyst per gram counts (routine salt flotation and McMaster counts) were performed by myself, Julie Cobean, Julia Whale, Evelin Rejman, Sarah Brisson, Adriana Rodriguez and Perryn Kruth. Whole mitochondrial genome PCR and sequencing was performed by me in conjunction with Julia Whale and Dr. Mian Hafeez. Sequencing of PCR samples was performed at the University of Guelph Laboratory Services (Guelph, Ontario, Canada) and results were obtained electronically. viii
9 TABLE OF CONTENTS ABSTRACT... ii DEDICATION... iv ACKNOWLEDGEMENTS... v DECLARATION OF WORK PERFORMED... viii TABLE OF CONTENTS... ix LIST OF TABLES... xiii LIST OF FIGURES... xiv LIST OF APPENDICES... xv ABBREVIATIONS... xvi CHAPTER 1: LITERATURE REVIEW INTRODUCTION APICOMPLEXA Brief introduction to apicomplexan pathogens Life cycles of the Eimeria and Isospora species implicated in enteric coccidiosis Methods of characterization RECLASSIFICATION OF MAMMALIAN ISOSPORA EIMERIID SPECIES CHARACTERIZED IN MUSTELIDS The family Mustelidae Eimeriid coccidia described from mustelids Eimeriid coccidia described from domestic ferrets Molecular characterization Clinical signs of disease in domestic ferrets Gross necropsy and histologic findings INTRODUCTION TO ENTERIC COCCIDIOSIS IN THE BLACK-FOOTED FERRET Natural history and conservation of the black-footed ferret in North America Coccidia identified from black-footed ferrets Morbidity, mortality and clinical signs associated with enteric coccidiosis in black-footed ferrets TREATMENT, PREVENTION AND CONTROL OF INFECTION BY EIMERIA SPP Current recommendations for treatment of eimeriid coccidia in carnivores ix
10 1.6.2 Current recommendations for anticoccidial treatment and prophylaxis in domestic and black-footed ferrets VACCINES AGAINST COCCIDIA Theory Species successes in anticoccidial vaccination RESEARCH GOALS AND OBJECTIVES Objectives Hypotheses Applications CHAPTER 2: MOLECULAR CHARACTERIZATION OF ENTERIC COCCIDIA FROM DOMESTIC FERRETS (MUSTELA PUTORIUS FURO) INTRODUCTION: MATERIALS & METHODS: Fecal samples Formalin fixed intestinal tissues Molecular characterization Phylogenetic analysis RESULTS: Fresh fecal samples Formalin fixed samples Molecular characterization Phylogenetic analysis DISCUSSION: CHAPTER 3: MORPHOLOGICAL AND MOLECULAR CHARACTERIZATION OF ENTERIC COCCIDIA ISOLATED FROM BLACK-FOOTED FERRETS (MUSTELA NIGRIPES) INTRODUCTION: MATERIALS AND METHODS: Fecal samples Formalin fixed intestinal tissues Molecular characterization RESULTS: Morphometric characterization Molecular characterization DISCUSSION: x
11 CHAPTER 4: NATURAL HISTORY OF ENTERIC COCCIDIOSIS IN THE BLACK-FOOTED FERRET (MUSTELA NIGRIPES) INTRODUCTION: MATERIALS AND METHODS: Toronto Zoo BFF breeding program Fecal oocyst evaluation Retrospective review of pathology records Prospective modified necropsy protocol Retrospective medical history review RESULTS: Fecal oocyst evaluation and retrospective medical history review Pathology Morbidity and mortality DISCUSSION: CHAPTER 5: EVALUATING THE DOMESTIC FERRET (MUSTELA PUTORIUS FURO) AS AN EXPERIMENTAL MODEL FOR ENTERIC COCCIDIOSIS IN THE BLACK-FOOTED FERRET (MUSTELA NIGRIPES) INTRODUCTION: MATERIALS AND METHODS: Animal care Oocyst preparation Experimental infections Animal welfare Hematology Morphologic and molecular characterization Necropsy protocol RESULTS: Oocyst shedding Morphologic and molecular characterization Clinical signs Hematology Necropsy DISCUSSION: xi
12 CHAPTER 6: WHOLE MITOCHONDRIAL GENOME SEQUENCES OF TWO EIMERIA SPECIES ISOLATED FROM DOMESTIC (MUSTELA PUTORIUS FURO) AND BLACK- FOOTED FERRETS (MUSTELA NIGRIPES) INTRODUCTION: MATERIALS & METHODS: Parasites DNA isolation from coccidia in feces Whole genome sequencing Phylogenetic analysis RESULTS: DISCUSSION: CHAPTER 7: CONCLUSIONS AND FUTURE DIRECTIONS REFERENCES APPENDICES xii
13 LIST OF TABLES Table 1.1 Morphometrics of Eimeria and Isospora (=Cystoisospora) species affecting mustelids Table 2.1 Amplification primers for nuclear 18S rdna and mitochondrial COI loci used in the identification of enteric coccidia from domestic ferrets Table 2.2 Summary of fecal samples from domestic ferrets submitted to two diagnostic laboratories from Table 2.3 Morphologic and molecular identification of coccidia from domestic ferrets Table 3.1 Amplification primers for nuclear 18S rdna, mitochondrial COI and COIII loci used in the identification of coccidia from black-footed ferrets Table 3.2 Morphologic and molecular characterization of coccidia from fecal and FFPE necropsy samples from black-footed ferrets Table 3.3 Morphometric characterization of Eimeria ictidea oocysts from black-footed ferrets Table 4.1 Eimeria ictidea shedding in black-footed ferret dam and kit family groups Table 4.2 Epidemiologic data for family groups of black-footed ferrets shedding Eimeria ictidea Table 4.3 Shedding of Eimeria ictidea in adult black-footed ferrets Table 4.4 Epidemiologic data for adult black-footed ferrets shedding Eimeria ictidea Table 4.5 Histologic findings from black-footed ferrets with enteric coccidiosis Table 4.6 Incidence of coccidial infections in black-footed ferrets at the Cheyenne Mountain Zoo Table 4.7 Yearly mortality associated with coccidiosis in black-footed ferrets at the Toronto Zoo Table 5.1 Prepatent period and oocyst shedding of Eimeria ictidea in experimentally infected domestic ferrets Table 5.2 Results of oral inoculation of domestic ferrets with oocysts of Eimeria ictidea Table 5.3 Distribution of coccidial life stages in intestinal tract of domestic ferrets orally inoculated with oocysts of Eimeria ictidea Table 6.1 PCR primers used to sequence the mitochondrial genome of Eimeria furonis Table 6.2 PCR primers used to sequence the mitochondrial genome of Eimeria ictidea Table 6.3 Coding regions in the mitochondrial genome of Eimeria furonis from a domestic ferret Table 6.4 Coding regions in the mitochondrial genome of Eimeria ictidea from a black-footed ferret Table 6.5 Pairwise comparison of coding regions in the mitochondrial genomes of Eimeria furonis and Eimeria ictidea xiii
14 LIST OF FIGURES Figure 1.1 Phylogeny of the Apicomplexa... 2 Figure 1.2 Classical life cycle of coccidian parasites... 4 Figure 1.3 Morphologic characteristics used for identification of eimeriid oocysts... 6 Figure 2.1 Life stages of Eimeria furonis within the small intestine of a domestic ferret Figure 2.2 Phylogenetic relationships of coccidia (Eimeria ictidea, Eimeria furonis and Isospora (=Cystoisospora) laidlawi) from domestic or black-footed ferrets Figure 3.1 Nuclear and mitochondrial genetic loci targeted by primers listed in Table Figure 3.2 Morphometrics of Eimeria ictidea from a black-footed ferret (Mustela nigripes) Figure 3.3 Nuclear 18S rdna sequences of Eimeria ictidea to newly generated (see Chapter 2) and published sequences of Eimeria furonis Figure 3.4 Mitochondrial cytochrome c oxidase subunit I sequences of Eimeria ictidea to sequences from other eimeriid parasites of carnivores Figure 4.1 Oocyst per gram counts and shedding period of Eimeria ictidea from black-footed ferret family groups from Figure 4.2 Sexual life stages of Eimeria ictidea in the small intestine of a black-footed ferret Figure 5.1 Exogenous life stages of Eimeria ictidea Figure 5.2 Endogenous life stages of Eimeria ictidea within the small intestine of an experimentally infected domestic ferret Figure 5.3 Distribution of sexual and asexual life stages of Eimeria ictidea along the intestinal tract of experimentally infected domestic ferrets Figure 6.1 Map of the mitochondrial genome of Eimeria furonis Figure 6.2 Map of the mitochondrial genome of Eimeria ictidea Figure 6.3 Comparison of the mitochondrial genomes of Eimeria furonis and Eimeria ictidea Figure 6.4 Phylogenetic relationships of coccidia from domestic and black-footed ferrets based on complete mitochondrial genome sequences xiv
15 LIST OF APPENDICES Appendix 1 Shedding of oocysts of Eimeria ictidea in black-footed ferret (Mustela nigripes) dam and kit family groups from Appendix 2a Hematology values for domestic ferrets (Mustela putorius furo) from days of age prior to experimental inoculation Appendix 2b Serum biochemistry values for domestic ferrets (Mustela putorius furo) from days of age prior to experimental inoculation Appendix 3a Hematology values for domestic ferrets (Mustela putorius furo) inoculated orally with Eimeria ictidea Appendix 3b Serum biochemistry values for domestic ferrets (Mustela putorius furo) inoculated orally with Eimeria ictidea Appendix 4 Domestic ferret (Mustela putorius furo) weekly monitoring sheet Appendix 5 Domestic ferret (Mustela putorius furo) 24 hour intensive monitoring sheet Appendix 6 Domestic ferret (Mustela putorius furo) infection trial standard operating procedures xv
16 ABBREVIATIONS ATP Adenosine triphosphate BFF Black-footed ferret(s) BI Bayesian inference bp Base pair CAPC Companion Animal Parasitology Council CDS Coding DNA sequence CITES Convention on International Trade in Endangered Species of Wild Fauna and Flora COI Cytochrome c oxidase subunit 1 COIII Cytochrome c oxidase subunit 3 CytB Cytochrome b DF Domestic ferret(s) DNA Deoxyribonucleic acid FFPE Formalin-fixed paraffin embedded tissue IUCN International Union on the Conservation of Nature L Length LSU Large subunit mt Mitochondrial NaOH Sodium hydroxide nu Nuclear OPG Oocyst per gram count PCR Polymerase chain reaction rdna Ribosomal DNA SI Shape index SND Single nucleotide difference SOP Standard operating procedure sp., spp. Species (singular, plural) SSP Species Survival Plan SSU Small subunit TMS Trimethoprim sulfadimethoxine USFWS United States Fish and Wildlife Service W Width xvi
17 CHAPTER 1: LITERATURE REVIEW 1.1 INTRODUCTION Black-footed ferrets (Mustela nigripes) are one of three wild ferret species worldwide. Although formerly distributed throughout the North American prairies, black-footed ferrets (BFF) had been extirpated from the majority of their range by the 1970s, and were declared extinct in the wild in Since 1986, a multi-institutional effort has been breeding this species in captivity with reintroduction back into the wild at select sites within Canada, the USA and Mexico. Coccidial enteritis is a major cause of death in young, captive black-footed ferrets (Bronson et al. 2007) but coccidiosis can affect all age classes (personal observation). As a result, fewer captive-bred ferrets may be reared successfully for release to the wild. The significance of coccidiosis in wild ferrets is unknown. Consequently, the prevention and control of coccidial outbreaks is an important part of blackfooted ferret captive breeding programs and management. This research is intended to improve the in situ and ex situ health of the black-footed ferret through the provision of a better understanding of the pathogenesis of enteric coccidiosis in this species, and to pave the way for the investigation of novel methods for disease treatment and control. 1.2 APICOMPLEXA Brief introduction to apicomplexan pathogens The phylum Apicomplexa comprises a large number of eukaryotic, intracellular, parasitic organisms many of which are of importance to human and veterinary medicine. As indicated by their name, these parasites are characterized by the presence of an apical complex at the anterior aspect of the infective stage of the life-cycle (Tenter et al. 2002). The taxonomic classifications of members of the Apicomplexa continue to be in a state of flux (reviewed by Adl et al., 2005; Cavalier-Smith, 2014; Tenter et al., 2002). For this reason, a more simplified taxonomic structure has been used in this review (see 1
18 Figure 1.1). The subclass Coccidia is a speciose group within the Apicomplexa with most genera falling into one of two major coccidian suborders within the Eucoccidiorida. To date, greater than 2,000 species of coccidia have been named (Duszynski, Upton, & Couch, n.d.; Upton, 2000). The adeleid coccidia (suborder Adeleorina) include monoxenous (single host) and heteroxenous (multiple hosts) parasites in genera such as Adelea, Haemogregarina, Hepatozoon, and Karyolysus. The eimeriorinid coccidia (suborder Eimeriorina) include the typical intestinal coccidia such as Eimeria, Isospora and Cyclospora species belonging to the family Eimeriidae as well as tissue (cyst forming) coccidia such as Cystoisospora, Besnoitia, Toxoplasma, and Sarcocystis species that belong to the family Sarcocystidae (Cox 1994) Figure 1.1 Phylogeny of the Apicomplexa. Numbers on branches and thickness indicate diversity (i.e., named species). Taxonomic groupings demonstrated by the phylogenetic tree: (1) subclass Coccidia (2) suborder Adeleorina (3) suborder Eimeriorina (4) family Eimeriidae and (5) family Sarcocystidae. Adapted from: Šlapeta J, Morin-Adeline V (2011) Apicomplexa Levine Sporozoa Leucart in The Tree of Life Web Project, 2
19 1.2.2 Life cycles of the Eimeria and Isospora species implicated in enteric coccidiosis The life cycle of Eimeria species is considered the classical coccidian life cycle, which is typically completed in one host (monoxenous) with many Eimeria species parasitizing only a single host species (stenoxenous) (Figure 1.2). The life cycle has two main phases of development; one that takes place within the host (endogenous) and the other that takes places outside of the host (exogenous). Classically, the endogenous stages of the Eimeria life cycle take place within the intestinal epithelium; however, some Eimeria species undergo extraintestinal endogenous development, such as Eimeria stiedae in rabbits, which replicates within the epithelium of the biliary tree. During the exogenous phase of the life cycle, unsporulated oocysts that are shed in the feces of the host sporulate within the environment, resulting in the formation of four sporocysts within each oocyst (tetrasporocystic). Each sporocyst contains two sporozoites (dizoic). Sporulation is affected by three main factors: temperature, moisture and aerobic conditions (Fayer 1980). Once ingested by the host, the wall of the sporulated oocyst is broken to release sporocysts from which the sporozoites (infective stage) excyst. The freed sporozoites penetrate the intestinal epithelial cells and undergo multiple mitotic divisions to form a single multinucleate meront. The meront then undergoes simultaneous cytokinesis to form first generation merozoites, which leave the host cell to infect new cells and undergo further asexual replications. The undifferentiated, uninucleate, tissue stage of the parasite within the intestinal epithelial cell is called a trophozoite. The number of cycles of asexual replication (merogony) is predetermined, after which the last generation of merozoites penetrate host cells and undergo sexual differentiation into male and female gamonts (gametogony). Each microgamont (male) undergoes simultaneous fission to produce numerous motile microgametes; each macrogamont (female) develops into a single mature macrogamete. Fertilization of a macrogamete by a motile microgamete results in formation of a zygote that is rapidly enclosed in a thick wall to form an unsporulated oocyst. Oocysts are shed with the host s feces into the environment, where they are protected from desiccation and chemical disinfection by the oocyst wall. Traditionally, Eimeria species 3
20 have been differentiated based on the host species or host genus affected, the site of endogenous life cycle development, and the microscopic cellular characteristics of the different life stages. Interestingly, experimental cross infection of Eimeria species from their natural host to a novel host of a taxonomically similar species has been successful in some cases (De Vos 1970; Levine and Ivens 1970; Haberkorn 1971), challenging the notion that Eimeria are truly stenoxenous parasites. Figure 1.2 Classical life cycle of coccidian parasites. This apicomplexan life cycle includes both sexual and asexual development. The three processes in the life cycle are merogony (asexual replication, A-D) followed by gametogony (formation of gametes, E-H) within the digestive tract of the host with release of unsporulated oocysts (I). Exogenous sporogony (I-L) results in the production of infective, sporulated oocysts (L). Adapted from Barta, 2001 with permission of the author. The life cycle of Isospora spp. is similar to that of species in the genus Eimeria (see Figure 1.2), but the number of sporocysts and sporozoites differ; sporulated oocysts contain two sporocysts (disporic), 4
21 each of which contains four sporozoites (tetrazoic). These characteristics are not unique to Isospora spp. because diasporic, tetrazoic sporulated oocysts are also found in the genera Besnoitia, Frenkelia, Hammondia, Sarcocystis and Toxoplasma. However, the sporocysts in the latter parasites are morphologically distinct in that they lack Stieda bodies Methods of characterization Morphological features Historically, eimeriid coccidia have been classified based on the cellular morphology of the different life stages (particularly the morphometrics of sporulated oocysts), where these stages occur in the host, and apparent host specificity (frequently assumed and not tested experimentally). The morphological features and dimensions of oocysts and their components are important diagnostic features because of the availability of these stages in clinical specimens; these characteristics can include: size (length [L]; width [W]; shape index [SI=L/W]), number of sporocysts; wall morphology; presence/absence of a micropyle, micropyle cap, residual body or polar granules for oocysts; size, number of sporozoites, wall morphology, presence/absence of Stieda body, substieda body, parastieda body or residual body for sporocysts; and presence/absence of refractile bodies for sporozoites (see Figure 1.3). Pertinent life cycle information includes: type of life cycle (monoxenous versus heteroxenous), tissue sites of merogony and gametogony (intestinal versus extraintestinal), and the presence or absence of extraintestinal hypobiotic stages (e.g., dormozoites or hypnozoites). Further information used to characterize coccidia that form tissue cysts generally includes details on life stages in the definitive and intermediate hosts, location and morphology of tissue cysts, route(s) of transmission among host species, and morphologic descriptions of merozoites (e.g., tachyzoites or bradyzoites) in tissue culture. 5
22 Figure 1.3 Morphologic characteristics used for identification of eimeriid oocysts. 1) Oocyst in cross section: ol - oocyst length; or - oocyst residual body; ow - oocyst width; pg - polar granule; row -rough outer wall. 2) The top of a hypothetical oocyst: mcd - depth of the micropyle cap; mcw - width of the micropyle cap; mw - width of the micropyle; sow - smooth outer wall. 3) Sporocyst in cross section: psb - parastieda body; sb - Stieda body; sl - sporocyst length; sp - sporozoite; sr - sporocyst residual body; srb - sporozoite refractile body; ssb - substieda body; sw - sporocyst width. From: Duszynski D, Wilber PG (1997) A guideline for the preparation of species descriptions in the Eimeriidae. Journal of Parasitology 83(2): , reproduced with permission of Allen Press Publishing Services Molecular characterization (genetic loci and methods) More recently, molecular techniques have been used to infer phylogenetic or evolutionary relationships among coccidia and to reclassify taxonomic assignments to better reflect the evolutionary history of these parasites. Molecular data can be more informative than phenotypic data because recent evolutionary divergence among coccidia is unlikely to be reflected in morphologic differences, but may be detectable using molecular data. The principle behind the use of molecular sequencing to describe evolutionary relationships is that nucleotide sequences, like morphological features, diverge over time under selective pressure; however, nucleotide sequences evolve at a more regular rate than do morphologic characteristics. Phenotypic data is thus less likely to detect recent evolutionary divergence. Sequences that are more similar are inferred to be more closely related and to have diverged more recently (Cox 1994). Molecular characterization can be performed using DNA, RNA or protein sequences. Most of the early molecular phylogenetic analyses of coccidia performed used ribosomal RNA sequences, usually by PCR amplification of ribosomal DNA (rdna) in the nuclear genome of the 6
23 parasites. Ribosomes contain both small and large RNA subunits; in eukaryotes the large ribosomal RNA consists of two forms, 5S and 28S, while the small ribosomal RNA exists only as 18S. Sequences from several genetic loci have been used for characterization of parasites, most commonly 18S rdna, 28S rdna and ribosomal internal transcribed spacer regions (ITS) from the nuclear genome and, more recently, mitochondrial cytochrome c oxidase subunits I (COI) and III (COIII); however, sequencing of nuclear 18S rdna (nu 18S rdna) has been the most prevalent in the literature by far. Early attempts to use 5S RNA sequences formed unlikely phylogenies and too few 28S ribosomal DNA sequences have been obtained to make this locus useful (Cox 1994; Tenter et al. 2002). The disadvantage of nu 18S rdna is that it is comparatively poor at distinguishing among closely related eimeriid coccidial species because of its conserved nature, but for that reason the nu18s rdna locus is useful for inferring relationships among species with greater evolutionary divergence. Although only exploited recently because of the paucity of suitable PCR primers, the mitochondrial COI locus appears to be more useful for distinguishing closely related eimeriid coccidia (Ogedengbe, Hanner, & Barta, 2011), but COI sequences are less useful for inferring more ancient relationships between highly divergent coccidial species. Consequently, the combined use of nu 18S rdna and mitochondrial COI sequencing has been recommended for improved species description and phylogenetic analysis (El-Sherry et al. 2013). Molecular characterization has also been used for diagnostic purposes, and is well-suited to the identification of coccidia when information on host specificity, parasite life cycle and life stages is not available as the molecular (genetic) data is the same for a given parasite during each of its life cycle stages. This information can be particularly useful in identifying the relationship between different life stages of heteroxenous parasites collected from different hosts (intermediate, definitive). Furthermore, for previously unidentified coccidia or those for which limited information is available, molecular characterization could be used to predict likely definitive hosts or parasite life cycle traits based on phylogenetic relationships to other known species. 7
24 1.3 RECLASSIFICATION OF MAMMALIAN ISOSPORA Recommendations have been made to reclassify the avian and mammalian Isospora into two separate genera based on life cycle, molecular phylogenetic studies and morphologic description of sporulated oocysts (Frenkel 1977; Barta et al. 2005). Due to their classical coccidian life cycle, presence of Stieda bodies within sporocysts, and close phylogenetic association with Eimeria species, the avian Atoxoplasma and Isospora have been retained in the genus Isospora (see Barta et al. 2005). Conversely, the presence of tissue life cycle stages, lack of Stieda bodies within sporocysts, and close phylogenetic association with other genera within the family Sarcocystidae, have required many mammalian Isospora to be reclassified as members of the genus Cystoisospora Frenkel 1977 (Frenkel 1977; Barta et al. 2005). Consequently, for the remainder of this thesis, Isospora species from mustelids will be referred to as Isospora (=Cystoisospora) to reflect their probable generic association. 1.4 EIMERIID SPECIES CHARACTERIZED IN MUSTELIDS The family Mustelidae The family Mustelidae, within the order Carnivora, comprises a group of approximately 59 carnivorous mammalian species within 22 genera. Native mustelids are found in terrestrial and aquatic environments on almost every continent, with the exception of Australia and Antarctica. The Mustelidae are classically divided into two subfamilies as defined by Wozencraft (2005): 1) Mustelinae (weasels, mink, ferrets, marten, wolverine), the larger subfamily, including the following genera: Arctonyx, Eira, Galictis, Gulo, Ictonyx, Lyncodon, Martes, Meles, Mellivora, Melogale, Mustela, Neovison, Poecilogale, Taxidea, and Vormela; and 2) Lutrinae (otters) including seven genera: Aonyx, Enhydra, Hydrictis, Lontra, Lutra, Lutrogale and Pteronura. More recently, molecular data suggest the Mustelidae should be separated into eight subfamilies, although this is not universally accepted (Koepfli et al. 2008; Larivière and Jennings 2009; Yu et al. 2011). 8
25 1.4.2 Eimeriid coccidia described from mustelids Ten named Eimeria species and twelve named Isospora (=Cystoisospora) species have been described in the Mustelidae and are summarized in Table 1.1. This table includes information on host range, life cycle and detailed morphologic data used to identify and classify the individual parasites. Two coccidial parasites isolated from the Libyan striped weasel (Ictonyx libyca) and the European polecat (Mustela putorius), initially ascribed to the genus Isospora: Isospora zorillae and Isospora putori, respectively, have since been reclassified as Sarcocystis spp. (see footnote to Table 2 of Yi-Fan et al., 2012). 9
26 Table 1.1 Morphologic characteristics of Eimeria and Isospora (=Cystoisospora) species affecting mustelids Coccidial species Cytoisospora eversmanni Cystoisospora pavlovskyi Eimeria baskanica^ Eimeria furonis Host genus and species Mustela eversmanii (Steppe polecat) Mustela putorius (European polecat) Mustela eversmanii Mustela putorius Mustela erminae (ermine) Mustela putorius Mustela putorius furo (dom. ferret) Mustela nigripes (BFF) Mustela vison (mink) Life cycle/ Oocyst shape and Location size Homoxenous L:18.5 (16 20) W: 14.8 (16 12) L/W: 1.3 ( ) Homoxenous L: 32.2 (29 36) W: 27.3 ( ) L/W: 1.2 ( ) Homoxenous Homoxenous Small intestine/ rectum (H 1927) Jejunum/ileum (BP 1993) Eimeria hiepei Mustela vison Homoxenous Bile duct Oval with tapered ends L: W: Spherical subspherical L: W: Spherical L: W: Oocyst description M: absent PG: absent OR: absent M: absent PG: absent OR: absent M: absent PG: absent OR: present OW: 2 layers M: absent PG: absent OR: absent OW: 2 layers (outer smooth) M: absent PG: absent OR: absent Sporocyst description Sporozoite description References L: 11.5 SRB: present Yi-Fan et al ( ) Svanbaev 1956 W: 9.8 Nukerbaeva & (9 11) Svanbaev 1973, L/W: ( ) SB: absent SR: present L: 19.5 SRB: present Yi-Fan et al (18 21) Svanbaev 1956 W: 14.4 Nukerbaeva & (12 15) Svanbaev 1973, L/W: ( ) SB: absent SR: present SR: absent Bean shaped Nukerbaeva & Svanbaev 1977 Spindloid L: 8-9 W: 4 SB: present SR: present L: 6 W: 4 SB: absent SR: absent Vermiform Blankenship-Paris et al Hoare 1927, 1935b Jolley et al Nukerbaeva & Svanbaev 1973,1977 Williams et al. 1988, 1992, 1996 Banana shaped Davis et al Grafner et al
27 Coccidial species Eimeria ictidea Eimeria irara Eimeria melis Eimeria mustelae Eimeria sablii Host genus and species Mustela eversmanni Mustela nigripes Mustela putorius Mustela putorius furo Eira barbara (tayra) Meles meles (European badger) Mustela vison Mustela nivalis (snow weasel) Martes zibellina (sable) Life cycle/ Location Homoxenous Small intestine Homoxenous Feces Homoxenous Homoxenous Duodenum/ileum Homoxenous Gut Oocyst shape and size Ovoid ellipsoid L: W: Ovoid L: W: Ellipsoid L: 20±0.18 W: 15.7±0.02 L/W:1.28±0.017 ( ) Spherical or Ellipsoid L: W: Spherical or subspherical L: W: 11.2 Eimeria sibirica Martes zibellina Homoxenous Ovoid L: avg 21.6 W: avg 18.0 L/W: 1:0.76 Oocyst description OW: 2 layers M: present PG: absent OR: absent OW: outer layer smooth M: absent PG: absent OR: absent OW: 2 layers (outer smooth) M: absent PG: present OR: present OW: 2 layers M: absent PG: present OR: absent OW: 2 layers M: absent OR: absent OW: 2 layers M: absent PG: absent OR: absent Sporocyst description Ovoid (irregular) L: 11.5 W:6.5 SB: present SR: present Ellipsoid L: W: 6.5 SB: present SR: present Ovoid L: 11.9±0.018 W: 6.5±0.08 L/W: 1.83 ( ) SB: present Ovoid L: 8 W: 5 SB: present SR: present Ovoid L: 5.6 W: 4.2 SR: present Ovoid L: W: SR: absent Sporozoite References description - Hoare 1927, 1935a, 1935b Jolley et al Litvenkova 1969 Svanbaev 1956 Tinar 1985 Williams et al. 1988, 1992 Elongate (one Carini & da end broader than Fonseca, 1938 the other) L: 9.0±0.05 W: 3.24±0.025 SRB: present Broad at one end and tapered at other L: 7 W: 3 Anwar et al Kotlan & Pospesch 1933 Glebezdin 1978 Iwanoff-Gobzem 1934 Levine 1948 Musaev & Veisov 1983 Tinar 1985 Elongate Nukerbaeva 1981 Elongate Nukerbaeva 1981 Yakimoff & Gousseff 1934 Yakimoff & Terwinsky 1930,
28 Coccidial species Eimeria vison (Eimeria mustelae) Host genus and species Mustela putorius Mustela putorius furo Mustela vison Life cycle/ Location Homoxenous Small intestine +/- large intestine Oocyst shape and size Ovoid L: W: 9-18 Oocyst description OW: 2 layers M: absent OR: sometimes present Sporocyst description Ovoid or Piriform L: 10 W: 5.5 SB: absent SR: present Sporozoite description Curved or Club shaped L: 9 W: 2.5 References Foreyt & Todd 1976 Foreyt et al Kingscote 1934, 1935 Levine 1948 McTaggart 1960 Nukerbaeva & Svanbaev 1973,1977 Tinar 1985 Umurzakov & Nukerbaeva 1985 Wolter 1961 Zimmermann 1959 Isospora africana Isospora altaica Ictonyx libyca (Libyan striped weasel) Mustela altaica (mountain weasel) Homoxenous Feces Homoxenous Gut Isospora goussevi Mustela nivalis Homoxenous Large intestine Spherical L: W: Oval or spherical L: W: L/W: 1.21 ( ) Ovoid L: 22.4 ( ) W: 17.4 ( ) L/W: 1.35 ( ) OW: 2 layers (outer smooth) M: absent PG: absent OR: absent OW: 2 layers M: absent PG: absent OR: absent OW: 1 layer PG: present OR: present Ovoid L: W: SB: absent SR: present Ovoid or spherical L: W: SR: present Ovoid L: 12.0 ( ) W: 7.0 ( ) SB: present SR: present Elongate L: 13.5 W: 3 Elongate Prasad 1961 * Svanbaev & Rachmatullina 1971 Musaev & Veisov
29 Coccidial species Isospora hoogstraali Isospora laidlawi Isospora lutrae Isospora martessii Host genus and species Ictonyx libyca Mustela putorius Mustela putorius furo Mustela vison Lutra lutra (European otter) Lutra canadensis (North American river otter) Martes zibellina Life cycle/ Location Homoxenous Feces Homoxenous Feces Intestinal contents Homoxenous Homoxenous Gut Oocyst shape and size Ellipsoid L: W: Ovoid L: W: Spherical L: 31.2 ( ) W: 29.6 (28-31) L/W: 1.04 ( ) Ovoid, short oval or spherical L: , 19.6, 16.8 W: , 16.8, 16.8 Isospora melis Meles meles Homoxenous Ovoid L: 32.8±0.34 W: 26.9±0.19 L/W:1.22 ( ) Oocyst description OW: 2 layers (outer smooth) M: absent PG: some OR: absent OW: 2 layers M: absent PG: absent OR: absent OW: 2 layers (outer smooth) M: absent PG: absent OR: absent OW: 2 layers M: absent OR: absent OW: 2 layers (outer smooth) M: absent PG: absent OR: absent Sporocyst description Ovoid L: W: SB: absent SR: present Ellipsoid L: 20.8 W: 14.4 SB: absent SR: present Ellipsoid L: 18.2 (17-19) W: 14.4 (14-16) L/W:1.28 ( ) Sb: absent ssb: absent SR: present Ovoid L: W: SR: present Ellipsoid L: 21.5±0.166 W: 14±0.12 L/W: 1.55 ( ) SR: absent Sporozoite description Club-shaped L: W: 4-6 References Prasad 1961 Sausage shaped Foreyt et al Hoare 1927 Levine 1948 McTaggart 1960 Nukerbaeva & Svanbaev 1973, 1974, 1977 Tinar 1985 Spindle- shaped L: 12.4 W: 2.5 SRB: present Torres et al Hoover et al Elongate Nukerbaeva 1981 Round at one end, other end tapered L: 14.2±1.16 W: 4.0±0.17 SRB: absent Anwar et al Glebezdin 1978 Kotlan & Pospesch 1933 Pellérdy
30 Coccidial species Isospora mustelae (nomen nudum) Host genus and species Life cycle/ Location Oocyst shape and size Martes martes * Ovoid L: 7 W: 2.25 Ovoid L: 20.6 ( ) W: 18.4 ( ) L/W: 1.1 ( ) Isospora nivalis Mustela nivalis Homoxenous Large intestine Unnamed Coccidia ^ Unnamed Coccidia ^ Unnamed Eimeria sp.^ Unnamed Eimeria sp.^ Unnamed Eimeria sp.^ Unnamed Eimeria sp.^ Unnamed Isospora sp.^ Unnamed Isospora sp.^ Mustela nigripes Mustela nigripes Mustela nigripes Mustela putorius furo Mustela nivalis Martes martes (marten) Mustela putorius furo Mustela putorius furo Oocyst Sporocyst Sporozoite References description description description M: present - - Galli-Valerio 1932 OW: 1 layer PG: absent OR: absent Ovoid L: 12.5 ( ) W: 8.0 ( ) SR: present Lemon or pear shaped Musaev & Veisov 1983 Urinary bladder Jolley et al Trachea, bronchus, bronchial glands Feces, intestinal contents Jolley et al Ovoid L: W: Jolley et al Williams et al Small intestine Blankenship-Paris et al Homoxenous? Musaev & Veisov Large intestine 1983 Homoxenous? Ovoid-ellipsoid L: ( ) W: 14.8 ( ) L/W: 1.36 ( ) Ovoid L: avg 21.6 W: avg 18.0 L/W: 1:0.76 OW: 1 layer PG: absent OR: absent OR: absent Ovoid or pear-shaped L: W: SR: present 4 sporocysts SR: present Elongate L: W: L: 12.6 W: 6.0 Yakimoff and Gousseff 1934 Feces Bell 1994 Feces Bell 1994 Legend: L = length; W = width; L/W = length-width ratio; avg = average; OW = oocyst wall; PG = polar granules; M = micropyle; SB = Stieda body; ssb = substieda body; OR = oocyst residuum; SR = sporocyst residuum; SRB = sporozoite refractile body; ^ = species inquirendae; - = no information provided by author(s); * = information obtained from secondary sources (primary reference could not be obtained). All measurements are in micrometers. Bolded references 14
31 are those from which morphometric data were assembled. Remaining references indicate other authors who have identified that parasite species in the same or similar host. 15
32 1.4.3 Eimeriid coccidia described from domestic ferrets Three species of coccidia were originally described from 50 domestic ferrets (Mustela putorius furo): Eimeria ictidea, Eimeria furonis, and Isospora (= Cystoisospora) laidlawi (Hoare, 1927). All three species were detected in feces from domestic ferrets at a research facility undergoing an outbreak of canine distemper. Sick ferrets appeared more frequently infected than healthy ones. As per Hoare (1927), none of the ferrets appeared to display clinical signs associated with protozoal infection. For each parasite, the author described morphology of sporulated oocysts isolated from feces and sporulation time (exogenous life stages). The pre-patent period (minimum duration of endogenous development) in an inoculated naïve ferret was described only for E. furonis and E. ictidea due to insufficient sample size of I. (=C.) laidlawi oocysts for an experimental infection trial. Sporulation of oocysts occurred within 5-6 days for E. furonis, 3 days for E. ictidea, and 4 days for I. (=C.) laidlawi. The sporulated oocysts of E. furonis were spherical, with a double outer wall with a thin, colourless outer layer and thick, yellowish inner layer, no micropyle or residual body, and measured on average µm (length [L] : ; width [W]: ; shape index [SI]: 1.07). Unsporulated oocysts contained a zygote with a diameter of 9.6 µm. Sporocysts were spindle-shaped with one end constricted/blunted, contained a residual body, and on average measured µm. Sporozoites were vermiform with one end narrower than the other, arranged head to tail, and had a central nucleus; a clear vacuole was identified in some at the broad end. The sporulated oocysts of E. ictidea were oval or elliptical, with a double outer wall with a thin, colourless outer layer and thick, yellowish inner layer, no micropyle or residual body, and measured on average µm (L: ; W: ; shape index: 1.35). The zygote in unsporulated oocysts was elongate with a diameter of µm when originally passed in feces, but became more spherical with time. Sporocysts were irregularly oval, with one end broad and the other more constricted, contained a residual body, and on average measured µm. Sporozoites were vermiform with one end narrower than the other, arranged head to tail, and had a central nucleus and a clear vacuole at the broad end. The sporulated oocysts of Isospora (=Cystoisospora) laidlawi were ovoid, with a double outer wall with a thin, colourless outer layer and thick, yellowish inner layer, no micropyle 16
33 or residual body, and measured on average µm (L: ; W: ). Unsporulated oocysts contained a spherical zygote with a diameter of 23.6 µm. Two sporocysts were identified, each containing 4 sporozoites and no Stieda body; sporocysts were elliptical, contained a residual body and on average measured on µm. Sporozoites were sausage shaped, with one end slightly pointed, and had a central nucleus and a clear vacuole identified at the pointed end. Sporozoites were arranged with pointed ends all at the same pole of the sporocyst. The pre-patent periods described for E. furonis and E. ictidea were 6 days and 7 days, respectively (Hoare, 1927). Since Hoare s initial description (Hoare, 1927; Hoare, 1935), multiple single case reports and outbreaks of severe clinical disease associated with intestinal coccidiosis have been reported in domestic ferrets. Blankenship-Paris et al. (1993) described a single case of a four-month-old domestic ferret that presented depressed, in thin body condition, dehydrated, and with pasty dark feces on the perineum. This ferret had been housed with its dam and another sibling; neither dam nor sibling showed clinical signs of enteric disease and both had negative fecal examination results on repeated evaluation. Routine fecal examination of the rest of the colony and necropsies on eight other ferrets in the colony revealed no evidence of coccidial infection. Enteric coccidiosis was determined to be the cause of disease in the fourmonth-old ferret based on necropsy findings, but the coccidia could not be speciated because diagnosis was made on histologic findings only. Sledge et al. ( 2011) described three separate outbreaks of severe enteric coccidiosis in domestic ferrets from one ferret rescue centre (group 1) and two shelters (groups 2 and 3), all affected by the same Eimeria sp. The morphologic characteristics of sporulated oocysts were only described for group 1; no coccidial oocysts were detected on direct smear or fecal flotation of diarrheic samples submitted from groups 2 and 3. Oocysts were identified as spherical, measuring µm in diameter, with four sporocysts each containing two sporozoites. Oocyst morphometrics, histopathologic findings and nu 18S rdna partial sequences from all three groups were used collectively to confirm the coccidial species identify in each outbreak as E. furonis. 17
