Malaria Sporozoites Traverse Host Cells within Transient Vacuoles
|
|
|
- Sheena Fox
- 5 years ago
- Views:
Transcription
1 Malaria Sporozoites Traverse Host Cells within Transient Vacuoles Veronica Risco-Castillo, Selma Topçu, Carine Marinach, Giulia Manzoni, Améliee. Bigorgne, Sylvie Briquet, Xavier Baudin, Maryse Lebrun, Jean-François Dubremetz, Olivier Silvie To cite this version: Veronica Risco-Castillo, Selma Topçu, Carine Marinach, Giulia Manzoni, Améliee. Bigorgne, et al.. Malaria Sporozoites Traverse Host Cells within Transient Vacuoles. Cell Host and Microbe, Elsevier, 2015, 18 (51), pp < /j.chom >. <hal > HAL Id: hal Submitted on 9 Nov 2015 HAL is a multi-disciplinary open access archive for the deposit and dissemination of scientific research documents, whether they are published or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers. L archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
2 Malaria sporozoites traverse host cells within transient vacuoles Veronica Risco-Castillo 1,4#, Selma Topçu 1,4, Carine Marinach 1,4, Giulia Manzoni 1, Amélie E. Bigorgne 1, Sylvie Briquet 1, Xavier Baudin 2, Maryse Lebrun 3, Jean-François Dubremetz 3, Olivier Silvie 1* 1 Sorbonne Universités, UPMC Univ Paris 06, INSERM U1135, CNRS ERL8255, Centre d Immunologie et des Maladies Infectieuses, F-75013, Paris, France. 2 Sorbonne Paris Cité, Univ Paris Diderot, CNRS, Institut Jacques Monod, ImagoSeine, UMR 7592, Paris F-75205, France 3 Université de Montpellier 2, CNRS, Dynamique des Interactions Membranaires Normales et Pathologiques, UMR 5235, F Montpelllier, France. 4 These authors contributed equally to this work *Corresponding author: Centre d Immunologie et des Maladies Infectieuses Faculté de Médecine Pierre et Marie Curie 91 Bd de l Hôpital, Paris, France olivier.silvie@inserm.fr Phone: , Fax: #Present address: Ecole Nationale Vétérinaire d'alfort (EnvA), Maisons-Alfort, France Running title: Perforin-dependent egress of malaria sporozoites Key words: Malaria; Plasmodium sporozoites; Perforin-like protein; Cell traversal; Lysosomes 1
3 SUMMARY Plasmodium sporozoites are deposited in the host skin by Anopheles mosquitoes. The parasites migrate from the dermis to the liver, where they invade hepatocytes through a moving junction (MJ) to form a replicative parasitophorous vacuole (PV). Malaria sporozoites need to traverse cells during progression through host tissues, a process requiring parasite perforin-like protein 1 (PLP1). We find that sporozoites traverse cells inside transient vacuoles that precede PV formation. Sporozoites initially invade cells inside transient vacuoles by an active MJ-independent process that does not require vacuole membrane remodeling or release of parasite secretory organelles typically involved in invasion. Sporozoites use ph sensing and PLP1 to exit these vacuoles and avoid degradation by host lysosomes. Next, parasites enter the MJ-dependent PV, which has a different membrane composition, precluding lysosome fusion. The malaria parasite has thus evolved different strategies to evade host cell defense and establish an intracellular niche for replication. 2
4 INTRODUCTION Malaria begins when Plasmodium sporozoites are deposited in the host skin by a female Anopheles mosquito. They rapidly travel to the liver and invade hepatocytes, where they differentiate into exo-erythrocytic forms (EEFs) and pathogenic merozoites inside a membrane-bound compartment, the parasitophorous vacuole (PV). Sporozoite progression through the host tissues following transmission by the mosquito relies on active gliding motility and the capacity of the parasite to migrate through cells (Ménard et al., 2013). During cell traversal (CT), sporozoites breach the host cell membrane and glide through the traversed cell cytoplasm (Mota et al., 2001). Reverse genetics studies have identified several parasite factors involved in sporozoite CT (Bhanot et al., 2005; Ishino et al., 2004, 2005; Kariu et al., 2006; Moreira et al., 2008; Talman et al., 2011). Among these factors, the Perforin-Like Protein 1 (PLP1, also called SPECT2), belongs to an evolutionary conserved family of pore-forming proteins characterized by the presence of a membrane attack complex/perforin (MACPF) domain (Kaiser et al., 2004). Recombinant forms of P. falciparum PLP1 protein or its MACPF domain were shown to have membrane lytic activity (Garg et al., 2013). It has been proposed that PLP1-mediated perforation of the host cell plasma membrane facilitates parasite entry into the traversed cell (Ishino et al., 2005), but the mechanisms of membrane rupturing during sporozoite CT have not been elucidated. The use of CT-deficient mutant P. berghei parasites, combined with intravital imaging approaches, established that CT allows migration of the parasites to the liver parenchyma following inoculation by the mosquito. In particular, plp1-knockout P. berghei sporozoites have reduced infectivity to rodents, associated with a lack of sporozoite CT activity in vitro and impaired parasite progression through the dermis and the liver sinusoidal barrier in vivo (Amino et al., 2008; Ishino et al., 2005; Tavares et al., 2013). CT was initially proposed to activate the parasite for productive invasion (Mota et al., 2002), notably based on the observation that sporozoites traverse several hepatocytes before establishing a PV, both in vitro and in vivo (Frevert et al., 2005; Mota et al., 2001). Nevertheless, CT-deficient sporozoites can productively invade hepatocytes in vitro as efficiently as WT parasites 3
5 (Amino et al., 2008; Ishino et al., 2004, 2005). In one study, plp1-deficient P. berghei sporozoites were reported to infect cells more rapidly than normal sporozoites, leading to the conclusion that CT retards rather than activates productive invasion (Amino et al., 2008). However, the kinetics of CT and productive invasion during the course of infection have not been studied in detail in any of these studies. Here we investigated the temporal and molecular mechanisms of CT and productive invasion during sporozoite infection. We found that CT precedes productive invasion, and that during CT Plasmodium sporozoites actively invade cells inside transient vacuoles, which are distinct from PVs. Plp1-knockout sporozoites fail to egress from transient vacuoles and are eliminated after fusion with the host cell lysosomes. Furthermore, treating cells with a selective inhibitor of lysosomal acidification abrogates sporozoite CT, reproducing the plp1- knockout phenotype. Our data reveal that Plasmodium sporozoites can actively invade cells inside two different vacuoles, and either use PLP1 and ph sensing to egress from transient non-replicative vacuoles, or remodel the PV membrane to escape degradation by the host cell lysosomal machinery. 4
6 RESULTS Sporozoite host cell traversal precedes productive invasion To analyze the kinetics of sporozoite CT and host cell infection, we took advantage of a GFP-expressing P. yoelii strain (Manzoni et al., 2014) and a robust experimental setup consisting of two related hepatocytic cell lines, HepG2/CD81 and parental HepG2 cells (Silvie et al., 2006a). HepG2/CD81 cells express the host entry factor CD81 and support P. yoelii CT and productive invasion, whereas the parental HepG2 cells lack CD81 and support P. yoelii CT but not productive invasion (Risco-Castillo et al., 2014; Silvie et al., 2003, 2006a). CT activity was monitored by flow cytometry using an established wound-repair assay based on uptake of a fluorescent dextran tracer by traversed cells (Mota et al., 2001) (Figure 1A). CT activity was maximal during the first hour of sporozoite incubation with cells, as shown by the rapid increase of dextran-positive cell numbers (Figure 1B), and was similar in HepG2 and HepG2/CD81 cells, as expected (Silvie et al., 2003, 2006a). The sporozoite invasion rate, defined as the percentage of GFP-positive cells, remained low in HepG2 cells throughout the assay, consistent with the transient intracellular localization of sporozoites during CT (Figure 1C). The percentage of GFP-positive HepG2/CD81 cells was also initially low and identical to that in HepG2 cells, and showed a marked increase only after a delay, which varied from 30 to 90 minutes depending on the experiments (Figure 1C). Detection of GFP-positive cells by FACS allows to quantify sporozoite invasion, but does not discriminate between sporozoite CT and productive invasion inside a PV. To distinguish productive from non-productive invasion events, cell cultures inoculated with sporozoites were dissociated by trypsin treatment at different time points, re-plated and cultured for an additional hours, before quantification of productive invasion events based on the number of developing EEFs (Figure 1A). This assay revealed that early invasion events were non-productive in HepG2/CD81 cells, whereas late invasion events coincided with parasite development into EEFs (Figure 1D). Collectively, these data 5
7 establish that early invasion events correspond to sporozoite CT activity, and are followed by a second phase of CD81-dependent productive invasion. Sporozoites form transient vacuoles during cell traversal Surprisingly, more than 50% of invaded (GFP-positive) HepG2 cells were dextrannegative at early time points, suggestive of parasite entry without membrane damage, whereas later during the course of infection most invaded cells were dextran-positive (Figure 2A). Furthermore, transmission electron microscopy (TEM) images of P. yoelii-infected HepG2 cells revealed the presence of a membrane around some sporozoites (Figure 2B-C). Because P. yoelii can traverse but not productively invade HepG2 cells, these results suggest that CT events may involve the formation of transient vacuoles. To test this hypothesis, we imaged PyGFP sporozoites incubated with HepG2 cells expressing a fluorescent marker of the plasma membrane, N20-mCherry, consisting of mcherry fused to the N-terminal region of neuromodulin (Zuber et al., 1989). Shortly after adding sporozoites to HepG2/N20-mCherry cells, intracellular GFP parasites could be observed enclosed in N20-mCherry-labeled vacuoles (Figure 2D), which were also stained with filipin, a cholesterol-binding agent that selectively labels the host cell but not the sporozoite membrane (Bano et al., 2007). A large proportion (40-50%) of intracellular sporozoites were contained inside filipin and N20-mCherry-labeled vacuoles at early time points (Figure 2E), corroborating the FACS results. In addition, we could image by spinning disk confocal microscopy sporozoite egress from N20-mCherry-labeled vacuoles (Figure 2F and Movie S1 and S2). These data provide direct evidence that Plasmodium sporozoites can traverse cells by forming transient vacuoles (TVs). PLP1-deficient sporozoites do not egress from transient vacuoles We then hypothesized that PLP1, which is required for CT (Ishino et al., 2005), may play a role in egress from TVs. We generated GFP-expressing plp1-deficient parasites in P. yoelii using the recent Gene Out Marker Out strategy (Manzoni et al., 2014) (Figure S1A 6
8 and S1B). PyΔplp1 mutants showed no defect during blood stage replication, transmission to mosquitoes and sporozoite production (Figure S1C-E). PyΔplp1 sporozoites were motile and developed into EEFs in vitro as efficiently as control parasites, but were poorly infective to mice in vivo, especially when administered through mosquito bites, the natural transmission route (Figure S2A-E). This loss of infectivity was associated with a complete abrogation of CT activity (Figure S2F-H). Surprisingly, in our in vitro invasion assays, the percentage of GFP-positive cells was much higher with PyΔplp1 sporozoites than with PyGFP, in both HepG2 and HepG2/CD81 cells (Figure 3A-B, curves). However, like PyGFP, PyΔplp1 sporozoites did not develop into EEFs inside HepG2 cells, showing that in the absence of CD81 all invasion events were nonproductive (Figure 3A, histograms). In HepG2/CD81 cells, PyΔplp1 formed similar numbers of EEFs as PyGFP (Figure 3B, histograms), despite higher invasion rates, indicating that invasion events were for a large part non-productive. EEF development coincided with late invasion events, as observed with PyGFP. Importantly, all PyΔplp1 sporozoites inside HepG2 were contained inside a vacuole, as evidenced by TEM (Figure 3C) and fluorescent labeling by filipin and N20-mCherry (Figure 3D and 3E). No egress of PyΔplp1 sporozoites was observed in live cell imaging experiments (Figure 3F and Movie S3). Similar results were obtained with P. berghei sporozoites in Hepa1-6 cells (Figure S3). Taken together, these data indicate that PLP1 is required for sporozoite egress from non-replicative TVs but not for entry into cells. Our results also show that abrogation of CT does not accelerate commitment to productive invasion, and that PyΔplp1 form both TVs and PVs in HepG2/CD81 cells. TVs are formed without rhoptry secretion or remodeling of the vacuole membrane Our data show that sporozoites can invade cells inside two types of vacuoles, nonreplicative TVs or replicative PVs. We further characterized the mechanism of formation of TVs, using the PyΔplp1 mutant, where abrogation of sporozoite egress results in the 7
9 accumulation of TVs inside cells. PyΔplp1 sporozoite invasion of HepG2 and HepG2/CD81 cells was prevented by exposure to cytochalasin D or anti-csp antibodies, which both inhibit sporozoite motility, (Figure 4A). This demonstrates that formation of TVs, like PVs, is an active process driven by the parasite motility, and not the result of passive uptake by the host cells. We have shown before that productive host cell invasion is associated with discharge of the sporozoite rhoptries, resulting in depletion of the rhoptry proteins RON2 and RON4 (Risco-Castillo et al., 2014). Interestingly, PyΔplp1 sporozoite rhoptries, when visible, appeared intact on TEM images of invaded HepG2 cells (Figure 3C), suggesting entry without rhoptry secretion. To corroborate this finding, we genetically engineered a PyΔplp1 parasite line expressing a mcherry-tagged version of RON4, and examined by fluorescence microscopy RON4-mCherry expression during sporozoite host cell invasion, using filipin staining to label intracellular vacuoles (Figure 4B). In HepG2 cells, where all the vacuoles correspond to TVs only, sporozoites still expressed apical RON4-mCherry, at all time points examined, in both PyΔplp1/RON4::mCherry and PyGFP/RON4::mCherry parasites (Figure 4B and 4C). In HepG2/CD81 cells, sporozoites inside vacuoles also expressed RON4- mcherry at early time points (30 min), when vacuoles correspond to TVs. Depletion of RON4-mCherry was observed at later time points, indicative of rhoptry discharge during productive invasion and formation of the PVs (Figure 4B and 4C). RON4 depletion was seen in a smaller proportion of the PyΔplp1/RON4::mCherry as compared to PyGFP/RON4::mCherry parasites, consistent with the fact that PLP1-deficient sporozoites predominantly form non-productive vacuoles. Altogether, these data confirm that formation of TVs, unlike PVs, occurs without rhoptry discharge. We next examined the presence of host proteins on the membrane of TVs versus PVs. We found that N20-mCherry and Basigin, an abundant transmembrane protein, were both included in the membrane of PyΔplp1 vacuoles inside HepG2 cells, which correspond to TVs (Figure 4D-F). Interestingly, a vast majority (>85%) of these vacuoles were also labeled with phalloidin, which binds to F-actin (Figure 4E and 4F). Similar results were obtained with 8
10 PyGFP sporozoites in HepG2 cells (Figure S4). This suggests that sporozoites include cortical cytoskeleton components during formation of TVs. In sharp contrast, all three markers were efficiently excluded from PVs in HepG2/CD81 cells (Figure 4F-G and Figure S4). These results indicate that host membrane proteins are excluded from the PV membrane (PVM) during productive invasion, whereas TVs are formed without remodeling of the vacuole membrane. PyΔplp1 non-replicative vacuoles are eliminated by host cell lysosomes PyΔplp1 sporozoites were retained inside vacuoles in HepG2 cells but failed to develop into EEFs, and were eliminated within 12 hours of infection (Figure 5A). In HepG2/CD81 cells, the number of infected cells also decreased over time but 20 to 50% of the parasites persisted and developed into EEFs (Figure 5A). We hypothesized that the host cell lysosomal machinery may be responsible for the elimination of PyΔplp1 non-replicative vacuoles. To test this hypothesis, we used the acidic organelle probe Lysotracker-red and antibodies against the lysosomal associated membrane protein 1 (LAMP1). About 80% of intracellular PyΔplp1 parasites were labeled by Lysotracker-red in HepG2 cells, whereas in HepG2/CD81 both Lysotracker-positive and Lysotracker-negative parasites could be found (Figure 5B and 5D). Similarly, LAMP1 staining was observed on most PyΔplp1 vacuoles inside HepG2 cells, and on a fraction of the parasites inside HepG2/CD81 cells (Figure 5C and 5D). Similar results were obtained with P. berghei in Hepa1-6 cells (Figure S3F). To further explore whether a similar phenomenon occurs in vivo, we examined liver cryosections from BALB/c mice injected with PyGFP or PyΔplp1 sporozoites. PyΔplp1 parasites detected in the liver parenchyma lacked the PVM marker UIS4 but were labeled by anti-lamp1 antibodies (Figure 5E and Figure S5A-B), corroborating results obtained in cell cultures (Figure 5D and Figure S5C-D). Conversely, only a minority of PyGFP parasites was LAMP1-positive in the liver, showing that a large proportion of WT parasites do not fuse with lysosomes in infected hepatocytes in vivo. 9
11 We also documented the degradation of non-replicative PyΔplp1 vacuoles by TEM analysis of infected HepG2 cells, which revealed accumulation of granular material inside the vacuole, suggestive of secondary lysosomes (Figure S5E). Treatment of cells with chloroquine (CQ), to inhibit lysosome acidification, enhanced PyΔplp1 sporozoite persistence in HepG2 cells (Figure 5F), but these sporozoites still failed to develop into EEFs (data not shown). Collectively, our results reveal that invaded PyΔplp1 parasites are efficiently recognized and eliminated by the host cell lysosomes in HepG2 cells, whereas in HepG2/CD81 cells some parasites successfully form a PV, via CD81, avoid lysosomal degradation and develop into EEFs. Accordingly, PyGFP and PyΔplp1 EEFs developing inside HepG2/CD81 cells were not labeled by Lysotracker-red (Figure S5F). PLP1-mediated sporozoite egress depends on lysosomal acidification In T. gondii, low ph promotes membrane binding and cytolytic activity of PLP1 (Roiko et al., 2014). We hypothesized that Plasmodium PLP1 activity might also be regulated by the ph, and that acidification of the vacuole upon fusion with lysosomes would activate PLP1 and parasite egress from TVs. To test this hypothesis, we incubated PyGFP sporozoites with host cells pre-treated with bafilomycin A1, a selective inhibitor of vacuolar-type H + -ATPases that blocks lysosomal acidification (Yoshimori et al., 1991). Remarkably, pre-treatment of cells with bafilomycin A1 suppressed sporozoite CT (Figure 6A-B). Concomitantly, we observed an increase in the number of PyGFP-invaded cells, in both HepG2/CD81 cells and HepG2 cells (Figure 6A-B, red bars). These results are reminiscent of the behavior of PyΔplp1 sporozoites (Figure 3 A-B). Similar numbers of EEFs were observed in bafilomycin A1-treated cells as in control cells (Figure 6C). However, in addition to EEFs, a population of non-developing sporozoites was observed in bafilomycin A1 treated cells (Figure 6D). These persisting intracellular sporozoites were found in both HepG2 and HepG2/CD81, and likely correspond to parasites that did not egress from non-replicative TVs yet avoided degradation owing to inhibition of lysosome function, as observed with PyΔplp1 mutant parasites in CQtreated cells. 10