34 Two cases of biliary coccidiosis with E. furonis have been reported in domestic ferrets. The first was in a nine-week-old male ferret from a research facility (Williams, Chimes, & Gardiner, 1996). The ferret presented with signs of hepatic disease and was negative for coccidia on fecal flotation and direct smears. Endogenous coccidial life stages were described from the gall bladder and liver on histologic examination. In tissue section, the oocysts were oval to spherical and measured µm. Meronts measured µm and contained up to 16 merozoites. The merozoites exhibited a doublelayered pellicle, prominent conoid, few rhoptries, and many micronemes anterior to the nucleus. Based on the morphologic description of the life stages in this case, the coccidia were identified by the authors as an Eimeria species, most likely E. furonis. Kaye et al. (2015) described a second case of biliary coccidiosis in an 18-month-old female pet domestic ferret with concurrent pure red cell aplasia. In this case, all endogenous coccidial life stages were observed on histologic examination of the epithelium of the extrahepatic biliary tree. The oocysts were ovoid and measured µm. Meronts measured µm and contained up to 16 merozoites, each measuring 2 5 µm. Based on the morphologic description of the life stages in this case and nu 18S rdna sequences, the pathogen was also determined to be E. furonis. Biliary coccidiosis has also been identified in mink (Mustela vison) with the etiologic agent identified as Eimeria hiepei (Davis, Chow & Gorham, 1953; Grafner, Graubmann & Dobbriner, 1967). Oocysts from Cystoisospora ohioensis have been reported from fecal samples collected from healthy domestic ferret kits in a large American ferret breeding operation that were raised on the same premise as juvenile domestic dogs (Patterson & Fox, 2007). The method of identification of this parasite was not described by Patterson & Fox. A second similar institution reported the presence of a Cystoisospora species, also thought to be C. ohioensis, in routine fecal examination of their ferret colony (Dr. Bambi Jasmin, personal communication). Coccidial identification in this case was performed by the Animal Health Diagnostic Center, at Cornell University. The significance of these findings is unknown as no clinical signs or histologic lesions have been described in domestic ferrets associated with shedding of 18
35 oocysts and the definitive host for C. ohioensis is the domestic dog. It is most likely that fecal identification of C. ohioensis represents a pseudoparasite in both of these cases or, perhaps, an undescribed Cystoisospora sp. that is morphologically indistinguishable from C. ohioensis. It is difficult to estimate the prevalence of enteric coccidia within the North American domestic ferret population. Fecal samples submitted to university or large veterinary diagnostic laboratories from domestic ferrets in Canada are uncommon, and samples positive for coccidia appear infrequently (Dr. Donald Martin, personal communication). Data from Idexx Vet Med Lab in Ludwigsburg, Germany was compiled to review the prevalence of coccidia and Giardia within fecal samples from domestic ferrets (Pantchev et al. 2011). The authors reported that of 284 fecal samples submitted from , 18 (6.3%) had detectable coccidial oocysts on fecal flotation. Oocysts were identified based on morphologic characteristics as E. ictidea, E. furonis, I. (=C.) laidlawi and another unidentified Isospora species. Comparative data from the same laboratory from included sample submissions from 253 ferrets, 21 (8.3%) of which were positive for coccidial oocysts on fecal flotation. Nine of the samples were positive for E. furonis, three were positive with both E. furonis and I.(=C.) laidlawi present, eight were positive only for I.(=C.) laidlawi, and one sample contained both E. furonis and E. ictidea; identification in all cases was based on morphologic characteristics. No statistically significant difference in the occurrence of coccidial oocysts was detected when data from the two periods were compared (Fisher s exact test; P=0.41) (Pantchev et al. 2011) Molecular characterization Molecular characterization of Eimeria furonis was first performed by Abe et al (2008) using oocysts purified from the feces of a single domestic ferret with clinical signs of coccidial enteritis. Small subunit ribosomal DNA (nu 18S rdna) primers CYC1FE (5ʹ-TAC CCA ATG AAA ACA GTT T-3 ) and CYC4RB (5 -CGT CTT CAA ACC CCC TAC TG-3 ) were used to amplify a 347 base pair (bp) fragment of nu 18S rdna. These primers were initially developed for molecular identification of Cyclospora species, but have since been shown to amplify nu 18S rdna from several Eimeria species (Matsubayashi 19
36 et al. 2005). The amplicon was sequenced (GenBank AB329724) and compared with previously published partial nu 18S rdna sequences from 40 Eimeria, two Isospora and four Cyclospora species. The resulting phylogram grouped E. furonis with E. alabamensis (cattle) and E. meleagrimitis (turkey). In the same study, the microscopic morphology of the oocysts was used to identify this coccidial species as E. furonis by comparison with published descriptions of E. furonis, E. ictidea and E. heipei by Hoare (1927), Hoare (1935) and Grafner, Graubmann & Dobbriner (1967), respectively. Nuclear 18S rdna was also used by Sledge et al. (2011) for molecular identification of the eimeriid coccidia implicated in the three distinct outbreaks of enteric disease in domestic ferrets. As described above, initial identification and speciation of the coccidia was performed using morphologic characteristics of the sporulated oocysts collected from feces in one of the three outbreaks being investigated; the oocysts were identified as E. furonis. Histologic sections of formalin fixed intestinal segments from ferrets from each of the three outbreaks contained multiple coccidial life stages. DNA was then isolated from stored, formalin-fixed tissues for further genetic analysis. Using the partial nu 18S rdna gene sequence reported by Abe et al. (2008) (GenBank AB329724), the following PCR primers were created: 5ʹ-ACA ATT GGA GGG CAA GTC TG-3ʹ and 5ʹ-GGCGAC AAG CCT GCT TGA AAC- 3ʹ. PCR amplification produced a 247 bp amplicon from each of the three groups. Analysis and sequencing of amplicons from all three groups showed 100% homology to nucleic acid sequences previously reported by Abe et al. (2008) for the gene encoding E. furonis nu 18S rdna. Coccidia were identified within hepatobiliary lesions in a domestic ferret receiving immunosuppressive therapy for red cell aplasia (Kaye et al. 2015). DNA was extracted from frozen liver, and a 247 bp fragment of the nu 18S rdna was amplified using the primers previously described by Sledge et al. (2011) and sequenced. Kaye et al. (2015) reported that the DNA sequence of the amplicon was 100% homologous to the published nu 18S rdna of E. furonis, and 95% homologous to the nu 18S rdna of E. myoxi (rodent), E. alabamensis (cattle) and I. robini (avian). 20
37 1.4.5 Clinical signs of disease in domestic ferrets Hoare (1927; 1935b), in his initial descriptions of enteric coccidiosis in domestic ferrets, observed that clinical signs of intestinal disease were not evident. The recent literature supports the finding of subclinical disease, but also describes signs ranging from mild transient diarrhea in young or stressed animals to more severe disease with dehydration, lethargy, depression, weight loss/emaciation, inappetence and death (Blankenship-Paris et al. 1993; Powers 2009; Sledge et al. 2011; Hoefer et al. 2012; Patterson et al. 2014). Rectal prolapse has also been reported in ferrets with enteric coccidiosis (Hillyer 1992; Hoefer et al. 2012). In one study, co-infection with coccidia and Lawsonia intracellularis (Desulfovibrio sp.) was diagnosed in 4 of 19 ferrets with proliferative bowel disease (Li et al. 1996). These ferrets presented with variable clinical signs including: diarrhea, lethargy, anorexia, weight loss, dehydration and emaciation. In the two reports of biliary coccidiosis, clinical signs conformed to those expected with hepatobiliary disease. Williams et al. (1996) described their case to have presented with emaciation, poor appetite, abdominal distension and icterus. Kaye et al. (2015) described a one week history of lethargy, inappetence and icterus, with serum biochemistry results consistent with cholestasis; later clinical signs in this case included melena, anemia and cachexia Gross necropsy and histologic findings The pathology of enteric coccidiosis in domestic ferrets was described by Hoare (1927; 1935b). Two healthy domestic ferrets were experimentally inoculated, one each with large numbers of mature oocysts of either E. furonis or E. ictidea that were isolated during his initial work. The inoculated ferrets were killed humanely for histologic examination of intestinal sections at the time of first detection of fecal oocyst shedding; no clinical signs of coccidiosis were detected in these ferrets prior to death. Infection with E. furonis resulted in invasion of the epithelium of the small intestine and rectum. Within the small intestine, the parasites were concentrated in the tips of the villi, but could be found to the level of the 21
38 opening of the crypts of Lieberkühn. In rectal sections, life stages were limited to the epithelial ridges between the openings of the glands of Lieberkühn. Organisms were located within the apical portion of the epithelial cells, and intensely infected regions exhibited multiple parasites within a single host cell. Both asexual and sexual life stages were present within the same sections. Hoare (1927) described similar histopathologic changes in naturally infected ferrets, but the proportion of asexual versus sexual life stages differed. In natural infections, sexual life stages were more numerous, whereas in experimental infections asexual life stages predominated; these findings would be expected to correlate with the stage of infection at which ferrets died or were humanely killed for tissue collection, and would not be reflective of differences between natural and experimental infection with this parasite. Hoare also described the morphology of the different endogenous stages, including trophozoite (3-4 µm), merozoite (stumpy, sausage shaped; L: 3-4 µm, W: 2 µm), macrogamete (spherical, 8 µm diameter with darkly staining globular inclusions of reserve material), and microgamete (described as similar to those of other Eimeria species). Two types of merogony are described from histologic sections, the first with stumpy merozoites as described above, and the second with merozoites with elongated curved bodies and a compact polar nucleus, measuring µm. This second merogonic generation was observed almost exclusively in the naturally infected ferrets and was associated with initiation of sexual differentiation and reproduction. The pathology of experimental and non-experimental infection with E. ictidea in domestic ferrets was also described by Hoare (1927; 1935b). Parasitic invasion of the epithelium was noted only in the small intestine, with patchy distribution of the parasite life stages throughout affected sections. Within the small intestinal villi, the parasites were again concentrated in the tips of the villi, with infected epithelial cells never containing more than one parasite. As each intracellular parasite grew it filled the entire host cell, displacing the nucleus to the base of the cell. Predominantly sexual life stages were detected in tissue sections, with few asexual generations observed. Interestingly, the parasites were arranged into age groups, with forms of the same life stage grouped together within the affected epithelial sections; this is in 22
39 contrast to E. furonis, where life stages of different maturities were found together in affected sections. Hoare described the morphology of the different endogenous stages of E. ictidea, including merozoites (free within the lumen: elongated, vermiform with one pointed end and a nucleus located at the rounded end, 11 µm 1 µm; within the epithelium: shortened and rounded, 3-4 µm diameter), macrogametes (elongated, 20 7 µm, occupying the entire host cell, with darkly staining globular inclusions of reserve material), and mature microgamonts (morphologically similar to those of other Eimeria species but larger than those of E. furonis). Of note, a tissue reaction was observed specifically in association with more developed life stages of E. ictidea (e.g., mature meronts, mature gamonts, unsporulated oocysts), which was not observed when cells contained earlier stages of development (e.g., trophozoites, immature gamonts). This tissue reaction was described by Hoare (1935a; 1935b) as the development of an annular constriction of the apical portion of the villus separating infected epithelial cells from unaffected cells. The constriction involved the epithelium but could also extend inwards into the core of the villus. These changes were associated with congestion of capillaries and extravasation of red blood cells within the constricted segment, and in some sections villar tip necrosis. In their case report of one domestic ferret, Blankenship-Paris et al. (1993) described the gross pathologic lesions associated with intestinal coccidiosis; in this case there was diffuse dilation and reddening of the small intestine, which was empty, and the colon contained dark watery material. Histologic lesions were confined to the ileum and jejunum. The jejunum exhibited thickening of the villi with a crypt to villus ratio of 1:5, mild granulomatous inflammation in the lamina propria, and large numbers of coccidial meronts, gamonts and oocysts within the enterocytes of the villar tips. The gross lesions described by Sledge et al. (2011) from 20 domestic ferrets are as follows: thin body condition with moderate to marked dehydration, perineal staining with diarrhea, moderate dilation of the small and large intestines, and the presence of pasty tan to tarry black digesta within the distal small intestine and colon. Other findings in one to a small number of ferrets included enlarged, pale tan livers; splenomegaly with dark red colouration; and multiple superficial gastric or duodenal ulcers. The 23
40 histologic lesions from 10 ferrets included moderate blunting and occasional fusion of jejunal and ileal villi, focal attenuation and erosion of the epithelium of the villar tips with exudation of fibrin, neutrophils and blood into the intestinal lumen in regions with severe erosion. Intact epithelial cells at the villus tips and, rarely, sloughed epithelial cells in the intestinal lumen contained numerous intracytoplasmic coccidia representing a range of asexual and sexual life stages (meronts, macrogamonts, microgamonts, and oocysts). The subjacent lamina propria of the small intestine and of the large intestine exhibited moderate lymphoplasmacytic infiltration with occasional neutrophils and congestion of blood vessels. Marked mucosal hemorrhage was identified in the most severely affected sections. Marked gross and histopathologic hepatobiliary lesions were described in a single ferret by Williams et al. (1996). On gross necropsy, the liver was pale and enlarged, with dilated, firm bile ducts and thickening of the gall bladder wall. Similar gross necropsy findings were described by Kaye et al. (2015): marked dilation and mural thickening of the entire biliary tree (including gall bladder, intrahepatic and extrahepatic bile ducts). On histopathology, Williams et al. (1996) noted that the marked thickening of the gallbladder wall was a result of cystic proliferation of mucosal glands, which were separated by tracts of fibrous connective tissue and marked granulomatous inflammation. Liver sections exhibited marked biliary hyperplasia, marked periductular fibrosis and moderate periportal lymphoplasmacytic cuffing. There was multifocal papillary proliferation of bile duct epithelium and dilation of the bile ducts, and within the ductular lumens there were moderate numbers of lymphocytes and plasma cells, small numbers of degenerate neutrophils, sloughed epithelial cells and debris. All endogenous coccidial life stages were present within the gall bladder and biliary epithelium, with meronts visible in 20% of the intact epithelial cells of the biliary tree and gallbladder, and oocysts free within the lumen of the intrahepatic bile ducts. Similar lesions were present in the case described by Kaye et al. (2015) and as well as in juvenile and adult farmed mink (Mustela vison) with hepatobiliary coccidiosis (Davis, Chow & Gorham, 1953). 24
41 1.5 INTRODUCTION TO ENTERIC COCCIDIOSIS IN THE BLACK-FOOTED FERRET Natural history and conservation of the black-footed ferret in North America Black-footed ferrets are one of only three wild ferret species worldwide; the other species are the European polecat (Mustela putorius) and the Siberian polecat or steppe polecat (Mustela eversmanii). They are the only native North American ferret species and the most endangered North American carnivore. They are nocturnal carnivores whose diet and lifestyle are highly dependent on local prairie dog (Cynomys sp.) populations. Prairie dogs comprise almost exclusively the diet for the BFF, who also use the complex burrow systems made by prairie dogs to escape their predators and raise their young (Santymire et al. 2014; USFWS BFF Recovery Program 2017). While formerly distributed throughout the North America prairie ecosystem, BFF were considered extinct by the late 1950s. In 1964 a single population was discovered in Mellette County, South Dakota. Progressive decline of this population in subsequent years resulted in the decision by United States Fish and Wildlife Service (USFWS) to initiate a captive breeding program for the species. From four females and five males were captured for this purpose. Despite successful breeding, no kits survived and the last adult ferret in this captive colony died in 1979; at that time BFF were again presumed extinct in the wild based on annual surveys of the initial capture site. In 1981, a dead BFF was discovered by a ranch dog outside of Meeteetse, Wyoming, allowing wildlife biologists to identify another colony of BFF. This colony flourished until 1985 when an outbreak of canine distemper in the BFF population and an outbreak of sylvatic plague in the local prairie dog population resulted in sharp population declines. From 1985 through 1987 all 24 of the remaining BFF were trapped and brought into captivity to re-initiate the captive breeding program. Six ferrets in this initial group died of canine distemper while in captivity and of the remaining 18 survivors, 7 bred successfully to create the founding population of the current captive breeding population. Today this captive breeding population consists of approximately 300 BFF distributed among multiple institutions (Santymire et al. 2014). 25
42 Since 1986, this multi-institutional effort has been breeding BFF in captivity with reintroductions back into the wild in 28 selected locations in Canada, the USA and Mexico. Currently, six facilities participate in the BFF Species Survival Plan (SSP): the Toronto Zoo, USFWS National Black-footed Ferret Conservation Center, National Zoo s Smithsonian Conservation Biology Institute, Louisville Zoological Garden, Cheyenne Mountain Zoo and the Phoenix Zoo (Black-footed Ferret Recovery Implementation Team 2011). As of 2011, over 8,000 BFF kits had been produced in captive breeding facilities (Black-footed Ferret Recovery Implementation Team 2011). Multiple infectious diseases pose a significant risk to the captive breeding and post-release survival of BFF, including canine distemper and sylvatic plague. Coccidiosis is recognized as a cause of significant juvenile morbidity and mortality in captive breeding programs, and can result in significant population losses (Bronson et al. 2007; Santymire et al. 2014; USFWS BFF Recovery Program 2017) Coccidia identified from black-footed ferrets Eimeria ictidea and Eimeria furonis have been identified in black-footed ferrets based on morphologic criteria (Jolley et al. 1994). Jolley et al. examined fecal samples from six captive BFF during a distemper outbreak as well as samples from wild BFF. They described one medium-sized ovoid, tetrasporic, dizoic oocyst with a double wall, presence of a polar body and lacking both an oocyst residual body and micropyle. The oocysts measured 23.2 µm (range ) by 15.5µm (range ), with a SI of The sporocysts were elongate with the presence of both sporocyst residuum and a Stieda body. Sporozoites contained prominent refractile bodies at the posterior end and were aligned anterior to posterior within sporocysts. These oocysts were shed by all six captive ferrets. On histopathology of intestinal sections, merogony and gametogony were observed within the villar epithelium throughout the small intestine, but were concentrated in the jejunum. Two morphologically distinct meronts were detected in these sections, one at the villar tips, which was larger and lacking in undifferentiated mass, and the other at the base of the villi or, rarely, in the intestinal crypts. Gametogony was predominantly 26
43 observed at the villar tips, and was noted throughout the small intestine. These organisms were considered consistent with Eimeria ictidea based on descriptions by Hoare (1927) from domestic ferrets. A second small spherical to subspherical, tetrasporic, dizoic oocyst was documented that had a pink double wall, a granular residual body, and lacked both oocyst polar body and micropyle. This smaller oocyst measured 12.6±1.2 µm ( ) by 11.9±0.9 µm ( ) with a SI of The sporocysts were elongate with the presence of a Stieda body, and sporozoites contained refractile bodies. Similar to the larger Eimeria species described above, merogony and gametogony were observed within the villar epithelium throughout the small intestine, with endogenous developmental stages most numerous in the jejunum. The meronts were small with 16 or fewer merozoites. Micro- and macrogamonts were observed clustered within the apical third of the villar epithelium, as were meronts. Jolley et al. (1994) determined these small spherical oocysts to be consistent with Eimeria furonis, as described by Hoare (1927) from domestic ferrets. Jolley et al. (1994) described a third type of coccidial oocyst occasionally detected in small numbers within the BFF fecal samples; the authors did not state whether this third type of oocyst was recovered from wild or captive BFF. The oocysts measured 37.0±1.3 µm ( ) by 22.3±2.3 µm ( ), with a SI of Attempts to sporulate collected oocysts were largely unsuccessful and corresponding endogenous stages were not identified on histopathologic examination of necropsied ferrets, precluding further morphologic identification of the parasite. It should be noted that coccidial oocysts with similar measurements had not been detected in wild or captive prey species available for ingestion by BFF (Jolley et al. 1994). Previous to this report, coccidial oocysts had been isolated from the feces of BFF in two captive populations (Carpenter & Hillman, 1979; Williams et al., 1988). The abstract by Carpenter & Hillman (1979) did not describe the oocysts, whereas Williams et al. (1988) stated that two Eimeria species (one with larger oocysts and one with smaller oocysts) were identified within the fecal samples, but they were 27
44 not identified further. Interestingly, Williams et al. reported both Eimeria species to be shed in the feces of all ferrets concurrently affected by distemper, and by approximately 30% of the clinically healthy ferrets at the time of investigation. Non-enteric coccidia have been reported from captive BFF in one facility by two authors (Jolley et al., 1994; Williams et al., 1988). Both reports, presumably describing the same case(s), noted the presence of endogenous coccidial life stages in histologic sections of respiratory tissue and merozoites of an unidentified coccidium in impression smears of the urinary bladder from BFF diagnosed with canine distemper. Meronts were observed within the epithelium of the trachea, a large bronchus and associated bronchial glands. Jolley et al. (1994) described the lesions as occurring in the same ferret, whereas in the earlier report by Williams et al. (1988) they are described as occurring in two different ferrets. There have been no subsequent published reports of systemic coccidiosis in black-footed ferrets, and no cases have been identified within the pathology database of the Toronto Zoo captive BFF population or by the current SSP pathologist (Dr. Michael M. Garner, personal communication). There is a significant information gap regarding the pre-patent periods and pathogenicity of both identified Eimeria species in BFF, and studies to further characterize the eimeriid coccidia of the BFF are lacking Morbidity, mortality and clinical signs associated with enteric coccidiosis in black-footed ferrets The clinical signs of enteric coccidiosis in black-footed ferrets include mucoid to hemorrhagic diarrhea, abdominal discomfort, lethargy, appetite loss, vomiting, and dehydration. In some cases, sudden death precedes the development of diarrhea. Both adult and juvenile BFF are affected by the disease, which causes significant morbidity and mortality in captive populations (Bronson et al. 2007). One retrospective study of the captive BFF population at the Smithsonian National Zoological Park determined that the most common cause of death in juvenile BFF (aged 30 days 11 months) was gastrointestinal pathology (52.4% of juvenile deaths), with 63.6% of these cases caused by enteric 28
45 coccidiosis (Bronson et al. 2007). Despite the significance of this disease to the captive population, its effect on morbidity and mortality in wild BFF populations is unknown. To the author s knowledge, no routine surveys of fecal parasites have been conducted on wild-born or captive released BFF during yearly spotlighting events at ferret release sites. However, samples may be collected opportunistically if fecal material is identified within the traps used to catch wild BFF during yearly surveys at release sites. Where fecal samples have been analyzed, a 13% prevalence of coccidiosis has been identified in wild born BFF (Dr. Rachel Santymire, personal communication). Fecal samples have been collected from BFF at four release sites within the USA: Wind Cave National Park (South Dakota); Badlands (South Dakota); Conata Basin (South Dakota) and Aubrey Valley (Arizona), and positive samples were identified only at the first site (Dr. Rachel Santymire, personal communication). Although radio-telemetry has been used at some release sites to determine sources of mortality and factors involved in survival, its use is not widespread. Furthermore, the nocturnal and fossorial lifestyle of the BFF is a significant impediment to the surveillance and monitoring of disease in this species. 1.6 TREATMENT, PREVENTION AND CONTROL OF INFECTION BY EIMERIA SPP Current recommendations for treatment of eimeriid coccidia in carnivores Described anticoccidial therapies for carnivores come from research in domestic cats and dogs infected by Cystoisospora species; these tissue coccidia (family Sarcocystidae) are only distantly related to the Eimeria species infecting the BFF and other ferrets. Current therapeutic recommendations by the Companion Animal Parasite Council (CAPC 2013) for treatment of described Cystoisospora species isolated from cats and dogs include the following: amprolium ( mg daily for 5 days in dogs; mg daily for 7-12 days in dogs; mg/kg daily for 7 days in cats), amprolium/sulfadimethoxine (150 mg/kg amprolium and 25 mg/kg sulfadimethoxine daily for 14 days in dogs), diclazuril (25 mg/kg for one dose in cats), furazolidone (8-20 mg/kg 1-2 times daily for 5 days in dogs and cats), ponazuril (20 mg/kg daily for 1-3 days in dogs and cats), quinacrine (10 mg/kg daily for 5 days in cats), 29
46 sulfadimethoxine (50-60 mg/kg daily for 5-20 days in dogs and cats), sulfadimethoxine/ormetoprim (55 mg/kg sulfadimethoxine and 11 mg/kg ormetoprim daily for 7-23 days in dogs), sulfaguanidine (150 or 200 mg/kg daily for 6 days, or mg/kg every 8 hours for 5 days in dogs and cats), toltrazuril (10-30 mg/kg daily for 1-3 days in dogs), trimethoprim/sulfonamide (30-60 mg/kg trimethoprim daily for 6 days if >4kg, mg/kg trimethoprim daily for 6 days if <4kg) (CAPC 2013). Notably, the use of all drugs listed by the CAPC is considered off-label, with the exception of sulfadimethoxine Current recommendations for anticoccidial treatment and prophylaxis in domestic and blackfooted ferrets Domestic ferrets Recommended daily oral treatment regimens for enteric coccidiosis in domestic ferrets include: amprolium (19 mg/kg once daily; 0.5 mg/kg), decoquinate (0.5 mg/kg), sulfadimethoxine (300 mg/kg in drinking water), or sulfadiazine-trimethoprim (30 mg/kg once daily), all administered for a minimum of two weeks (Bell, 1994; Patterson & Fox, 2007; Patterson et al., 2014). Both the aforementioned coccidiostats, amprolium and decoquinate, are sold in large formats and are ideal for use in larger operations such as breeding facilities, research facilities or rescue centers. Other anticoccidial therapies used in domestic ferrets include toltrazuril (20 mg/kg) and ponazuril (30-50 mg/kg) once daily. It should be noted that all anticoccidial therapy used in domestic ferrets is considered off-label drug use. Multiple follow up fecal examinations should be performed after the treatment regimen is complete and large groups may need to be treated multiple times. Routine cage cleaning is also important to decrease the environmental oocyst burden and prevent re-infection and, in the case of coccidial outbreaks, ferrets should be transferred to clean cages multiple times during the course of anticoccidial therapy. Disinfectants, such as bleach or quaternary ammonium compounds, or dry heat should be used for effective environmental decontamination (Patterson et al., 2014). 30
47 Species Survival Plan recommendations for black-footed ferrets Treatment and prophylaxis of enteric coccidiosis with oral sulfadimethoxine was previously recommended by the BFF Species Survival Plan (SSP). However, due to a suspicion of decreasing efficacy of treatment, ponazuril has been recommended recently for treatment. Due to the perceived exquisite sensitivity of BFF to enteric coccidia, the current SSP recommendation for treatment is oral ponazuril at 30 mg/kg once if ferrets are to be transported, anesthetized, stressed or are otherwise suffering from another illness or injury (even in the absence of clinical signs or fecal shedding). The same single oral dose of 30 mg/kg is also recommended for kits at weaning (30-35 days of age), post weaning (40-45 days of age), and prior to anesthesia for initial examination and vaccines (50-60 days of age). Large, crowded or otherwise stressed litters should be administered 30 mg/kg orally once every 7-10 days during the period of stress. For treatment of coccidial diarrhea diagnosed by fecal examination, 30 mg/kg orally once every 7 days for two doses, or 50 mg/kg orally once daily for 3 days in food (repeated in 7 days) is recommended. In BFF with clinical signs of dehydration, administration of subcutaneous or intravenous fluid therapy has been performed. Additional therapy with other antibiotics is sometimes provided in cases with severe clinical signs or where secondary or primary bacterial enteritis is suspected. There is no pharmacokinetic or pharmacodynamic information available for the use of anticoccidial drugs in BFF or other Mustelidae, and thus it is unknown whether the current dose or frequency of administration is truly appropriate for treatment of coccidiosis. In 2 to 3-month-old piglets administered a single dose of ponazuril orally at 5 mg/kg, peak serum concentration occurred at 42 hours (36-48 hr), and elimination half-life was ~5.6 days (Zou et al. 2014). In llamas, administered ponazuril as a single dose of 20 mg/kg orally, peak serum concentration occurred at 84 hours, and elimination half-life was ~5.6 days (Prado et al., 2011). In domestic cows administered ponazuril as a single 5 mg/kg dose orally, peak serum concentration occurred at 48 hours and elimination half-life was 58 hours (Dirikolu et al., 2009). The relevance of serum drug concentrations for treating an intestinal infection that lacks extraintestinal life stages is likely minimal because the highest drug dose will reach the site of concern (intestines) and systemic distribution is not required. 31
48 Furthermore, no safety or efficacy studies have been performed in any ferret species to validate the current uses of either sulfadimethoxine or ponazuril for treatment, nor have the current recommended treatment lengths been validated. However, anecdotal information based on current usage would indicate that they are safe at the current dosages and frequencies of administration as no adverse effects have been reported. A recent efficacy study in shelter dogs and cats showed that oral ponazuril (50 mg/kg) administered once daily for 3 days was effective for treatment of infection with Cystoisospora, as determined by a reduction in or cessation of fecal oocyst shedding at 4 and 8 days post treatment. Treatment efficacy in this study was inversely correlated to fecal oocyst counts at the initiation of treatment (Litster et al. 2014). Interestingly, efficacy of this dose compared to the other two treatment groups (single 50 mg/kg or 20 mg/kg oral dose) did not seem to differ but no statistical analysis was performed. Given the ubiquitous use of ponazuril in captive breeding facilities, and concerns regarding resistance of coccidia species to sulfadimethoxine therapy, information on minimum effective doses and dose regimes would be necessary to inform appropriate future SSP treatment and management plans, and to minimize development of drug resistance. 1.7 VACCINES AGAINST COCCIDIA Theory The development of resistance of protozoal parasites to chemotherapeutic agents has resulted in a shift towards the development of vaccines for the protection of domestic livestock. Immunity to enteric coccidiosis in avian and mammalian species involves both humoral and cell mediated responses. Eimeria spp. infection in sheep, rats, poultry and other species generally results in a protective immune response against subsequent re-infections (Catchpole et al. 1993; Shi et al. 2000). Interestingly, this is not the case for some host parasite interactions; for example, a recent report indicated that primary infection with E. ninakohlyakimovae in goat kids did not provide protective immunity against subsequent challenge with the same parasite (Ruiz et al. 2013). 32
49 Vaccines can be divided into four general categories: live vaccines, inactivated/killed vaccines, subunit vaccines and recombinant vaccines. Live vaccines are orally administered using small numbers of infectious oocysts or oocysts from strains with low pathogenicity and result in patent, but ideally subclinical, infections in the host that will elicit a protective immune response. Such live vaccines can be produced using attenuated forms of the pathogen of interest, for example, in chickens using precocious strains of Eimeria spp. These precocious strains undergo a reduced number of merogonic replications within the host cells and thus fewer oocysts are shed in the feces of vaccinated animals. This reduction in endogenous merogonic cycles reduces the amount of damage to the intestinal epithelium as well as reducing the number of oocysts contaminating the environment. Another strategy has been to use live parasites with truncated life cycles. An example of this is the Toxoplasma gondii vaccine developed to prevent abortion in sheep. This parasite was passaged multiple times through a mouse host, resulting in an inability to produce tissue cysts (Meeusen et al. 2007). This is desirable as the cyst stage of this parasite, normally inhibited by the immune system, can be reactivated during periods of stress or immunocompromise. The potential drawbacks of live vaccines include: 1) the ability to produce and isolate adequate numbers of coccidial oocysts to meet vaccine production requirements; 2) the potential development of clinical disease in the host as a result of inoculation; 3) the need for all susceptible individuals to receive the vaccine simultaneously to prevent fecal-oral inoculation of unvaccinated animals with high doses of the infective agent likely to be present in a shared environment through fecal shedding. Inactivated vaccines are produced when the microbe of interest is killed via application of heat, radiation or chemical treatment prior to inoculation into the host species. While safer, because they cannot induce disease in the inoculated patient, inactivated vaccines stimulate a reduced immune response compared with live vaccines and are consequently less effective. Subunit vaccines contain single or multiple antigens of importance in initiating the host immune response, rather than the entire pathogen of concern. Subunit vaccines cannot induce disease in the immunized host, but are more difficult to produce 33
50 because they require a detailed understanding of host immune response to infection. Recombinant vaccines involve the genetic modification of a vector (virus or bacteria), one capable of infecting the host of interest, to contain DNA of the pathogen of interest. These vectors induce an immune response in the vaccinated host but, as with subunit vaccines, cannot induce disease. However, recombinant vaccines are again difficult to produce because they require an in depth understanding of the life cycle stages, genes and antigens targeted by the host immune response to infection. There are currently no recombinant vaccines marketed in Canada for use in veterinary medicine against protozoal disease. Creation of effective vaccines against protozoal parasites is complicated by parasite antigenic diversity during the different life cycle stages and among protozoal species and strains of the same species (Meeusen et al. 2007). Although most parasites induce some level of immunity in their host species, the immunological response to different parasite life stages and species has been poorly characterized for most coccidia. Furthermore, many parasites have developed mechanisms to evade host immune responses or to continue survive and replicate in and transmission by previously infected hosts. Our limited understanding of the immune responses against coccidial antigens has restricted commercial vaccine production to live or attenuated vaccines (Meeusen et al. 2007). A notable disadvantage of anticoccidial vaccines is that they need to be developed for each coccidial species of interest because of the species-specific nature of the immune responses; this is a considerable limitation compared with anticoccidial drugs that can have a much wider spectrum of action (Vermeulen 2005). While the requirement for mass production of vaccine is a limiting factor for vaccines developed for the agricultural industry, this drawback would be less important for production of a vaccine to be used in an endangered species Species successes in anticoccidial vaccination The first successful immunization against coccidiosis was reported in 1918 in dogs (Hall & Wigdor). In this report, a dog that had previously recovered from coccidial infection with Diplospora 34
51 bigemina, was fed three increasing doses of live non-attenuated coccidial culture (at 14, 32 and 48 days post recovery from primary infection), which resulted in no development of clinical signs and no oocyst shedding for days after each challenge. Subsequently, immunization of dogs and cats against coccidia, with protection lasting up to seven months, was reported by Andrews (1926). Immunization of albino rats to eimeriid infection after administration of three or more sublethal doses of Eimeria nieschulzi via gastric intubation was reported by Morehouse (1938); further experiments showed that sporozoites did not enter the host intestinal epithelium in immunized rats given a challenge dose (Morehouse 1938). Similar findings were reported in chickens immunized against Eimeria tenella that had 50% fewer intra-epithelial sporozoites following challenge compared to naïve birds (Augustine and Danforth 1986). Conversely, chickens previously inoculated with Eimeria acervulina exhibited more intracellular sporozoites after challenge than naïve birds, but sporozoites were not observed to develop in previously immunized birds (Augustine and Danforth 1986). These findings provide further evidence that the immune response to Eimeria spp. may differ among host species. Vaccination against Eimeria species has been most successful and is most widely used in the poultry industry, particularly in breeder and layer flocks. Almost all vaccines marketed for poultry are live vaccines (attenuated and non-attenuated). Vaccination against other apicomplexan parasites in domestic mammals has also been achieved but has been generally less effective for disease prevention and is less widely available. Marketed killed and inactivated (attenuated) vaccines include those containing killed tachyzoites of Neospora caninum for cattle (Neoguard, Merck Animal Health), and chemically inactivated merozoites of Sarcocystis neurona for horses (EPM Vaccine, Fort Dodge no longer in production). A subunit vaccine for Babesia canis in dogs uses cultured antigen (Pirodog, Merial). Available live vaccines include a vaccine against Toxoplasma gondii in sheep (Ovilis Toxovax, Intervet) that uses an attenuated temperature sensitive strain (S48). 35
52 1.8 RESEARCH GOALS AND OBJECTIVES Objectives a) To determine and characterize (morphologically and molecularly) the enteric coccidial species currently affecting the black-footed ferret population b) To describe the natural history of enteric coccidiosis in captive black-footed ferrets, including pre-patent period, shedding frequency and burdens, and morbidity and mortality rates c) To compare molecular, morphologic and life history characteristics of enteric coccidial species identified in domestic ferrets to those in black-footed ferrets d) To validate domestic ferrets as an experimental model for intestinal coccidiosis in the blackfooted ferret Hypotheses a) Multiple Eimeria species will be isolated from the black-footed ferret population b) The Eimeria species identified from black-footed ferrets will be the same as those previously described in domestic ferrets c) A single pathogenic Eimeria species will be implicated in recorded outbreaks of clinical coccidiosis during the period of study d) Domestic ferrets can act as an experimental model of intestinal coccidiosis for black-footed ferrets Applications The goal of this project is to better characterize the enteric coccidia of the endangered blackfooted ferret in order to set the stage for improved disease prevention and treatment. To the author s knowledge, this project is the first attempt to isolate and perform molecular characterization of the coccidial species endemic in the black-footed ferret population. This information will be used to compare these species to known coccidia from domestic ferrets and other related mammals. As experimental work 36
53 cannot be carried out on enteric coccidiosis in the BFF due to its endangered status, if the domestic ferret can be validated as an experimental model, studies of the patterns of anticoccidial resistance and development of immunity against Eimeria spp. can be undertaken in vivo. The ultimate goal would be the development of an autogenous vaccine used to improve survival of ferret kits and reduce morbidity and mortality associated with coccidiosis in BFF captive breeding programs. Based on clinical experience, stressful life events such a breeding, weaning and transfer between institutions appear to increase the risk of coccidial outbreaks in adult BFF. As such, vaccination could assist in reducing disease outbreaks in BFF associated with various management activities. There is no data on the significance of coccidiosis in wild populations and limited means of disease surveillance following release; vaccination during captiverearing or pre-release conditioning of BFF would be an ideal method of reducing the potential effects of this disease in released and free-living BFF. Increasing the numbers of ferrets being released to the wild and releasing ferrets immune to the subsequent threat of coccidiosis would support the goals of the conservation initiative for the black-footed ferret. 37
54 CHAPTER 2: MOLECULAR CHARACTERIZATION OF ENTERIC COCCIDIA FROM DOMESTIC FERRETS (MUSTELA PUTORIUS FURO) This chapter has been submitted for publication as: Adriana R. Pastor, Dale A. Smith and John R. Barta (2017). Molecular Characterization of Enteric Coccidia from Domestic Ferrets (Mustela putorius furo). Vet Parasitol: Regional Studies and Reports (In review) ABSTRACT: Combined morphometric and molecular characterization of coccidia that infect domestic ferrets (Mustela putorius furo) was completed to improve the diagnostic specificity of enteric coccidiosis in this host. Coccidia positive fecal samples (n=11) and formalin fixed paraffin embedded intestinal tissues (n=3) from domestic ferrets were collected from diagnostic laboratories in Canada and Europe. An average of 3.5 and 13 domestic ferret fecal samples per year were coccidia-positive when tested by Canadian and European diagnostic laboratories, respectively, during the period Oocyst morphometrics and sequence genotyping at two loci (nuclear 18S rdna [nu 18S rdna] and mitochondrial cytochrome c oxidase subunit I [mt COI]) were conducted on all samples. The first nu 18S rdna and mt COI sequences for Isospora (=Cystoisospora) laidlawi, and the first mt COI sequence for Eimeria furonis were generated during this study. Phylogenetic analysis of the mitochondrial COI sequences demonstrated that E. furonis was most closely related to E. cf ictidea isolated from a blackfooted ferret (Mustela nigripes) and that I. (=C.) laidlawi was closely related to C. canis and C. felis. The identifications provided by diagnostic laboratories of the specific parasite species present in a sample showed poor agreement with their identifications based on genotyping obtained in this study. Molecular techniques appear to be essential for accurate determination of coccidial species responsible for individual and group outbreaks of coccidiosis and for further understanding of eimeriid host-parasite relationships. Key words: coccidia; Cystoisospora laidlawi; domestic ferret; Eimeria furonis; Eimeria ictidea; Mustela putorius furo 38
55 2.1 INTRODUCTION: Coccidia are host-specific parasites of the phylum Apicomplexa, with greater than 2000 species named to date (Duszynski et al. 2000; Upton 2000). The eimeriorinid coccidia (suborder Eimeriorina) include typical intestinal coccidia such as Eimeria, Isospora and Cyclospora species belonging to the family Eimeriidae as well as tissue (cyst-forming) coccidia such as Cystoisospora, Besnoitia, Toxoplasma and Sarcocystis species that belong to the family Sarcocystidae (see Cox, 1994). Enteric coccidia affect both domestic ferrets (Mustela putorius furo) and their wild counterparts. In his initial descriptions of enteric coccidiosis in domestic ferrets, Hoare (1927, 1935b) did not observe clinical signs of intestinal disease associated with infection. More recently, it has been recognized that enteric coccidiosis can result in clinical signs ranging from mild transient diarrhea to more severe disease with dehydration, lethargy, depression, weight loss/emaciation, inappetence and death (Blankenship-Paris et al. 1993; Powers 2009; Sledge et al. 2011; Hoefer et al. 2012; Patterson et al. 2014). Rectal prolapse has also been reported in ferrets with enteric coccidiosis (Hillyer 1992; Hoefer et al. 2012). Disease appears to be most common in young or stressed animals. In one study, co-infection with coccidia and Lawsonia intracellularis (Desulfovibrio sp.) was diagnosed in 4 of 19 ferrets with proliferative bowel disease (Li et al. 1996); these ferrets presented with clinical signs including diarrhea, lethargy, anorexia, weight loss, dehydration and emaciation. Two cases of biliary coccidiosis have also been reported in domestic ferrets; infection was associated with biliary epithelial hyperplasia, cholecystitis and cholangiohepatitis (Williams et al. 1996; Kaye et al. 2015). Three species of coccidia affecting domestic ferrets were originally described and named by Hoare (1927): Eimeria ictidea, Eimeria furonis, and Isospora (=Cystoisospora) laidlawi. The three species were detected in feces from 50 domestic ferrets involved in an outbreak of canine distemper at a research facility. For each parasite, the author described the morphology of sporulated oocysts isolated from feces as well as sporulation time (exogenous life stages). All subsequent reports of morphologic diagnoses of these coccidia have been based on Hoare s original descriptions. The pre-patent period 39
56 (minimum duration of endogenous development) in inoculated naïve ferrets was described for E. furonis and E. ictidea as 6 and 7 days, respectively (Hoare 1935b). The pre-patent period for I. (=C.) laidlawi was not determined because the number of oocysts available was insufficient for an experimental infection trial. Hoare (1927) described the sporulated oocysts of E. furonis as follows: spherical; double outer wall with a thin, colourless outer layer and a thick, yellowish inner layer; no micropyle or residual body; and measuring on average µm (length [L]: ; width [W]: ; shape index [SI]: 1.07). Sporocysts were spindle-shaped with one end constricted/blunted, contained a residual body, and on average measured µm. Sporozoites were vermiform with one end narrower than the other, arranged head to tail, and each had a central nucleus; a clear vacuole was identified in some sporozoites at their broad posterior end. The sporulated oocysts of E. ictidea were described as follows: oval or elliptical, with a double outer wall with a thin, colourless outer layer and a thick, yellowish inner layer; no micropyle or residual body; and measuring on average µm (L: ; W: ; SI: 1.35). Sporocysts were irregularly oval, with one end broad and the other more constricted, contained a residual body, and measured µm on average. Sporozoites were vermiform with one end narrower than the other, arranged head to tail in the sporocysts, and had a central nucleus and a clear vacuole at their broad posterior end. The sporulated oocysts of I. (=C.) laidlawi were ovoid, with a double outer wall with a thin, colourless outer layer and a thick, yellowish inner layer; had no micropyle or residual body; and measured on average µm (L: ; W: ). A SI of 1.17 can be calculated from the original mean dimensions. Two sporocysts were identified, each containing four sporozoites and no Stieda body; sporocysts were elliptical, contained a residual body and measured µm on average. Sporozoites were sausage shaped, with one end slightly pointed, and had a central nucleus and a clear 40
57 vacuole identified at the pointed end. Sporozoites were arranged with pointed ends all at the same pole of the sporocyst. Oocysts identified as Cystoisospora ohioensis have been reported from fecal samples collected from healthy domestic ferret kits in a large American ferret breeding operation that also housed juvenile domestic dogs (Patterson and Fox, 2007). The method of identification of this parasite was not described. A second similar institution reported the presence of Cystoisospora (=Isospora) species, also thought to be C. ohioensis, in routine fecal examination of their colony (Dr. Bambi Jasmin, personal communication). Identification in this case was performed by the Animal Health Diagnostic Center at Cornell University and was based on morphometrics using light microscopy. The significance of these findings is unknown, but no clinical signs or histologic lesions were described in the ferrets shedding these oocysts. The definitive hosts for C. ohioensis are canids, including the domestic dog. More recently, molecular techniques have been used for the more precise identification of coccidia. Nucleotide sequences, like morphological features, diverge over time under selective pressure; however, recent evolutionary divergence among coccidia is more likely to be reflected in molecular, as compared to morphologic, differences. Thus, nucleotide sequences that are more similar are inferred to be more closely related and to have diverged more recently (Cox 1994). Molecular characterization of ferret coccidia has only been performed for one species, Eimeria furonis. Abe et al. (2008) extracted DNA from oocysts from the feces of a single domestic ferret with clinical signs resulting from coccidial enteritis. Using primers initially developed for molecular identification of Cyclospora species (see Matsubayashi et al., 2005), small subunit ribosomal DNA (nu 18S rdna) primers CYC1FE (5ʹ-TAC CCA ATG AAA ACA GTT T-3 ) and CYC4RB (5 -CGT CTT CAA ACC CCC TAC TG-3 ) were used to amplify a 347 base pair fragment of nu 18S rdna. The amplicon was sequenced (GenBank AB329724) and compared with previously published partial nu 18S rdna sequences from 40 Eimeria, two Isospora and four Cyclospora species. The resulting phylogram 41
58 grouped E. furonis with E. alabamensis (cattle) and E. meleagrimitis (turkey). In the same study, the microscopic morphology of the oocysts was used to identify this coccidial species as Eimeria furonis by comparison with Hoare s (1927, 1935b) published descriptions of Eimeria furonis and Eimeria ictidea. Sledge et al. (2011) also used nu 18S rdna to identify Eimeria furonis as the cause of three distinct outbreaks of enteric disease in domestic ferrets. Initial identification was performed using morphometrics of sporulated oocysts collected from feces in one of the three outbreaks being investigated. Formalin fixed, paraffin embedded intestinal segments from ferrets from each of the outbreaks contained multiple coccidial life stages when examined by light microscopy. PCR amplification of a 247 base pair (bp) amplicon of the nu 18S rdna was generated from DNA isolated from stored, formalin-fixed tissues for further genetic analysis. Analysis and sequencing of amplicons from all three groups showed 100% identity to sequences previously reported by Abe et al. (2008) for the gene encoding E. furonis nu 18S. In 2015, Kaye et al. identified coccidia within hepatobiliary lesions in a domestic ferret receiving immunosuppressive therapy for red cell aplasia. DNA was extracted from frozen liver, and a fragment of the nu 18S rdna was amplified using the primers previously described by Sledge et al.(2011). The authors reported that the DNA sequence of the amplicon had 100% identity to the published nu 18S rdna sequence of E. furonis, and 95% identity to the nu 18S rdna of E. myoxi (rodent), E. alabamensis (cattle) and Isospora robini (avian) (Kaye et al. 2015). It is difficult to estimate the current prevalence of enteric coccidia within the North American domestic ferret population, and no studies have been conducted to do so. Fecal samples submitted to veterinary diagnostic laboratories from domestic ferrets in Canada are uncommon, and samples positive for coccidia appear infrequently (Dr. Donald Martin, personal communication). Conversely, in Europe, the prevalence of coccidia within the domestic ferret population appears to be higher, based on submissions to a large veterinary diagnostic laboratory in Germany. Data from Idexx Vet Med Lab in Ludwigsburg, Germany was compiled to review the prevalence of coccidia and Giardia within fecal samples from domestic ferrets (Pantchev et al. 2011). The authors reported that of 284 fecal samples 42
59 submitted from , 18 (6.3%) had detectable coccidial oocysts on fecal flotation. Based on morphologic characteristics, oocysts were identified as E. ictidea, E. furonis, I. laidlawi (herein referred to as I. (=C.) laidlawi as noted above) and another unidentified Isospora species. Comparative data from the same laboratory from included sample submissions from 253 ferrets, 21 (8.3%) of which were positive for coccidial oocysts on fecal flotation. Nine of the samples were identified as containing E. furonis, three contained both E. furonis and I. (=C.) laidlawi, eight contained only I. (=C.) laidlawi, and one sample contained both E. furonis and E. ictidea; identification in all cases was again based on oocyst morphometrics. No statistically significant difference in the occurrence of coccidial oocysts was detected when data from the two periods were compared (Fisher s exact test; P=0.41) (Pantchev et al. 2011). The purpose of the present study was to perform a more detailed molecular characterization of the coccidial species isolated from domestic ferrets, to estimate prevalence of the different coccidial species within the Canadian domestic ferret population, and to associate morphologic and molecular characteristics of a greater range of enteric coccidial species in order to improve diagnostic accuracy. 2.2 MATERIALS & METHODS: Fecal samples Multiple diagnostic laboratories within Ontario, Canada 1, and a major European diagnostic laboratory 2 were solicited for fecal samples from domestic ferrets shedding coccidial oocysts. Fecal samples were diagnosed positive for coccidia based on fecal flotation and light microscopic identification of Eimeria or Cystoisospora species. Eleven samples were collected during the study period (from ) and preserved in potassium dichromate (2.5% w/v), eight from Europe and three from Canada. Centrifugal flotation with saturated salt solution (Ryley et al. 1976) was used to isolate oocysts from fecal samples for genomic DNA extraction. Genomic DNA extraction and purification were performed using a 1 Animal Health Laboratory, Guelph, ON; Antech Diagnostics Canada Ltd, Mississauga, ON; IDEXX Canada, Markham, ON 2 Vet Med Labor GmbH, Division of IDEXX Laboratories, Ludwigsburg, Germany 43
60 QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer s instructions. After addition of DNAzol to the samples (Qiagen, Hilden, Germany), samples were vortexed using 0.5 mm glass beads (Biospec Products Inc., Bartlesville, OK, USA) prior to extraction in order to fracture the oocyst walls and release the sporocysts. Concentrations of the resultant DNA were estimating using a Nanodrop 2000 spectrophotometer (NanoDrop Products, Wilmington, DE, USA) and stored at 4 C for immediate use or 20 C for later use. For each laboratory, the number of domestic ferret fecal sample submissions, numbers diagnosed positive for coccidial oocysts, and number of each coccidial species identified in positive samples were tabulated for each of the years Formalin fixed intestinal tissues Major diagnostic pathology services across Canada 3 were contacted to identify cases of enteric coccidiosis identified on necropsy of domestic ferrets. Cases were considered positive based on the presence of asexual or sexual life stages of the parasites in intestinal sections. The histologic sections on each positive case were reviewed, re-described and organisms measured (AP, DAS). Gross necropsy reports for all cases were also reviewed to identify any clinical correlates associated with enteric coccidiosis. DNA was extracted from ten 5-6 µm scrolls of formalin fixed paraffin embedded tissue (FFPE) using the QIAamp DNA FFPE Tissue Kit (Qiagen), as per manufacturer instructions Molecular characterization Regions from the nu 18S rdna and mitochondrial cytochrome c oxidase subunit I (mt COI) DNA were amplified by polymerase chain reaction (PCR) from each sample using the primers listed in Table 2.1. PCR amplification was performed for all samples in a volume of 25 µl containing ~100 ng of 3 Animal Health Centre, Abbotsford, BC; Animal Health Laboratory, Guelph, ON; Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, QC; Histovet Surgical Pathology, Guelph, ON; IDEXX Canada, Markham, ON; Prairie Diagnostic Services Inc, Saskatoon, SK 44
61 genomic DNA, 1 PCR buffer, 1.5 mm MgCl 2, 0.2 mm deoxyribonucleotide triphosphates (dntps), 400 nm of each primer, and 1 U of Invitrogen Platinum Taq DNA Polymerase (Thermo Fisher Scientific, Toronto, ON, Canada). Reactions were performed on a Bio-Rad T100 PCR thermal cycler (Bio-Rad Laboratories, Singapore). Samples were denatured and Taq polymerase activated at 95 C for 3 min, then subjected to 35 cycles of 94 C for 30s, anneal at C (see Table 2.1 for specific anneal conditions for the various primer pairs) for 30s, and extension at 72 C for 30-75s (see Table 2.1), followed by a final extension at 72 C for 7 min. Suitable DNA (i.e., genomic DNA from an Eimeria or Sarcocystis sp.) was included in the PCR reactions to act as a positive control for the reaction chemistry. All amplification products were subjected to electrophoretic separation using 1.5% submarine agarose gel, stained with ethidium bromide, and visualized on an ultraviolet transilluminator (Spectronics Corporation, New York, NY, USA). DNA band size was determined by comparison with a 1 kb DNA ladder (GeneRuler 1kb Plus DNA ladder, Thermo Fisher Scientific, Waltham, MA, USA). Bands were excised with a new sterile scalpel blade and PCR products were purified from the gel using a QIAquick Gel Extraction Kit (Qiagen). PCR products were cycle sequenced using an ABI Prism 7000 Sequence Detection System (Applied Biosystems Inc., Foster City, CA, USA) by the Molecular Biology Unit of the Laboratory Services Division, University of Guelph (Guelph, ON, Canada) using the amplification primers to obtain sequences in both directions. The resulting chromatograms were aligned and analyzed with Geneious Ver or later (Biomatters Limited, Auckland, New Zealand) and high quality consensus sequences generated. The resulting consensus sequences were searched from within Geneious against publically available sequences on the BLAST server (blast.ncbi.nlm.nih.gov/blast.cgi) using the blastn search algorithm against the nr/nt database (GenBank+EMBL+DDBJ+RefSeq AA or DNA). Resultant new nucleotide sequences were submitted to GenBank. 45
62 2.2.4 Phylogenetic analysis To determine the phylogenetic affinities of the newly obtained sequences with sequences from related apicomplexan taxa, representative nu 18S rdna and mt COI sequences were downloaded from GenBank with special reference to sequences from parasites that infect members of the order Carnivora. Nuclear 18S and mt COI sequences were aligned independently using MAFTT v7.017 (Katoh et al. 2002) executed from within Geneious and then concatenated into a combined nu18s rdna/ mt COI dataset. Multiple sequences from a single parasite were used to generate consensus sequences for each locus as described by Ogedengbe et al. (2017). Aligned sequences were trimmed to the length of the largest newly generated nu 18S sequence. Phylogenetic trees were generated using Bayesian Inference (BI) using MrBayes Ver (Huelsenbeck and Ronquist 2001) executed from within Geneious; the combined nu 18S and mt COI alignment was partitioned to permit locus-appropriate substitution models to be applied to each partition. For the nu 18S sequence partition, the general time reversible (GTR) substitution model (nst=6) with gamma rate variation (i.e. a GTR+G+I model) was applied. For the mt COI sequence partition, the codon (M1) substitution model (using translation table 4 [i.e. metmt ]) was used instead of the GTR with the remaining parameters remaining the same. The resulting tree was rooted using a pair of adeleid coccidia (Hepatozoon spp.) as the taxonomic outgroup. All BI analyses were run for a chain length of 1,000,000 with tree sampling every 1,000 following a burn-in of 100,000 with default settings of 4 heated chains and heated chain temp of
63 2.3 RESULTS: Fresh fecal samples From inclusive, the Canadian diagnostic parasitology laboratory 4 received an average of (range: ) domestic ferret fecal samples yearly; the European parasitology laboratory 5 received a yearly average of 230 samples (range: ). The number of fecal samples diagnosed as positive for coccidial oocysts per year, on fecal flotation, during this time averaged 3.5 (range 0-8) and 13.0 (range 6-20), for the Canadian and European laboratories, respectively. The diagnosing laboratories used oocyst morphometrics to identify the species of coccidia present. Almost all coccidia-positive submissions to the Canadian laboratory were identified as containing an I. (=C.) species based on light microscopy. Coccidia in only three samples from the Canadian laboratory were identified as E. furonis, one in each of 2010, 2012 and E. ictidea was not identified in any samples submitted to the Canadian laboratory. Approximately equal numbers of coccidia-positive samples from the European laboratory were identified as E. furonis and I. (=C.) laidlawi each year. Only two samples from the European laboratory contained oocysts that were identified as Eimeria ictidea using morphometrics, one from each of 2011 and Laboratory submissions to both laboratories are summarized in Table 2.2. Twelve fecal samples preserved in potassium dichromate were received for analysis by the authors. Eleven samples had previously been identified as containing a single coccidial species, five containing E. furonis, two containing E. ictidea and four containing I. (=C.) laidlawi. A final sample had been identified as containing a mix of E. furonis and Cystoisospora canis. Results of microscopic and molecular characterization of these samples are summarized in Table IDEXX Canada, Markham, ON 5 Vet Med Labor GmbH, Division of IDEXX Laboratories, Ludwigsburg, Germany 47
64 2.3.2 Formalin fixed samples Only three cases of coccidiosis were identified in domestic ferrets within the databases of the five diagnostic laboratories that participated in the retrospective study. Histologic sections of intestine were received from these three cases, which originated in Ontario 6 and Quebec 7. The Quebec sample (P2010-I) was collected in 2010 and the Ontario samples ( and ) in 1993 and 2017, respectively. On gross necropsy, the small intestinal contents of case P2010-I were described as pasty, mucoid yellow-brown feces with some blood. For case , the small intestines were described as empty, but melena was present within the terminal portion of the large intestine. Scant intestinal contents and dark brown fecal material in the colon were described in case In all cases, endogenous developmental stages of coccidia were visible in histological sections (Figure 2.1 is exemplary of the findings from one case). Hematoxylin and eosin stained sections from P2010-I contained two affected regions of small intestine. The intestinal mucosa of the first region contained numerous asexual life stages and moderate numbers of sexual life stages, as well as a small number of oocysts free within the lumen. The second section contained tissues that were poorly preserved; nonetheless, 0-4 oocysts per 400 field were identifiable within the intestinal lumen. Two regions of affected small intestine were identified from after screening of all submitted sections; both contained low numbers of sexual and asexual endogenous stages. Within one region there were small numbers of meronts within the intestinal mucosa and lamina propria. The second region had small numbers of oocysts within cells of the epithelium and lamina propria as well as free within the intestinal lumen. In case multiple sections of jejunum contained numerous coccidian meronts, gamonts 6 Animal Health Laboratory, Guelph, ON 7 Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, QC 48
65 and oocysts within intestinal villi; within the ileum, scattered epithelial cells also contained these various life stages. Average length and width of oocysts were measured from slide sections for all cases. For P2010- I, oocyst average length and width were determined from seven oocysts to be 9.4 µm (range ) and 7.5 µm (range ) respectively, with a SI of 1.25 (range ). Average length and width of oocysts measured from were determined from 5 oocysts to be µm (range ) and 23.3 µm (range ) respectively, with a SI of 1.23 (range ). For the third case, , only 2 oocysts were identified and average length and width of oocysts measured 9.82 µm (range ) and 8.45 µm (range ) respectively, with a SI of 1.16 (range ) Molecular characterization DNA was successfully extracted from all twelve fecal samples and two cases with formalin fixed tissue samples. Attempts at amplification of DNA extracted from sample , using the primer pairs listed in Table 2.1, were unsuccessful. Molecular identification results and GenBank accession numbers for the remaining samples are summarized in Table 2.3. Both the nu 18S rdna and mt COI sequences from I. (=C.) laidlawi were unique when compared with available sequences from other Cystoisospora species within the public databases. However, sequences from I. (=C.) laidlawi were most similar to sequences from C. canis and C. felis and somewhat more divergent from sequences from members of the C. ohioensis species complex. Two apparent genotypes of E. furonis were identified based on nu 18S and mt COI sequencing results. Genotype 1, represented by EU sample 9014, had 100% identity to previously published sequences of the nu 18S locus from two isolates from Japan (GenBank AB and AB329724). Genotype 2, represented by EU sample 907 and Canadian sample , had 99.4% identity at the nu 18S locus (3 single nucleotide differences [SNDs] over 561 base pair region [bp]) to the three sequences above belonging to E. furonis genotype 1. Pairwise alignment of mt COI sequences from both genotypes identified only 2 SNDs (99.6% pairwise identity over 513 bp region). Partial mt COI 49
66 sequences of E. furonis from both genotypes were only distantly related (94.1% pairwise identity; 30 SNDs over 513 bp and 90.5% pairwise identity; 49 SNDs over 513 bp respectively) to publicly available sequences from Eimeria ictidea from the black-footed ferret (Mustela nigripes) (GenBank KT203399) and Eimeria mephitidis from the striped skunk (Mephitis mephitis) (GenBank KT203398), the only other Eimeria species infecting members of the Carnivora for which sequence was available Phylogenetic analysis A phylogenetic reconstruction based on concatenated partial nu 18S rdna and mt COI sequences of E. furonis, I. (=C.) laidlawi and related coccidia is illustrated in Figure 2.2. The combined 18S/COIbased tree demonstrates that the two Eimeria species from ferrets form a well-supported monophyletic group that branches among a collection of other eimeriid coccidia that infect mammals. The sarcocystid parasite of the domestic ferret, I. (=C.) laidlawi, was found to group as the sister taxon to C. canis that together formed a monophyletic group with the closely related C. felis; all three of these closely related Cystoisopora species possess comparatively large, egg-shaped oocysts that are similar morphologically. 2.4 DISCUSSION: The present work has generated the first nu 18S rdna and mt COI sequences for Cystoisospora laidlawi, and the first mt COI sequence for Eimeria furonis; both isolated from the domestic ferret. In this study, histologic presence of organisms and microscopic identification of oocysts shed in feces have been correlated with published and novel nu 18S and mt COI sequences. Eimeria ictidea was not identified in any Canadian sample and this coccidium was reported in only 2 of 1840 fecal samples submitted from across the European Union (EU) to IDEXX Germany during , suggesting that E. ictidea is not a frequent cause of enteric coccidiosis in domestic ferrets in Canada or the EU. 50
67 During the study period ( ), almost twice as many domestic ferret fecal submissions were made to the European, as compared to the Canadian, diagnostic laboratory; however, the prevalence of coccidia-positive samples was similar. The methodology used in this report cannot be used to determine the actual prevalence of enteric coccidial infection (coccidiasis) or disease (coccidiosis) within the domestic ferret population. Fecal samples may be submitted to laboratories either as a result of investigation into enteric disease, or as part of a routine health examination. Thus, without historic information accompanying each sample one can simply identify the proportion of positive samples and compare the frequency of the finding of different coccidial species. Prospective surveys of fecal samples from healthy and sick domestic ferrets, with greater sample size, would be necessary to determine the true prevalence of these parasites within the population and to infer their clinical significance. Comparatively few mitochondrial COI sequences have been generated for apicomplexan parasites compared with other genetic loci; the majority of published sequences obtained from Apicomplexa are from nu 18S. The disadvantage of using nu 18S rdna sequences for parasite identification is that they are poor at distinguishing among closely related eimeriid coccidia due to the highly conserved nature of the nuclear ribosomal RNA locus. In contrast, mt COI sequences appear to be more useful for distinguishing closely related coccidian species (Ogedengbe et al., 2011) but are less useful than nu 18S rdna sequences for inferring more ancient relationships among more distantly related coccidia. Consequently, the combined use of nu 18S rdna and mt COI sequencing has been recommended for improved species description and phylogenetic analysis (El-Sherry et al. 2013). For these reasons, both nu 18S and mt COI sequences were analysed in the present study. Despite adequate quantities of DNA extracted from the Ontario laboratory sample ( ), successful amplification did not result with any primer pair (Table 2.1). Potential reasons for this include degradation of formalin-fixed DNA into fragments too small for amplification with the desired primers, perhaps as a result of extended length of time in formalin prior to paraffin embedding or length of time stored as FFPE tissue (23 years), or insufficient parasite DNA within the paraffin scrolls. The primer pairs 51
68 used appear to be useful for most eimeriid coccidia (Ogedengbe 2015), and successfully amplified both Eimeria species from DNA isolated from oocysts, so it is unlikely that failure to amplify DNA from this sample resulted from an inability of the primers used to recognize the parasite seen on section. Two genotypes of E. furonis were identified in this study. Genotype 1, was identified only from samples originating from domestic ferrets in Europe, but exhibited 100% identity based on nu 18S sequencing with previously published sequences from both Japan and the USA. Genotype 2 was identified from samples originating from domestic ferrets in both Canada and Europe. The small number of single nucleotide differences between the two genotypes at two genetic loci in different genomes are consistent with intraspecific variation (i.e. strain variation). As might have been expected because of their morphological and host similarities, nu 18S and mt COI sequences of E. furonis were determined to be most similar to an Eimeria species (E. ictidea) previously isolated from black-footed ferrets (Mustela nigripes); these eimeriid coccidia formed a monophyletic group that was distinct from other eimeriid coccidia infecting mammals in the phylogenetic analyses based on combined nu 18S rdna and mt COI sequences. Similarly, the nu 18S rdna and mt COI sequences of I. (=C.) laidlawi are most similar to sequences from two other Cystoisospora species of carnivores (C. canis and C. felis) that both have large, egg-shaped oocysts comparable to those of I. (=C.) laidlawi. Both morphometrics and genotyping support the close relationships among these three sarcocystid coccidia of carnivores. These molecular data confirm that transfer of Isospora laidlawi to the genus Cystoisospora by Barta et al. (2005) is warranted. The previous light microscopic identifications of coccidial species in 3 of the 11 fecal samples were not in agreement with the molecular findings. These results were not surprising because light microscopy has been shown to be an insensitive tool for distinguishing among apicomplexan parasites at both the genus and species level. Furthermore, re-evaluation of these samples by the authors revealed that many of the samples that were identified incorrectly based on morphometrics contained primarily 52
69 unsporulated oocysts, making accurate identification based on microscopic appearance highly challenging. These findings further underscore the importance of molecular methods in accurate parasite identification. In the absence of molecular tools, accurate measurement of oocyst size, shape, and determination of SI can be useful for differentiating among species of Eimeria and Cystoisospora; however, this can only be performed accurately on sporulated oocysts from feces. Interestingly, the size and shape indices of oocysts of E. furonis measured in histologic sections did not match those previously described by Hoare (1927) for the same oocysts in feces, despite molecular confirmation of identity. Thus, measurements of oocysts in histologic sections are not recommended for use in coccidial identification. Our observations highlight the utility of molecular methods for identifying enteric coccidia infecting domestic ferrets and suggest that diagnoses based on morphological methods should perhaps be limited to broad determinations of disease etiology (i.e., coccidiosis or coccidiasis ). Using molecular techniques we were able to differentiate morphologically similar coccidial species isolated from the feces of domestic ferrets and specifically identify parasites seen in histological sections of ferret intestine. Molecular techniques thus appear to be essential for determining the coccidial species responsible for individual and group outbreaks of coccidiosis and for further understanding of eimeriid host-parasite relationships. ACKNOWLEDGEMENTS: Many thanks to Julia Whale and Alex Leveille for their assistance and encouragement during the course of this project. The authors would like to recognize the contributions of Dr. Donald Martin (IDEXX Canada), and Drs. Nikola Pantchev and Majda Globokar (IDEXX Germany) for the contributions of data and samples to this project. The authors would also like to recognize the Laboratoire de Pathologie (Service de diagnostic, Faculté de médecine vétérinaire, St. Hyacinthe, Quebec) and the Animal Health Laboratory (Guelph, Canada) for contributions of samples and data to this project. Finally, this project was made possible through funding by the Toronto Zoo Residency Research Fund to DAS/AP and partial 53
70 funding from a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (400566) to JRB. 54