12 Collectively, our data support a model where Plasmodium sporozoites, during CT, actively invade cells inside transient non-replicative vacuoles, independently of host entry factors and without forming a moving junction (Figure 7). Sporozoites use ph sensing and PLP1 to egress from these non-replicative vacuoles and avoid degradation by the host cell lysosomal machinery. Subsequently, parasites enter a MJ-dependent PV that supports parasite liver stage development. 11
13 DISCUSSION Malaria sporozoites can invade cells either transiently during CT, or by establishing a resident PV where they further develop into EEFs. Here we show that transmigrating sporozoites do not necessarily breach the host cell membrane at the time of invasion, as currently believed, but enter cells inside transient vacuoles, from which they subsequently egress using PLP1 and ph sensing. Our FACS and microscopy data demonstrate that a large proportion of early CT events occur after the formation of TVs, whereas late traversal events are associated with membrane rupture before complete sealing of a primary vacuole. This might be due to variations in parasite motility over time or reflect the timing of secretion and/or activation of PLP1. Apicomplexan zoites productively invade host cells through a MJ, a structure composed in part by RON proteins secreted from the parasite rhoptries (Besteiro et al., 2011). The MJ anchors the invading parasite to the host cell, and serves as a molecular sieve that selectively excludes host proteins from the membrane of the nascent vacuole, resulting in protection from the host cell lysosomes (Mordue et al., 1999). Although the nature of the Plasmodium sporozoite MJ remains elusive, our data show that productive invasion is associated with depletion of sporozoite RON proteins and exclusion of several host proteins from the PVM. In contrast, we observed no sign of depletion of RON4 from sporozoites during formation of TVs. Although we cannot formally exclude partial rhoptry secretion during non-productive invasion, these results, combined with the TEM images, strongly suggest that rhoptries are not discharged during entry inside TVs. In addition, we provide evidence that host membrane proteins as well as cortical F-actin are incorporated in the membrane of TVs. This suggests that molecular partitioning occurs during productive invasion only, supposedly at the moving junction, but not during TV formation. Collectively, our data illustrate that TVs are formed without rhoptry secretion or remodeling of the vacuole membrane, two characteristic features of MJ-dependent productive invasion. From these data we conclude that formation of TVs results from active MJ-independent sporozoite invasion, which is different from the classical mechanism of PV formation in Apicomplexa. 12
14 Analysis of the invasion kinetics of PyGFP and PyΔplp1 sporozoites indicates that most non-productive events occur earlier than productive host cell entry. This observation suggests that vigorous sporozoite motility allows parasite internalization inside TVs, whereas productive invasion inside the PV is only possible after activation of the parasite. It has been proposed that CT activates sporozoites for commitment to productive invasion (Mota et al., 2002). However, CT-deficient P. berghei (Ishino et al., 2004, 2005) and P. yoelii (this study) sporozoites infect hepatocytes with normal efficiency in vitro, showing that prior contact with the host cell cytoplasm is not required for parasite activation. Another study reported that CT retards productive invasion, based on the observation that PbΔplp1 sporozoites invade cells more rapidly than normal parasites (Amino et al., 2008). We also observed that PyΔplp1 and PbΔplp1 sporozoites invade cells more rapidly than control parasites, yet our data clearly show that these early events are non-productive. Productive invasion occurs after a significant delay in both WT and CT-deficient parasites, indicating that CT itself has no impact on parasite activation. The delayed onset of productive invasion that we observed in vitro is consistent with the physiological need for the parasite to migrate from the injection site in the skin to its replication site in the liver in vivo. In this regard, it has been shown that P. yoelii sporozoites leave the inoculation site in the skin up to one hour or more after intradermal injection (Yamauchi et al., 2007). PLP1-deficient sporozoites, similarly to WT parasites, invade cells by forming TVs but fail to egress and are retained inside non-replicative vacuoles that fuse with lysosomes, resulting in a dramatic reduction of infectivity in vivo. Not surprisingly, they remain capable of forming EEFs in vitro, as observed before with CT-deficient P. berghei lines (Bhanot et al., 2005; Ishino et al., 2004, 2005; Kariu et al., 2006; Moreira et al., 2008; Talman et al., 2011). It should be noted that in vitro only a small proportion (less than 10%) of the PyΔplp1 sporozoites invade cells and get trapped inside TVs during the early stages of infection. Most parasites remain extracellular and can eventually commit to the second phase of productive invasion upon activation, explaining why EEF numbers are not reduced in vitro with the Δplp1 mutants. Alternatively, we cannot exclude that some sporozoites may also form a 13
15 junction post-invasion, from within a primary non-replicative vacuole, to form a secondary replicative PV (Figure 7). In such a scenario, the MJ may serve primarily for molecular partitioning, to modify the vacuole membrane and avoid its recognition by the host cell lysosomes. Along this line, a recent study showed that T. gondii tachyzoites internalized inside macrophages by phagocytosis can then actively invade from within the phagosomal compartment to form a PV (Zhao et al., 2014). We uncovered here a role for PLP1 in sporozoite egress from TVs during CT, revealing that parasite egress and cell traversal are intricate mechanisms. Many pathogens use pore-forming proteins to disrupt host membranes during infection, including for escaping from vacuolar compartments. For example, Listeria monocytogenes uses the pore-forming toxin Listeriolysin O (LLO) to egress from phagolysosomes and reach the infected cell cytosol to replicate (Hamon et al., 2012). Several apicomplexan PLPs are implicated in parasite egress events. Plasmodium PLP2 was recently shown to play a role in permeabilizing the erythrocyte membrane during egress of P. falciparum and P. berghei gametocytes (Deligianni et al., 2013; Wirth et al., 2014). PLP1 was reported to play a role in egress of P. falciparum merozoites from infected erythrocytes (Garg et al., 2013). Intriguingly, previous proteomic studies in P. falciparum have detected PLP1 at the sporozoite stage only (PlasmoDB.org), and PLP1-deficient P. berghei and P. yoelii parasites show no defect during erythrocytic growth, ruling out any important role of PLP1 during the blood stages, at least in rodent malaria parasites. In T. gondii, TgPLP1 mediates the rapid egress of tachyzoites from the host cell after parasite replication, and is involved in the permeabilization of both the PVM and the host cell membrane (Kafsack et al., 2009). Sporozoites must switch off their CT machinery once they have invaded a cell by forming a PV, to avoid the rupture of the PVM. This may be achieved through control of PLP1 secretion from the micronemes and/or through regulation of the protein activity. Here we show that treating cells with bafilomycin A1, an inhibitor of lysosomal acidification, suppresses sporozoite egress from TVs and cell traversal. This reveals that the parasite uses ph sensing to activate PLP1-dependent egress and avoid degradation by the host cell 14
16 lysosomal machinery. Proteins with MACPF domains are typically secreted as monomers, bind to their target membrane, oligomerize, then undergo a conformational change that leads to the formation of a pore (Dunstone and Tweten, 2012). Various pore-forming proteins are regulated by the ph, including Listeria LLO and Toxoplasma PLP1 (Roiko et al., 2014; Schuerch et al., 2005). Although the mechanism underlying Plasmodium PLP1 regulation by ph remains to be defined, our data support a model where Plasmodium sporozoites use ph sensing to detect the fusion of the vacuole with the lysosomes, activate PLP1 and egress from the vacuole. During productive invasion, modification of the PVM by molecular partitioning at the moving junction precludes its fusion with the host cell lysosomes, preventing activation of PLP1 and egress from the PV. Alternatively, remodeling of the PVM during parasite entry may alter the binding properties of PLP1 and render the PVM refractory to PLP1 lytic activity. In conclusion, this study provides insights into temporal and molecular mechanisms of cell traversal versus productive invasion during the early stages of malaria. Our data reveal that Plasmodium sporozoites actively invade cells inside two types of vacuoles, and use two different strategies, egress from the vacuole or remodeling of the vacuole membrane, to escape degradation by the host cell lysosomes. These findings illustrate how the malaria parasite evades the host cell defense mechanisms to ensure its safe migration from the skin to the liver and the establishment of a suitable intracellular niche for replication. 15
17 EXPERIMENTAL PROCEDURES Experimental animals and ethics statement Female Swiss and BALB/c mice (6 8 weeks old, from Janvier) were used for parasite infections. All animal work was conducted in strict accordance with the Directive 2010/63/EU of the European Parliament and Council On the protection of animals used for scientific purposes. The protocol was approved by the Charles Darwin Ethics Committee of the University Pierre et Marie Curie, Paris, France (permit number Ce5/2012/001). Parasites and cell lines We used reference P. yoelii 17XNL (clone 1.1) and P. berghei ANKA (clone 15cy1) parasites. Control GFP-expressing PyGFP and PbGFP parasite lines (Manzoni et al., 2014) were obtained after integration of a GFP expression cassette at the dispensable P230p locus. Anopheles stephensi mosquitoes were fed on P. yoelii or P. berghei-infected mice using standard methods (Ramakrishnan et al., 2013), and kept at 24 C and 21 C, respectively. P. yoelii and P. berghei sporozoites were collected from the salivary glands of infected mosquitoes or days post-feeding, respectively. Hepatoma cell lines were cultured at 37 C under 5% CO 2 in DMEM supplemented with 10% fetal calf serum and antibiotics (Life Technologies), as described (Silvie et al., 2007). Stable expression of mcherry fused to the N-terminal 20 amino acids of neuromodulin (N20-mCherry) was achieved by cell transduction with a lentiviral vector (Vectalys), following the manufacturer s instructions. Targeted PLP1 gene deletion in P. yoelii and P. berghei PyΔplp1 and PbΔplp1 mutant parasites were generated using a Gene Out Marker Out strategy (Manzoni et al., 2014). P. yoelii 17XNL and P. berghei ANKA WT parasites were transfected with pyplp1 and pbplp1 targeting constructs, respectively, using standard transfection methods (Janse et al., 2006). GFP-expressing parasite mutants were isolated by flow cytometry after positive and negative selection rounds, as described (Manzoni et al., 16
18 2014). Correct construct integration was confirmed by analytical PCR using specific primer combinations. For mcherry tagging of P. yoelii RON4, drug-selectable marker-free PyΔplp1 parasites were transfected with a PyRON4 targeting vector, as described (Risco-Castillo et al., 2014), and recombinant parasites were isolated by flow cytometry. Details on construct design and parasite transfections are provided as Supplemental Experimental Procedures. Sporozoite cell traversal and invasion assays Sporozoite CT and invasion were monitored by flow cytometry (Prudêncio et al., 2008). Briefly, hepatoma cells (5 x 10 4 per well in collagen-coated 96-well plates) were incubated with GFP-expressing sporozoites (5 x 10 3 to 3 x 10 4 per well) in the presence of 0.5 mg/ml rhodamine-conjugated dextran (Life technologies). At different time points, cell cultures were washed, trypsinized and analyzed on a Guava EasyCyte 6/2L bench cytometer equipped with 488 and 532 nm lasers (Millipore), for detection of GFP-positive and dextran-positive cells. For inhibition of lysosome acidification, cells were treated with 1 μm bafilomycin A1 or 100 μm chloroquine (Sigma) for 2 or 12 hours, respectively, or with the solvent alone (DMSO) as a control. Cultures were washed before addition of sporozoites. In some experiments, invasion assays were performed in the presence of 10 μg/ml NYS1 anti-csp antibody (Charoenvit et al., 1987), 25 μg/ml MT81 anti-cd81 antibody (Silvie et al., 2006b) or 1 μg/ml cytochalasin D (Sigma). To study the kinetics of productive invasion events, sporozoite-infected cell cultures were trypsinized at different time points, replated in 96-well plates and further cultured for hours. Cells were then fixed with 4% PFA and the number of EEFs was determined by fluorescence microscopy. Fluorescence microscopy For imaging experiments, cells were plated in Ibidi 96-well μ-plates (Biovalley), and imaged on a Zeiss Axio Observer.Z1 inverted fluorescence microscope equipped with LD Plan- Neofluar 40X/0.6 Corr Ph2 M27 and Plan-Apochromat 63X/1.40 Oil DIC M27 objectives. Images acquired using the Zen 2012 software (Zeiss) were processed with ImageJ or 17
19 Photoshop CS6 software (Adobe) for adjustment of contrast. To assess liver stage development, HepG2/CD81 cells were infected with P. yoelii WT, PyGFP or PyΔplp1 sporozoites and cultured for 6 to 36 hours before fixation with 4% PFA. Cells were then permeabilized with Triton X-100, and the parasites were stained using antibodies specific for Plasmodium HSP70 (Tsuji et al., 1994) and UIS4 (Sicgen). Nuclei were stained with Hoechst (Life Technologies). For visualization of cell membranes, infected cultures were fixed with 4% PFA and labeled with filipin (Sigma), phalloidin-tritc (Sigma), and/or anti-basigin antibodies (8A6, Abcam). For quantitative analysis, at least 40 parasites were examined per condition. For lysosome visualization, hepatoma cells infected with GFP-expressing sporozoites were incubated with 60 nm Lysotracker Red DND-99 (Life Technologies) for 30 min before fluorescence microscopy imaging. LAMP1 immunostaining was performed on fixed cells, using monoclonal antibodies specific for human (H4A3, Abcam) or mouse (1D4B, Abcam) LAMP1. For immunostaining of mouse liver sections, BALB/c mice were injected in the tail vein with 1 x 10 6 PyGFP or PyΔplp1 sporozoites, and euthanized three hours later. The liver was removed, immediately frozen in liquid nitrogen and cut into 7 μm cryosections. Liver sections were fixed in 4% paraformaldehyde, permeabilized in 1% Triton X100 and analyzed by immunofluorescence using antibodies against mouse LAMP1 (1D4B, Abcam) and parasite CSP (Charoenvit et al., 1987). Spinning disk confocal microscopy HepG2 cells expressing the N20-mCherry membrane marker were plated in Ibidi 8-well μ- slides (Biovalley). After addition of PyGFP or PyΔplp1 sporozoites, cultures were placed onto a spinning disk microscope system in a controlled chamber at 37 C under 5% CO 2. We used a CSU22 spinning-disk confocal system (Yokogawa) mounted on a DMI 6000 inverted microscope (Leica), equipped with a Plan-Apochromat 100X/1.40 Oil objective and a cooled EMCCD camera QuantEM 512SC (Photometrics), and driven by Metamorph 7 software (Molecular Devices). Images were recorded every 5 seconds during 15 min, and processed with ImageJ for adjustment of contrast. 18
20 Transmission Electron Microscopy HepG2 cell cultures were incubated with PyΔplp1 sporozoites for 45 min or 5 hours before fixation with 2.5% glutaraldehyde in 0.15 M cacodylate buffer. Samples were then treated with 1% osmium tetroxide, dehydrated in a series of ethanol concentrations, and embedded in EPON resin mixture. Ultrathin sections (50 to 60 nm) were observed with a Jeol 1200EXII (Tokio, Japon) transmission electron microscope. Images were recorded with a Quemesa 11 Mpixel camera and the item software (Olympus Soft Imaging Solutions, Munster, Germany). Statistical analysis Statistical significance was assessed by non-parametric analysis using the Mann-Whitney U, Kruskal Wallis and log rank (Mantel-Cox) tests. Multiple comparisons were performed by two-way ANOVA followed by Bonferroni post-test. All statistical tests were computed with GraphPad Prism 5 (GraphPad Software). In vitro experiments were performed at least three times, with a minimum of three technical replicates per experiment. In vivo experiments in mice were only performed once or twice, as indicated, to minimize animal usage. 19
21 AUTHOR CONTRIBUTIONS V.R.C., S.T. and C.M. designed and performed experiments and analyzed the data; G.M., A.E.B. and S.B. performed experiments; X.B. performed spinning disk microscopy; M.L. analyzed the data; J.F.D. performed electron microscopy and analyzed the data; O.S. supervised the project, designed experiments and analyzed the data, and wrote the manuscript with contributions from all authors. ACKNOWLEDGMENTS We thank Jean-François Franetich, Maurel Tefit, Thierry Houpert and Sylvie Minard for rearing the mosquitoes; Bénédicte Hoareau-Coudert (Flow Cytometry Core CyPS) for parasite sorting by flow cytometry; Julius Hafalla and Arnaud Moris for helpful discussions. We acknowledge the ImagoSeine facility, member of the France BioImaging infrastructure supported by the Agence Nationale de la Recherche (ANR-10-INSB-04). This work was funded by the European Union (FP7 Marie Curie grant PCIG10-GA , FP7 PathCo Collaborative Project HEALTH-F ), the Agence Nationale de la Recherche (ANR-10-PDOC ) and the Laboratoire d'excellence ParaFrap (ANR-11- LABX-0024). GM was supported by a DIM Malinf doctoral fellowship awarded by the Conseil Régional d'ile-de-france. 20
22 REFERENCES Amino, R., Giovannini, D., Thiberge, S., Gueirard, P., Boisson, B., Dubremetz, J.-F., Prévost, M.-C., Ishino, T., Yuda, M., and Ménard, R. (2008). Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3, Bano, N., Romano, J.D., Jayabalasingham, B., and Coppens, I. (2007). Cellular interactions of Plasmodium liver stage with its host mammalian cell. Int J Parasitol 37, Besteiro, S., Dubremetz, J.F., and Lebrun, M. (2011). The moving junction of apicomplexan parasites: a key structure for invasion. Cell Microbiol 13, Bhanot, P., Schauer, K., Coppens, I., and Nussenzweig, V. (2005). A surface phospholipase is involved in the migration of plasmodium sporozoites through cells. J Biol Chem 280, Charoenvit, Y., Leef, M.F., Yuan, L.F., Sedegah, M., and Beaudoin, R.L. (1987). Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect. Immun. 55, Deligianni, E., Morgan, R.N., Bertuccini, L., Wirth, C.C., Silmon de Monerri, N.C., Spanos, L., Blackman, M.J., Louis, C., Pradel, G., and Siden-Kiamos, I. (2013). A perforin-like protein mediates disruption of the erythrocyte membrane during egress of Plasmodium berghei male gametocytes. Cell. Microbiol. 15, Dunstone, M.A., and Tweten, R.K. (2012). Packing a punch : the mechanism of pore formation by cholesterol dependent cytolysins and membrane attack complex / perforin-like proteins. Curr. Opin. Struct. Biol. 22, Frevert, U., Engelmann, S., Zougbede, S., Stange, J., Ng, B., Matuschewski, K., Liebes, L., and Yee, H. (2005). Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol 3, e192. Garg, S., Agarwal, S., Kumar, S., Yazdani, S.S., Chitnis, C.E., and Singh, S. (2013). Calcium-dependent permeabilization of erythrocytes by a perforin-like protein during egress of malaria parasites. Nat. Commun. 4,