71 Table 2.1 Amplification primers for nuclear 18S rdna and mitochondrial COI loci, anneal temperatures (Ta), extension times and expected PCR product sizes used in the identification of enteric coccidia from domestic ferrets (Mustela putorious furo) Gene Target Primer Pairs Primer Sequence (5ʹ-3ʹ) Size (bp) Ta ( C) Anneal (sec) Reference nu 18S rdna CYC1FE TACCCAATGAAAACAGTTT Matsubayashi et al. (2005) CYC4RB CGTCTTCAAACCCCCTACTG Matsubayashi et al. (2005) Cocci_18S_595F CCGCGGTAATTCCAGCTCCAAT Present study Cocci_18S_847R GCTGMAGTATTCAGGGCGACAA Present study Lank_18S_224F TCATAGTAACCGAACGGATC Ogedengbe (2015) Api_SSU_2733R CGGAATTAACCAGACAAATC Mathew et al. (2000) mt COI COI_10F GGWDSWGGWRYWGGWTGGAC Ogedengbe et al. (2011) COI_500R CATRTGRTGDGCCCAWAC Ogedengbe et al. (2011) COI 272F CAATTCTAYGATGCCGCWTT Present study COI_500R CATRTGRTGDGCCCAWAC Ogedengbe et al. (2011) Sdae-COI_260F GATCTTTATGTTYTTRATGCC Ogedengbe (2015) Sdae-COI_1147R CATTACCCATAACYACACC Ogedengbe (2015) 55
72 Table 2.2 Summary of fecal samples from domestic ferrets (Mustela putorius furo) submitted to two diagnostic laboratories from No. fecal samples positive for coccidia/ No. samples submitted (percentage positive) No. samples positive for Cystoisospora sp. No. samples positive for Eimeria furonis No. samples positive for Eimeria ictidea Year Canada Europe Canada Europe Canada Europe Canada Europe /140 (2.1) 6/214 (2.8) /160 (1.2) 14/214 (6.5) /127 (6.3) 20/213 (9.4) /114 (0) 17/215 (7.9) /108 (2.8) 10/231 (4.3) /81 (2.5) 16/270 (5.9) /127 (4.7) 12/234 (5.1) /108 (3.7) 9/249 (3.6) Total 28 (2.9) 104 (5.6) Average/ year Legend: Numbers in brackets refer to the percent of the total number of fecal samples submitted. 56
73 Table 2.3 Morphologic and molecular identification of coccidia from domestic ferrets (Mustela putorius furo) Sample ID Source External Lab Morphologic Diagnosis Morphologic Diagnosis (ARP) Molecular Diagnosis mt COI GenBank Accession nu 18S rdna GenBank Accession FFPE enteric coccidia Histologic sample P2010-I FFPE enteric coccidia Histologic sample E. furonis Identical to MF Identical to MF FFPE NP Histologic sample E. furonis Same as MF Same as MF feces NP E. furonis * E. furonis MF MF feces E. furonis E. furonis E. furonis MF MF feces I. (=C.) laidlawi no oocysts visualized I. (=C.) laidlawi MF MF A feces E. ictidea Cystoisospora sp. I. (=C.) laidlawi Identical to MF Identical to MF feces E. furonis no oocysts visualized E. furonis Identical to MF Identical to MF feces E. furonis E. furonis E. furonis Identical to MF Identical to MF feces E. furonis E. furonis E. furonis MF MF feces I. (=C.) laidlawi Cystoisospora sp. I. (=C.) laidlawi Same as MF Same as MF feces I. (=C.) laidlawi no oocysts visualized I. (=C.) laidlawi Same as MF Same as MF feces I. (=C.) laidlawi Cystoisospora sp. I. (=C.) laidlawi Same as MF Same as MF CAN feces C. canis + E. furonis Cystoisospora sp. I. (=C.) laidlawi MF MF Legend: FFPE = formalin fixed paraffin embedded intestinal sections; - = unsuccessful; * = morphologic diagnosis performed by JRB; same as = 100% sequence identity with listed GenBank entry over entire sequence length; identical to = 100% sequence identity, but shorter sequence, than listed GenBank entry 57
74 25 µm Figure 2.1 Life stages of Eimeria furonis within the small intestinal epithelium of a domestic ferret (Mustela putorius furo). Asexual life stages: merozoites (black circle). Sexual life stages: oocyst (solid black arrow), macrogamonts (open arrows with labels), microgamont (dotted black arrow). Hematoxylin and eosin staining; scale bar = 25μm. 58
75 Figure 2.2 Phylogenetic relationships of coccidia (Eimeria ictidea, Eimeria furonis and Isospora (=Cystoisospora) laidlawi) from domestic (Mustela putorius furo) or black-footed (Mustela nigripes) ferrets based on partial nuclear 18S rdna and mitochondrial COI sequences of these parasites and related apicomplexan parasites. A summary of the sources of the molecular data for the remaining taxa included in this phylogenetic analysis are found in Supplementary Table 1 of Ogedengbe et al. (2017). Bayesian support is indicated for each node; horizontal distance is proportional to hypothesized evolutionary change (scale indicates sequence divergence of 10%). 59
76 CHAPTER 3: MORPHOLOGICAL AND MOLECULAR CHARACTERIZATION OF ENTERIC COCCIDIA ISOLATED FROM BLACK-FOOTED FERRETS (MUSTELA NIGRIPES) ABSTRACT Black-footed ferrets (BFF, Mustela nigripes) are the only ferret species native to North America, and have been identified as endangered since Starting in 1986, a multi-institutional effort has been breeding this species in captivity with successful reintroductions back into the wild. Coccidiosis is recognized as a cause of significant juvenile morbidity and mortality in captive breeding programs, and can result in significant population losses. Little is known about the etiology of enteric coccidiosis in BFF. Coccidia positive fecal samples (n=12) and formalin fixed paraffin embedded intestinal tissues (n=11) were obtained from BFF in the Toronto Zoo and Louisville Zoo Species Survival Plan (SSP) populations. Oocyst morphometrics and sequence genotyping at three loci (nuclear 18S rdna, mitochondrial cytochrome c oxidase subunit I and mitochondrial cytochrome c oxidase subunit III) were conducted. Results suggest that the same Eimeria species, E. ictidea, was the cause of enteric coccidiosis in both SSP populations, in both juvenile and adult age classes. Wider research is indicated to determine whether these findings are representative of the larger captive and wild BFF populations. 3.1 INTRODUCTION: Black-footed ferrets (BFF) are one of only three wild ferret species worldwide; the other two being the European polecat (Mustela putorius) and the Siberian polecat or steppe polecat (Mustela eversmanii). The BFF, the only native North American ferret species, was formerly distributed throughout the North America prairie ecosystem, but were considered extinct by the late 1950s. In 1964 a single population was discovered in Mellette County, South Dakota. Progressive decline of this population in subsequent years resulted in the decision by United States Fish and Wildlife Service to initiate a captive breeding program for the species. From four females and five males were captured for this 60
77 purpose. Despite successful breeding, no kits survived and the last adult ferret in this captive colony died in 1979; BFF were again presumed extinct in the wild based on annual surveys of the initial capture site. In 1981, a dead BFF was discovered by a ranch dog outside of Meeteetse, Wyoming, allowing wildlife biologists to identify another colony of BFF. This colony flourished until 1985 when an outbreak of canine distemper in this wild BFF population and an outbreak of sylvatic plague in the local prairie dog population resulted in sharp population declines. From 1985 through 1987, all 24 of the remaining BFF were trapped and brought into captivity to re-initiate the captive breeding program. Six ferrets in this initial group died of canine distemper while in captivity, and seven of the remaining eighteen survivors are the founding population of the current captive breeding population. Today this captive breeding population consists of approximately 300 BFF distributed among multiple institutions (Santymire et al. 2014). Since 1986, a multi-institutional effort has been breeding BFF in captivity with reintroductions back into the wild in selected locations in Canada, the USA and Mexico. Currently, six facilities participate in the BFF Species Survival Plan (SSP): the Toronto Zoo, United States Fish and Wildlife Service's National Black-footed Ferret Conservation Center, National Zoo s Smithsonian Conservation Biology Institute, Louisville Zoo, Cheyenne Mountain Zoo and the Phoenix Zoo (Black-footed Ferret Recovery Implementation Team 2011). In order to provide the best genetic matches, BFF are transferred among the six institutions for breeding. Approximately kits are produced annually between the six SSP facilities, with ~200 of these kits allocated for release to the wild yearly (Santymire et al. 2014). As of 2011, over 8,000 BFF kits had been produced in captive breeding facilities (Black-footed Ferret Recovery Implementation Team 2011). Multiple infectious diseases pose a significant risk to the captive breeding and post-release survival of BFF, including canine distemper and sylvatic plague (Santymire et al. 2014; USFWS BFF Recovery Program 2017). Coccidiosis is recognized as a cause of significant juvenile morbidity and mortality in captive breeding programs, and can result in significant population losses (Bronson et al. 61
78 2007; Santymire et al. 2014; USFWS BFF Recovery Program 2017). While the effects of the disease on the wild population are not clear, a prevalence of approximately 13% has been reported based on fecal samples collected from wild BFF born at release sites. (Dr. R. Santymire, personal commication) Coccidia are eukaryotic, host-specific parasites of the phylum Apicomplexa, affecting numerous hosts within a wide taxonomic range. Two species of coccidia, Eimeria ictidea Hoare 1927 and Eimeria furonis Hoare 1927, have been identified in black-footed ferrets based on morphometrics (Jolley et al. 1994). Jolley et al. examined fecal samples from six captive BFF during a distemper outbreak as well as samples from wild BFF. They described one medium-sized ovoid, eimeriid oocyst with a double wall, presence of a polar body and lacking both an oocyst residual body and micropyle. Oocysts of this Eimeria species (sp.) measured µm (range ), with a shape index (SI) of The sporocysts were elongate with the presence of both sporocyst residuum and a Stieda body. Sporozoites contained prominent refractile bodies at the posterior end and were aligned anterior to posterior within sporocysts. These oocysts, shed by all six captive ferrets, were considered consistent with Eimeria ictidea based on descriptions by Hoare (1927). On histopathologic examination of intestinal sections, parasites undergoing merogony and gamogony were observed within the villar epithelium throughout the small intestine, but were concentrated in the jejunum (Hoare 1935b); parasite life stages were not described from other tissues/organs A second small spherical to subspherical, eimeriid oocyst was also documented in the captive ferrets by Jolley et al. (1994); this second species had a pink double oocyst wall, a granular residual body, and lacked both oocyst polar body and micropyle. This smaller species measured µm (range ) with a SI of The sporocysts were elongate and possessed a Stieda body, and sporozoites contained refractile bodies. Similar to the larger Eimeria sp. described above, merogonic and gamogonic stages were observed within the villar epithelium throughout the small intestine, but were most numerous in the jejunum. Jolley et al. (1994) concluded these small spherical oocysts were consistent with E. furonis described by Hoare (1927) from domestic ferrets. 62
79 Jolley et al. (1994) described a third type of coccidial oocyst occasionally detected in small numbers within BFF fecal samples; however, the authors did not state whether this third oocyst morphotype was recovered from wild or captive animals. The oocysts measured µm (range ), with a SI of Attempts to sporulate collected oocysts were unsuccessful and corresponding endogenous stages were not identified on histopathologic examination of necropsied ferrets, precluding further morphologic identification of the parasite. It should be noted that coccidial oocysts with similar measurements had not been detected in wild or captive prey species available for ingestion by BFF, making it unlikely that this coccidial species would have been a pseudoparasite (Jolley et al. 1994). Previous to this report by Jolley, coccidial oocysts had been isolated from the feces of BFF in two captive populations (Carpenter & Hillman, 1979; Williams et al., 1988). The abstract by Carpenter & Hillman (1979) did not describe the oocysts, whereas Williams et al. (1988) stated that two Eimeria sp. (one with larger oocysts and one with smaller oocysts) were observed within the fecal samples, but they were not identified further. Interestingly, Williams et al. reported both Eimeria sp. to be shed in the feces of all ferrets concurrently affected by distemper, and by approximately 30% of the clinically healthy ferrets at the time of investigation. Non-enteric coccidia have also been reported by two authors from captive BFF at one facility (Jolley et al., 1994; Williams et al., 1988). Both reports, which presumably described the same case(s), noted the presence of endogenous coccidial life stages in histologic sections of respiratory tissue and merozoites of an unidentified coccidium in impression smears of the urinary bladder from BFF diagnosed with canine distemper. Meronts were observed within the epithelium of the trachea, a large bronchus and associated bronchial glands. In the later report Jolley et al. (1994) described the lesions as occurring in the same ferret, whereas in the earlier report by Williams et al. (1988) they are described as occurring in two different ferrets. Paraffin blocks containing formalin fixed tissues from these cases have since been discarded, precluding further attempts at parasite identification with molecular methods. Subsequent to 63
80 these reports, further cases of systemic coccidiosis in BFF have neither been published, nor identified within the pathology database of the Toronto Zoo captive BFF population, nor by the current SSP pathologist (Dr. Michael M. Garner, personal communication). Previous characterization of coccidia from black-footed ferrets has been based on host species, affected tissues in the host, and morphometric characterization of life stages in histologic sections and oocyst characteristics using light microscopy. It is known that morphologically similar Eimeria species are not necessarily conspecific and may vary in host specificity and pathogenicity. Molecular characterization is thus required to accurately identify coccidia to the species level. No molecular characterization of coccidian parasites from black-footed ferrets has been performed to date. There is a significant information gap regarding which parasite species are implicated in morbidity and mortality events associated with enteric coccidiosis in BFF, and whether different coccidia are associated with this disease in adult versus juvenile age classes, or in different SSP institutions. Studies to further characterize the eimeriid coccidia of the BFF are warranted to improve the management of this disease in the captive population. The objectives of this research were to morphologically and molecularly characterize coccidia associated with enteric disease in BFF at the Toronto Zoo and in other SSP facilities. 3.2 MATERIALS AND METHODS: Fecal samples Twelve fecal samples were collected during the study period (from ) and preserved in potassium dichromate (2.5% w/v aqueous); seven from the Toronto Zoo and five from the Louisville Zoo. Centrifugal flotation with saturated salt solution (Ryley et al. 1976) was used to isolate and concentrate oocysts from fecal samples for light microscopic examination and genomic DNA extraction. One to two drops of the supernatant from the centrifugal flotation were placed directly on a slide and beneath a coverslip. The morphology and dimensions of sporulated oocysts were documented using a 64
81 Provis AX70 photomicroscope (Olympus Canada, Richmond Hill, ON, Canada) fitted with a digital imaging device (Infinity3-1C, Lumenera Corporation, Ottawa, ON, Canada) controlled using isolution Lite image analysis software (Hoskin Scientific, Burlington, ON, Canada) operated at a total magnification of Morphologic features noted for each oocyst included: oocyst wall morphology; number of sporocysts; presence or absence of a micropyle, micropyle cap, residual body, and polar granules. For sporocysts: size, number of sporozoites per sporocyst, and presence or absence of Stieda body and sporocyst residuum were noted. Alignment of sporozoites within the sporocyst and presence/absence of refractile bodies within sporozoites were also described. The sporulated oocyst length and width measurements (in µm) were then used to calculate the SI for each measured oocyst. Morphologic and morphometric features were compared to previously published values for E. furonis and E. ictidea from domestic and black-footed ferrets. Genomic DNA extraction and purification were performed using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer s instructions, as described in Chapter 2 (Materials & Methods) Formalin fixed intestinal tissues The pathology records of the Toronto Zoo were searched from for cases of BFF diagnosed with enteric coccidiosis on histopathology. For each case, slides of histologic sections from all submitted intestinal segments were reviewed to confirm the presence of sexual and/or asexual life stages within the intestinal epithelium. Scrolls (5-6 µm) were cut from the paraffin blocks containing affected intestinal sections, and DNA extracted from the formalin fixed paraffin-embedded tissue (FFPE), using the QIAamp DNA FFPE Tissue Kit (Qiagen, Toronto, Ontario), as per manufacturer s instructions. 65
82 3.2.3 Molecular characterization Molecular characterization of coccidial isolates was performed on oocysts purified from fresh fecal samples (isolated as described above) that were collected from juvenile and adult ferrets from , and DNA extracted from FFPE samples of BFF intestine containing parasite life stages. Regions from the nuclear 18S (SSU) rdna (nu 18S rdna), mitochondrial cytochrome c oxidase subunit I (mt COI) DNA and mitochondrial cytochrome c oxidase subunit III (mt COIII) DNA were amplified by polymerase chain reaction (PCR) from each sample using the primers listed in Table 3.1 and methodology described in the Materials & Methods section of Chapter 2. Table 3.1 also contains the specific anneal conditions used for the various primer pairs. Genomic DNA from an Eimeria species of poultry was included in the PCR reactions to act as a positive control for the reaction chemistry. A representative selection of the newly generated nucleotide sequences resulting from the above were submitted to GenBank. DNA obtained from oocysts collected from fecal samples during the first year of the study (2014) was used to generate a complete mitochondrial genome (see Chapter 6 for details) using primer pairs and sequencing primers summarized in Table 3.1. All subsequent samples collected in 2015 and 2016 had shorter mt COI and mt COIII sequences obtained to permit genotyping of all collected oocysts at these two loci. The location of each primer in the nu 18S, mt COI and mt COIII genetic locus is illustrated in Figure RESULTS: From coccidia-positive fecal samples were obtained from twelve BFF ferrets/ferret groups from the Toronto Zoo and Louisville Zoo SSP populations (see Table 3.2). Nine samples were from single housed adults between the ages of 1-5 years (6:3 Male/Female). Two samples were from mixed groups; one pooled fecal sample from four adults (FERA-1; 1:3 M/F) and one fecal sample from a family 66
83 group consisting of a dam and five kits (2:3 M/F). One fecal sample was collected from a juvenile male ferret at the time of necropsy. Eleven BFF with enteric coccidiosis were identified in the Toronto Zoo necropsy reports from , and all were confirmed by histological re-evaluation (Table 3.2). Both juvenile (n=9; 3:6 M/F) and adult ferrets (n=2; 2:0 M/F) were represented Morphometric characterization Twelve coccidia-positive fecal samples were identified from adult and juvenile BFF from by on site laboratories at either the Toronto Zoo or the Louisville Zoo. Fecal flotation and light microscopic re-examination of the samples, identified coccidial oocysts in 10 of these 12 samples. Morphometric characterization was performed on six samples in which there was adequate quantity and quality of sporulated oocysts for examination. These included three samples from singlehoused adults, one from a juvenile at the time of necropsy, one of pooled feces from a group of adult ferrets, and one of pooled feces from a family group (dam and kits). Two of the three samples from single-housed adults were from the same ferret on different dates in 2016; the dates of collection were separated by a period in which shedding of oocysts was not identified on routine, repeated fecal examinations. Oocysts were elliptical with a colourless double wall, and contained four sporocysts, each with two sporozoites. Sporocysts were ovoid and both Stieda body and residual body were present. Sporozoites exhibited an anterior to posterior alignment within the sporocysts, and refractile bodies were identified (Figure 3.2). Results for length, width and shape index of sporulated oocysts, including range and average values, are summarized in Table 3.3 and Figure 3.2. The average measurements, based on the results of all 148 oocysts measured, were: length µm ( ), width µm ( ), and shape index 1.30 ( ). The same measurements were performed on 59 sporocysts from a single ferret (Noodle), and results are as follows: average length µm ( ), average width 7.38 µm ( ), and average SI 1.76 ( ). In one sample (Mohawk-2), sporozoites were visible free on the slide. 67
84 Measurement of three of these provided an average length of µm ( ) and an average width of 3.41 µm ( ) Molecular characterization Molecular characterization was successfully performed on oocysts from seven of 10 fecal samples containing coccidial oocysts, and FFPE tissue from nine of the 11 necropsy cases (see Table 3.2). Attempts at amplification of DNA extracted from necropsy samples Z and Z137-14, using the primer pairs listed in Table 3.1, were unsuccessful. Similarly, attempts at PCR and sequencing of DNA extracted from fecal oocysts from two Toronto Zoo BFF, Jenna and Ruckus, were unsuccessful. Molecular identification results for the remaining samples are summarized in Table 3.2. Only one Eimeria species, E. ictidea, was identified in all enteric coccidiosis cases diagnosed at necropsy in both juvenile and adult BFF at the Toronto Zoo from This same species was identified in all Toronto and Louisville Zoo BFF fecal samples that were sequenced successfully (n=8), with the exception of a single case from Louisville. This Louisville ferret was identified as having a rodent pseudoparasite (Eimeria species) in the submitted fecal sample; the eimeriid pseudoparasite had 98.6% sequence identity at the mt COI locus to the murine coccidium Eimeria falciformis. All sequences generated for E. ictidea exhibited 100% sequence identity at the mt COI and COIII loci. Novel nu 18S rdna, mt COI and mt COIII sequences were generated for E. ictidea from both geographic locations and deposited in GenBank (Accessions MF860826, MF860827, MF860823, MF860825, MF860822, MF860824). Sequences were compared to those previously published for related eimeriid coccidia. The nu 18S rdna sequence from Eimeria ictidea isolated from the Toronto Zoo BFF had 97.36% identity (14 single nucleotide differences) to the previously published sequences from isolates of E. furonis from domestic ferrets (Mustela putorius furo) in Japan (GenBank AB and AB329724) and newly generated sequences from Canadian and European isolates (GenBank MF MF774680, see Chapter 2 and Figure 3.3). In contrast, nu 18S rdna sequence of E. furonis from domestic ferrets (see Chapter 2) showed 99.53% to 100% identity (0 to 3 SND) to the Japanese 68
85 sequences. Comparison of newly generated partial sequences of the mt COI region from E. ictidea from BFF to isolates of E. furonis (GenBank MF MF774036) from DF and E. mephitidis (GenBank KT203398) from the striped skunk (Mephitis mephitis), the only carnivore Eimeria sp. for which a mt COI sequence was previously available, reveals only 94.15% and 90.84% sequence identity, respectively, with these other Eimeria spp. of carnivores (Figure 3.4). 3.4 DISCUSSION: This work presents the first nu 18S rdna, mt COI and mt COIII sequences (nu 18S rdna - MF860826, MF860827; mt COI - MF860823, MF860825; mt COIII - MF860822, MF860824) generated from an intestinal eimeriid parasite of the BFF, referred to here as E. ictidea, collected from multiple BFF of different ages from two separate captive populations (Toronto Zoo, Toronto, Ontario, Canada and Louisville Zoo, Louisville, Kentucky, USA). The morphometric description of coccidial oocysts from BFF in this work are consistent with previous descriptions of E. ictidea from mustelids, including BFF, the Steppe polecat, the European polecat and domestic ferrets (Hoare 1927; Svanbaev 1956; Jolley et al. 1994). Thus, I propose the name E. ictidea for the enteric coccidium described from BFF, reflecting the similarity in morphology, host species and location of infection in intestinal tissues, yet recognizing the absence of species identification by molecular techniques. Molecular characterization of parasites that agree with the description of E. ictidea morphologically from various mustelid host species would allow not only for determination of whether the parasites are conspecific, but would also provide insight into the potential for crosstransmission among related mustelid hosts. DNA extraction from FFPE samples allowed successful PCR and sequencing of small DNA fragments (220 bp) in nine of the eleven cases in which the technique was attempted. Age of the samples did not appear to be the major factor associated with successful extraction of good quality DNA; the two samples for which it was unsuccessful were the most recent (2014) and oldest (1998) cases.consequently, it may be possible to use banked FFPE tissues from historic necropsy cases from other SSP institutions 69
86 and necropsies of wild-born or re-introduced ferrets to determine the identity of the coccidial species underlying disease in these cases and to better characterize the disease in the greater BFF captive and wild populations. Williams et al. (1988) were contacted regarding their historic FFPE samples, but formalin blocks were no longer available for these cases and thus comparisons could not be made. Banked FFPE samples were requested from other SSP institutions, however, the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) restrictions on the international transport of DNA from endangered species did not allow for sample acquisition during the period in which this research was conducted. Evaluation of FFPE samples from the Toronto Zoo indicate that the same Eimeria species has been implicated in deaths associated with enteric coccidiosis from , as well as episodes of clinical disease in ferrets in the Toronto Zoo population from Samples from coccidia-positive BFF at the Louisville Zoo in 2016 also contained the same Eimeria species. Finding the same parasite at multiple SSP locations was expected because BFF are transferred among institutions on a yearly basis for breeding and potential release. Consequently, these parasites have repeated opportunities to move between institutions in infected hosts or on contaminated cage materials to become established at a new location. Furthermore, the stress of transport and transfer to a new environment may precipitate shedding of endemic coccidia and increase the risk of a coccidial outbreak; this concern is reflected in the SSP recommendations for prophylactic treatment of all BFF with anti-coccidial medication prior to shipment (USFWS BFF Recovery Program 2017). A single BFF from the Louisville SSP population, not showing clinical signs consistent with coccidiosis, was identified as having a rodent Eimeria species in the submitted fecal sample. Morphometric characterization of oocysts in this sample was not performed due to the paucity of visible oocysts; however, examination at 100 suggested that the oocysts in the sample were ovoid in shape and of comparable size to oocysts identified in other BFF samples. The finding of a rodent Eimeria in a BFF fecal sample is not unexpected as whole rodents comprise a significant part of the captive BFF diet. The oocysts shed by the BFF were most likely acquired through ingestion of an infected prey item and thus 70
87 most likely represent pseudoparasitism. Molecular characterization was, however, required to differentiate this from a case of true enteric coccidiasis. Reports from the first captive BFF population, derived from South Dakota, indicate the presence of an unidentified species of enteric coccidium (Carpenter and Hillman 1979) in this group before its demise in No reports containing morphometric descriptions of the coccidia from this group were found on literature review, and all parasites of this group have been lost with their hosts. All subsequent reports on enteric coccidiosis in BFF are from ferrets derived from the second founder group from Wyoming in the 1980s. The frequent transfer of ferrets among SSP institutions within the captive breeding program and to different release sites within North America would be expected to result in the same Eimeria species being found in all populations. The exception to this would be the potential for cross-transfer of other eimeriid parasites to wild BFF from sympatric mustelid species, such as the longtailed weasel (Mustela frenata). Jolley et al. described two other species of enteric coccidia from this second captive population in 1994; the first was similar to E. furonis of domestic ferrets, and the second a large coccidian parasite of unknown genus. Neither of these parasites was identified in the Toronto and Louisville Zoo populations during the course of this study. In order to determine whether these parasites persist within the present-day BFF populations and their impact on this species, more detailed examination of coccidia-positive fecal samples from captive and wild BFF populations is recommended. Furthermore, the molecular identification of enteric coccidia from historic and future necropsy samples of wild and captive BFF could aid in determining the presence of, and contribution to, mortality events by these additional coccidia species. ACKNOWLEDGEMENTS: The authors would like to recognize the Wildlife Health Centre staff at the Toronto Zoo, for their assistance with the collection of fecal samples from the BFF from The authors would also like to recognize the Louisville Zoo, for their contribution of samples to this project. Finally, this project was made possible through funding by the Toronto Zoo Residency Research Fund to DAS/AP and partial 71
88 funding from a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (400566) to JRB. 72
89 Table 3.1 Amplification primers used to sequence the nuclear 18S rdna, mitochondrial COI and COIII loci of Eimeria ictidea originating from fecal and formalin-fixed paraffin embedded tissue samples from black-footed ferrets (Mustela nigripes), including anneal temeratures (Ta), extension times and expected PCR product sizes Gene Target Primer Pairs Primer Sequence (5ʹ-3ʹ) Size (bp) Ta ( C) Anneal (sec) Reference 18SrDNA Sarco_18S_123F TATCAGCTTTCGACGGTAGTGTATT Ogedengbe et al. (2016) ERIB10_REV CTTCCGCAGGTTCACCTACGG mt COI T_Eim_COI_272F CAATTCTAYGATGCCGCWTT Chapter 2 (Table 2.1) COX1-500R CATRTGRTGDGCCCAWAC Ogedengbe et al. (2011) COI-400F GGDTCAGGTRTTGGTTGGAC El-Sherry et al. (2013) COI-1202R CAAKRAYHGCACCAAGAGATA El-Sherry et al. (2013) mt COIII WG-MT_4140F AGAAAACCTAAAATCATCATGT Ogedengbe et al. (2015) Eimeriid_CO3_799R AAGTGAGTTCGCATGTTTAC Ogedengbe et al. (2015) Figure 3.1 Nuclear and mitochondrial genetic loci targeted by primers listed in Table 3.1 and used to characterize Eimeria ictidea originating from black-footed ferrets (Mustela nigripes) 73
90 10 µm 5 µm Figure 3.2 A+B) Features and cellular contents of Eimeria ictidea from a black-footed ferret (Mustela nigripes). Legend: Oocyst: dotted thin white arrow = polar granule. Sporocyst: dotted thin black arrow = Stieda body; thick white arrow= sporozoite refractile body; thick black arrow= residuum; scale bars as indicated. C) Shape index, length and width measurements of sporulated oocysts of Eimeria ictidea from black-footed ferrets (Mustela nigripes). Legend: indicates the mean. Dotted oval indicates one standard deviation around the mean. 74
91 Identity Eimeria ictidea MF (Guanella LZ) Eimeria ictidea MF (Mystery MTZ) Eimeria furonis MF (Type 1) Eimeria furonis AB (Type 1) Eimeria furonis AB (Type 1) Eimeria furonis MF (Type 2) Eimeria furonis MF (Type 2) Figure 3.3 Comparison of nuclear 18S rdna sequence alignment of Eimeria ictidea from two black-footed ferrets (Mustela nigripes) to newly generated (see Chapter 2) and published sequences of Eimeria furonis from domestic ferrets (Mustela putorius furo). Identity Eimeria mephitidis KT Eimeria ictidea MF (Guanella LZ) Eimeria ictidea MF (Mystery MTZ) Eimeria furonis MF (Type 1) Eimeria furonis MF (Type 2) Figure 3.4 Comparison of mitochondrial cytochrome c oxidase subunit I sequence alignment of Eimeria ictidea from two black-footed ferrets (Mustela nigripes) to sequences from other eimeriid parasites of carnivores. 75
92 Table 3.2 Morphologic and molecular characterization of coccidia from fecal and FFPE necropsy samples from black-footed ferrets (Mustela nigripes) Sample ID Sample Source Year Age (years) Sex Source Microscopic Description^ Molecular Diagnosis* Z Toronto Zoo M FFPE rare asexual stages - Z Toronto Zoo M FFPE sexual and asexual stages E. ictidea Z Toronto Zoo F FFPE rare asexual stages E. ictidea Z Toronto Zoo F FFPE sexual and asexual stages E. ictidea Z Toronto Zoo F FFPE sexual and asexual stages E. ictidea Z Toronto Zoo F FFPE sexual and asexual stages E. ictidea Z Toronto Zoo F FFPE sexual and asexual stages E. ictidea Z Toronto Zoo M FFPE sexual and asexual stages E. ictidea Z Toronto Zoo M FFPE sexual and asexual stages E. ictidea Z Toronto Zoo F FFPE sexual and asexual stages E. ictidea Z Toronto Zoo M FFPE fresh feces sexual and asexual stages POS, E. cf. ictidea - E. ictidea FERA_1 Toronto Zoo 2014 > 1 M/F fresh feces POS, E. cf. ictidea E. ictidea Noodle Toronto Zoo M fresh feces POS, E. cf. ictidea E. ictidea Ruckus Toronto Zoo F fresh feces POS - Mystery Toronto Zoo M fresh feces POS E. ictidea Mohawk Toronto Zoo M fresh feces POS, E. cf. ictidea E. ictidea Jenna Toronto Zoo F fresh feces POS - Thrope Louisville Zoo M fresh feces NEG - FloJean Louisville Zoo F fresh feces NEG - Rigatoni Louisville Zoo M fresh feces POS rodent Eimeria Guanella +kits Louisville Zoo ; 0.15 F; kits 2M/3F fresh feces POS, E. cf. ictidea E. ictidea Clive Louisville Zoo M fresh feces POS E. ictidea Legend: FFPE= formalin fixed paraffin embedded. Sex M/F = samples from family groups containing both sexes. - = PCR and sequencing unsuccessful. ^ = life stages identified on histologic section. * = mitochondrial COI and/or COIII sequencing results. Presence (POS) or absence (NEG) of oocysts and morphological identification of oocysts based on measurements, when 76
93 Table 3.3 Morphometric (length, width, shape index) characterization of Eimeria ictidea oocysts from fecal samples from black-footed ferrets (Mustela nigripes) Sample ID FERA - 1 ^ Z Noodle Mohawk -1 Mohawk - 2 Guanella* Total Number of oocysts Length (µm) ( ) ( ) ( ) ( ) ( ) ( ) ( ) Width (µm) ( ) ( ) ( ) ( ) ( ) ( ) ( ) Shape index 1.35 ( ) 1.34 ( ) 1.27 ( ) 1.24 ( ) 1.39 ( ) 1.22 ( ) 1.30 ( ) Legend: ^= mixed adult group; * = dam and kit group 77
94 CHAPTER 4: NATURAL HISTORY OF ENTERIC COCCIDIOSIS IN THE BLACK- FOOTED FERRET (MUSTELA NIGRIPES) ABSTRACT Black-footed ferrets (BFF, Mustela nigripes), the only native North American ferret species, are endangered throughout their former geographic range. An intensive captive breeding program produces animals to supplement re-established wild populations. Coccidial enteritis is a major cause of morbidity in young, captive ferrets, but the disease also affects adults. Limited information is available on the pathogenesis of intestinal coccidiosis in captive BFF, and characterization of the natural history of the disease for improved prevention and management is imperative. The objectives of this research were to determine morbidity and mortality rates in the Toronto Zoo captive BFF population, as well as characterizing the natural history of the disease in this species through evaluation of shedding patterns, body tissues affected, pre-patent period, and periods of enhanced host susceptibility to infection. Coccidia-associated mortality in BFF at the Toronto Zoo from averaged 0.53% yearly in adults (range %) and 1.95% in juveniles (range %). Clinical signs and histologic lesions in Toronto Zoo BFF were similar to those described in previous publications. A seasonal influence on oocyst shedding was identified in adult BFF, and ferrets appeared to maintain persistent infection with E. ictidea, shedding coccidia in multiple years. A larger multi-institutional study is required to better elucidate the natural history of enteric coccidiosis in this species. 4.1 INTRODUCTION: Black-footed ferrets (BFF, Mustela nigripes) are the only native North American ferret species and are endangered throughout their former geographic range. When the last remaining truly wild population underwent serious decline as a result of disease, the decision was made by the by United States Fish and Wildlife Service to capture the remaining 24 animals and establish a captive breeding program; this occurred between 1985 and Only seven of the captured ferrets bred successfully, and are the 78
95 founders of the current North American BFF population (USFWS BFF Recovery Program 2017). The captive population, which now numbers approximately 300 individuals is distributed among and managed by six collaborating facilities; these include the Toronto Zoo, United States Fish and Wildlife Service's National Black-Footed Ferret Conservation Center, National Zoo s Smithsonian Conservation Biology Institute, Louisville Zoo, Cheyenne Mountain Zoo and the Phoenix Zoo (Black-footed Ferret Recovery Implementation Team 2011; Santymire et al. 2014). Since 1991, BFF have been released into reintroduced into the wild at multiple sites within their former range and over 8,000 BFF kits had been produced in captive breeding facilities as of 2011(Black-footed Ferret Recovery Implementation Team 2011). Twenty-eight BFF reintroduction sites currently exist throughout North America; however, there continues to be a need to support wild populations as only a four of the re-established groups are truly self-sustaining. Enteric coccidiosis is recognized as a cause of significant morbidity and mortality in captive breeding programs, affecting both juvenile and adult animals (Bronson et al. 2007; USFWS BFF Recovery Program 2017). Two Eimeria species, Eimeria ictidea and Eimeria furonis, have been identified from cases of entric coccidiosis in BFF (Jolley et al. 1994). Jolley et al. examined fecal samples from both wild and captive BFF, and provided detailed morphologic descriptions of the oocysts of both Eimeria spp., as well as descriptions of the intestinal pathology associated with infection. Asexual and sexual life stages of both of the aforementioned Eimeria spp. were identified on histologic section within the villar epithelium throughout the small intestine, but were concentrated in the jejunum. Intestinal sections from BFF infected with E. ictidea exhibited two morphologically distinct meronts, one at the villar tips, which was larger and lacking in undifferentiated mass, and the other at the base of the villi or, rarely, in the intestinal crypts; gamogony was predominantly observed at the villar tips, and was noted throughout the small intestine. 79