23 Hamon, M.A., Ribet, D., Stavru, F., and Cossart, P. (2012). Listeriolysin O: The Swiss army knife of Listeria. Trends Microbiol. 20, Ishino, T., Yano, K., Chinzei, Y., and Yuda, M. (2004). Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol 2, E4. Ishino, T., Chinzei, Y., and Yuda, M. (2005). A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol 7, Janse, C.J., Ramesar, J., and Waters, A.P. (2006). High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc 1, Kafsack, B.F.C., Pena, J.D.O., Coppens, I., Ravindran, S., Boothroyd, J.C., and Carruthers, V.B. (2009). Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science 323, Kaiser, K., Camargo, N., Coppens, I., Morrisey, J.M., Vaidya, A.B., and Kappe, S.H. (2004). A member of a conserved Plasmodium protein family with membrane-attack complex/perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol Biochem Parasitol 133, Kariu, T., Ishino, T., Yano, K., Chinzei, Y., and Yuda, M. (2006). CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol 59, Manzoni, G., Briquet, S., Risco-Castillo, V., Gaultier, C., Topcu, S., Ivanescu, M.L., Franetich, J.F., Hoareau-Coudert, B., Mazier, D., and Silvie, O. (2014). A rapid and robust selection procedure for generating drug-selectable marker-free recombinant malaria parasites. Sci Rep 4, Ménard, R., Tavares, J., Cockburn, I., Markus, M., Zavala, F., and Amino, R. (2013). Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol 11,
24 Mordue, D.G., Desai, N., Dustin, M., and Sibley, L.D. (1999). Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med 190, Moreira, C.K., Templeton, T.J., Lavazec, C., Hayward, R.E., Hobbs, C. V, Kroeze, H., Janse, C.J., Waters, A.P., Sinnis, P., and Coppi, A. (2008). The Plasmodium TRAP / MIC2 family member, TRAP-Like Protein ( TLP ), is involved in tissue traversal by sporozoites. 10, Mota, M.M., Pradel, G., Vanderberg, J.P., Hafalla, J.C., Frevert, U., Nussenzweig, R.S., Nussenzweig, V., and Rodriguez, A. (2001). Migration of Plasmodium sporozoites through cells before infection. Science 291, Mota, M.M., Hafalla, J.C., and Rodriguez, A. (2002). Migration through host cells activates Plasmodium sporozoites for infection. Nat Med 8, Prudêncio, M., Rodrigues, C.D., Ataíde, R., and Mota, M.M. (2008). Dissecting in vitro host cell infection by Plasmodium sporozoites using flow cytometry. Cell. Microbiol. 10, Ramakrishnan, C., Delves, M.J., Lal, K., Blagborough, A.M., Butcher, G., Baker, K.W., and Sinden, R.E. (2013). Laboratory maintenance of rodent malaria parasites. Methods Mol Biol 923, Risco-Castillo, V., Topcu, S., Son, O., Briquet, S., Manzoni, G., and Silvie, O. (2014). CD81 is required for rhoptry discharge during host cell invasion by Plasmodium yoelii sporozoites. Cell Microbiol 16, Roiko, M.S., Svezhova, N., and Carruthers, V.B. (2014). Acidification Activates Toxoplasma gondii Motility and Egress by Enhancing Protein Secretion and Cytolytic Activity. PLoS Pathog. 10, e Schuerch, D.W., Wilson-Kubalek, E.M., and Tweten, R.K. (2005). Molecular basis of listeriolysin O ph dependence. Proc. Natl. Acad. Sci. U. S. A. 102, Silvie, O., Rubinstein, E., Franetich, J.-F., Prenant, M., Belnoue, E., Rénia, L., Hannoun, L., Eling, W., Levy, S., Boucheix, C., et al. (2003). Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat. Med. 9,
25 Silvie, O., Greco, C., Franetich, J.F., Dubart-Kupperschmitt, A., Hannoun, L., van Gemert, G.J., Sauerwein, R.W., Levy, S., Boucheix, C., Rubinstein, E., et al. (2006a). Expression of human CD81 differently affects host cell susceptibility to malaria sporozoites depending on the Plasmodium species. Cell Microbiol 8, Silvie, O., Charrin, S., Billard, M., Franetich, J.F., Clark, K.L., van Gemert, G.J., Sauerwein, R.W., Dautry, F., Boucheix, C., Mazier, D., et al. (2006b). Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J Cell Sci 119, Silvie, O., Franetich, J.F., Boucheix, C., Rubinstein, E., and Mazier, D. (2007). Alternative invasion pathways for Plasmodium berghei sporozoites. Int J Parasitol 37, Talman, A.M., Lacroix, C., Marques, S.R., Blagborough, A.M., Carzaniga, R., Ménard, R., and Sinden, R.E. (2011). PbGEST mediates malaria transmission to both mosquito and vertebrate host. Mol. Microbiol. 82, Tavares, J., Formaglio, P., Thiberge, S., Mordelet, E., Van Rooijen, N., Medvinsky, A., Ménard, R., and Amino, R. (2013). Role of host cell traversal by the malaria sporozoite during liver infection. J. Exp. Med. 210, Tsuji, M., Mattei, D., Nussenzweig, R.S., Eichinger, D., and Zavala, F. (1994). Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 80, Wirth, C.C., Glushakova, S., Scheuermayer, M., Repnik, U., Garg, S., Schaack, D., Kachman, M.M., Weißbach, T., Zimmerberg, J., Dandekar, T., et al. (2014). Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cell. Microbiol. 16, Yamauchi, L.M., Coppi, A., Snounou, G., and Sinnis, P. (2007). Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 9, Yoshimori, T., Yamamoto, A., Moriyama, Y., Futai, M., and Tashiro, Y. (1991). Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 266,
26 Zhao, Y., Marple, A.H., Ferguson, D.J.P., Bzik, D.J., and Yap, G.S. (2014). Avirulent strains of Toxoplasma gondii infect macrophages by active invasion from the phagosome. Proc. Natl. Acad. Sci. U. S. A. 111, Zuber, M.X., Strittmatter, S.M., and Fishman, M.C. (1989). A membrane-targeting signal in the amino terminus of the neuronal protein GAP-43. Nature 341,
27 FIGURE LEGENDS Figure 1. Kinetics of P. yoelii cell traversal and cell invasion. A. Invasion/infection assay. Cell cultures were incubated for 10 to 180 min with GFP-expressing sporozoites in the presence of rhodamine-labeled dextran, trypsinized and either directly analyzed by FACS, to determine the percentage of traversed (dextran-positive) and invaded (GFP-positive) cells, or replated and further incubated for 24 to 48 h, to determine the number of EEF-infected cells by fluorescence microscopy (productive infection). B-C. HepG2 and HepG2/CD81 cells (5 x 10 4 ) were incubated at 37 C with PyGFP sporozoites (3 x 10 4 ) in the presence of rhodamineconjugated dextran, and analyzed by FACS to determine the percentage of traversed (dextran-positive) cells (B) and invaded (GFP-positive) cells (C). Results are expressed as the mean percentage (+/- SD) of triplicate wells. Statistical significance was assessed using two-way ANOVA followed by Bonferroni test. *** p< D. HepG2/CD81 cell cultures were incubated with PyGFP sporozoites for 10 to 120 minutes, dissociated and replated and cultured for an additional 24 hours, to determine the number of EEFs by fluorescence microscopy. Figure 2. Sporozoites form transient vacuoles during cell traversal. A. HepG2 cells were incubated with PyGFP sporozoites in the presence of rhodamine-labeled dextran for 15 or 120 minutes. Cells were then trypsinized and analyzed by FACS to determine the proportion of dextran-negative cells among infected (GFP-positive) cells. B-C. Electron micrographs of PyGFP sporozoites inside HepG2 cells, 1 hour post-infection. A vacuole membrane surrounds the parasite in B but not in C. The insets show at higher magnification the parasite plasma membrane (arrow) and the vacuole membrane (arrowheads). Rhoptries are indicated with asterisks. Bars, 2 μm. D. HepG2 cells expressing the fluorescent plasma membrane protein N20-mCherry (red) were incubated with PyGFP sporozoites (green) for 30 min, fixed and labeled with filipin (blue). Bars, 10 μm. E. HepG2/N20-mCherry cells were incubated with PyGFP sporozoites for 30 or 120 min before fixation and labeling with filipin, and the presence or absence of a vacuole was determined by fluorescence microscopy. F. Time- 26
28 lapse confocal microscopy of a PyGFP sporozoite (green) in a HepG2/N20-mCherry cell (membranes labeled in red). Images were extracted from Movie S1. A constriction of the parasite is indicated with an arrowhead. Bars, 10 μm. See also Movies S1-2. Figure 3. PyΔplp1 sporozoites accumulate inside non-replicative vacuoles. A-B. HepG2 (A) or HepG2/CD81 (B) cells (5 x 10 4 ) were incubated with PyGFP or PyΔplp1 sporozoites (3 x 10 4 ) for 10 to 120 minutes, trypsinized, and either directly analyzed by FACS to quantify invaded (GFP-positive) cells (lines), or replated and cultured for an additional 24 hours before quantification of EEFs by fluorescence microscopy (bars). Shown are the mean values (± SD) of triplicate wells. Statistical significance was assessed using two-way ANOVA followed by Bonferroni test. ** p<0.01; *** p<0.001; ns, non significant. C. Electron micrographs of PyΔplp1 sporozoites in HepG2 cell. The insets show at higher magnification the parasite plasma membrane (arrow) and the vacuole membrane (arrowheads). The parasite rhoptries are indicated with asterisks. Bars, 1 μm. D. HepG2/N20-mCherry cells (red) were incubated with PyΔplp1 sporozoites (green) for 30 min, fixed and labeled with filipin (blue). Bars, 10 μm. E. HepG2/N20-mCherry cells were incubated with PyΔplp1 sporozoites for 30 or 120 min before fixation and labeling with filipin, and the presence or absence of a vacuole was determined by fluorescence microscopy. F. Time-lapse confocal microscopy of a PyΔplp1 sporozoite (green) in a HepG2/N20-mCherry cell (membranes labeled in red). Images were extracted from Movie S3. Bars, 10 μm. See also Figures S1-3 and Movies S3-7. Figure 4. Sporozoites form TVs without rhoptry secretion or remodeling of the vacuole membrane. A. HepG2 and HepG2/CD81 were incubated for 2 hours with PyΔplp1 sporozoites in the presence of anti-pycsp antibody or cytochalasin D, and analyzed by FACS to determine the percentage of invaded cells, in comparison to control wells without inhibitors. B. Transgenic PyΔplp1/RON4::mCherry and PyGFP/RON4::mCherry sporozoites were incubated with HepG2 or HepG2/CD81 cells for 60 or 120 min before fixation and filipin 27