96 Extraintestinal coccidia have also been reported from captive BFF at one facility (Jolley et al., 1994; Williams et al., 1988). The authors identified the presence of endogenous coccidial life stages in histologic sections of respiratory tissue and in impression smears of the urinary bladder from BFF diagnosed with canine distemper. No subsequent reports of systemic coccidiosis in BFF have been published or identified within the pathology database of the Toronto Zoo captive BFF population or by the current SSP pathologist (Dr. Michael M. Garner, personal communication). Recent investigations into the etiologic agents of enteric coccidiosis in BFF at the Toronto Zoo have identified a single Eimeria species associated with all cases of enteric coccidiosis and associated mortality in juvenile and adult BFF from Furthermore, this pathogen was identified in fecal samples, based on morphologic and molecular characterization, from adult and juvenile BFF in another zoological collection (Louisville Zoo, Kentucky, USA) (see Chapter 3). This coccidium is morphologically consistent with Hoare s original description of E. ictidea (1927) and is referred to henceforth as Eimeria ictidea. There is a significant information gap regarding the pathogenicity of E. ictidea in BFF. The objectives of this research were to determine morbidity and mortality rates in the Toronto Zoo and additional captive BFF SSP populations, as well as characterizing the natural history of the disease in this species through evaluation of shedding patterns, body tissues affected, pre-patent period and periods of enhanced host susceptibility to infection. 4.2 MATERIALS AND METHODS: Toronto Zoo BFF breeding program At the Toronto Zoo black-footed ferret breeding program, all adult ferrets are housed individually, with the exception of dams and kits. After the birth of the kits, dams are housed with their offspring from whelp date until removal at approximately 4-6 months of age. Routine monthly fecal 80
97 examinations (direct examination and flotation) are performed in house for all ferrets in the breeding program, based on SSP recommendations, to evaluate for the presence of coccidia Fecal oocyst evaluation Family groups: From , daily fecal examination for coccidial oocysts was initiated for all group-housed dams and kits. In 2014, fecal samples were collected daily from all dams and kits from weaning (30 days after whelping) to 72 days post whelping. Based on 2014 data, in 2015 this surveillance was extended from weaning (35 days post whelping) to 135 days of age. Furthermore, fecal samples were collected from the dam for an additional 14 days after removal of kits. In 2016, no fecal samples were collected from dam and kit groups at the Toronto Zoo but samples were submitted from one group of dam and kits from another SSP population at the Louisville Zoo (Kentucky, USA). Adults: From daily fecal samples were also collected from all adult ferrets identified as shedding coccidial oocysts on their monthly routine fecal examination and from clinically ill BFF. Samples were collected for days after initial positive sample identification. In 2016, fecal samples were also submitted from four coccidia-positive adult ferrets from the Louisville Zoo population; samples were collected for 7 days post initial identification of shedding. Individual fecal samples were analyzed via flotation using the McMaster method, followed by routine flotation in saturated salt solution (Dryden et al. 2005), to determine the presence or absence of oocysts and oocyst burden (oocysts per gram of feces; OPG). Temporal trends in oocyst shedding were monitored. Coccidia-positive ferrets were evaluated visually on a daily basis for presence of clinical signs consistent with infection. Infected juvenile ferrets and adult ferrets were treated with oral ponazuril or toltrazuril regardless of the presence of clinical signs, as per the black-footed ferret SSP recommendations. Based on these recommendations, ponazuril is typically administered orally at
98 mg/kg once daily for 3-7 days until clinical signs have resolved or oocyst shedding has been significantly reduced (USFWS BFF Recovery Program 2017) Retrospective review of pathology records The pathology records of the Toronto Zoo were searched from for cases of BFF diagnosed with enteric coccidiosis on histopathology. For each case, gross necropsy reports were reviewed, and slides of histologic sections from all submitted intestinal segments re-examined to confirm the presence of sexual and/or asexual life stages within the intestinal epithelium and describe the histologic lesions associated with presence of the parasite life stages Prospective modified necropsy protocol During the study period, , necropsy protocols for all BFF were modified to improve detection of coccidial life stages and better to determine which portions of the intestinal tract were affected. The entire length of the intestine from duodenum to anus was measured, and intestinal contents were flushed with 12 ml of sterile saline into a sterile container. Intestinal contents were preserved in 2.5% potassium dichromate solution (mixed 1:1 with intestinal contents v/v) for molecular diagnostics. Paired, 2-cm long intestinal samples were collected from all sections of small and large bowel: duodenum (1), jejunum (6), ileum (1), and colon (2). The eight small intestinal samples were collected at equal distances from the pyloric sphincter to the beginning of the colon, and the distance from the pylorus noted for each. Colon samples were taken at 25% and 75% of the length of the colon. One sample from each pair was preserved in Serra solution (100% ethanol (60%, v/v); 37% formaldehyde (30%, v/v); glacial acetic acid (10%, v/v) and the second sample was frozen. Representative tissues from all internal organs, as well as additional intestinal samples, skin, muscle and brain, were also collected and preserved in 10% buffered formalin. Histopathologic examination was performed on all tissues collected. 82
99 4.2.5 Retrospective medical history review Medical histories of all BFF held by the Toronto Zoo since the initiation of the SSP program were reviewed for data on frequency of occurrence of shedding of coccidial oocysts in adults and juveniles, as well as any association of shedding with clinical signs, and administration of anticoccidial treatment. Data was tabulated yearly for adult and juvenile ferrets to determine annual morbidity and mortality rates associated with enteric coccidiosis. Medical records and pathology reports were solicited from the other SSP institutions to determine comparative morbidity and mortality rates associated with enteric coccidiosis in BFF at other facilities. Both morbidity and mortality rates were calculated as incidence/attack rates, with yearly adult population size or number of family groups (dam and kits) as the denominator for morbidity rates, and number of yearly deaths in each age class as the denominator for mortality rates. 4.3 RESULTS: Fecal oocyst evaluation and retrospective medical history review Family groups: Fecal samples were collected from seven groups of dams and kits housed together at the Toronto and the Louisville Zoos from All data from first to last day of collection for all family groups is listed in Appendix 1, selected pertinent data for each group is presented in Table 4.1. Five groups of dams and kits were sampled in 2014, and one group in each of 2015 and Shedding occurred no earlier than 55 days of kit-age in any of the groups, and was identified from days of age (Table 4.1, Table 4.2, Figure 4.1). In 2014, fecal oocyst shedding was identified in three of the five surveyed groups. In two of the three groups (dams Poppy and Bumblefoot), changes to fecal colour and consistency were identified concurrently with periods of oocyst shedding; both groups shed higher numbers of oocysts than the other dam and kit groups in 2014 and Both Poppy and Bumblefoot had had litters in the previous one and 83
100 two years prior to this study, respectively; based on medical record review these dams and their litters were also diagnosed as shedding coccidial oocysts that were too numerous to count on direct exam and fecal flotation. Clinical signs in the previous years included dark tarry, hemorrhagic or soft mucoid feces, and reduced appetite; both groups received treatment with toltrazuril (Baycox Coccidiocide Solution 2.5%; Bayer Inc., Mississauga, Canada) and trimethoprim sulfamethoxazole (Novo-Trimel; Teva Canada Ltd., Scarborough, Canada) (TMS). One of four kits from Poppy s 2013 litter (Z113-13) died of enteric coccidiosis three days after the group was diagnosed as shedding coccidial oocysts and the initiation of treatment with TMS. In 2015, low grade fecal oocyst shedding (<14 oocysts per gram of feces) without associated clinical signs was noted in the Fiddlesticks group on three days during a seven day period, from days of kit-age, and again for a single day at 128 days of kit-age. The dam had been diagnosed and treated for enteric coccidiosis in 2014, at which time she exhibited clinical signs of loose green feces to hemorrhagic diarrhea, lethargy and dehydration. In 2016 she was diagnosed as shedding low numbers of coccidia, exhibited no clinical signs and did not receive treatment prior to resolution of shedding. In 2016, Guanella and kits shed oocysts over a nine day period and daily fecal oocyst shedding ranged from ,714 OPG. Combined treatment with ponazuril (first four days of shedding), sulfadimethoxine injectable (first two days of shedding; additional product information not available), amoxicillin oral (first two days of shedding; additional product information not available), penicillin injectable (first two days of shedding; additional product information not available) and subcutaneous fluids (first two days of shedding; additional product information not available) was administered to this group. Previous medical history was not available for this female for review. In 2014 and 2015, fecal oocyst shedding in all groups in the Toronto Zoo population started in the three week period from the last week of July to mid-august. In 2016, shedding was first identified in the Louisville Zoo group in mid-july. 84
101 Adults: Seven single-housed adult BFF (5:2 M/F) were detected to have shed coccidia during the study period (Table 4.3). Shedding periods lasted from 2-10 days, and oocyst per gram counts ranged from ,274 (Table 4.4). Clinical signs were identified in four of the seven ferrets, and consisted of loss/reduction of appetite (n=2), weight loss (n=1), lethargy (n=1), blood in feces (n=1), loose or runny feces (n=3), soft mucoid feces (n=1), green colour of feces (n=2). Five of the seven adults received treatment after detection of oocyst shedding, two of which received treatment in the absence of clinical signs. Treatment consisted of oral toltrazuril in four cases, toltrazuril in combination with trimethoprim sulfamethoxazole in one case (Mohawk-A), and ponazuril and sulfadimethoxine (manufacturer s information not available) in one case (Clive) (Table 4.3). Three of the adults in this study, Mohawk, Mystery and Jenna, shed oocysts during multiple different periods in Mohawk shed oocysts in May, July and September of 2016; data from the first two periods are reported in Tables 4.3 and 4.4. Mystery shed oocysts in June and July of Clinical signs were observed only during the first shedding period and included poor appetite and hemorrhagic to soft mucoid feces. Jenna shed oocysts in July, September and November of 2016, and again in February and May of Although clinical signs were not detected in association with the initial period of shedding in July 2016 (see Table 4.4), depressed mentation and hemorrhagic mucoid feces were identified in the subsequent two shedding periods. In both Mohawk and Jenna, oocysts were not detected in feces on multiple recheck and routine monthly fecal examination between shedding periods. Ruckus, the fourth ferret, shed low numbers of oocysts for two days in 2016 while housed alone, and had been reported to have shed oocysts during a 30 day period in 2014 while housed in a family group with her kits. Diarrhea to soft mucoid feces and loss of appetite were reported in Clinical signs in 2016 consisted of small amounts of runny feces for a two day period, two days after oocyst shedding was no longer detected. Treatment consisted of toltrazuril and TMS in 2014, and toltrazuril again in Similarly, Noodle shed low numbers of oocysts in July 2015, and had been identified as shedding low numbers of oocysts during September of the previous year. 85
102 The final ferret, Rigatoni, shed oocysts in feces in low numbers ( OPG) over 5 days. All positive samples were pooled for molecular diagnostics, and sequencing results showed 99.0% identity (7 single nucleotide differences; SNDs) at the mt COI locus with a pair of rodent Eimeria species (i.e., GenBank HM771682, JQ993704) (see Chapter 3). This ferret did not show clinical signs associated with shedding and was not treated. Previous medical history was not available for Clive, the single adult ferret from the Louisville Zoo. Based on sample collection dates and medical record review for the adult BFF for , shedding occurred during spring, summer and fall with three ferrets shedding in May (Jenna, Ruckus and Mohawk), one ferret shedding in June (Mystery), three ferrets shedding in July (Mohawk, Jenna, Noodle), one ferret shedding in August (Clive), two ferrets shedding in September (Noodle, Jenna) and one ferret shedding in November (Jenna) Pathology Eleven BFF with enteric coccidiosis were identified in the Toronto Zoo necropsy reports from (Table 4.5). Cases were identified from inclusive, and all were confirmed by histological re-evaluation (Chapter 3, Table 3.2). Both juvenile (n=9; 3:6 M/F) and adult ferrets (n=2; 2:0 M/F) were represented. Gross Pathology: Gross necropsy findings were similar across the 11 cases and included: mucoid to fluid luminal contents (n=7; 63.6%), beige to white pasty coating of the mucosal surface of the small intestine (n=6; 54.5%) and colon (n=4; 36.4%), gaseous dilation of intestinal segments (n=3; 27.2%), segmental enteritis and hemorrhage (n=1; 9.1%). In one case, Z228-98, no gross lesions were identified within the intestines. Impression smears of luminal contents or scrapings of intestinal mucosa were performed in four cases and coccidia were identified in all four. Histopathology: 86
103 Both sexual and asexual life stages were identified within the small intestinal segments in all cases except Z and Z106-02, in which only rare asexual parasite life stages were identified (Table 4.5; Figure 4.2). Other histologic lesions seen in intestinal segments containing coccidia included lymphoplasmacytic inflammation of the lamina propria (n=4), neutrophilic infiltration of the lamina propria (n=2), villar necrosis (n=2), villar atrophy or blunting (n=3), and thrombi within the villar tips (n=1). Additional necropsy diagnoses included cholangiolar hyperplasia, multiple hepatobiliary cysts with suppuration, renal adenocarcinoma, apocrine gland adenocarcinoma (Z228-98); concurrent clostridial enteritis (Z143-99); presumptive Salmonella sp. septicemia (Z106-02); interstitial pneumonitis (Z108-03); myocardial mineralization, interstitial pneumonia and nephritis, periportal hepatitis and bacteremia (Z124-12); and suppurative esophagitis (Z137-14). From , three black-footed ferrets were necropsied using the detailed protocol described above. Only one of the three cases, Z137-14, was diagnosed with enteric coccidiosis based on histopathology. Two duodenal, five jejunal, two ileal and two colonic sections were collected at measured lengths from the pylorus. Parasite life stages were identified within the mucosal epithelium of all intestinal segments extending from the distal duodenum (10-12 cm aboral to pylorus) through to the distal colon ( cm aboral from pylorus). The distal duodenal section contained asexual life stages only, with a single focus of epithelial cells containing meronts. Sexual life stages (microgamonts, macrogamonts, unsporulated oocysts) were identified within villar epithelial cells in all remaining sections of the small intestine, with numerous oocysts in the bowel lumen. Mild lymphoplasmacytic inflammation of the lamina propria was associated with the jejunal and ileal lesions, and blunting of the villi was identified within one jejunal segment. The colonic sections contained small to moderate numbers of sexual life stages, identified within both superficial and deep crypt epithelium, with occasional life stages identified near the germinal cells. Large numbers of oocysts and bacteria were identified within the 87
104 lumen of these colonic sections, and both sections contained abscesses within the crypts. The proximal duodenum (0-2 cm) was the only section of the intestines not containing parasitic life stages Morbidity and mortality Annual morbidity rates for enteric coccidiosis at the Cheyenne Mountain Zoo, and mortality rates from enteric coccidiosis in Toronto Zoo BFF are summarized in Tables 4.6 and 4.7. During , yearly incidence of coccidiosis in adult BFF at the Cheyenne Mountain Zoo averaged 6.9% (range %). For family groups, consisting of juvenile ferrets housed with their dams, yearly incidence of enteric coccidiosis averaged 11.5% (range %). From , coccidia-associated mortality in adult BFF at the Toronto Zoo averaged 0.53% yearly (range %), with an average total mortality rate of 14.1% per year (range %). For juvenile ferrets (under 1 year of age) during the same period, coccidia-associated mortality accounted for an average of 13.3% of deaths yearly (range 0-100%), with an overall average mortality rate of 17.0% per year (range %) from all causes. Multiple additional SSP institutions provided partial medical and pathology data sets for use in this study, which were not sufficiently detailed to permit computation of morbidity and mortality rates for those populations. 4.4 DISCUSSION: The work described here supports previous clinical findings regarding the impact, through both morbidity and mortality, associated with enteric coccidiosis in BFF. No previous studies have been undertaken to determine morbidity and mortality rates associated with enteric coccidiosis across BFF SSP institutions. 88
105 In a retrospective mortality study of captive BFF from at the Smithsonian s National Zoological Park, Bronson et al. (2007) reported that gastrointestinal disease was the most common cause of death in juvenile BFF (52.4%), with 33.3% of juvenile mortality cases in the study caused by enteric coccidiosis. While the data is not directly comparable, the findings reported here also reflect enteric coccidiosis as a common cause of death in juveniles, with increased mortality associated with the disease compared with adult counterparts. All Toronto Zoo mortalities in both juvenile and adult age classes, for which necropsy tissues were available have been attributed to infection with a single coccidia species, Eimeria ictidea (see Chapter 3). Multiple SSP institutions provided partial medical and pathology data sets for use in this study, which were not sufficiently detailed to permit computation of morbidity and mortality rates for those populations. In future, it would be useful to determine whether morbidity and mortality rates associated with enteric coccidiosis vary among SSP institutions, as this may allow for improved identification of host, parasite and environmental factors that increase risk. Clinical signs reported here are consistent with those described from both BFF and domestic ferrets with enteric coccidiosis (Sledge et al. 2011; Santymire et al. 2014; USFWS BFF Recovery Program 2017). Changes to fecal colour and consistency were the most common abnormalities identified at the time of first detection of oocyst shedding. While clinical signs in the cases described here do not always correlate directly with the quantity of oocysts shed, individuals shedding higher number of oocysts showed clinical signs more frequently than those shedding lower numbers. In this study, oocyst shedding from single-housed adult BFF ranged from ,274 oocysts per gram. Daily fecal samples produced from individual adult ferrets range in size from approximately 1-16 grams. In light of these findings, during peak shedding from ~ to oocysts can be shed into the environment in one day, providing a massive infective dose. The large numbers of oocysts shed, 89
106 combined with confinement in a small enclosure space, and hardiness of Eimeria oocysts in the environment, would be expected to markedly increase risk of infection in captive BFF. Oocyst shedding from family groups ranged from 0 371,714 OPG. The wide variability seen in OPG counts between days, as seen in Figure 4.1 and Table 4.1, may be accounted for by the staggered initiation and resolution of shedding by different ferrets. It is most likely that the source of infection in these family groups is shedding by the dams, some of which were identified to shed in multiple years, although environmental contamination cannot be excluded. Shedding in the adult ferrets was clustered during particular time periods, specifically May, July, and September. If shedding is associated with stress and immunosuppression, activities such as breeding, whelping, electro-ejaculation of male ferrets, and shipment/transfer could act as stressors. Whelping, which occurs primarily in May and June, could also act as a stressor to other ferrets in the facility, either through social cues or as there would be associated changes in husbandry protocols. The September cluster could be associated with the transfer of ferrets between institutions; kits are pulled during this time and adult ferrets are moved among institutions thus changing the population dynamics of each SSP site. A large cluster of shedding was recorded in July. With the exception of weaning of kits, no other major stressors are expected to occur during this time thus the increase in shedding by adults in this group cannot be easily explained. Interestingly, shedding was not identified in ferrets from March through April, which is the typical breeding season and when ferrets are introduced for breeding, a presumably stressful time. Shedding was not noted from December through April, which could reflect reduced environmental burdens due to low humidity levels as would be expected in a Nordic climate during the winter (which would kill oocysts and thus block transmission), or may be consistent with reduced stress during this period. The results from single-housed adults are in contrast to the dam and juvenile ferret groups in which oocyst shedding appeared to be correlated to a period of days of kit age. These results are consistent with reports from other facilities of increased incidence of shedding by kits after 70 days of age (USFWS BFF Recovery Program 2017). 90
107 Retrospective and prospective review of histologic sections of intestines from affected BFF at the Toronto Zoo showed the presence parasitic life stages in epithelial cells of both the small and large intestines. Neither Hoare (1935b) nor Jolley et al. (1994) mentioned the presence of parasites in the large intestine of experimentally infected domestic ferrets or naturally infected BFF, respectively. In the study described here, asexual and sexual life stages were identified within the epithelial cells of the small intestinal villi from base to tip, and were most numerous in jejunum. This matches the description by Jolley et al. (1994); however, Hoare (1935a, b) found E. ictidea to be present primarily in the villar tips. Jolley et al. (1994) also described two morphologically distinct meronts of E. ictidea within the small intestinal sections, one at the villar tip that was larger and lacking in undifferentiated mass, and the other at the base of the villi or in the intestinal crypts; these findings were not echoed in this study, as merogonic stages were identified throughout the intestinal epithelium from villus to the base of the crypt, and no visual differences between meronts in any location were identified. Hoare (1935a, b) also described a resulting annular constriction of the villus separating the affected and non-affected segments; this constriction was neither seen in the cases described here nor mentioned by Jolley et al (1994). Whether these differences result from E. ictidea from BFF and E. ictidea from domestic ferrets being different parasites, or from differences in tissue tropism of a single parasite in two different hosts, cannot be ascertained from the available information. Histologic lesions such as necrosis, hemorrhage, villar atrophy and inflammation associated with the presence of parasitic life stages were rare. These changes are normally elicited by the host immune system (inflammation) and the parasite (cellular rupture to release life stages resulting in hemorrhage and necrosis) in response to infection. In light of the fact that acute death occurred in a number of these ferrets (Z113-13, Z117-13, Z118-13, Z119-13, Z137-14), in the absence of secondary disease processes, and with the intestinal epithelium intact but containing myriad parasitic life stages, an alternative mechanism for mortality associated with the infection must be proposed. It is possible that these parasites elaborate exotoxins during their life cycle, and when at high density result in sudden death of the host with minimal 91
108 tissue changes. The presence of parasitic life stages occupying the majority of both small and large intestinal epithelial cells could also potentially impair fluid and protein movement in and out of the mucosa; however, clinical signs associated with malabsorptive diarrhea were not identified in any of these cases. The presence of bacteria within the blood or other organ tissues was not identified in any cases, and consequently sepsis is unlikely to be the cause of death. Black-footed ferrets appear to maintain persistent infection with E. ictidea. Adult BFF in the Toronto Zoo population shed coccidia in multiple years, and in two adult ferrets multiple times in the same year. While the coccidia seen in all cases were not confirmed as E. ictidea using molecular techniques, morphologic similarities and a lack of additional Eimeria spp. identified on molecular work undertaken suggest that only one species of parasite is and has been present in the collection. Two dams that had been identified as infected based on routine fecal screening in previous years presumably acted as the source of infection to their litters of kits in multiple years. While continued environmental contamination cannot be ruled out, these findings imply a failure of the immune response of the BFF to clear infection with E. ictidea, or even to protect against sufficient replication of organisms to result in clinical disease. Based on clinical experience and review of the literature, BFF appear to be much more sensitive to infection with E. ictidea compared with their domestic counterparts. In domestic ferrets, subclinical shedding of oocysts appears to be the most common, with rare reports in the literature of overt disease and that only in juveniles (Blankenship-Paris et al. 1993; Abe et al. 2008). However, a single report exists of three separate clinical outbreaks of Eimeria furonis infection in domestic ferrets under intensive management, with increased morbidity and mortality affecting all ages classes (Sledge et al. 2011). The role of genetics in the apparent increased susceptibility of BFF to enteric coccidiosis is unknown, but the current captive BFF population is derived from seven founders, and inbreeding depression or familial genetic susceptibility may play a role in their increased susceptibility to disease caused by E. ictidea. 92
109 Black-footed ferrets diagnosed with enteric coccidiosis during the course of the study were treated with either ponazuril or toltrazuril, sulfonamide drugs or, often, a combination of the two groups of therapeutic agents. Toltrazuril and ponazuril are triazine coccidiocides, with proven efficacy against both asexual and sexual life stages of mammalian and avian Eimeria spp. (Mehlhorn and Aspo ck 2008). The sulfonamides are antimicrobial drugs that exhibit coccidiostatic or coccidiocidal effects depending on dose; they act by blocking folate synthesis and have effects on first and second generation meronts (asexual life stages), as well as potentially acting on sexual life stages (Mehlhorn and Aspo ck 2008). Based on the limited data available from this study and the fact that treatment was initiated in almost all adult BFF and family groups at the time of oocyst detection, regardless of the presence of clinical disease, the effects of treatment on duration of clinical signs cannot be effectively evaluated. It appears subjectively that adult ferrets treated with toltrazuril, and in one case a combination of toltrazuril and TMS, showed reduction in oocyst shedding after 3-5 days of oral anti-coccidial therapy (see Table 4.3). The effects of treatment with either sulfonamides or triazines would be expected to reduce oocyst shedding; consequently, the duration and amount of oocyst shedding reported in this study may not accurately characterize the natural course of disease. Perceived resistance to sulfa drugs has been reported from multiple SSP facilities. In light of this, and their potential negative effects on ferret reproduction (e.g., prevention of embryo implantation in the uterus and impairment of sperm development), sulfonamides are no longer recommended by the SSP for treatment of coccidia in this species (USFWS BFF Recovery Program 2017). The frequent and widespread use of triazines in the management of enteric coccidiosis in BFF presents a risk for development of resistance to this drug class in the future. Neither pharmacokinetic (PK) nor pharmacodynamic (PD) studies have been published to validate the dose and frequency of dosage in either class of drugs in BFF, and consequently, it is unclear whether this perceived failure of some ferrets to respond to treatment is based on true resistance versus inappropriate dosing. The only work evaluating ponazuril in BFF evaluated serum levels of ponazuril after a single oral dose of 50 mg/kg, and reported 93
110 therapeutic levels for 10 days after administration (USFWS BFF Recovery Program 2017). No information was provided on number or age of ferrets that participated in the study, or on how the determination of what were therapeutic levels was made. Furthermore, as life cycles of the coccidia affecting BFF are limited to the gastrointestinal tract and do not exhibit tissue stages, the validity and usefulness of assessing blood levels of ponazuril in determining appropriate dosage and dose schedules is questionable. Further work to determine the PK and PD of triazines in ferrets is warranted to provide safe and efficacious treatment, and to reduce the risk of development of resistance. Furthermore, the creation and validation of a model for enteric coccidiosis in a related species would allow for in vivo studies of drug resistance. 94
111 OPG Age of Kits Poppy Bumblefoot Calico Aubrey Ruckus Fiddlesticks Guanella Figure 4.1 Oocyst per gram counts and shedding period of Eimeria ictidea from black-footed ferret (Mustela nigripes) family groups from
112 25 µm Figure 4.2 Small intestinal epithelium of a black-footed ferret (Mustela nigripes) containing sexual life stages of Eimeria ictidea. Legend: Solid black arrow = oocyst. Hatched arrow = macrogamont. Outlined arrow = microgamont. Hematoxylin and eosin staining; scale bar = 25 µm. 96
113 Table 4.1 Shedding of oocysts of Eimeria ictidea in black-footed ferret (Mustela nigripes) dam and kit family groups from Numbers of Oocysts Shed (oocysts per gram of feces) Dam Identity Collection Year Age of kits (days) Poppy* Bumblefoot* Calico* Aubrey* Ruckus* Fiddlesticks* Guanella^ < < < < < Legend: < 14 = oocyst positive samples with less than 14 oocyst per gram of feces; - = no sample recorded for this date; underline = last sampling date; + = coccidia present but OPG count not performed; * = Toronto Zoo ferret; ^ = Louisville Zoo ferret; thick outer border = days treatment was received; = range of sequential dates between previous and subsequent number during which OPG counts were performed and samples contained 0 oocysts 97
114 Table 4.2 Summary of epidemiologic data for family groups of black-footed ferrets (Mustela nigripes) shedding oocysts of Eimeria ictidea Dam Identity Poppy * 2014 Bumblefoot * 2014 Aubrey * 2014 Fiddlesticks * 2015 Guanella ^ 2016 Number of kits Kit age (days) at time of shedding Shedding period (days) OPG min OPG max < Clinical signs YES YES NO NO NO Treated - YES YES NO YES Legend: * = Toronto Zoo ferret; ^ = Louisville Zoo ferret; OPG = oocysts per gram of feces; - = missing data. 98
115 Table 4.3 Shedding of oocysts of Eimeria ictidea in single-housed adult black-footed ferrets (Mustela nigripes) Numbers of Oocysts Shed (oocysts per gram of feces) Collection Year Ferret Identity Noodle* Ruckus* Mohawk-A* Mohawk-B* Mystery* Jenna* Clive^ Age (years) Legend: < 1 = oocyst positive samples with less than 1 oocyst per gram of feces; underline = last sampling date; + = coccidia present but OPG count not performed; * = Toronto Zoo ferret; ^ = Louisville Zoo ferret; thick outer border = days treatment was received
116 Table 4.4 Summary of epidemiologic data for single housed adult black-footed ferrets (Mustela nigripes) shedding oocysts of Eimeria ictidea Noodle* Ruckus* Mohawk-A* Mohawk-B* Mystery* Jenna* Clive^ Sex M F M M M F M Age (years) Shedding period (days) OPG min OPG max Clinical signs YES YES NO NO YES NO YES Treated NO YES YES YES YES YES YES Legend: * = Toronto Zoo ferret; ^ = Louisville Zoo ferret; M = male; F= female; OPG = oocysts per gram of feces; - = missing data NOTE: Mohawk-A* and Mohawk-B* refer to two separate episodes of oocyst shedding by the same ferret 100
117 Table 4.5 Histologic findings from necropsies of black-footed ferrets (Mustela nigripes) with enteric coccidiosis. Number of Sections Affected Ferret ID Year Age (years) Sex Coccidia in Intestinal Sections Small Intestine a Large Intestine a Z M rare asexual stages S - 0/1, A - 1/1 S - 0/1, A - 0/1 Z M sexual and asexual stages S - 2/4, A - 2/4 S - 0/3, A - 0/3 Z F rare sexual stages S - 1/4, A - 0/4 S - 0/1, A - 0/1 Z F sexual and asexual stages S - 1/2, A - 1/2 S - 0/1, A - 0/1 Z F sexual and asexual stages S - 2/3, A - 2/3 S - 1/1, A - 1/1 Z F sexual and asexual stages S - 2/4, A - 0/4 none Z F sexual and asexual stages S - 7/7, A - 0/7 none Z M sexual and asexual stages S - 5/6, A - 5/6 S - 1/1, A - 0/1 Z M sexual and asexual stages S - 5/6, A - 4/6 S - 1/1, A - 0/1 Z F sexual and asexual stages S - 4/5, A - 3/5 none Z M sexual and asexual stages S - 10/11, A - 9/11 S - 2/2, A - 0/2 Legend: a = x/n, where x is number of sections containing sexual or asexual lifestages, n is the number of sections examined; S = sexual life stages; A= asexual life stages 101
118 Table 4.6 Yearly incidence of coccidial infection in black-footed ferrets (Mustela nigripes) at the Cheyenne Mountain Zoo Cheyenne Mountain Zoo Year Adult Family /16 (6.25) /19 (42.11) /21 (0.00) 1/4 (25.00) /21 (0.00) 0/7 (0.00) /23 (0.00) 0/8 (0.00) /24 (8.33) 1/4 (25.00) /25 (0.00) 0/6 (0.00) /26 (11.54) 0/7 (0.00) /25 (4.00) 0/9 (0.00) /25 (4.00) 0/8 (0.00) /28 (0.00) 0/5 (0.00) /30 (13.33) 0/9 (0.00) /35 (0.00) 3/5 (60.00) /7 (28.57) Mean annual (%) Legend: - = missing data; x/n=; where x is the number of ferrets shedding coccidial oocysts and n is the total number of adult ferrets, or family groups in a given year; () = incidence expressed as a percentage 102
119 Table 4.7 Yearly mortality rate and incidence of mortality associated with coccidial infection in black-footed ferrets (Mustela nigripes) at the Toronto Zoo Total Mortality Coccidia Other Causes Year Kit Adult Kit Adult /15 (0.00) 0/23 (0.00) 3/15 (20.00) 4/23 (17.39) /38 (0.00) 1/19 (5.26) 8/38 (21.05) 9/19 (47.34) /47 (0.00) 1/19 (5.26) 16/47 (34.04) 1/19 (5.26) /34 (0.00) 0/15 (0.00) 4/34 (11.76) 3/15 (20.00) /32 (0.00) 0/16 (0.00) 5/32 (15.63) 1/16 (6.25) /50 (2.00) 0/20 (0.00) 4/50 (8.00) 2/20 (10.00) /27 (7.41) 0/18 (0.00) 3/27 (11.11) 1/18 (5.55) /20 (0.00) 0/16 (0.00) 6/20 (30.00) 2/16 (12.50) /16 (0.00) 0/15 (0.00) 4/16 (25.00) 2/15 (13.33) /30 (0.00) 0/16 (0.00) 2/30 (6.67) 0/16 (0.00) /19 (0.00) 0/15 (0.00) 4/19 (21.05) 2/15 (13.33) /34 (0.00) 0/16 (0.00) 11/34 (32.35) 3/16 (18.75) /17 (0.00) 0/16 (0.00) 0/17 (0.00) 1/16 (6.25) /17 (0.00) 0/16 (0.00) 3/17 (17.65) 3/16 (18.75) /11 (0.00) 0/16 (0.00) 1/11 (9.09) 2/16 (12.50) /11 (9.09) 0/17 (0.00) 1/11 (9.09) 3/17 (17.65) /24 (16.67) 0/17 (0.00) 4/24 (16.67) 3/17 (17.65) /26 (3.84) 0/17 (0.00) 3/26 (11.54) 2/17 (11.76) /4 (0.00) 0/17 (0.00) 0/4 (0.00) 2/17 (11.76) /11 (0.00) 0/17 (0.00) 2/11 (18.18) 1/17 (5.88) Mean annual (%) Legend: x/n=; where x is the number of ferrets that died with coccidial infection or of other causes and n is the total number of adult ferrets or kits in a given year; () = incidence expressed as a percentage 103
120 CHAPTER 5: EVALUATING THE DOMESTIC FERRET (MUSTELA PUTORIUS FURO) AS AN EXPERIMENTAL MODEL FOR ENTERIC COCCIDIOSIS IN THE BLACK-FOOTED FERRET (MUSTELA NIGRIPES) ABSTRACT: The purpose of this study was to determine whether the domestic ferret (Mustela putorius furo) is susceptible to an isolate of Eimeria ictidea originating from black-footed ferrets (BFF, Mustela nigripes), and thus could act as a suitable experimental model in which to investigate the pathogenesis and management of this disease. A pilot study was performed with 10 male, intact juvenile domestic ferrets. Ferrets were administered an oral inoculum containing either a high dose ( oocysts), moderate dose ( oocysts), or saline control, and observed for shedding of oocysts and development of clinical signs. Seven of ten ferrets developed patent infection, all of which had received the high dose inoculum. The prepatent period was 7-9 days, and duration of shedding varied from 1-7 days. Clinical signs were identified in six of the seven infected ferrets and were consistent with those previously described for enteric coccidiosis in domestic and BFF. Parasite life stages were identified within the intestines of four of the seven ferrets with patent infection, and were limited to the distal jejunum and ileum. The demonstrated ability to produce patent infections in domestic ferrets following oral inoculation of a high dose of E. ictidea ( oocysts) isolated from BFF provides an avenue for future experimental investigations into the control and treatment of enteric coccidiosis in this endangered species. 5.1 INTRODUCTION: Black-footed ferrets (BFF, Mustela nigripes) are one of only three ferret species worldwide. While formerly distributed throughout the North American prairies, black-footed ferrets were declared extinct in the wild in the 1980s. Since 1986, a multi-institutional consortium has been breeding this species in captivity with reintroductions back into the wild within their historic range in selected locations in Canada, the USA and Mexico. Introduced colonies of BFF are present in Arizona, Colorado, Kansas, 104
121 Montana, New Mexico, South Dakota, Utah, Wyoming and Chihuahua (Mexico). Reintroduction attempts in Saskatchewan, Canada, have been unsuccessful to date. Multiple infectious diseases pose a significant risk to the captive breeding and post-release survival of BFF, including distemper and sylvatic plague (Santymire et al. 2014; USFWS BFF Recovery Program 2017). Coccidiosis is a recognized cause of juvenile and adult morbidity and mortality in captive breeding programs, and can result in significant losses (Bronson et al. 2007; Santymire et al. 2014; USFWS BFF Recovery Program 2017). The effect of the disease on wild populations is unknown. Clinical signs of coccidiosis include mucoid to hemorrhagic diarrhea, abdominal discomfort, lethargy, appetite loss, vomiting and dehydration. Recent investigations into diseases affecting BFF at the Toronto Zoo have identified a single Eimeria species, E. ictidea, associated with all cases of enteric coccidiosis in juvenile and adult BFF from (see Chapter 3). This same Eimeria species was identified retrospectively as the cause of juvenile and adult mortalities in previous years (1999 through 2014 inclusive) (Chapters 3 and 4). Furthermore, this pathogen was identified in fecal samples, based on morphologic and molecular characterization, from adult and juvenile BFF in another zoological collection (Louisville Zoo, Kentucky, USA) (see Chapter 3). Enteric coccidiosis also occurs in domestic ferrets (Mustela putorius furo), with three morphologically distinct species of coccidia: Eimeria ictidea, Eimeria furonis, and Isospora (= Cystoisospora) laidlawi. Both of the aforementioned Eimeria species have been identified in black-footed ferrets based on morphologic criteria, but molecular characterization was needed to confirm whether the same species of parasite infects both ferret species (see Chapter 2 and 3). To this end, nuclear and mitochondrial sequences for E. furonis and for I.=(C.) laidlawi were generated (Chapter 2), expanding the existing limited sequence data from the nuclear 18S rrna locus of Eimeria furonis. Molecular characterization of E. ictidea from domestic ferrets was not possible because samples containing this parasite were not available for study; consequently, it is unclear whether the same coccidium affects both domestic and black-footed ferrets. 105
122 There is no published information describing the pre-patent periods and pathogenicity of enteric coccidia in BFF and, given the conservation status of the BFF, experimental work cannot be undertaken in the natural host. The purpose of this study was to determine whether the domestic ferret is susceptible to E. ictidea isolated from BFF; if susceptible, the domestic ferret could act as a suitable experimental model in which to investigate the pathogenesis, prevention, and treatment of coccidiosis caused by E. ictidea. 5.2 MATERIALS AND METHODS: Animal care Ten juvenile, male, intact ferrets of 48 (n=6) or 50 (n=4) days of age were obtained from a commercial source (Marshall BioResources, North Rose, New York, USA) and were housed in the University of Guelph, Central Animal Facility, Isolation Facility. Ferret weights on arrival ranged from g (average= g). All ferrets were housed individually in wire bottom cages of cm size, and were divided in equal numbers between two non-adjoining rooms. They received ad libitum access to Envigo Teklad Certified Global Ferret Diet (Madison, Wisconsin, USA) and water, changed daily. Room temperature was maintained at C and a 16 hour light, 8 hour dark photoperiod was provided. All personnel working with the ferrets were required to wear personal protective equipment including: disposable facemasks, gloves, gowns, and bouffant caps. Shoes were provided for use in each room. This study was carried out in accordance with the recommendations in the Canadian Council on Animal Care guidelines. The protocol was approved by the Animal Care Committee of the University of Guelph (Animal Use Protocol: 3289), and by both the Animal Welfare Committee and Animal Care and Research Committee of the Toronto Zoo. An initial physical examination and blood collection were performed on each ferret by the principal investigator (ARP) one day after arrival to assess health status prior to enrollment in the study. Ferrets were mask induced with isoflurane (Isoflurane USP, Fresenius Kabi, Richmond Hill, Ontario) in 106
123 oxygen, placed on a heat disc (SnuggleSafe, Lenric C21 Ltd, Littlehampton, United Kingdom), weighed, examined, and blood was collected from the jugular vein for routine CBC and biochemical profiles. All ferrets subsequently underwent an acclimation period of two weeks. During this time, fecal samples were collected daily from each ferret and examined for the presence of coccidial oocysts using a standard salt flotation technique (Dryden et al. 2005) to ensure that all individuals were free of coccidia prior to initiation of experimental work. Any ferret positive for coccidia was to be removed from the study Oocyst preparation Oocysts used for inoculation originated from fecal samples from two naturally infected BFF. These samples were stored in potassium dichromate for four weeks prior to oocyst purification and use in this infection trial. Stored fecal samples were mixed with distilled water and passed through a small sieve to remove debris. The strained contents were transferred to a 50 ml conical vial and topped up to 50 ml with additional distilled water. Samples were centrifuged (Sorvall ST40R Centrifuge, Thermo Scientific) at 2800 rpm (1315 G) for 10 minutes at 12 C. A drop of supernatant was evaluated microscopically at 100 for the presence of oocysts. If oocysts were observed, the supernatant was poured off into a second 50 ml conical tube, and again topped up to 50 ml with distilled water and re-centrifuged under the same conditions. Otherwise the supernatant was discarded. The pellets from both the first and second tubes were combined with saturated salt solution at a 1:4 ratio by volume. Oocysts were floated in the salt solution by centrifugation at 1500 rpm (377 G) for 10 minutes at 12 C. The top 5 ml of supernatant were collected and transferred to a clean 50 ml conical tube, topped up to 40 ml with distilled water, and washed via centrifugation at 2800 rpm (1315 G) for 10 minutes. After the wash step, the supernatant was again checked for presence of oocysts and discarded if no oocysts were observed. The pellet was collected and the presence of oocysts confirmed by examination of a drop placed on a clean glass slide at 100. Once verified, the contents of the pellet of concentrated oocysts was placed in a 250 ml storage container and mixed with approximately 200 ml of sterile saline (0.9% sodium chloride; Hospira, 107
124 Montreal, Quebec) prior to storage for two to four weeks at 4 C until inoculation. Prior to inoculation, a McMaster count was performed to determine the number of oocysts per ml in order to determine appropriate volume of inoculum Experimental infections Part 1: Five ferrets were randomly assigned to each of the control and infection groups. After the acclimation period, on day 0, four ferrets in the infection group were inoculated orally with a high dose oocyst suspension ( oocysts in 0.25 ml of saline) mixed into 1 ml of FerreTone Skin & Coat Supplement (United Pet Group Inc., Blacksburg, Virginia, USA); a fifth ferret was inoculated with a moderate dose oocyst suspension ( oocysts in 0.25 ml of saline) in the same volume of FerreTone. Ferrets in the control group were inoculated with a placebo (0.25 ml of saline) in 1 ml of FerreTone. Inoculation was performed by offering the oocyst suspension or placebo to the ferrets in a plastic container. Fecal samples were collected daily from each inoculated ferret for 14 days post-inoculation. Samples were analyzed via fecal flotation using the McMaster method, followed by routine flotation in saturated salt solution (Dryden et al. 2005) to determine the presence or absence of oocysts and oocyst burden (oocysts per gram of feces [OPG]).Temporal trends in oocyst shedding were monitored. Ferrets were evaluated visually twice daily for the presence of clinical signs of coccidial disease. The first of every two ferrets identified to shed oocysts was to be humanely killed at the time of peak oocyst shedding (i.e., the first day that fecal oocyst counts remained static or declined) and necropsied to confirm the presence of and describe parasitic replication in the intestinal mucosa. Any remaining animals that shed oocysts were to be monitored throughout the 14 day period following inoculation in order to determine the duration and intensity of oocyst shedding; for these individuals the total number oocysts shed during patency was determined. 108
125 Part 2: All ferrets from the infection group that did not shed oocysts during Part 1 (n=4) and all but one ferret from the previous control group (n=4) were orally inoculated with the high dose oocyst suspension ( in 1 ml of saline) mixed with an equal volume of FerreTone. Consequently, between phases 1 and 2, all but one ferret were inoculated at least once with the BFF coccidia, in order to increase experimental animal numbers and determine if ferret age played a role in susceptibility to infection. One ferret from the previous control group was inoculated with a lower dose ( oocysts in 0.75 ml of 0.9% saline mixed with 1 ml FerreTone) of oocysts that had been collected from the single domestic ferret that shed in Part 1; oocysts were purified as described above for the initial inocula. Fecal collection and analysis were performed as previously. As in Part 1, one in every two ferrets sequentially identified to be shedding oocysts in feces was killed humanely at the time of peak shedding and a complete necropsy examination performed. The remainder of the ferrets observed to be shedding were monitored for the full 14 days of the trial, after which they were killed humanely and necropsied, and total number of oocysts shed during patency was determined. All ferrets that did not shed coccidial oocysts during the infection trial were rehomed at the end of the trial Animal welfare Ferrets were evaluated twice daily for development of clinical signs of coccidial disease and any animal showing clinical disease was to be treated, as determined by a veterinarian, with supportive care, including fluid therapy. A grading system for clinical signs, including intervention points and removal criteria, was created for use during daily evaluation (see Appendices 3 and 4). Animals whose clinical signs could not be ameliorated without the use of specific anticoccidial therapy were to be euthanized. Should the inoculation in Part 1 have resulted in clinical disease that required extensive treatment and/or necessitated euthanasia, a lower number of oocysts would be used for subsequent inoculation in Part 2. Ferrets to be euthanized were anesthetized by mask induction with isoflurane in oxygen, a 1 ml blood 109
126 sample was collected from the cranial vena cava, and then an intracardiac dose of potassium chloride (2 meq/kg) was administered Hematology Blood was collected from all ferrets under isoflurane anesthesia at the time of pre-trial health examination and again at the time of humane killing. Blood was collected via jugular venipuncture initially due to the small size of the ferrets at arrival, and then by cranial vena cava venipuncture or cardiocentesis prior to euthanasia. Complete blood count and serum biochemistries were performed by the Animal Health Laboratory of the University of Guelph, Guelph Ontario Morphologic and molecular characterization Morphologic and molecular characterization of oocysts shed by the domestic ferrets during the course of the infection trial was performed to ensure that the ferrets were shedding the same species of Eimeria with which they were inoculated. Oocysts were concentrated from positive fecal samples as described above. A drop of concentrated oocyst solution was viewed, photographed and measured at 400 and 600 for comparison with previously determined morphometrics of Eimeria ictidea oocysts (Chapter 3). Regions from the mitochondrial cytochrome c oxidase subunit I and III (mt COI and mt COIII) DNA were amplified by polymerase chain reaction (PCR) from each sample using primer pairs 400F/1202R and -172F/799R, respectively. For all PCR reactions samples were denatured at 95 C for 5 min, then subjected to 35 cycles of 94 C for 30s, anneal at 52 C for 30s, and extension at 72 C for 60s, followed by a final extension at 72 C for 7 min. PCR, gel electrophoresis and sequencing methods used were as described in the Materials & Methods section of Chapter 2. The resulting consensus sequences were searched from within Geneious against previous sequences for E. ictidea produced by the authors and against publically available sequences on the 110
127 BLAST server (blast.ncbi.nlm.nih.gov/blast.cgi) using the blastn search algorithm against the nr/nt database (GenBank+EMBL+DDBJ+RefSeq AA or DNA) Necropsy protocol All humanely killed ferrets underwent a complete necropsy (Appendix 6) using the modified protocol described in Materials & Methods section of Chapter RESULTS: Initial physical examination was unremarkable with the exception of mild to moderate bilateral ceruminous discharge within the external ear canal of all ferrets. Complete blood count and serum biochemistry results for all ferrets were within normal reference intervals for juvenile domestic ferrets (Appendices 2a and b) (Fox 2014). Six days after arrival, a single ferret (103) in the control group developed mild upper respiratory signs consisting of sneezing and clear nasal and ocular discharge; these clinical signs were associated with mild dehydration and decreased food and water consumption. The ferret was treated with subcutaneous fluid therapy (10 ml Plasmalyte-A subcutaneous, Baxter, Alliston, Ontario), heat and supportive care and all clinical signs resolved within three days. This ferret was deemed healthy to participate in the remainder of the clinical trial. A second ferret (105) in the control group developed unilateral purulent ocular discharge 14 days after arrival, one day prior to placebo inoculation. The ferret was treated topically twice daily for five days with Isathal ophthalmic gel (fusidic acid 10 mg/g, Dechra Veterinary Products Inc., Point-Claire, Quebec), and the discharge resolved, but reoccurred within 2 days of treatment cessation. Ocular examination showed mild conjunctivitis, but no evidence of corneal lesions, and fluorescein staining did not indicate the presence of corneal ulceration. The ferret was treated for an additional six days with tobramycin ophthalmic solution (3 mg/ml, Sandoz Tobramycin 0.3%, Boucherville, Quebec), after which clinical signs resolved completely. No coccidial oocysts were shed in feces from any of the ferrets during the two week acclimation period. 111
128 5.3.1 Oocyst shedding All ferrets readily ingested the inoculum with either placebo or concentrated oocysts. In Part 1, one ferret (203) in the infection group, which had received the high dose ( oocysts), shed oocysts on day 8 and day 9 after inoculation (Tables 5.1, 5.2). This ferret was 71 days of age at the time shedding was initially identified. The ferret was killed humanely 11 days post inoculation, later than had been outlined in the protocol, as processing of fecal samples had been delayed by two days resulting in late detection of oocyst shedding in this individual. Oocysts were not identified in the feces of the three remaining ferrets that received the high inoculation dose, the single ferret that received the lower dose ( oocysts), or in the ferrets within the control group. In Part 2, six of eight ferrets inoculated with the high dose ( oocysts) shed oocysts during the 14 day observation period (Tables 5.1, 5.2). Four of these ferrets were from the previous control group. One of the ferrets previously inoculated with the high dose inoculum in Phase 1 that had not shed oocysts, did shed oocysts after being inoculated a second time with the same dose during Phase 2. The ferret that had previously received the low dose ( oocysts) of oocysts in Phase 1 also shed after inoculation with the high dose in Phase 2. Three ferrets did not shed oocysts after high dose inoculation in Phase 2, one of these had been part of the previous control group; the other two had received the high dose inoculation previously in Phase 1. The pre-patent period ranged from 7-9 days (Table 5.1), with equivalent numbers of ferrets commencing shedding on each of days 7 through 9. All six ferrets were between 91 and 93 days of age at the time shedding was initially identified. Oocyst per gram counts and shedding trends for all individuals are shown in Table 5.1. Total oocyst shedding during patency was <14 oocysts, 8,904 oocysts and 172,291 oocysts, for ferrets 201, 104 and 105 respectively. The two ferrets for which the prepatent period was 9 days only shed oocysts for one day and in low numbers. Oocysts were not identified in the feces of the three remaining ferrets, two of which received the high inoculation dose ( oocysts) and the third that received the lower inoculation dose ( oocysts) (Table 5.2). 112
129 5.3.2 Morphologic and molecular characterization In all seven ferrets that shed oocysts, the morphologic features and measurements (length, width, shape index) of the shed oocysts were consistent with those of the E. ictidea administered in the inoculum (Figure 5.1). Molecular confirmation of the identity of the oocysts shed was successful in 3 out of the 7 ferrets (102, 103, 203); samples from the four remaining ferrets did not contain adequate quantity or quality of DNA for confirmation Clinical signs Clinical signs associated with patent infection were identified in 6 of 7 ferrets (Table 5.2). These signs included: weight loss (n=5), diarrhea (n=1), mucoid soft feces (n=2), feces containing blood (n=2), and malodorous feces (n=1). Appetite reduction was noted in two ferrets from the infection group in Part 1 between 6-8 days post infection; however, no oocyst shedding was detected from either ferret during this time Hematology CBC and serum biochemistry values from ferrets collected during pre-trial health screening are displayed in Appendices 1a and 1b. Values obtained for ferrets euthanized during or after the experimental trial are displayed in Appendices 3a and 3b. Minor variances from reference range values for CBC and serum biochemistry were identified in six of the seven ferrets with patent infection. In all six ferrets for which a complete serum biochemistry was obtained immediately prior to death, creatinine kinase (CK) values were elevated (see Appendix 3b). Ferret 103 exhibited a mild hypoalbuminemia (20; ref g/l) on ante-mortem serum biochemistry (see Appendix 3b). 113
130 5.3.5 Necropsy No gross or histopathologic lesions were present and coccidia could not be identified in sections of intestine from the single ferret (203) humanely killed in Part 1. For the ferrets humanely killed in Part 2, no evidence of diarrhea, hematochezia, or mucoid fecal material was identified grossly. Ferret 105, killed at the termination of the experiment but still shedding low numbers of oocysts in its feces, exhibited a 7 cm region of congested mucosa within the distal jejunum. Coccidial life stages were identified in small intestinal sections from four of the seven ferrets that were identified to shed oocysts at some point prior to necropsy (Figure 5.2, Tables 5.2 and 5.3). Affected sections included jejunum in all four animals, as well as ileum in one, and were collected from 114 to 218 cm aboral from the pylorus (see Figure 5.3). Coccidia were not identified in sections of duodenum, proximal jejunum or large intestine; however, oocysts were identified within fecal material in the lumen of the large intestine from one ferret (103). Of the sections of intestine examined for each ferret, the number of sections containing parasites ranged from one to eight: 1 section in ferret 102, 2 sections in ferret 201, 4 sections in ferret 105, 8 sections in ferret 103. A mix of sexual and asexual life stages was observed within the enterocytes in small intestinal sections from ferret 103, the remainder of the ferrets showed either asexual (102, 201) or sexual (105) life stages in affected segments. Pathologic changes and additional histologic findings in small intestinal sections of ferrets with enteric coccidia included rare regions of blunting of the villi and sloughing of the epithelium associated with hemorrhage and inflammation. The primary lesions identified were subjectively increased numbers of eosinophils, lymphocytes and plasma cells within the lamina propria of the small intestine, and similarly increased neutrophils, lymphocytes and plasma cells within the lamina propria of the large intestine. Neutrophils were rarely present in intestinal crypts and glands. Other gross necropsy findings included two ferrets with renal cortico-medullary cysts, and one ferret with mild thickening of the esophageal mucosa midway along the esophageal length. No histologic changes were identified within a sample of esophagus taken from this region. 114
131 5.4 DISCUSSION: The findings of this study show that domestic ferrets are susceptible to infection with the enteric coccidium Eimeria ictidea isolated from black-footed ferrets. Both morphometric and molecular diagnostic methods were used to confirm that ferrets were shedding oocysts of the same species with which they were inoculated. Molecular characterization was successful in three of the seven ferrets that developed patent infections, and as no other coccidial species was identified during pre-trial observation, morphometry was considered to be confirmatory in the remaining four animals. We have referred to the eimeriid coccidium affecting BFF, and used in this experimental trial, as E. ictidea based on morphometric similarity of their oocysts with those of E. ictidea as described from domestic ferrets (see Chapter 3). There is limited published information on infection of domestic ferrets with E. ictidea, outside of Hoare s original descriptions (1927; 1935a, b), which form the basis for all subsequent identifications of E. ictidea in domestic ferrets and in BFF. Attempts to obtain exemplars of E. ictidea from domestic ferrets to characterize using molecular techniques were unsuccessful (Chapter 2). Multiple diagnostic laboratories in Canada and Europe were solicited for coccidia-positive fecal samples from domestic ferrets, but no samples of E. ictidea were received over a 4 year period ( ). Eimeria ictidea was identified, based on microscopic examination, in only two samples submitted to a European diagnostic laboratory from It is unproven whether the E. ictidea described from domestic ferrets and the E. ictidea identified from black-footed ferrets, and used in this experimental work, are the same or are simply morphologically indistinguishable Eimeria species. However, the consistency in morphology, host genus and location of infection within the intestinal tissues, combined with the successful cross-transmission of this parasite to domestic ferrets described in the present study, suggests they are likely conspecific. 115
132 The pre-patent period (minimum duration of endogenous development) for infection with E. ictidea in the inoculated domestic ferrets ranged from 7-9 days (see Table 5.1); the pre-patent period for this parasite in the BFF, the natural host for this coccidium, is unknown. Hoare experimentally infected naïve domestic ferrets with E. ictidea derived from naturally occurring infection in this species (Hoare 1935b). The inoculated ferrets shed oocysts after a pre-patent period of 7 days, consistent with the 7-9 days seen in the work described here with E. ictidea. Shedding of oocysts was identified over a period of 1-7 days (see Table 5.1) and intensity ranged from less than 14 up to 15,624 OPG. These results may be skewed, with erroneously low duration of shedding and number of oocysts shed, as three of the seven ferrets were humanely killed at or prior to the expected peak of oocyst shedding for tissue collection and histologic examination in order to increase the probability of identifying parasite life stages within the intestinal sections. Shedding periods were similar to those identified in adult single-housed BFF, which ranged from 2-9 days; however, oocyst per gram counts from the domestic ferrets were consistently lower than OPG counts from BFF ( ,274 OPG) infected with the same parasite (see Chapter 4). Furthermore, the total number of oocysts shed by individual domestic ferrets (14-172,291 oocysts) during patency was reduced compared to BFF, despite similar length of shedding period (see Chapter 4). The domestic ferrets in this study were naïve individual juveniles, whereas the BFF were adults 1-5 years of age; some of which were showing clinical signs at the time of oocyst shedding. The relative influences of age, species, and previous exposure to the parasite on our observations are unknown. Two different fecal flotation methods were used on all samples to increase the probability of oocyst detection. The McMaster method was used to provide accurate OPG counts for quantification of oocyst shedding; however, this method had a minimum detection limit of ~13 oocysts per gram (13.33 OPG calculated) because it is based on dilution of the initial sample with flotation media (saturated salt). In samples with few oocysts, oocysts may be missed or to be present in numbers below this detection limit. Routine salt flotation is, in contrast, performed using the entire sample allowing for detection of 116
133 small numbers of oocysts. Consequently, in cases where oocyst per gram counts were low, shedding was identified on routine salt flotation but not by the McMaster method and recorded as positive but below the detection limit of the enumeration method. Subclinical to clinical disease occurred in six of the seven ferrets that developed patent infection, with weight loss being the most frequent clinical sign. Other clinical signs were typical of enteric coccidiosis including diarrhea, hematochezia, and mucoid and/or soft feces. These clinical signs are similar to those previously described for black-footed ferrets infected with this parasite (USFWS BFF Recovery Program, 2017; Chapter 4) and for domestic ferrets with enteric coccidiosis (Sledge et al. 2011). Interestingly, development of clinical disease was not described in the naïve domestic ferret inoculated by Hoare (1935) with E. ictidea derived from naturally occurring infection. Based on review of the literature, severe clinical disease resulting from intestinal coccidiosis is rare in domestic ferrets. Black-footed ferrets, however, appear more susceptible to disease development and more frequently show significant clinical signs. No domestic ferret required treatment for clinical coccidiosis during the course of this study. It is possible that the more pronounced clinical signs associated with enteric coccidiosis in BFF may result from the limited genetic diversity in a population derived from so few individuals, and increased susceptibility of BFF to other diseases such as sylvatic plague have been described in comparison with their domestic counterparts (Williams et al. 1994). Although unlikely, recent acquisition of E. ictidea from a related host species, such as the domestic ferret, could have resulted in increased pathogenicity and severity of clinical disease from infection with this parasite in BFF. Minor variances from reference range values for CBC and serum biochemistry were identified in six of the seven ferrets with patent infection, but only in one case (ferret 103), did this appear to be correlated with infection/disease. In this animal, a mild hypoalbuminemia (20; ref g/l) was noted (Appendix 3b). On histopathology, large regions of the small intestine contained parasite life stages; however, inflammation, lysis of epithelial cells and necrosis of affected areas that could be expected to result in protein loss into the intestinal lumen were not identified. 117
134 Creatine kinase values were elevated in all six ferrets for which a complete serum biochemistry was obtained immediately prior to death; these findings are consistent with release from CK rich tissues, such a skeletal muscle, during venipuncture and manual restraint. The pathologic lesions identified within the intestinal sections of ferrets euthanized at the time of oocyst shedding were similar to those identified in affected BFF, but in most cases were less locally extensive or widespread throughout the small intestine than those observed in necropsy cases of BFF (see Chapter 4). Coccidia were seen in the histologic sections of four ferrets, all three ferrets that were actively shedding oocysts at the time of necropsy (102, 103, 105), and one of four in which necropsies were performed after oocyst shedding had ceased (201). Although the primary objective of the examination of histologic sections from affected ferrets was to identify coccidial life stages, attempts were made to describe the pathologic changes associated with the presence of the parasite. Despite necropsies being performed almost immediately after death, and the use of Serra solution fixative to improve parasite and tissue preservation, the villi and villar epithelium of the trimmed sections were frequently distorted or absent, and consequently accurate commentary on these areas was precluded and was made only on visible components of the lamina propria and crypts or glands. A deliberate decision was made not to kill and collect samples from the saline inoculated control ferrets after Phase 1, and those not shedding oocysts during Phase 1 or 2, thus no age-matched intestinal sections were available for comparison. It is difficult to comment on the significance of the inflammatory cells observed in the lamina propria of the small and large intestinal segments, or the proliferative rate of the crypt epithelium. The only changes identified which may be considered significant are the presence of neutrophils within the crypts and glands of small and large intestinal sections, respectively, but these lesions were rare and not associated with the presence of parasitic life stages. 118
135 While no parasitic life stages were identified in intestinal sections from ferrets 104, 203 and 205, one of the three ferrets, ferret 104, exhibited lymphoplasmacytic inflammation and blunting of jejunal villi in one section (36-38 cm aboral from pylorus). These findings might be expected as the most extensive histologic lesions would occur associated with lysis of the intestinal epithelial cells as oocysts were shed into the feces, after which new intestinal epithelial cells would re-cover the denuded villar surface. Thus, for those cases in which histologic examination was performed after shedding had ceased, presence of the protozoal life stages in the intestines would be expected to be significantly reduced or absent. Hoare (1935a, b) described a particular reaction to the presence of parasitic life stages of Eimeria ictidea in the small intestine of domestic ferrets, in which only the villar tips were affected and there was resultant annular constriction of the villus separating the affected and non-affected segments. These particular changes were not identified in any of the ferrets in this study, and had not been noted retrospectively in naturally infected BFF (Chapter 4). While patent infection and intestinal disease could be experimentally created in domestic ferrets, without equivalent experimental work in BFF, it is difficult to fully compare the susceptibility to infection and to the development of disease between the two species. Eimeria species tend to be host specific, thus if E. ictidea from BFF is not conspecific with E. ictidea in domestic ferrets, it might be expected that the domestic ferret would be less susceptible to infection and the development of disease than is the BFF. Even if the two parasites are identical, natural passaging through the BFF may alter affinity for the domestic ferret. It appears that the infectious dose of oocysts of E. ictidea derived from BFF required to initiate a patent infection in domestic ferrets is high. The ferrets that developed patent infections were administered an inoculum containing sporulated oocysts and, even with this extremely high inoculating dose, only a proportion of inoculated ferrets became infected. Neither the ferret that received the low dose 119
136 inoculum nor the ferret that received the passaged oocysts from Part 1 of the study shed oocysts during the 14 day period post inoculation. The latter finding was unexpected, as fresh passaged oocysts would be expected to contain larger proportions of viable oocysts and be comparatively more infective than oocysts that had been stored for 2-4 weeks prior to inoculation. The only publication describing oral inoculation of Eimeria species in domestic ferrets (Hoare 1935b) did not quantify the number of oocysts administered. A study performed in 16 farmed juvenile mink (Mustela vison) administered 2,000 sporulated oocysts of each of three coccidial species (I. laidlawi, E. vison, and an unknown Eimeria species) resulting in patent infection with one of the three species (Foreyt et al. 1977), as determined by the presence of oocysts on fecal examination. The authors did not reveal which type of oocysts resulted in the infection. The number of oocysts required to result in infection in BFF is unknown. Based on the authors observations of over 100,000 oocysts per gram of feces being shed by black-footed ferrets into cages of <1m 2 floor space (see Chapter 4), we estimated that under normal caged conditions animals would likely ingest thousands of oocysts over a short period of time. This was in part why a large number of oocysts (up to 1,000,000 as available from our store of viable oocysts) was administered to each domestic ferret in order to increase the probability that infection and shedding would result. Furthermore, for the parasite to persist within the ferret population, the total number of oocysts shed into the environment would have to be several times higher than the infective dose required to generate a patent infection, otherwise the parasite would be expected to die out. If E. ictidea of domestic ferrets and E. ictidea of BFF are conspecific, the difference in oocyst shedding between the host species during patent infection could potentially explain the low prevalence of E. ictidea reported from the domestic ferret population (as seen in Chapter 2). Both humoral and cell mediated immunity are involved in the immune response to coccidia. The role of maternal derived antibodies in combatting protozoal infection in mustelids has not been studied, 120
137 but in carnivores maternal antibodies to viruses can last up to 16 weeks (Chappuis 1998). In poultry, maternal Eimeria-specific IgG is transferred via the egg yolk to offspring. In one study, breeding hens were infected with a single species of Eimeria days prior to lay. Their hatched chicks were challenged by inoculation with oocysts of the same and a related Eimeria species, and showed reduced oocyst shedding compared to age matched controls, indicative of passive transfer of immunity (Smith et al. 1994). It is possible then, that the presence or absence of maternal antibodies may be a factor in the age at which ferrets are susceptible to coccidial infection and the development of disease. The facility from which the domestic ferrets were acquired has not previously detected Eimeria species on routine fecal screening (Dr. Bambi Jasmin, personal communication); consequently, it is unlikely that they would have received maternal immunity to or been exposed to this parasite and thus can be considered to be naive. Despite a theoretical lack of maternal immunity, there did appear to be an effect of age on susceptibility to infection. In Part 1, when the ferrets were approximately 70 days of age, only 1 of 4 ferrets inoculated with the high dose of oocysts developed patent infection. In comparison, in Part 2, when the ferrets were days of age, 3 of 4 ferrets that had been in the saline control group for Part 1 developed patent infections, as did 2 of 4 ferrets that had been inoculated in Part 1 but had not shed oocysts. One of these previously inoculated ferrets was the individual that had received the lower dose of oocysts. Thus, it appears that patent infection could be produced more easily in the older ferret kits. However, the two ferrets that had previously been inoculated exhibited the shortest shedding periods (1 day) and lowest oocyst per gram counts, and parasite life stages in these cases were rare (201) to absent (205) on histologic examination of numerous sections of intestine. It is thus possible that the primary inoculation resulted in abbreviated infections or infections in which so few oocysts were shed that infection was not detected. Our observation of endogenous stages in the intestinal tissues of some of the ferrets following cessation of oocyst shedding suggests that the pre-patent period and duration of patency may vary considerably from animal to animal; consequently, it is possible that some of the kits would 121
138 have ultimately shed a few oocysts from the primary inoculum if followed beyond 14 days postinoculation. Whether through an aborted or undetected infection, previous exposure of these kits to the parasite probably generated partial immunity against E. ictidea and therefore, the intensity of infection upon challenge in previously exposed domestic ferret kits was reduced. The authors acknowledge the limitations of this initial pilot study; however, it was proven that patent infection with E. ictidea isolated from black-footed ferrets could be generated in a novel host, the domestic ferret. Further studies will be required to investigate the effect of age on susceptibility to infection, as well as the possibility of development of immunity after exposure and its role in reducing parasite replication and disease in subsequent infection. With so few BFF in existence the use of BFF for in vivo infection trials cannot be contemplated; consequently, refining the domestic ferret infection model will be essential for carrying out research specifically intended to help manage coccidiosis in the endangered black-footed ferret. 122
139 25 µm Figure 5.1 Exogenous life stages of Eimeria ictidea shed from a domestic ferret (Mustela putorius furo) experimentally inoculated with oocysts originating from black-footed ferrets (Mustela nigripes). Unsporulated oocyst (solid black arrow). Sporulated oocyst (solid white arrow). Bright field microscopy, scale bar = 25 µm.