29 staining. Apical RON4-mCherry fluorescence is indicated with arrowheads. Bars, 10 μm. C. HepG2 and HepG2/CD81 cells were incubated with PyGFP/RON4::mCherry or PyΔplp1/RON4::mCherry sporozoites, labeled with filipin, and the proportion of RON4- depleted sporozoites inside filipin-positive vacuoles was determined by fluorescence microscopy. D. HepG2/N20-mCherry and HepG2/CD81/N20-mCherry cells were incubated with PyΔplp1 or PyGFP sporozoites for 30 or 120 min, respectively, fixed, and labeled with filipin. Bars, 10 μm. E-F. HepG2 (E) and HepG2/CD81 (F) cells were incubated for 120 min with PyΔplp1 (E) or PyGFP (F) sporozoites, respectively, fixed, and labeled with filipin and either phalloidin-tritc or anti-basigin antibodies. Bars, 10 μm. G. Cell cultures processed as in D-F were examined by fluorescence microscopy to determine the proportion of labeled vacuoles among filipin-positive TVs (PyΔplp1 sporozoites in HepG2 cells) versus PVs (PyGFP sporozoites in HepG2/CD81 cells). See also Figure S4. Figure 5. Non-replicative PyΔplp1 vacuoles are eliminated by the host cell lysosomes. A. HepG2 or HepG2/CD81 cells (5 x 10 4 ) were incubated with PyΔplp1 sporozoites (3 x 10 4 ) for 1 to 12 h, and analyzed by FACS to determine the percentage of infected (GFP-positive) cells. B. HepG2 and HepG2/CD81 were incubated with GFP-expressing PyΔplp1 sporozoites (green) for 4 h, then labeled with Lysotracker-red and examined by fluorescence microscopy. Bars, 10 μm. C. HepG2 and HepG2/CD81 were incubated with GFP-expressing PyΔplp1 sporozoites (green) for 5 h, then fixed and labeled with antibodies against LAMP1 (red) and Hoechst (blue). Bars, 10 μm. The insets show LAMP1-negative (i and ii) and LAMP1- positive (iii and iv) parasites. D. HepG2/CD81 and HepG2 cells were incubated with PyΔplp1 sporozoites for 4 h and labeled as in B and C. The proportion of Lysotracker- or LAMP1- positive parasites was then determined by fluorescence microscopy. At least 100 infected cells were examined per condition. E. Liver sections from BALB/c mice infected with PyGFP or PyΔplp1 sporozoites were labeled with antibodies against CSP, UIS4 or LAMP1, and analysed by fluorescence microscopy to determine the proportion of parasites expressing UIS4 and LAMP1 among PyGFP (n = 52) and PyΔplp1 (n = 56) parasites. F. HepG2 cells 28
30 were treated with chloroquine (CQ) for 12 hours before addition of PyΔplp1 sporozoites. The percentage of infected (GFP-positive) cells in treated versus untreated cultures was determined by FACS at 4 and 20 hours post-infection. See also Figure S5. Figure 6. Blocking lysosomal acidification inhibits sporozoite egress from transient vacuoles. A-B. HepG2/CD81 (A) or HepG2 cells (B) (3 x 10 4 ) were pre-treated with bafilomycin A1 or solvent alone (control), then incubated with PyGFP sporozoites (1 x 10 4 ) in the presence of rhodamine-conjugated dextran, and analyzed by FACS to determine the percentage of traversed (dextran-positive) cells (lines) and invaded (GFP-positive) cells (bars). Results are expressed as the mean percentage (+/- SD) of triplicate wells. Statistical significance was assessed using two-way ANOVA followed by Bonferroni test (GFP-positive cells: non significant at 15 and 30 min, p<0.001 at 60, 90 and 120 min). C-D. HepG2/CD81 or HepG2 cells treated like in A and B were incubated for 24 hours before analysis by FACS (C) and fluorescence microscopy (D), to determine the percentage of infected cells and the proportion of replicative forms (EEFs). Figure 7. A model of host cell invasion by malaria sporozoites. Plasmodium sporozoites invade cells actively inside two types of vacuoles. A. Sporozoites initially enter cells actively inside a transient vacuole (1), without forming a junction, and subsequently egress using PLP1 (2). PLP1-mediated membrane rupture may occur before complete sealing of the primary vacuole (3). PLP1 activity depends on lysosomal acidification, and results in parasite cell traversal and escape from lysosomal degradation. PLP1-deficient parasites cannot breach the vacuole membrane and are trapped inside non-replicative vacuoles, which are eliminated after fusion with the host cell lysosomes (4). B. Sporozoites eventually switch to productive invasion through a moving junction (5), a process that requires the host entry factor CD81 and results in the formation of the PV. Productive invasion is associated with remodeling of the vacuole membrane, precluding its fusion with lysosomes, and leads to parasite liver stage development inside the PV (6). We cannot exclude that some sporozoites 29
31 may enter cells through the non-productive invasion pathway and form a junction intracellularly (7), resulting in the remodeling of the initial non-replicative vacuole into a replicative PV. 30
32
33
34
35
36
37
38
Hepatitis C virus entry and cell-cell transmission : implication for viral life cycle and antiviral treatment
 Hepatitis C virus entry and cell-cell transmission : implication for viral life cycle and antiviral treatment Fei Xiao To cite this version: Fei Xiao. Hepatitis C virus entry and cell-cell transmission
Hepatitis C virus entry and cell-cell transmission : implication for viral life cycle and antiviral treatment Fei Xiao To cite this version: Fei Xiao. Hepatitis C virus entry and cell-cell transmission
Plasmodium yoelii Sporozoites with Simultaneous Deletion of P52 and P36 Are Completely Attenuated and Confer Sterile Immunity against Infection
 INFECTION AND IMMUNITY, Aug. 2007, p. 3758 3768 Vol. 75, No. 8 0019-9567/07/$08.00 0 doi:10.1128/iai.00225-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Plasmodium yoelii Sporozoites
INFECTION AND IMMUNITY, Aug. 2007, p. 3758 3768 Vol. 75, No. 8 0019-9567/07/$08.00 0 doi:10.1128/iai.00225-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Plasmodium yoelii Sporozoites
Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis
 Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis A. Reagents: 1. DMEM or RPMI DMEM (4.5g/L glucose) RPMI 1640 Cellgro #MT-10-017-CM Cellgro #MT-10-040-CM 2. Giemsa
Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis A. Reagents: 1. DMEM or RPMI DMEM (4.5g/L glucose) RPMI 1640 Cellgro #MT-10-017-CM Cellgro #MT-10-040-CM 2. Giemsa
Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development
 Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development Shiroh Iwanaga, Izumi Kaneko, Tomomi Kato, Masao Yuda* Department of Medical Zoology, Mie University School
Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development Shiroh Iwanaga, Izumi Kaneko, Tomomi Kato, Masao Yuda* Department of Medical Zoology, Mie University School
Gliding Motility Assay for P. berghei Sporozoites
 Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding
 Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding Ashley M. Vaughan, 1 Ahmed S.I. Aly, 1 and Stefan H.I. Kappe 1,2, * 1 Seattle Biomedical Research Institute, Seattle, WA 98109, USA
Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding Ashley M. Vaughan, 1 Ahmed S.I. Aly, 1 and Stefan H.I. Kappe 1,2, * 1 Seattle Biomedical Research Institute, Seattle, WA 98109, USA
ACCEPTED. Parasitology Unit, Max Planck Institute for Infection Biology, Berlin, Germany
 EC Accepts, published online ahead of print on 30 January 2009 Eukaryotic Cell doi:10.1128/ec.00347-08 Copyright 2009, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
EC Accepts, published online ahead of print on 30 January 2009 Eukaryotic Cell doi:10.1128/ec.00347-08 Copyright 2009, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts
 Blackwell Publishing LtdOxford, UKMMIMolecular Microbiology0950-382X 2005 The Authors; Journal compilation 2005 Blackwell Publishing Ltd? 200559513691379Original ArticleA protein that mediates malarial
Blackwell Publishing LtdOxford, UKMMIMolecular Microbiology0950-382X 2005 The Authors; Journal compilation 2005 Blackwell Publishing Ltd? 200559513691379Original ArticleA protein that mediates malarial
Arrested oocyst maturation in Plasmodium parasites. lacking type II NADH:ubiquinone dehydrogenase
 Supplemental Information for: Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase Katja E. Boysen and Kai Matuschewski Contents: - Supplemental Movies 1 and
Supplemental Information for: Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase Katja E. Boysen and Kai Matuschewski Contents: - Supplemental Movies 1 and
The silent path to thousands of merozoites: the Plasmodium liver stage
 The silent path to thousands of merozoites: the Plasmodium liver stage Miguel Prudêncio*, Ana Rodriguez and Maria M. Mota* Abstract Plasmodium sporozoites are deposited in the skin of their vertebrate
The silent path to thousands of merozoites: the Plasmodium liver stage Miguel Prudêncio*, Ana Rodriguez and Maria M. Mota* Abstract Plasmodium sporozoites are deposited in the skin of their vertebrate
A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development
 A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development Christine Lehmann 1, Anna Heitmann 1, Satish Mishra 2, Paul-Christian Burda 3, Mirko Singer 4, Monica Prado
A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development Christine Lehmann 1, Anna Heitmann 1, Satish Mishra 2, Paul-Christian Burda 3, Mirko Singer 4, Monica Prado
Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity
 Molecular & Biochemical Parasitology 156 (2007) 32 40 Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity Kota Arun
Molecular & Biochemical Parasitology 156 (2007) 32 40 Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity Kota Arun
A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S.