140 25 µm 25 µm Figure 5.2 Life stages of Eimeria ictidea within the small intestinal epithelium of an experimentally infected domestic ferret (Mustela putorius furo). A) Sexual life stages (micro- and macrogamonts - white arrows) crowding the villar enterocytes; all stages are found between the nucleus and luminal surface of infected enterocytes. Hematoxylin and eosin staining, scale bar = 25µm. B) At higher magnification, meronts (black arrows) and gamonts (open arrows) are crowded between the enterocyte nuclei and brush border. Hematoxylin and eosin staining, scale bar = 25µm. 124
141 Number of Ferrets Affected duod jej 1 jej 2 jej 3 jej 4 jej 5 ileum colon 1 colon 2 SI Intestinal Section Containing Life Stages of Eimeria ictidea Asexual life stages Sexual life stages Figure 5.3 Presence and location of sexual and asexual life stages of Eimeria ictidea within the intestinal epithelium of domestic ferrets (Mustela putorius furo) (n=7) that developed patent infection after experimental inoculation with oocysts originating from black-footed ferrets (Mustela nigripes). Legend: duod = duodenum; jej= jejunum. Sequential numbers for jejunal and colonic sections represent the order, aboral from the pylorus, from which the samples were collected. SI = additional section(s) of small intestine whose aboral sequence was not recorded. 125
142 Table 5.1 Prepatent period and oocyst shedding patterns in domestic ferrets (Mustela putorius furo) experimentally inoculated with oocysts of Eimeria ictidea originating from black-footed ferret (Mustela nigripes) that developed patent infections Oocysts shed per gram of feces Ferret Identity Day post inoculation < * * , ,173.3 < < * 11 < * 12 0 < < * < 14* 0* Legend: *ferret euthanized as of this date; < 14 = oocyst positive samples with less than 14 oocysts per gram of feces 126
143 Table 5.2 Results of oral inoculation of domestic ferrets (Mustela putorius furo) with oocysts of Eimeria ictidea originating from black-footed ferrets (Mustela nigripes). Results: Part 1 Results: Part 2 Ferret Identity Inoculum Oocyst Shedding Presence of clinical disease* Coccidia present in sections Inoculum Oocyst Shedding Presence of clinical disease* Coccidia present in sections 101 Saline N N oocysts a N N Saline N N oocysts Y N Y^ 103 Saline N N oocysts Y Y Y^ 104 Saline N N oocysts Y Y N 105 Saline N N oocysts Y Y Y oocysts N N oocysts Y Y Y oocysts N N oocysts N N oocysts Y Y N oocysts N N oocysts N N oocysts N N oocysts Y Y N^ Legend: * includes any of the following: weight loss, diarrhea, mucoid feces, malodorous feces, inappetence; N = no; Y= yes; - = necropsy not performed; a = oocysts collected after passage through ferret 203; ^ = shedding oocysts at time of necropsy; = not shedding oocysts at time of necropsy 127
144 Table 5.3. Distribution of coccidial life stages in domestic ferrets (Mustela putorius furo) orally inoculated with oocysts of Eimeria ictidea originating from black-footed ferrets (Mustela nigripes) Ferret Identity Intestinal level Duodenum N N N N N N N Jejunum 1 N N N N N N N Jejunum 2 N N N N N N N Jejunum 3 N S, A N N N N N Jejunum 4 N S, A N N N N N Jejunum 5 N S, A N S A N N Ileum N S, A N N N N N Colon 1 N N a N N N N N Colon 2 N N a N N N N N Unmeasured small intestine b,c S - 0/5 A - 1/5 S - 4/5 A - 2/5 S - 0/5 A - 0/5 S - 2/6 A - 0/6 S - 0/6 A - 0/6 S - 0/6 A - 0/6 S - 0/8 A - 0/8 Unmeasured large intestine b,c S - 0/1 A - 0/1 S - 0/1 A - 0/1 S - 0/1 A - 0/1 S - 0/2 A - 0/2 S - 0/1 A - 0/1 none S - 0/1 A - 0/1 Legend: N = no parasite life stages; S = sexual life stages present; A = asexual life stages present; a = oocysts present in feces; b = additional sections of intestine for which the location measured from the pylorus was not obtained; c = x/n, where x is number of sections containing sexual or asexual lifestages, n is the number of sections examined 128
145 CHAPTER 6: WHOLE MITOCHONDRIAL GENOME SEQUENCES OF TWO EIMERIA SPECIES ISOLATED FROM DOMESTIC (MUSTELA PUTORIUS FURO) AND BLACK- FOOTED FERRETS (MUSTELA NIGRIPES) ABSTRACT: The complete mitochondrial (mt) genomes of Eimeria furonis and Eimeria ictidea (Eimeriidae, Coccidia, Apicomplexa) originating from single fecal samples from a domestic (Mustela putorius furo) and a black-footed ferret (Mustela nigripes), respectively, were sequenced. Both mt genomes were circular-mapping with lengths of 6,165 base pairs (Eimeria furonis - GenBank: MF795598) and 6,182 base pairs (Eimeria ictidea - GenBank: KT203399). Genome organization and gene contents were comparable with those of other publically available mt genomes from a variety of Eimeria species and related coccidia; there were three complete coding DNA sequence regions encoding cytochrome c oxidase subunit I, cytochrome c oxidase subunit III and cytochrome B, and 33 regions encoding fragmented rdna. Alignment of these mt genome sequences demonstrates a relatively high (94.5%, 340 single nucleotide differences [SNDs]) pairwise sequence identity between these Eimeria spp. infecting ferrets; the majority of the SNDs resulted in synonymous codon changes with no changes to their protein products. Alignment of the newly sequenced mt genomes demonstrates, and phylogenetic reconstructions support, the monophyly of these Eimeria spp. of ferrets, with another Eimeria sp. of carnivores as the sister taxon to this clade. 6.1 INTRODUCTION: Coccidia are protozoal, eukaryotic, host-specific parasites of the phylum Apicomplexa, and can be divided into two major taxonomic suborders, the eimerioirinid and adeleid coccidia. The eimeriorinid coccidia include both the typical intestinal coccidia (e.g., Eimeria, Isospora, Cyclospora) species belonging to the family Eimeriidae as well as tissue or cyst-forming coccidia (e.g., Cystoisospora, 129
146 Besnoitia, Toxoplasma, Sarcocystis) of the family Sarcocystidae (see Cox, 1994). Ten species of Eimeria and twelve species of Isospora (=Cystoisospora) have been described in the Mustelidae (see Chapter 1, Table 1.1). Eimeria furonis has been reported in the European polecat (Mustela putorius), domestic ferret (DF; Mustela putorius furo), black-footed ferret (BFF; Mustela nigripes), and mink (Mustela vison) (Hoare 1927; Nukerbaeva and Svanbaev 1973; Jolley et al. 1994). Eimeria ictidea has been reported in the Steppe polecat (Mustela eversmanii), as well as the European polecat, domestic ferret and black-footed ferret (Hoare 1927; Svanbaev 1956; Jolley et al. 1994). These reports are based on the morphometric characteristics of oocysts identified in the feces of the aforementioned host species without the use of molecular techniques to confirm specific parasite identities. Recently, sequences of the mitochondrial cytochrome c oxidase subunit I gene (mt COI) and nuclear small subunit ribosomal DNA (nu 18S rdna) of E. furonis originating from a domestic ferret (nu 18S rdna: GenBank MF MF774680; mt COI: GenBank MF MF774036), and E. ictidea originating from a black-footed ferret (nu 18S rdna: GenBank MF MF860827; mt COI: GenBank MF860823, MF860825), were generated (see Chapters 2 and 3). The parasite originating from the black-footed ferret was identified as E. ictidea based on morphologic similarity to the original descriptions of E. ictidea from domestic ferrets; however, sequence-based genotyping of E. ictidea from domestic ferrets has not been completed and, consequently, it has not been demonstrated unequivocally that the two parasites are conspecific. In the present work, the complete mitochondrial genomes of E. furonis from the DF and E. ictidea from the BFF are described and compared with the mitochondrial genomes of related coccidia. 6.2 MATERIALS & METHODS: Parasites Two isolates of morphologically distinct Eimeria species were used in this study. Isolate one, identified morphologically and by nu 18S rdna and mt COI sequences as Eimeria furonis, was obtained 130
147 from a fecal sample from a DF that was submitted for routine ova and parasite examination to a European diagnostic laboratory 8. Isolate two, identified morphologically and by nu 18S rdna and mt COI sequences as Eimeria ictidea, was obtained from a fecal sample from a BFF and was collected during routine cage cleaning in a captive breeding facility (see Chapters 3 and 4). Fecal collection techniques for the BFF were reviewed and approved by both the Animal Welfare Committee and the Animal Care and Research Committee of the Toronto Zoo DNA isolation from coccidia in feces Genomic DNA was isolated from fecal derived coccidial oocysts as described section of the Chapter 2 Materials & Methods. Parasite DNA concentration was estimated using a Nanodrop 2000 spectrophotometer (NanoDrop Products, Wilmington, DE, USA) and DNA was stored at 4 C for immediate use or 20 C for later use Whole genome sequencing Mitochondrial whole genome amplification for both Eimeria species was initiated using sets of mt-specific primers that generated overlapping polymerase chain reaction (PCR) fragments (Tables 6.1 and 6.2). PCR amplification was performed for all samples in a volume of 25 µl containing ~100 ng of genomic DNA, 1 PCR buffer, 3 mm MgCl 2, 0.6 mm deoxyribonucleotide triphosphates (dntps), 500 nm of each primer and 4 U of Invitrogen Platinum Taq DNA Polymerase (Thermo Fisher Scientific, Toronto, ON, Canada). PCR reactions were performed on a Bio-Rad T100 PCR thermal cycler (Bio-Rad Laboratories, Singapore), using settings as described previously in the Materials & Methods section of Chapter 2. Table 6.1 details the specific anneal conditions used for the various primer pairs. Genomic DNA from either Eimeria maxima or Eimeria tenella acted as a positive control for the reaction chemistry. Gel electrophoresis, purification and sequencing of the PCR amplification products, were 8 Vet Med Labor GmbH, Division of IDEXX Laboratories, Ludwigsburg, Germany 131
148 performed as described in Chapter 2. The resulting chromatograms were aligned and analyzed with Geneious Ver or later (Biomatters Limited, Auckland, New Zealand) and high quality consensus sequences generated. The completed mt genome sequences were annotated by comparison with previously annotated mt genomes from other Eimeria species (e.g., Eimeria innocua - KR ) and the annotated mt genomes deposited in GenBank Phylogenetic analysis To determine the phylogenetic affinities of the newly obtained sequences with sequences from related apicomplexan taxa, representative whole mt genome sequences from eimeriid coccidia were downloaded from GenBank. A complete mt genome sequence from an unnamed Choleoeimeria sp. was used to root the ingroup taxa; several small genomic rearrangements in the Choleoeimeria sp. sequence required some rearrangement of the genome sequence to unambiguously align homologous regions across the complete mt genomes. Whole genome sequences were aligned using MAFTT v7.017 (Katoh et al. 2002) executed from within Geneious; the resulting alignment was examined by eye to adjust start and stop codon positions in aligned coding DNA sequence [CDS] regions (i.e., mt COI, mitochondrial cytochrome c oxidase subunit III gene [mtcoiii], mitochondrial cytochrome b gene [CytB]). Phylogenetic trees were generated using Bayesian Inference (BI) using MrBayes Ver (Huelsenbeck and Ronquist 2001) executed from within Geneious. The aligned complete mt genomes were partitioned into coding (i.e., CDS) and noncoding regions so that region-specific models of nucleotide substitution could be applied. Characters in the non-coding region were analysed with the general time reversible (GTR) model (Tavaré 1986) with the following parameters: nucmodel=4by4; nst=6; rates=invgamma (i.e. GTR+I+G). Characters in the coding regions were analysed using the codon nucleotide model (i.e., lset nucmodel=codon rates=gamma ngammacat=4) using metazoan mitochondrial translation (i.e., code=metmt). 132
149 All BI analyses were run for a chain length of 1,000,000 with tree sampling every 1,000 following a burn-in of 100,000 with default settings of 4 heated chains and heated chain temp of RESULTS: The whole mt genome sequences of the single isolates of E. furonis and E. ictidea were, respectively, 6165 base pairs (bp) (Figure 6.1, GenBank: MF795598) and 6182 bp (Figure 6.2, GenBank: KT203399). Content and organization of both mt genomes consisted of three protein-coding genes (mt COI, mt COIII and CytB) interspersed with large and small subunit ribosomal DNA (rdna) fragments. Details of the various CDS and rdna fragments are summarized in Table 6.3 (for E. furonis) and Table 6.4 (for E. ictidea). Pairwise alignment of the mt genome sequences from E. furonis and E. ictidea demonstrated a relatively high pairwise sequence identity (94.6%, 333 single nucleotide differences [SNDs]) between these two parasites. The bulk of the SNDs (67.6%, 225/333) were clustered within the three CDS regions that encode CytB, mt COI and mt COIII (see Figure 6.3 and Table 6.5). However, the majority of these SNDs (82.6%, 186/225) were synonymous codon changes that resulted in no changes to the protein products. Only 41 SNDs were involved in 34 amino acid changes distributed among the three CDS. The 33 rdna fragments comprised 2108 and 2109 bp, respectively, of the mt genomes of E. furonis and E. ictidea. Pairwise comparison of these rdna fragments demonstrated high (98.6%, 30 SNDs) sequence identity between the two parasites. The remaining 778 and 794 bp, respectively, of the mt genomes of E. furonis and E. ictidea were intergenic stretches between the various rdna and CDS regions; these intergenic regions were more variable that other regions of the genomes with 78 SNDs (almost 10% sequence divergence). Additionally, all indels were restricted to these variable intergenic regions. The BI phylogeny generated from aligned, complete mt genomes (Figure 6.4) supported the close relationship between E. furonis and E. ictidea within a clade of Eimeria species that include the only three sequences available for Eimeria spp. of carnivores. Eimeria mephitidis from the striped skunk (Mephitis 133
150 mephitis; Family Mephitidae) was the sister taxon to the two Eimeria species of ferrets (Family Mustelidae). 6.4 DISCUSSION: This work generated the first complete mt genomes from coccidia that infect domestic and blackfooted ferrets (Carnivora; Mustelidae). Eimeria mephitidis from the striped skunk Mephitis mephitis (Carnivora; Mephitidae), is the only other Eimeria species from a carnivore for which a complete mt genome has been reported. Comparatively few eimeriid coccidia, only 26 Eimeria species, have been described from carnivores; there are 14 named species from the mustelids, four from the procyonids, four from the ursids, three from the herpestids, and one from the viverrids (Duszynski et al. 2000). The majority of coccidia that infect the digestive tract of carnivores belong to the family Sarcocystidae, including monoxenous or facultatively heteroxenous Cystoisospora species or heteroxenous parasites in the genera Sarcocystis, Hammondia and Neospora. So far as is known, none of the parasites in the Sarcocystidae possess typical apicomplexan mt genomes with 3 complete CDS and many rdna fragments (Ogedengbe 2015). The mt genomes from the two Eimeria sp. of mustelid origin demonstrate the same structural organization (i.e., the order and number of CDS and rdna fragments) and circular mapping as the mt genomes from other Eimeria spp. and other closely related eimeriid coccidia such as Isospora, Cyclospora and Lankesterella species. Despite the ability of the eimeriid sequences to be mapped circularly, the physical form of Eimeria spp. mt genomes may be a linear concatemer of multiple genome copies as demonstrated for Eimeria tenella (Hikosaka et al. 2011). As in the mt genomes of other eimeriid coccidia (Ogedengbe et al. 2013, 2014), the CDS for mt COIII demonstrated the highest sequence divergence between E. furonis and E. ictidea; the mt COI CDS was somewhat more conserved and CytB CDS demonstrated the fewest SNDs. As expected, based on limited sequence divergence between E. furonis and E. ictidea, a BI phylogenetic analysis using aligned, complete mt genome sequences generated a tree that placed these 134
151 two Eimeria species that infect mustelids within a well-supported monophyletic group. The sister taxon for these ferret parasites was the only other Eimeria species from carnivores for which a complete mt genome is available, E. mephitidis, which infects hosts belonging to a different family of carnivores. Eimeriid parasites that infect closely-related definitive hosts are commonly found in a single or limited number of clades based on mitochondrial and nuclear genetic loci (Ogedengbe et al., in press). Sequencing of the mt genomes and at least one nuclear genetic locus (i.e., nu 18S rdna) from additional Eimeria species infecting carnivores will be required to determine if all carnivore-specific Eimeria species share a common ancestor. 135
152 Table 6.1 PCR primer pairs and resulting fragments used for sequencing the mitochondrial genome sequence of an isolate of Eimeria furonis originating from a fecal sample from a domestic ferret (Mustela putorius furo) Fragment Primer names Primer sequences (5ʹ-3ʹ) Size (bp) Anneal Temp References 1 WG-MT_4140F AGAAAACCTAAAATCATCATGT Ogedengbe et al. (2015) Eim_CO3_799R AAGTGAGTTCGCATGTTTAC Ogedengbe et al. (2015) 2 Eim_COI_19F ACTGCYGCAAACCATAARGAA Present study Api_LSUG_UNI_R AGATAGGGAACAAACTGYCTCAA Present study 3 WG_MT_5416F GGTCCAGATAAGCGATCTCATG Ogedengbe et al. (2013) Eim_COI_1436R CACATTGTGTTCARATAAGTTA Present study 4 WG-MT_6219F GCATCCATCTACAGCTGCGG Ogedengbe et al. (2013) WG-MT_344R GTAGGAATCTRAATTCCCAACC Ogedengbe et al. (2013) 5 Api_LSUE UNI_F AGGTGCTCAGGGTCTTACCG Present study WG_MT_63R CTGGTATGGATGGATAACACT Ogedengbe et al. (2015) 6 Lank_COB-30F CCAGGCCAACTGAACTCGTT Present study q_eim_coi_221r GGCATAACTACAAAGAARATCATA Present study 7 Cocci_MT_WG_F TACACCTAGCCAACACGAT Ogedengbe et al. (2014) q_eim_coi_221r GGCATAACTACAAAGAARATCATA Present study 136
153 Table 6.2 PCR primer pairs and resulting fragments used for sequencing the mitochondrial genome sequence of an isolate of Eimeria ictidea originating from a fecal sample from a black-footed ferret (Mustela nigripes) Fragment Primer names Primer sequences (5ʹ-3ʹ) Size (bp) Anneal Temp References 1 WG_MT_63R CTGGTATGGATGGATAACACT Ogedengbe et al. (2015) WG-MT_4140F AGAAAACCTAAAATCATCATGT Ogedengbe et al. (2015) 2 Cocci_MT_WG_F TACACCTAGCCAACACGAT Ogedengbe et al. (2014) q_eim_coi_221r GGCATAACTACAAAGAARATCATA Present study 3 WG-MT_3658F CTGGCGAGAAGGGAAGTGTG Ogedengbe et al. (2013) Eim_CO3_799R AAGTGAGTTCGCATGTTTAC Ogedengbe et al. (2015) 4 Lank_COB-30F CCAGGCCAACTGAACTCGTT Present study WG_MT_4072R GGTTGTTTCCATCTCGACTC Ogedengbe et al. (2013) 137
154 Table 6.3 Coding regions within the mitochondrial genome of the eimeriid parasite Eimeria furonis from a domestic ferret (Mustela putorius faro) 138
155 Table 6.4 Coding regions of the mitochondrial genome of the eimeriid parasite Eimeria ictidea originating from a black-footed ferret (Mustela nigripes). Protein coding regions (CDS) Sequence size (bp) Start position (bp) Stop position (bp) Direction Translation start codon Translation stop codon Cytochrome c oxidase subunit I (COI) Forward ATG TAA Cytochrome c oxidase subunit III (COIII) Forward TTA TAA Cytochrome b (CytB) Forward ATG TAA Ribosomal DNA fragments (rdna) Product SSUrRNA forward RNA9; SSU/8 SSUrRNA forward SSUA; SSU/4 (partial) SSUrRNA forward RNA23t LSUrRNA forward RNA20 (partial); LSU LSUrRNA forward LSUF; LSU11 LSUrRNA forward LSUG; LSU/12 LSUrRNA reverse LSU SSUrRNA forward RNA14; SSU/1 LSUrRNA reverse LSUC; LSU/4 SSUrRNA forward SSU SSUrRNA reverse SSUF; SSU/12 LSUrRNA forward RNA10; LSU/13 (partial) LSUrRNA forward RNA11; LSU/5 SSUrRNA forward SSUD; SSU/10 SSUrRNA forward RNA17; SSU3 SSUrRNA forward RNA15; SSU LSUrRNA forward RNA13; LSU/10 LSUrRNA forward RNA6; LSU/15 LSUrRNA reverse LSUD; LSU/8 LSUrRNA reverse RNA16 (partial) SSUrRNA reverse RNA8; SSU/5 LSUrRNA forward RNA2; LSU/2 LSUrRNA reverse LSUA; LSU/1 SSUrRNA reverse RNA19; SSU/7 LSUrRNA forward RNA1; LSU/6 LSUrRNA reverse LSUB; LSU/3 LSUrRNA reverse RNA3; LSU/7 LSUrRNA reverse RNA18; LSU/14 SSUrRNA reverse SSUB; SSU/6 LSUrRNA reverse RNA7 LSUrRNA reverse LSUE; LSU/9 SSUrRNA reverse SSUE; SSU/11 (partial) SSUrRNA reverse RNA5/SSU9 139
156 Table 6.5 Pairwise comparison of coding DNA and concatenated rdna fragment sequences between the mitochondrial genomes of Eimeria furonis originating from a domestic ferret (Mustela putorius furo) and Eimeria ictidea originating from a black-footed ferret (Mustela nigripes). Total length Nucleotide Total amino Amino acid (nucleotides) identity acids identity COI CDS % (95) % (12) COIII CDS % (76) % (17) CytB CDS % (54) % (5) rdna fragments % (32) n/a n/a Legend: Numbers in brackets indicate the number of single nucleotide differences; n/a = not applicable 140
157 Figure 6.1 Circular mapping and organization of the mitochondrial genome content of Eimeria furonis showing three protein-coding genes (COI, COIII and CytB) interspersed with large and small subunit rrna fragments. 141
158 Figure 6.2 Circular mapping and organization of the mitochondrial genome content of Eimeria ictidea showing three protein-coding genes (COI, COIII and CytB) interspersed with large and small subunit rrna fragments. 142
Phylum:Apicomplexa Class:Sporozoa
 Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Wildlife Health Centre Bios
 Wildlife Health Centre Bios Meet the Toronto Zoo s Wildlife Health Centre Team Dr. Christopher Dutton, BSc, BVSc, MSc, DipIACZM, DipIECZM (ZHM) Head of Veterinary Services Dr. Christopher Dutton is the
Wildlife Health Centre Bios Meet the Toronto Zoo s Wildlife Health Centre Team Dr. Christopher Dutton, BSc, BVSc, MSc, DipIACZM, DipIECZM (ZHM) Head of Veterinary Services Dr. Christopher Dutton is the
Protozoa. Apicomplexa Sarcomastigophora Ciliophora. Gregarinea Coccidia Piroplasma
 Protozoa Apicomplexa Sarcomastigophora Ciliophora Gregarinea Coccidia Piroplasma Coccidia characterized by thick-walled oocysts excreted in feces In Humans Cryptosporidium Isospora Cyclospora Sarcocystis
Protozoa Apicomplexa Sarcomastigophora Ciliophora Gregarinea Coccidia Piroplasma Coccidia characterized by thick-walled oocysts excreted in feces In Humans Cryptosporidium Isospora Cyclospora Sarcocystis
Coccidia. Nimit Morakote, Ph.D.
 Coccidia Nimit Morakote, Ph.D. 1 Learning objectives After class, students will be able to: Describe morphology, life cycle, signs and symptoms, prevention and control, laboratory diagnosis and treatment
Coccidia Nimit Morakote, Ph.D. 1 Learning objectives After class, students will be able to: Describe morphology, life cycle, signs and symptoms, prevention and control, laboratory diagnosis and treatment
Diagnosis, treatment and control: dealing with coccidiosis in cattle
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Diagnosis, treatment and control: dealing with coccidiosis in cattle Author : Adam Martin Categories : Vets Date : January
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Diagnosis, treatment and control: dealing with coccidiosis in cattle Author : Adam Martin Categories : Vets Date : January
Ahead of print online version
 Folia Parasitologica 60 [3]: 232 236, 2013 ISSN 0015-5683 (print), ISSN 1803-6465 (online) Institute of Parasitology, Biology Centre ASCR http://folia.paru.cas.cz/ A new species of Choleoeimeria (Apicomplexa:
Folia Parasitologica 60 [3]: 232 236, 2013 ISSN 0015-5683 (print), ISSN 1803-6465 (online) Institute of Parasitology, Biology Centre ASCR http://folia.paru.cas.cz/ A new species of Choleoeimeria (Apicomplexa:
Protozoan Parasites of Veterinary importance 2017
 Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Apicomplexa of Intestinal Pathology
 LECTURES #4, #5 & #6: APICOMPLEXA 1 Apicomplexa of Intestinal Pathology Cryptosporidium, Eimeria, Cystoisospora General Characteristics of Apicomplexa A. Morphology by stage Zoite o Tear-shaped (cylindrical
LECTURES #4, #5 & #6: APICOMPLEXA 1 Apicomplexa of Intestinal Pathology Cryptosporidium, Eimeria, Cystoisospora General Characteristics of Apicomplexa A. Morphology by stage Zoite o Tear-shaped (cylindrical
Joerg Kinne, Mansoor Ali*, Ulrich Wernery, and J. P. Dubey
 J. Parasitol., 88(3), 2002, pp. 548 552 American Society of Parasitologists 2002 CLINICAL LARGE INTESTINAL COCCIDIOSIS IN CAMELS (CAMELUS DROMEDARIUS) IN THE UNITED ARAB EMIRATES: DESCRIPTION OF LESIONS,
J. Parasitol., 88(3), 2002, pp. 548 552 American Society of Parasitologists 2002 CLINICAL LARGE INTESTINAL COCCIDIOSIS IN CAMELS (CAMELUS DROMEDARIUS) IN THE UNITED ARAB EMIRATES: DESCRIPTION OF LESIONS,
Apicomplexans Apicomplexa Intro
 Apicomplexans Apicomplexa Intro Cryptosporidium Apicomplexan Select Characteristics Gliding motility Apical Complex organelle for invasion of host cell Life cycle alternates b/w sexual and asexual phases
Apicomplexans Apicomplexa Intro Cryptosporidium Apicomplexan Select Characteristics Gliding motility Apical Complex organelle for invasion of host cell Life cycle alternates b/w sexual and asexual phases
A NEW SPECIES OF GENUS EIMERIA (APICOMPLEXA: EUCOCCIDIORIDA) FROM GOAT.
 A NEW SPECIES OF GENUS EIMERIA (APICOMPLEXA: EUCOCCIDIORIDA) FROM GOAT. B.V. More 1, H.A.Kamble. 2 S.V. Nikam 3, 1 Department of Zoology, Ramkrishna Paramhansa Mahavidyalaya, Osmanabad. (M.S.) India. 2
A NEW SPECIES OF GENUS EIMERIA (APICOMPLEXA: EUCOCCIDIORIDA) FROM GOAT. B.V. More 1, H.A.Kamble. 2 S.V. Nikam 3, 1 Department of Zoology, Ramkrishna Paramhansa Mahavidyalaya, Osmanabad. (M.S.) India. 2
Sam R. Telford, Jr The Florida Museum of Natural History, University of Florida, Gainesville, Fl32611, USA
 Systematic Parasitology 23: 203-208, 1992. 0 1992 Kluwer Academic Publishers. Printed in the Netherlands. An eimeriid species (Apicomplexa: Eimeriidae) that parasitises the gallbladder and bile-duct of
Systematic Parasitology 23: 203-208, 1992. 0 1992 Kluwer Academic Publishers. Printed in the Netherlands. An eimeriid species (Apicomplexa: Eimeriidae) that parasitises the gallbladder and bile-duct of
Systemic Apicomplexans. Toxoplasma
 Systemic Apicomplexans Toxoplasma Protozoan Groups Historically, protozoa have been grouped by mode of motility. Flagellates Hemoflagellates Trypanosoma cruzi Leishmania infantum Mucoflagellates Tritrichomonas
Systemic Apicomplexans Toxoplasma Protozoan Groups Historically, protozoa have been grouped by mode of motility. Flagellates Hemoflagellates Trypanosoma cruzi Leishmania infantum Mucoflagellates Tritrichomonas
LABORATORY. The Protozoa. At the Bench
 LABORATORY Laboratory 8, Page 1 8 The Protozoa Introduction: The protozoa are unicellular animals that are classified on the basis of the organelles used for locomotion (flagella, pseudopodia, cilia or
LABORATORY Laboratory 8, Page 1 8 The Protozoa Introduction: The protozoa are unicellular animals that are classified on the basis of the organelles used for locomotion (flagella, pseudopodia, cilia or
PLASMODIUM MODULE 39.1 INTRODUCTION OBJECTIVES 39.2 MALARIAL PARASITE. Notes
 Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Canine and Feline Distemper. Description. The following chart indicates the animals which are susceptible to infection by canine and feline distemp
 Canine and Feline Distemper Description Canine and feline distemper are diseases affecting many wild and domestic carnivo The following chart indicates the animals which are susceptible to infection by
Canine and Feline Distemper Description Canine and feline distemper are diseases affecting many wild and domestic carnivo The following chart indicates the animals which are susceptible to infection by
Cr 2 O 7. Key words: coccidia - apicomplexa - Eimeria - peacock - Pavo cristatus - Egypt. Materials and Methods
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 105(8): 965-969, December 2010 965 Eimeria pavoaegyptica sp. nov. (Apicomplexa: Eimeriidae) in faeces of Indian peacocks, Pavo cristatus Linnaeus, 1758 (Galliformes:
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 105(8): 965-969, December 2010 965 Eimeria pavoaegyptica sp. nov. (Apicomplexa: Eimeriidae) in faeces of Indian peacocks, Pavo cristatus Linnaeus, 1758 (Galliformes:
DOWNLOAD OR READ : VETERINARY CLINICAL PARASITOLOGY PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : VETERINARY CLINICAL PARASITOLOGY PDF EBOOK EPUB MOBI Page 1 Page 2 veterinary clinical parasitology veterinary clinical parasitology pdf veterinary clinical parasitology Use these links
DOWNLOAD OR READ : VETERINARY CLINICAL PARASITOLOGY PDF EBOOK EPUB MOBI Page 1 Page 2 veterinary clinical parasitology veterinary clinical parasitology pdf veterinary clinical parasitology Use these links
Course Curriculum for Master Degree in Internal Medicine/ Faculty of Veterinary Medicine
 Course Curriculum for Master Degree in Internal Medicine/ Faculty of Veterinary Medicine The Master Degree in Internal Medicine/Faculty of Veterinary Medicine is awarded by the Faculty of Graduate Studies
Course Curriculum for Master Degree in Internal Medicine/ Faculty of Veterinary Medicine The Master Degree in Internal Medicine/Faculty of Veterinary Medicine is awarded by the Faculty of Graduate Studies
Observations on Eimeria species of Dasyprocta leporina (Linnaeus, 1758) (Rodentia: Dasyproctidae) from the state of Pará, North Brazil
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 99: 000-000, 2004 1 Observations on Eimeria species of Dasyprocta leporina (Linnaeus, 1758) (Rodentia: Dasyproctidae) from the state of Pará, North Brazil Ralph
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 99: 000-000, 2004 1 Observations on Eimeria species of Dasyprocta leporina (Linnaeus, 1758) (Rodentia: Dasyproctidae) from the state of Pará, North Brazil Ralph
Parasitology Amoebas. Sarcodina. Mastigophora
 Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Biology of toxoplasmosis
 1 Biology of toxoplasmosis E. Petersen 1 and J. P. Dubey 2 1 Statens Seruminstitut, Copenhagen, Denmark 2 U.S. Department of Agriculture, Beltsville, USA History Toxoplasma gondii is a coccidium, with
1 Biology of toxoplasmosis E. Petersen 1 and J. P. Dubey 2 1 Statens Seruminstitut, Copenhagen, Denmark 2 U.S. Department of Agriculture, Beltsville, USA History Toxoplasma gondii is a coccidium, with
EFFECTS OF SEASON AND RESTRICTED FEEDING DURING REARING AND LAYING ON PRODUCTIVE AND REPRODUCTIVE PERFORMANCE OF KOEKOEK CHICKENS IN LESOTHO
 EFFECTS OF SEASON AND RESTRICTED FEEDING DURING REARING AND LAYING ON PRODUCTIVE AND REPRODUCTIVE PERFORMANCE OF KOEKOEK CHICKENS IN LESOTHO By SETSUMI MOTŠOENE MOLAPO MSc (Animal Science) NUL Thesis submitted
EFFECTS OF SEASON AND RESTRICTED FEEDING DURING REARING AND LAYING ON PRODUCTIVE AND REPRODUCTIVE PERFORMANCE OF KOEKOEK CHICKENS IN LESOTHO By SETSUMI MOTŠOENE MOLAPO MSc (Animal Science) NUL Thesis submitted
Giardia and Apicomplexa. G. A. Lozano UNBC
 Giardia and Apicomplexa G. A. Lozano UNBC NINE Protozoan diseases/parasites Ciliphora, Ichthyophthirius, Ick Sarcomastigophora, Giardia, giardiasis Apicomplexa: Eimeria, Toxoplasma, Sarcocystis, Cryptosporidium.
Giardia and Apicomplexa G. A. Lozano UNBC NINE Protozoan diseases/parasites Ciliphora, Ichthyophthirius, Ick Sarcomastigophora, Giardia, giardiasis Apicomplexa: Eimeria, Toxoplasma, Sarcocystis, Cryptosporidium.