 VI. Malaria A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S. because they were resistant to malaria & other diseases 3. Many
VI. Malaria A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S. because they were resistant to malaria & other diseases 3. Many
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/70169
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/70169
Motility precedes egress of malaria parasites from oocysts
 RESEARCH ARTICLE Motility precedes egress of malaria parasites from oocysts Dennis Klug*, Friedrich Frischknecht* Integrative Parasitology, Center for Infectious Diseases, Heidelberg University Medical
RESEARCH ARTICLE Motility precedes egress of malaria parasites from oocysts Dennis Klug*, Friedrich Frischknecht* Integrative Parasitology, Center for Infectious Diseases, Heidelberg University Medical
Automated classification of Plasmodium sporozoite movement patterns reveals a shift towards productive motility during salivary gland infection
 Automated classification of Plasmodium sporozoite movement patterns reveals a shift towards productive motility during salivary gland infection Stephan Josef Hegge, Mikhail Kudryashev, Ashley Smith, Friedrich
Automated classification of Plasmodium sporozoite movement patterns reveals a shift towards productive motility during salivary gland infection Stephan Josef Hegge, Mikhail Kudryashev, Ashley Smith, Friedrich
Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites
 Mediators of Inflammation, Article ID 362605, 6 pages http://dx.doi.org/10.1155/2014/362605 Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites Hong Zheng, Zhangping Tan, and
Mediators of Inflammation, Article ID 362605, 6 pages http://dx.doi.org/10.1155/2014/362605 Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites Hong Zheng, Zhangping Tan, and
Quantitative Dynamics of Plasmodium yoelii Sporozoite Transmission by Infected Anopheline Mosquitoes
 INFECTION AND IMMUNITY, July 2005, p. 4363 4369 Vol. 73, No. 7 0019-9567/05/$08.00 0 doi:10.1128/iai.73.7.4363 4369.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Quantitative
INFECTION AND IMMUNITY, July 2005, p. 4363 4369 Vol. 73, No. 7 0019-9567/05/$08.00 0 doi:10.1128/iai.73.7.4363 4369.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Quantitative
INVESTIGATING THE MOTILITY OF PLASMODIUM
 INVESTIGATING THE MOTILITY OF PLASMODIUM by Natasha Vartak A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Science Baltimore, Maryland April,
INVESTIGATING THE MOTILITY OF PLASMODIUM by Natasha Vartak A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Science Baltimore, Maryland April,
Plasmodium Pre-Erythrocytic Stages: Biology, Whole Parasite Vaccines and Transgenic Models
 American Journal of Immunology, 2012, 8 (3), 88-100 ISSN 1553-619X 2012 Science Publication doi:10.3844/ajisp.2012.88.100 Published Online 8 (3) 2012 (http://www.thescipub.com/aji.toc) Plasmodium Pre-Erythrocytic
American Journal of Immunology, 2012, 8 (3), 88-100 ISSN 1553-619X 2012 Science Publication doi:10.3844/ajisp.2012.88.100 Published Online 8 (3) 2012 (http://www.thescipub.com/aji.toc) Plasmodium Pre-Erythrocytic
PRINCIPAL INVESTIGATOR: Dr. Jetsumon (Sattabongkot) Prachumsri
 AD (Leave blank) Award Number: W81XWH-07-2-0090 TITLE: Proteomic Study of Human Malaria Parasite Plasmodium Vivax Liver Stages for Development of Vaccines and Drugs PRINCIPAL INVESTIGATOR: Dr. Jetsumon
AD (Leave blank) Award Number: W81XWH-07-2-0090 TITLE: Proteomic Study of Human Malaria Parasite Plasmodium Vivax Liver Stages for Development of Vaccines and Drugs PRINCIPAL INVESTIGATOR: Dr. Jetsumon
alaria Parasite Bank Collection sites of P. falciparum isolates PARASITE BIOLOGY
 M alaria Parasite Bank established in 1992 is a supporting unit for research activities on different aspects of malaria. The main objective of establishing this facility is to strengthen researches at
M alaria Parasite Bank established in 1992 is a supporting unit for research activities on different aspects of malaria. The main objective of establishing this facility is to strengthen researches at
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5870/1679/dc1 Supporting Online Material for Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes Chung-hui Yang, Priyanka
www.sciencemag.org/cgi/content/full/319/5870/1679/dc1 Supporting Online Material for Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes Chung-hui Yang, Priyanka
Parasitology Departement Medical Faculty of USU
 Malaria Mechanism of infection Parasitology Departement Medical Faculty of USU Introduction Malaria parasites Phylum Order Suborder Family Genus Species : : Apicomplexa : Eucoccidiida : Haemosporida :
Malaria Mechanism of infection Parasitology Departement Medical Faculty of USU Introduction Malaria parasites Phylum Order Suborder Family Genus Species : : Apicomplexa : Eucoccidiida : Haemosporida :
PLASMODIUM MODULE 39.1 INTRODUCTION OBJECTIVES 39.2 MALARIAL PARASITE. Notes
 Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Inheritance of coat and colour in the Griffon Bruxellois dog
 Inheritance of coat and colour in the Griffon Bruxellois dog R Robinson To cite this version: R Robinson. Inheritance of coat and colour in the Griffon Bruxellois dog. Genetics Selection Evolution, BioMed
Inheritance of coat and colour in the Griffon Bruxellois dog R Robinson To cite this version: R Robinson. Inheritance of coat and colour in the Griffon Bruxellois dog. Genetics Selection Evolution, BioMed
Developmental Biology of Sporozoite-Host. Malaria: Implications for Vaccine Design. Javier E. Garcia, Alvaro Puentes and Manuel E.
 Developmental Biology of Sporozoite-Host Interactions in Plasmodium falciparum Malaria: Implications for Vaccine Design Javier E. Garcia, Alvaro Puentes and Manuel E. Patarroyo Clin. Microbiol. Rev. 2006,
Developmental Biology of Sporozoite-Host Interactions in Plasmodium falciparum Malaria: Implications for Vaccine Design Javier E. Garcia, Alvaro Puentes and Manuel E. Patarroyo Clin. Microbiol. Rev. 2006,
Biotecnologicas (IIB-INTECH), Universidad Nacional de San Martin, Av. General Paz 5445, Predio INTI, edificio 24 (1650), Buenos Aires, Argentina
 [Frontiers in Bioscience 17, 726-744, January 1, 2012] Plasmodium sporozoite motility: an update Georgina N. Montagna 1, Kai Matuschewski 1, Carlos A. Buscaglia 2 1 Parasitology Unit, Max Planck Institute
[Frontiers in Bioscience 17, 726-744, January 1, 2012] Plasmodium sporozoite motility: an update Georgina N. Montagna 1, Kai Matuschewski 1, Carlos A. Buscaglia 2 1 Parasitology Unit, Max Planck Institute
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12234 Supplementary Figure 1. Embryonic naked mole-rat fibroblasts do not undergo ECI. Embryonic naked mole-rat fibroblasts ( EF) were isolated from eight mid-gestation embryos. All the
doi:10.1038/nature12234 Supplementary Figure 1. Embryonic naked mole-rat fibroblasts do not undergo ECI. Embryonic naked mole-rat fibroblasts ( EF) were isolated from eight mid-gestation embryos. All the
David A Wilkinson, Olivier Duron, Colette Cordonin, Yann Gomard, Beza Ramasindrazana, Patrick Mavingui, Steven M Goodman, Pablo Tortosa
 The bacteriome of bat flies (Nycteribiidae) from the Malagasy region: a community shaped by host ecology, bacterial transmission mode, and host-vector specificity. David A Wilkinson, Olivier Duron, Colette
The bacteriome of bat flies (Nycteribiidae) from the Malagasy region: a community shaped by host ecology, bacterial transmission mode, and host-vector specificity. David A Wilkinson, Olivier Duron, Colette
Blood protozoan: Plasmodium
 Blood protozoan: Plasmodium Dr. Hala Al Daghistani The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans: four species are associated The Plasmodium spp.
Blood protozoan: Plasmodium Dr. Hala Al Daghistani The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans: four species are associated The Plasmodium spp.
Malaria remains the most important parasitic disease. Review Article
 Review Article Pre-erythrocytic Stage of Malaria Infection and the Molecular Targets Available for Interventions Dickson Adah 1,2, Yi Jun Yang 1,2, Quan Liu 1,2, Limei Qin 1, Li Qin 1, Xiaoping Chen 1
Review Article Pre-erythrocytic Stage of Malaria Infection and the Molecular Targets Available for Interventions Dickson Adah 1,2, Yi Jun Yang 1,2, Quan Liu 1,2, Limei Qin 1, Li Qin 1, Xiaoping Chen 1
Blood protozoan: Plasmodium
 Blood protozoan: Plasmodium The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans:four species are associated The Plasmodium spp. life cycle can be divided
Blood protozoan: Plasmodium The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans:four species are associated The Plasmodium spp. life cycle can be divided
The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands
 The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands Fabian E. Saenz 1,2, Bharath Balu 1, Jonah Smith 2, Sarita R. Mendonca 1,2, John H. Adams
The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands Fabian E. Saenz 1,2, Bharath Balu 1, Jonah Smith 2, Sarita R. Mendonca 1,2, John H. Adams
Time-Lapse Imaging of Red Blood Cell Invasion by the Rodent Malaria Parasite Plasmodium yoelii
 Time-Lapse Imaging of Red Blood Cell Invasion by the Rodent Malaria Parasite Plasmodium yoelii Kazuhide Yahata 1 *, Moritz Treeck 2,3, Richard Culleton 1,4, Tim-Wolf Gilberger 2,5, Osamu Kaneko 1 * 1 Department
Time-Lapse Imaging of Red Blood Cell Invasion by the Rodent Malaria Parasite Plasmodium yoelii Kazuhide Yahata 1 *, Moritz Treeck 2,3, Richard Culleton 1,4, Tim-Wolf Gilberger 2,5, Osamu Kaneko 1 * 1 Department
Evaluation of the hair growth and retention activity of two solutions on human hair explants
 activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign
 A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
INFLUENCE OF CONTAMINATION OF ENVIRONMENT AND BREEDING CONDITIONS ON DEVELOPMENT OF COCCIDIOSIS IN CHICKENS
 INFLUENCE OF CONTAMINATION OF ENVIRONMENT AND BREEDING CONDITIONS ON DEVELOPMENT OF COCCIDIOSIS IN CHICKENS Muriel Naciri, P. Yvoré, L. Conan To cite this version: Muriel Naciri, P. Yvoré, L. Conan. INFLUENCE
INFLUENCE OF CONTAMINATION OF ENVIRONMENT AND BREEDING CONDITIONS ON DEVELOPMENT OF COCCIDIOSIS IN CHICKENS Muriel Naciri, P. Yvoré, L. Conan To cite this version: Muriel Naciri, P. Yvoré, L. Conan. INFLUENCE
Marissa Vignali*, Cate Speake* and Patrick E Duffy*
 Minireview Malaria sporozoite proteome leaves a trail Marissa Vignali*, Cate Speake* and Patrick E Duffy* Addresses: *Malaria Program, Seattle Biomedical Research Institute, Seattle, Washington 98109,
Minireview Malaria sporozoite proteome leaves a trail Marissa Vignali*, Cate Speake* and Patrick E Duffy* Addresses: *Malaria Program, Seattle Biomedical Research Institute, Seattle, Washington 98109,
A n estimated 3.3 billion people were at risk of malaria infection in There is as of yet no licensed
 OPEN SUBJECT AREAS: PARASITOLOGY MOLECULAR BIOLOGY Received 27 March 2014 Accepted 23 June 2014 Published 11 July 2014 Correspondence and requests for materials should be addressed to A.S.I.A. (aaly@tulane.