Course Curriculum for Master Degree in Poultry Diseases/Veterinary Medicine
 Course Curriculum for Master Degree in Poultry Diseases/Veterinary Medicine The Master Degree in Poultry Diseases /Veterinary Medicine, is awarded by the Faculty of Graduate Studies at Jordan University
Course Curriculum for Master Degree in Poultry Diseases/Veterinary Medicine The Master Degree in Poultry Diseases /Veterinary Medicine, is awarded by the Faculty of Graduate Studies at Jordan University
ANTICOCCIDIALS USED FOR THE THERAPY OF COCCIDIOSIS IN CHICKENS, TURKEYS AND GEESE
 ANTICOCCIDIALS USED FOR THE THERAPY OF COCCIDIOSIS IN CHICKENS, TURKEYS AND GEESE Guideline Title Anticoccidials used for the Therapy of Coccidiosis i n Chickens, Turkey and Geese Legislative Basis Directive
ANTICOCCIDIALS USED FOR THE THERAPY OF COCCIDIOSIS IN CHICKENS, TURKEYS AND GEESE Guideline Title Anticoccidials used for the Therapy of Coccidiosis i n Chickens, Turkey and Geese Legislative Basis Directive
Coccidiosis in macropods and other species
 Coccidiosis in macropods and other species Author: Derek Spielman Wildlife Assistance and Information Foundation; Sydney School of Veterinary Science, the University of Sydney Abstract This presentation
Coccidiosis in macropods and other species Author: Derek Spielman Wildlife Assistance and Information Foundation; Sydney School of Veterinary Science, the University of Sydney Abstract This presentation
Key words: Coccidia, Choleoeimeria rochalimai, fine structure, gall bladder epithelium, Hemidactylus mabouia, Brazil
 FOLIA PARASITOLOGICA 47: 91-96, 2000 Ultrastructural study of meronts and gamonts of Choleoeimeria rochalimai (Apicomplexa: Eimeriidae) developing in the gall bladder of the gecko Hemidactylus mabouia
FOLIA PARASITOLOGICA 47: 91-96, 2000 Ultrastructural study of meronts and gamonts of Choleoeimeria rochalimai (Apicomplexa: Eimeriidae) developing in the gall bladder of the gecko Hemidactylus mabouia
1) Most common, infectious, pathogenic animal (zoonotic) parasite of humans; estimated that 13% of humans are infected
 XX Phylum Apicomplexa (Chapter 8) 2005 A. Characteristics 1. All are parasitic 2. APICAL COMPLEX a. Group of organelles used to invade host cells b. Visible only with electron microscopy Picture Slide
XX Phylum Apicomplexa (Chapter 8) 2005 A. Characteristics 1. All are parasitic 2. APICAL COMPLEX a. Group of organelles used to invade host cells b. Visible only with electron microscopy Picture Slide
HISTOPATHOLOGY. Introduction:
 Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine
 Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine The Master Degree in Theriogenology & Artificial Insemination /Faculty of Veterinary Medicine
Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine The Master Degree in Theriogenology & Artificial Insemination /Faculty of Veterinary Medicine
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Most clients are well aware that puppies
 D i a g n o s t i c s P A R A S I T O L O G Y Michael W. Dryden, DVM, MS, PhD, & Patricia A. Payne, DVM, PhD Kansas State University Fecal Examination Techniques Intestinal parasites are both a real and
D i a g n o s t i c s P A R A S I T O L O G Y Michael W. Dryden, DVM, MS, PhD, & Patricia A. Payne, DVM, PhD Kansas State University Fecal Examination Techniques Intestinal parasites are both a real and
Outline 1/13/15. Range is mostly surrounding Puerto Rico Important for Tourism and ecological balance
 1/13/15 Prevalence of Toxoplasma gondii in Antillean manatees (Trichechus manatus manatus) and investigating transmission from feral cat feces in Puerto Rico Heidi Wyrosdick M.S. Candidate University of
1/13/15 Prevalence of Toxoplasma gondii in Antillean manatees (Trichechus manatus manatus) and investigating transmission from feral cat feces in Puerto Rico Heidi Wyrosdick M.S. Candidate University of
MURDOCH RESEARCH REPOSITORY
 MURDOCH RESEARCH REPOSITORY This is the author s final version of the work, as accepted for publication following peer review but without the publisher s layout or pagination. The definitive version is
MURDOCH RESEARCH REPOSITORY This is the author s final version of the work, as accepted for publication following peer review but without the publisher s layout or pagination. The definitive version is
Coccidia. Toxoplasma gondii, Sarcocystis spp., Isospora belli, Cryptosporidium spp., Cyclospora cayetanenesis. Nimit Morakote, Ph.D.
 Coccidia Toxoplasma gondii, Sarcocystis spp., Isospora belli, Cryptosporidium spp., Cyclospora cayetanenesis Nimit Morakote, Ph.D. 1 เอกสารประกอบการบรรยายน จ ดทาสาหร บกระบวนว ชา 317331, ภาค เร ยนท 2 ป
Coccidia Toxoplasma gondii, Sarcocystis spp., Isospora belli, Cryptosporidium spp., Cyclospora cayetanenesis Nimit Morakote, Ph.D. 1 เอกสารประกอบการบรรยายน จ ดทาสาหร บกระบวนว ชา 317331, ภาค เร ยนท 2 ป
Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51
 Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual reproduction Intestinal
Protozoan Parasites: Lecture 20 - Heteroxenous Coccidia - Part 1 Pages 39-51 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual reproduction Intestinal
A COCCIDIAN IN HAEMOGAMASID MITES; POSSIBLE VECTORS OF ELLEIPSISOMA THOMSONI FRANCA, 1912
 Masson, Paris, 1987. Ann. Parasitol. Hum. Comp., 1987, 62, n 2, pp. 107-116. A COCCIDIAN IN HAEMOGAMASID MITES; POSSIBLE VECTORS OF ELLEIPSISOMA THOMSONI FRANCA, 1912 H. A. MOHAMED, D. H. MOLYNEUX, K.
Masson, Paris, 1987. Ann. Parasitol. Hum. Comp., 1987, 62, n 2, pp. 107-116. A COCCIDIAN IN HAEMOGAMASID MITES; POSSIBLE VECTORS OF ELLEIPSISOMA THOMSONI FRANCA, 1912 H. A. MOHAMED, D. H. MOLYNEUX, K.
Rodent Husbandry and Care 201 Cynthia J. Brown and Thomas M. Donnelly
 EXOTIC PET MANAGEMENT FOR THE TECHNICIAN Preface Michelle S. Schulte and Agnes E. Rupley xi Rodent Husbandry and Care 201 Cynthia J. Brown and Thomas M. Donnelly This article reviews the husbandry, care
EXOTIC PET MANAGEMENT FOR THE TECHNICIAN Preface Michelle S. Schulte and Agnes E. Rupley xi Rodent Husbandry and Care 201 Cynthia J. Brown and Thomas M. Donnelly This article reviews the husbandry, care
of Nebraska - Lincoln
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 2006
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 2006
American Journal of Animal and Veterinary Sciences. Introduction. Original Research Paper
 American Journal of Animal and Veterinary Sciences Original Research Paper Eimeria Legionensis and Eimeria kofoidi (Apicomplexa: Eimeriidae) Infection and Associated Lesions in Naturally Infected Red-Legged
American Journal of Animal and Veterinary Sciences Original Research Paper Eimeria Legionensis and Eimeria kofoidi (Apicomplexa: Eimeriidae) Infection and Associated Lesions in Naturally Infected Red-Legged
Lecture 11 Wednesday, September 19, 2012
 Lecture 11 Wednesday, September 19, 2012 Phylogenetic tree (phylogeny) Darwin and classification: In the Origin, Darwin said that descent from a common ancestral species could explain why the Linnaean
Lecture 11 Wednesday, September 19, 2012 Phylogenetic tree (phylogeny) Darwin and classification: In the Origin, Darwin said that descent from a common ancestral species could explain why the Linnaean
Protozoologica INTRODUCTION. Acta Protozool. (2008) 47: 69 76
 Acta Protozoologica Acta Protozool. (2008) 47: 69 76 A New Species of Coccidia (Apicomplexa: Sarcocystidae) from the Slendertailed Meerkat Suricata suricatta (Scheber, 1776) from South Africa Amal K. EL-GAYAR
Acta Protozoologica Acta Protozool. (2008) 47: 69 76 A New Species of Coccidia (Apicomplexa: Sarcocystidae) from the Slendertailed Meerkat Suricata suricatta (Scheber, 1776) from South Africa Amal K. EL-GAYAR
Fact sheet. All animals, particularly herbivores, appear to be natural hosts for coccidian species with a high degree of host specificity observed.
 Coccidia in k angaroos Fact sheet Introductory statement Coccidians are protozoan parasites which infect the intestinal tract of many animals. Within kangaroos, coccidia infections can lead to clinical
Coccidia in k angaroos Fact sheet Introductory statement Coccidians are protozoan parasites which infect the intestinal tract of many animals. Within kangaroos, coccidia infections can lead to clinical
Some aspects of wildlife and wildlife parasitology in New Zealand
 Some aspects of wildlife and wildlife parasitology in New Zealand Part 3/3 Part three: Kiwis and aspects of their parasitology Kiwis are unique and unusual in many ways. For a comprehensive and detailed
Some aspects of wildlife and wildlife parasitology in New Zealand Part 3/3 Part three: Kiwis and aspects of their parasitology Kiwis are unique and unusual in many ways. For a comprehensive and detailed
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign
 A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
STUDY OF EIMERIA INTRICATA IN GOAT AND SHEEP FROM BEED DISTRICT, MAHARASHTRA STATE INDIA
 STUDY OF EIMERIA INTRICATA IN GOAT AND SHEEP FROM BEED DISTRICT, MAHARASHTRA STATE INDIA More B.V., Kamble H.A. and Nikam S.V. 1 Department of Zoology, Ramkrishna Paramhansa Mahavidyalaya, Osmanabad. (M.S.),
STUDY OF EIMERIA INTRICATA IN GOAT AND SHEEP FROM BEED DISTRICT, MAHARASHTRA STATE INDIA More B.V., Kamble H.A. and Nikam S.V. 1 Department of Zoology, Ramkrishna Paramhansa Mahavidyalaya, Osmanabad. (M.S.),
A Study of Coccidiosis in Livestock in the Island of Dominica. Joshua Santelises. Study Abroad Texas A&M University. Dr.
 A Study of Coccidiosis in Livestock in the Island of Dominica Joshua Santelises Study Abroad 2012 Texas A&M University Dr. Thomas Lacher Dr. Jim Woolley Abstract The following experiment was done to investigate
A Study of Coccidiosis in Livestock in the Island of Dominica Joshua Santelises Study Abroad 2012 Texas A&M University Dr. Thomas Lacher Dr. Jim Woolley Abstract The following experiment was done to investigate
Dominance/Suppression Competitive Relationships in Loblolly Pine (Pinus taeda L.) Plantations
 Dominance/Suppression Competitive Relationships in Loblolly Pine (Pinus taeda L.) Plantations by Michael E. Dyer Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and Stand University
Dominance/Suppression Competitive Relationships in Loblolly Pine (Pinus taeda L.) Plantations by Michael E. Dyer Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and Stand University
Ahead of print online version
 Folia Parasitologica 61 [3]: 195 200, 2014 ISSN 0015-5683 (print), ISSN 1803-6465 (online) doi: 10.14411/fp.2014.018 Institute of Parasitology, Biology Centre ASCR http://folia.paru.cas.cz/ Four new species
Folia Parasitologica 61 [3]: 195 200, 2014 ISSN 0015-5683 (print), ISSN 1803-6465 (online) doi: 10.14411/fp.2014.018 Institute of Parasitology, Biology Centre ASCR http://folia.paru.cas.cz/ Four new species
The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 95(1): 43-47, Jan./Feb. 2000 The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 95(1): 43-47, Jan./Feb. 2000 The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
Intestinal Apicomplexans
 Itestial Apicomplexas Cystoisospora (= Isospora) Protozoa Groups Historically, protozoa have bee grouped by mode of motility. Flagellates Hemoflagellates Trypaosoma cruzi Leishmaia ifatum Mucoflagellates
Itestial Apicomplexas Cystoisospora (= Isospora) Protozoa Groups Historically, protozoa have bee grouped by mode of motility. Flagellates Hemoflagellates Trypaosoma cruzi Leishmaia ifatum Mucoflagellates
Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49
 Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual
Protozoan Parasites: Lecture 21 Apicomplexans 3 Heteroxenous Coccidia - Part 1 Pages 37-49 Tissue cyst -forming Coccidia General Taxonomy Apicomplexa Heteroxenous Two host life cycles Asexual & sexual
Isospora ticoticoi n. sp. (Apicomplexa: Eimeriidae) from the Rufouscollared Sparrow Zonotrichia capensis in South America
 Acta Protozoologica Acta Protozool. (2009) 48: 345 349 Isospora ticoticoi n. sp. (Apicomplexa: Eimeriidae) from the Rufouscollared Sparrow Zonotrichia capensis in South America Lianna M. C. BALTHAZAR 1,
Acta Protozoologica Acta Protozool. (2009) 48: 345 349 Isospora ticoticoi n. sp. (Apicomplexa: Eimeriidae) from the Rufouscollared Sparrow Zonotrichia capensis in South America Lianna M. C. BALTHAZAR 1,
University of Canberra. This thesis is available in print format from the University of Canberra Library.
 University of Canberra This thesis is available in print format from the University of Canberra Library. If you are the author of this thesis and wish to have the whole thesis loaded here, please contact
University of Canberra This thesis is available in print format from the University of Canberra Library. If you are the author of this thesis and wish to have the whole thesis loaded here, please contact
Project title: Evaluation of the prevalence of coccidia in Ontario suckling. piglets and identification of a preventive treatment
 Project title: Evaluation of the prevalence of coccidia in Ontario suckling piglets and identification of a preventive treatment Final report: July 6, 2007 Principal Investigator: Andrew Peregrine, Department
Project title: Evaluation of the prevalence of coccidia in Ontario suckling piglets and identification of a preventive treatment Final report: July 6, 2007 Principal Investigator: Andrew Peregrine, Department
Apicomplexan Parasites: Environmental Contamination and Transmission
 Polish Journal of Microbiology 2004, Vol. 53, Suppl., 67 73 Apicomplexan Parasites: Environmental Contamination and Transmission EDWARD SIÑSKI 1 and JERZY M. BEHNKE 2 1 Department of Parasitology, Institute
Polish Journal of Microbiology 2004, Vol. 53, Suppl., 67 73 Apicomplexan Parasites: Environmental Contamination and Transmission EDWARD SIÑSKI 1 and JERZY M. BEHNKE 2 1 Department of Parasitology, Institute
Biology of Isospora spp. from Humans, Nonhuman Primates, and Domestic Animals
 CLINICAL MICROBIOLOGY REVIEWS, Jan. 1997, p. 19 34 Vol. 10, No. 1 0893-8512/97/$04.00 0 Copyright 1997, American Society for Microbiology Biology of Isospora spp. from Humans, Nonhuman Primates, and Domestic
CLINICAL MICROBIOLOGY REVIEWS, Jan. 1997, p. 19 34 Vol. 10, No. 1 0893-8512/97/$04.00 0 Copyright 1997, American Society for Microbiology Biology of Isospora spp. from Humans, Nonhuman Primates, and Domestic
Coccidia in a Shelter Setting Video Transcript July 2013
 Coccidia in a Shelter Setting Video Transcript July 2013 This transcript has been automatically generated and may not be 100% accurate. This text may not be in its final form and may be updated or revised
Coccidia in a Shelter Setting Video Transcript July 2013 This transcript has been automatically generated and may not be 100% accurate. This text may not be in its final form and may be updated or revised
Protozoan Parasites: Lecture 20 Apicomplexans II Coccidia Part II & Cryptosporidium Pages 28-36
 Protozoan Parasites: Lecture 20 Apicomplexans II Coccidia Part II & Cryptosporidium Pages 28-36 Coccidia: Life cycle & treatment/control effectiveness? Asexual stages Sexual stages Prophylactic drugs Current
Protozoan Parasites: Lecture 20 Apicomplexans II Coccidia Part II & Cryptosporidium Pages 28-36 Coccidia: Life cycle & treatment/control effectiveness? Asexual stages Sexual stages Prophylactic drugs Current
AVIAN COCCIDIOSIS. One of the most potentially destructive diseases in domestic poultry production. Most costly of all poultry diseases.
 AVIAN COCCIDIOSIS One of the most potentially destructive diseases in domestic poultry production. Most costly of all poultry diseases. Strictly a gut infection in chickens and turkeys. All avian species
AVIAN COCCIDIOSIS One of the most potentially destructive diseases in domestic poultry production. Most costly of all poultry diseases. Strictly a gut infection in chickens and turkeys. All avian species
Large Animal Topics in Parasitology for the Veterinary Technician Jason Roberts, DVM This presentation is designed to review the value veterinary
 Large Animal Topics in Parasitology for the Veterinary Technician Jason Roberts, DVM This presentation is designed to review the value veterinary technicians can add to mixed or large animal practices
Large Animal Topics in Parasitology for the Veterinary Technician Jason Roberts, DVM This presentation is designed to review the value veterinary technicians can add to mixed or large animal practices
Professor Joe Camp June 2018
 Giardia in dogs Professor Joe Camp June 2018 How does a dog get Giardia? Why is it in so many kennels? Why is it so hard to get rid of? What can you do in a large kennel (including shelter kennels)? Giardia
Giardia in dogs Professor Joe Camp June 2018 How does a dog get Giardia? Why is it in so many kennels? Why is it so hard to get rid of? What can you do in a large kennel (including shelter kennels)? Giardia
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Cystic echinococcosis in a domestic cat: an Italian case report
 13th NRL Workshop, Rome, 24-25 May, 2018 Cystic echinococcosis in a domestic cat: an Italian case report Istituto Zooprofilattico Sperimentale (IZS) of Sardinia National Reference Laboratory for Cistic
13th NRL Workshop, Rome, 24-25 May, 2018 Cystic echinococcosis in a domestic cat: an Italian case report Istituto Zooprofilattico Sperimentale (IZS) of Sardinia National Reference Laboratory for Cistic
Cryptosporidium: Cryptosporidium: Director, UK Cryptosporidium Reference Unit the global challenge in monit toring
 Cryptosporidium: still cryptic after all these years Dr Rachel Chalmers Cryptosporidium: Director, UK Cryptosporidium Reference Unit the global challenge in monit toring urtesy of the Francis A. Countway
Cryptosporidium: still cryptic after all these years Dr Rachel Chalmers Cryptosporidium: Director, UK Cryptosporidium Reference Unit the global challenge in monit toring urtesy of the Francis A. Countway
Medical Parasitology (EEB 3895) Lecture Exam #2
 1 Name November 2016 Medical Parasitology (EEB 3895) Lecture Exam #2 Read through the exam once before you begin. Read the questions CAREFULLY; be certain to provide all of the information requested. In
1 Name November 2016 Medical Parasitology (EEB 3895) Lecture Exam #2 Read through the exam once before you begin. Read the questions CAREFULLY; be certain to provide all of the information requested. In
Drd. OBADĂ MIHAI DORU. PhD THESIS ABSTRACT
 UNIVERSITY OF AGRICULTURAL SCIENCES AND VETERINARY MEDICINE ION IONESCU DE LA BRAD IAŞI FACULTY OF VETERINARY MEDICINE SPECIALIZATION MICROBIOLOGY- IMUNOLOGY Drd. OBADĂ MIHAI DORU PhD THESIS ABSTRACT RESEARCHES
UNIVERSITY OF AGRICULTURAL SCIENCES AND VETERINARY MEDICINE ION IONESCU DE LA BRAD IAŞI FACULTY OF VETERINARY MEDICINE SPECIALIZATION MICROBIOLOGY- IMUNOLOGY Drd. OBADĂ MIHAI DORU PhD THESIS ABSTRACT RESEARCHES
PREVALENCE AND PATHOLOGY OF RABBIT COCCIDIOSIS IN NAIROBI COUNTY, KENYA.
 PREVALENCE AND PATHOLOGY OF RABBIT COCCIDIOSIS IN NAIROBI COUNTY, KENYA. A research project submitted in partial fulfillment for the award of the degree of Bachelor of Veterinary Medicine, UON. Investigator:
PREVALENCE AND PATHOLOGY OF RABBIT COCCIDIOSIS IN NAIROBI COUNTY, KENYA. A research project submitted in partial fulfillment for the award of the degree of Bachelor of Veterinary Medicine, UON. Investigator:
On the correct name for some subfamilies of Mustelidae ABSTRACT. Key-Words: Mustelidae; Subfamilies; Guloninae; Ictonychinae.
 Volume 54(##):### ###, 2014 Guloninae or Martinae? Zorillinae, Galictinae or Ictonychinae? On the correct name for some subfamilies of Mustelidae (Mammalia, Carnivora) Fabio Oliveira do Nascimento 1,2,3
Volume 54(##):### ###, 2014 Guloninae or Martinae? Zorillinae, Galictinae or Ictonychinae? On the correct name for some subfamilies of Mustelidae (Mammalia, Carnivora) Fabio Oliveira do Nascimento 1,2,3
Cryptosporidium spp. Oocysts
 Sampling and Source Tracking of Cryptosporidium spp. Oocysts June 28, 2005 Kristen L. Jellison, Ph.D. Department of Civil & Environmental Engineering Lehigh University Bethlehem, Pennsylvania Ultimate
Sampling and Source Tracking of Cryptosporidium spp. Oocysts June 28, 2005 Kristen L. Jellison, Ph.D. Department of Civil & Environmental Engineering Lehigh University Bethlehem, Pennsylvania Ultimate
Veterinary Surgical Pathology and Necropsy Services
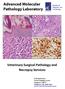 Veterinary Surgical Pathology and Necropsy Services 61 Biopolis Drive, Proteos Building Level 6 Singapore 138673 Telephone: (65) 6586 9629 http://www.imcb.a star.edu.sg/php/ittd i histo.php Advanced Molecular
Veterinary Surgical Pathology and Necropsy Services 61 Biopolis Drive, Proteos Building Level 6 Singapore 138673 Telephone: (65) 6586 9629 http://www.imcb.a star.edu.sg/php/ittd i histo.php Advanced Molecular
Parasitological laboratory อ.น.สพ.ดร.กฤษฎา ข าพ ล 17/09/2561
 Parasitological laboratory อ.น.สพ.ดร.กฤษฎา ข าพ ล 17/09/2561 Diagnosis Diagnostic techniques: radiography, anatomical pathology, necropsy, microscopic examination of tissue sections, clinical pathology,
Parasitological laboratory อ.น.สพ.ดร.กฤษฎา ข าพ ล 17/09/2561 Diagnosis Diagnostic techniques: radiography, anatomical pathology, necropsy, microscopic examination of tissue sections, clinical pathology,
Anti-protozoan study of a medicinal herb, Bidens pilosa
 1 2017 JITMM Anti-protozoan study of a medicinal herb, Bidens pilosa Meng-Ting Yang, Tien-Fen Kuo, Yueh-Chen Wu, Cicero L.T. Chang and Wen-Chin Yang Taiwan International Graduate Program Molecular and
1 2017 JITMM Anti-protozoan study of a medicinal herb, Bidens pilosa Meng-Ting Yang, Tien-Fen Kuo, Yueh-Chen Wu, Cicero L.T. Chang and Wen-Chin Yang Taiwan International Graduate Program Molecular and
Hepatic Coccidiosis of the Domestic Rabbit Oryctolagus cuniculus domesticus L. in Saudi Arabia
 World Journal of Zoology 3 (1): -35, 2008 ISSN 1817-98 IDOSI Publications, 2008 Hepatic Coccidiosis of the Domestic Rabbit Oryctolagus cuniculus domesticus L. in Saudi Arabia Ebtesam M. Al-Mathal Department
World Journal of Zoology 3 (1): -35, 2008 ISSN 1817-98 IDOSI Publications, 2008 Hepatic Coccidiosis of the Domestic Rabbit Oryctolagus cuniculus domesticus L. in Saudi Arabia Ebtesam M. Al-Mathal Department
9 Parasitology 9 EXERCISE EQA. Objectives EXERCISE
 0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
BEHAVIOUR OF THE DOMESTIC DOG (Canis familiaris)
 THE INFLUENCE OF CEREBRAL LATERALISATION ON THE BEHAVIOUR OF THE DOMESTIC DOG (Canis familiaris) A thesis submitted for the Degree of DOCTOR OF PHILOSOPHY by Luke Aaron Schneider B. A. (Hons) School of
THE INFLUENCE OF CEREBRAL LATERALISATION ON THE BEHAVIOUR OF THE DOMESTIC DOG (Canis familiaris) A thesis submitted for the Degree of DOCTOR OF PHILOSOPHY by Luke Aaron Schneider B. A. (Hons) School of
BLOOD PARASITES MORPHOTYPES OF ROCK LIZARDS OF ARMENIA
 PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY C h e m i s t r y a n d B i o l o g y 2015, 2, p. 45 49 B i o l o g y BLOOD PARASITES MORPHOTYPES OF ROCK LIZARDS OF ARMENIA T. K. HARUTYUNYAN, F. D. DANIELYAN,
PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY C h e m i s t r y a n d B i o l o g y 2015, 2, p. 45 49 B i o l o g y BLOOD PARASITES MORPHOTYPES OF ROCK LIZARDS OF ARMENIA T. K. HARUTYUNYAN, F. D. DANIELYAN,
Black-footed Ferret Mustela nigripes
 COSEWIC Assessment and Addendum on the Black-footed Ferret Mustela nigripes in Canada EXTIRPATED 2009 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected
COSEWIC Assessment and Addendum on the Black-footed Ferret Mustela nigripes in Canada EXTIRPATED 2009 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
cyst&' appeared to be of two kinds-one smaller and Smnith "is inclined to regard these epithelial cell parasites as
 COCCIDIA IN SUBEPITHELIAL INFECTIONS OF THE INTESTINES OF BIRDS PHILIP B. HADLEY From the Agricultural Experiment Station of the Rhode Island State College' Received for publication, July 10, 1916 In an
COCCIDIA IN SUBEPITHELIAL INFECTIONS OF THE INTESTINES OF BIRDS PHILIP B. HADLEY From the Agricultural Experiment Station of the Rhode Island State College' Received for publication, July 10, 1916 In an
DOWNLOAD OR READ : VETERINARY CLINICAL EPIDEMIOLOGY PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : VETERINARY CLINICAL EPIDEMIOLOGY PDF EBOOK EPUB MOBI Page 1 Page 2 veterinary clinical epidemiology veterinary clinical epidemiology pdf veterinary clinical epidemiology Use these links
DOWNLOAD OR READ : VETERINARY CLINICAL EPIDEMIOLOGY PDF EBOOK EPUB MOBI Page 1 Page 2 veterinary clinical epidemiology veterinary clinical epidemiology pdf veterinary clinical epidemiology Use these links
Introduction to Biorisk and the OIE Standard
 Introduction to Biorisk and the OIE Standard World Association of Veterinary Laboratory Diagnosticians 18 th International Symposium, Sorrento, Italy 7 th -10 th June 2017 2015 Dr. Anthony Fooks Member,
Introduction to Biorisk and the OIE Standard World Association of Veterinary Laboratory Diagnosticians 18 th International Symposium, Sorrento, Italy 7 th -10 th June 2017 2015 Dr. Anthony Fooks Member,
Eimeria idmii sp. n. (Apicomplexa: Eimeriidae) from the Arabian Mountain Gazelle, Gazella gazella, in Saudi Arabia
 J. Helminthol. Soc. Wash. 59(1), 1992, pp. 120-124 Eimeria idmii sp. n. (Apicomplexa: Eimeriidae) from the Arabian Mountain Gazelle, Gazella gazella, in Saudi Arabia O. B. MOHAMMED1 AND H. S. HUSSEIN2'3
J. Helminthol. Soc. Wash. 59(1), 1992, pp. 120-124 Eimeria idmii sp. n. (Apicomplexa: Eimeriidae) from the Arabian Mountain Gazelle, Gazella gazella, in Saudi Arabia O. B. MOHAMMED1 AND H. S. HUSSEIN2'3
Species: Panthera pardus Genus: Panthera Family: Felidae Order: Carnivora Class: Mammalia Phylum: Chordata
 CHAPTER 6: PHYLOGENY AND THE TREE OF LIFE AP Biology 3 PHYLOGENY AND SYSTEMATICS Phylogeny - evolutionary history of a species or group of related species Systematics - analytical approach to understanding
CHAPTER 6: PHYLOGENY AND THE TREE OF LIFE AP Biology 3 PHYLOGENY AND SYSTEMATICS Phylogeny - evolutionary history of a species or group of related species Systematics - analytical approach to understanding
Project Protocol Number UNIVERSITY OF HAWAII INSTITUTIONAL ANIMAL CARE &USE COMMITTEE 2002 VERTEBRATE ANIMAL USE PROTOCOL FORM
 Project Protocol Number UNIVERSITY OF HAWAII INSTITUTIONAL ANIMAL CARE &USE COMMITTEE 2002 VERTEBRATE ANIMAL USE PROTOCOL FORM The applicant is responsible for providing complete and accurate information.
Project Protocol Number UNIVERSITY OF HAWAII INSTITUTIONAL ANIMAL CARE &USE COMMITTEE 2002 VERTEBRATE ANIMAL USE PROTOCOL FORM The applicant is responsible for providing complete and accurate information.
May 17, SWBAT explain why scientists classify organisms SWBAT list major levels of hierarchy
 May 17, 2017 Aims: SWBAT explain why scientists classify organisms SWBAT list major levels of hierarchy Agenda 1. Do Now 2. Class Notes 3. Guided Practice 4. Independent Practice 5. Practicing our AIMS:
May 17, 2017 Aims: SWBAT explain why scientists classify organisms SWBAT list major levels of hierarchy Agenda 1. Do Now 2. Class Notes 3. Guided Practice 4. Independent Practice 5. Practicing our AIMS:
Cumbria Biodiversity Data Centre Cumbria Mammal Group
 Cumbria Biodiversity Data Centre Cumbria Mammal Group Cumbria Mammal Atlas Cumbria Biodiversity Data Centre and Cumbria Mammal Group November 17 Copyright Notice Maps are copyright Cumbria Biodiversity
Cumbria Biodiversity Data Centre Cumbria Mammal Group Cumbria Mammal Atlas Cumbria Biodiversity Data Centre and Cumbria Mammal Group November 17 Copyright Notice Maps are copyright Cumbria Biodiversity
MANAGEMENT OF SEVERE HEPATIC COCCIDIOSIS IN DOMESTIC RABBITS
 MANAGEMENT OF SEVERE HEPATIC COCCIDIOSIS IN DOMESTIC RABBITS B. Bibin Becha* and S.S. Devi Avian Disease Diagnostic Laboratory, Manjadi, P.O., Thiruvalla, Kerala 689 105 Received : 28.11.2013 Accepted
MANAGEMENT OF SEVERE HEPATIC COCCIDIOSIS IN DOMESTIC RABBITS B. Bibin Becha* and S.S. Devi Avian Disease Diagnostic Laboratory, Manjadi, P.O., Thiruvalla, Kerala 689 105 Received : 28.11.2013 Accepted
of Nebraska - Lincoln
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 6-1985
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 6-1985
DOWNLOAD OR READ : MOLECULAR PATHOLOGY AND THE DYNAMICS OF DISEASE PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : MOLECULAR PATHOLOGY AND THE DYNAMICS OF DISEASE PDF EBOOK EPUB MOBI Page 1 Page 2 molecular pathology and the dynamics of disease molecular pathology and the pdf molecular pathology
DOWNLOAD OR READ : MOLECULAR PATHOLOGY AND THE DYNAMICS OF DISEASE PDF EBOOK EPUB MOBI Page 1 Page 2 molecular pathology and the dynamics of disease molecular pathology and the pdf molecular pathology
Prevalence of Endoparasites in Peacocks (Pavo cristatus) Prevalenţa endoparazitozelor la Păuni (Pavo cristatus)
 Prevalence of Endoparasites in Peacocks (Pavo cristatus) Prevalenţa endoparazitozelor la Păuni (Pavo cristatus) Adriana TITILINCU, Viorica MIRCEAN, BEJAN A., Anamaria IOVU, Roxana UNGUREANU, COZMA V. University
Prevalence of Endoparasites in Peacocks (Pavo cristatus) Prevalenţa endoparazitozelor la Păuni (Pavo cristatus) Adriana TITILINCU, Viorica MIRCEAN, BEJAN A., Anamaria IOVU, Roxana UNGUREANU, COZMA V. University
JEFFERSON COLLEGE COURSE SYLLABUS VAT114 PRINCIPLES OF CLINICAL MEDICINE II. 4 Credit Hours
 JEFFERSON COLLEGE COURSE SYLLABUS VAT114 PRINCIPLES OF CLINICAL MEDICINE II 4 Credit Hours Prepared by: Dana Nevois, RVT, BS Revised 06/08 John Keck, Dean of Career & Technical Education VAT114 PRINCIPLES
JEFFERSON COLLEGE COURSE SYLLABUS VAT114 PRINCIPLES OF CLINICAL MEDICINE II 4 Credit Hours Prepared by: Dana Nevois, RVT, BS Revised 06/08 John Keck, Dean of Career & Technical Education VAT114 PRINCIPLES
Introduction. Syst Parasitol (2014) 89:83 89 DOI /s
 Syst Parasitol (2014) 89:83 89 DOI 10.1007/s10-014-9510-7 Coccidial dispersion across New World marsupials: Klossiella tejerai Scorza, Torrealba & Dagert, 1957 (Apicomplexa: Adeleorina) from the Brazilian
Syst Parasitol (2014) 89:83 89 DOI 10.1007/s10-014-9510-7 Coccidial dispersion across New World marsupials: Klossiella tejerai Scorza, Torrealba & Dagert, 1957 (Apicomplexa: Adeleorina) from the Brazilian
For Vets General Information Prevalence of Tox Prevalence of opl Tox asm opl asm Humans Hum Animals Zoonotic Risk & Other Ris Zoonotic Risk & Ot
 For Vets General Information Toxoplasma gondii is a protozoal parasite capable of infecting any warm-blooded animal, including humans. Wild and domestic cats are the only known definitive hosts of Toxoplasma;
For Vets General Information Toxoplasma gondii is a protozoal parasite capable of infecting any warm-blooded animal, including humans. Wild and domestic cats are the only known definitive hosts of Toxoplasma;
Cryptosporidiosis in Cattle
 Cryptosporidiosis in Cattle The Moredun Foundation News Sheet Vol. 6, No. 1, February 2014 Beth Wells BSc, PhD Sarah Thomson BSc, MRes Moredun Research Institute Key points Cryptosporidiosis is the disease
Cryptosporidiosis in Cattle The Moredun Foundation News Sheet Vol. 6, No. 1, February 2014 Beth Wells BSc, PhD Sarah Thomson BSc, MRes Moredun Research Institute Key points Cryptosporidiosis is the disease
AARJMD VOLUME 1 ISSUE 19 (MARCH 2014) ISSN : A Peer Reviewed International Journal of Asian Academic Research Associates AARJMD
 A Peer Reviewed International Journal of Asian Academic Research Associates AARJMD ASIAN ACADEMIC RESEARCH JOURNAL OF MULTIDISCIPLINARY PERCENTAGE PREVALENCE OF EIMERIAN SPECIES IN AWASSI SHEEP IN NORTHERN
A Peer Reviewed International Journal of Asian Academic Research Associates AARJMD ASIAN ACADEMIC RESEARCH JOURNAL OF MULTIDISCIPLINARY PERCENTAGE PREVALENCE OF EIMERIAN SPECIES IN AWASSI SHEEP IN NORTHERN
PCR detection of Leptospira in. stray cat and
 PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
COMMISSION DELEGATED REGULATION (EU)
 L 296/6 Official Journal of the European Union 15.11.2011 COMMISSION DELEGATED REGULATION (EU) No 1152/2011 of 14 July 2011 supplementing Regulation (EC) No 998/2003 of the European Parliament and of the
L 296/6 Official Journal of the European Union 15.11.2011 COMMISSION DELEGATED REGULATION (EU) No 1152/2011 of 14 July 2011 supplementing Regulation (EC) No 998/2003 of the European Parliament and of the
UNIT III A. Descent with Modification(Ch19) B. Phylogeny (Ch20) C. Evolution of Populations (Ch21) D. Origin of Species or Speciation (Ch22)
 UNIT III A. Descent with Modification(Ch9) B. Phylogeny (Ch2) C. Evolution of Populations (Ch2) D. Origin of Species or Speciation (Ch22) Classification in broad term simply means putting things in classes
UNIT III A. Descent with Modification(Ch9) B. Phylogeny (Ch2) C. Evolution of Populations (Ch2) D. Origin of Species or Speciation (Ch22) Classification in broad term simply means putting things in classes