OPEN SUBJECT AREAS: PARASITOLOGY MOLECULAR BIOLOGY Received 27 March 2014 Accepted 23 June 2014 Published 11 July 2014 Correspondence and requests for materials should be addressed to A.S.I.A. (aaly@tulane.
THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT PRAKASH SRINIVASAN
 THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT BY PRAKASH SRINIVASAN Submitted in partial fulfillment of the requirements For the degree of
THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT BY PRAKASH SRINIVASAN Submitted in partial fulfillment of the requirements For the degree of
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii. Yates, Lauren A.
 A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland sporozoites
 https://doi.org/10.1186/s12936-018-2431-1 Malaria Journal RESEARCH Open Access Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland
https://doi.org/10.1186/s12936-018-2431-1 Malaria Journal RESEARCH Open Access Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland
Comparative Plasmodium gene overexpression reveals distinct perturbation of sporozoite transmission by profilin
 MBoC ARTICLE Comparative Plasmodium gene overexpression reveals distinct perturbation of sporozoite transmission by profilin Yuko Sato a,b, *, Marion Hliscs a,c, Josefine Dunst a,d, Christian Goosmann
MBoC ARTICLE Comparative Plasmodium gene overexpression reveals distinct perturbation of sporozoite transmission by profilin Yuko Sato a,b, *, Marion Hliscs a,c, Josefine Dunst a,d, Christian Goosmann
Famacha scores should not be handled as numerical data
 Famacha scores should not be handled as numerical data Maurice Mahieu To cite this version: Maurice Mahieu. Famacha scores should not be handled as numerical data. Veterinary Parasitology, Elsevier, 2017,
Famacha scores should not be handled as numerical data Maurice Mahieu To cite this version: Maurice Mahieu. Famacha scores should not be handled as numerical data. Veterinary Parasitology, Elsevier, 2017,
Malaria. This sheet is from both sections recording and includes all slides and diagrams.
 Malaria This sheet is from both sections recording and includes all slides and diagrams. Malaria is caused by protozoa family called plasmodium (Genus) mainly affect blood system specially RBCs and each
Malaria This sheet is from both sections recording and includes all slides and diagrams. Malaria is caused by protozoa family called plasmodium (Genus) mainly affect blood system specially RBCs and each
Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis
 Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis Brandy L. Salmon, Anna Oksman, and Daniel E. Goldberg* Howard Hughes Medical Institute, Departments
Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis Brandy L. Salmon, Anna Oksman, and Daniel E. Goldberg* Howard Hughes Medical Institute, Departments
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland X Approved for public release; distribution unlimited
 Award Number: W8XWH--- TITLE: Defining the Role of Autophagy Kinase ULK Signaling in Therapeutic Response of Tuberous Sclerosis Complex to Inhibitors PRINCIPAL INVESTIGATOR: Reuben J. Shaw, Ph.D. CONTRACTING
Award Number: W8XWH--- TITLE: Defining the Role of Autophagy Kinase ULK Signaling in Therapeutic Response of Tuberous Sclerosis Complex to Inhibitors PRINCIPAL INVESTIGATOR: Reuben J. Shaw, Ph.D. CONTRACTING
Downloaded from:
 Al-Khattaf, FS; Tremp, AZ; El-Houderi, A; Dessens, JT (2016) The Plasmodium alveolin IMC1a is stabilised by its terminal cysteine motifs and facilitates sporozoite morphogenesis and infectivity in a dosedependent
Al-Khattaf, FS; Tremp, AZ; El-Houderi, A; Dessens, JT (2016) The Plasmodium alveolin IMC1a is stabilised by its terminal cysteine motifs and facilitates sporozoite morphogenesis and infectivity in a dosedependent
SOAR Research Proposal Summer How do sand boas capture prey they can t see?
 SOAR Research Proposal Summer 2016 How do sand boas capture prey they can t see? Faculty Mentor: Dr. Frances Irish, Assistant Professor of Biological Sciences Project start date and duration: May 31, 2016
SOAR Research Proposal Summer 2016 How do sand boas capture prey they can t see? Faculty Mentor: Dr. Frances Irish, Assistant Professor of Biological Sciences Project start date and duration: May 31, 2016
Phylum:Apicomplexa Class:Sporozoa
 Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
CONTRACTING ORGANIZATION: Rutgers, The State University of New Jersey Newark, NJ
 AWARD NUMBER: W81XWH-13-1-0429 TITLE: Using "Click Chemistry" to Identify Potential Drug Targets in Plasmodium PRINCIPAL INVESTIGATOR: Dr. Purnima Bhanot CONTRACTING ORGANIZATION: Rutgers, The State University
AWARD NUMBER: W81XWH-13-1-0429 TITLE: Using "Click Chemistry" to Identify Potential Drug Targets in Plasmodium PRINCIPAL INVESTIGATOR: Dr. Purnima Bhanot CONTRACTING ORGANIZATION: Rutgers, The State University
Visit ABLE on the Web at:
 This article reprinted from: Lessem, P. B. 2008. The antibiotic resistance phenomenon: Use of minimal inhibitory concentration (MIC) determination for inquiry based experimentation. Pages 357-362, in Tested
This article reprinted from: Lessem, P. B. 2008. The antibiotic resistance phenomenon: Use of minimal inhibitory concentration (MIC) determination for inquiry based experimentation. Pages 357-362, in Tested
EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS WORK-PROGRAMME PROPOSAL Version 2 VISAVET. Universidad Complutense de Madrid
 EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL Directorate D Animal Health and Welfare Unit D1- Animal health and Standing Committees EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS
EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL Directorate D Animal Health and Welfare Unit D1- Animal health and Standing Committees EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS
Malaria in the Mosquito Dr. Peter Billingsley
 Malaria in the Mosquito Senior Director Quality Systems and Entomology Research Sanaria Inc. Rockville MD. 1 Malaria: one of the world s foremost killers Every year 1 million children die of malaria 250
Malaria in the Mosquito Senior Director Quality Systems and Entomology Research Sanaria Inc. Rockville MD. 1 Malaria: one of the world s foremost killers Every year 1 million children die of malaria 250
Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent
 Supplementary materials Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent Shankar Thangamani 1, Haroon Mohammad 1, Mostafa Abushahba 1, Maha Hamed 1, Tiago Sobreira
Supplementary materials Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent Shankar Thangamani 1, Haroon Mohammad 1, Mostafa Abushahba 1, Maha Hamed 1, Tiago Sobreira
A Unique Approach to Managing the Problem of Antibiotic Resistance
 A Unique Approach to Managing the Problem of Antibiotic Resistance By: Heather Storteboom and Sung-Chul Kim Department of Civil and Environmental Engineering Colorado State University A Quick Review The
A Unique Approach to Managing the Problem of Antibiotic Resistance By: Heather Storteboom and Sung-Chul Kim Department of Civil and Environmental Engineering Colorado State University A Quick Review The
BIO Parasitology Spring 2009
 BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 10 Malaria-Life Cycle a. Micro and macrogametocytes in mosquito stomach. b. Ookinete
BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 10 Malaria-Life Cycle a. Micro and macrogametocytes in mosquito stomach. b. Ookinete
A role for apical membrane antigen 1 during invasion of hepatocytes
 JBC Papers in Press. Published on December 15, 2003 as Manuscript M311331200 A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. Olivier Silvie a,
JBC Papers in Press. Published on December 15, 2003 as Manuscript M311331200 A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. Olivier Silvie a,
Boosting Bacterial Metabolism to Combat Antibiotic Resistance
 Boosting Bacterial Metabolism to Combat Antibiotic Resistance The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published
Boosting Bacterial Metabolism to Combat Antibiotic Resistance The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published
Plasmodium sporozoites acquire virulence and. immunogenicity during mosquito hemocoel transit
 IAI Accepts, published online ahead of print on 30 December 2013 Infect. Immun. doi:10.1128/iai.00758-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 Plasmodium sporozoites
IAI Accepts, published online ahead of print on 30 December 2013 Infect. Immun. doi:10.1128/iai.00758-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 Plasmodium sporozoites
9 Parasitology 9 EXERCISE EQA. Objectives EXERCISE
 0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
Seasonal Variations of yeso sika Deer Skin and its Vegetable Tanned Leather
 Seasonal Variations of yeso sika Deer Skin and its Vegetable Tanned Leather Shigeharu Fukunaga, Akihiko Yoshie, Ikuo Yamakawa, Fumio Nakamura Laboratory of Animal By-product Science, Graduate School of
Seasonal Variations of yeso sika Deer Skin and its Vegetable Tanned Leather Shigeharu Fukunaga, Akihiko Yoshie, Ikuo Yamakawa, Fumio Nakamura Laboratory of Animal By-product Science, Graduate School of
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12 1. NAME OF THE VETERINARY MEDICINAL PRODUCT HALOCUR 0.5 mg/ml oral solution for calves 2. Qualitative and quantitative composition Active substance Halofuginone
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12 1. NAME OF THE VETERINARY MEDICINAL PRODUCT HALOCUR 0.5 mg/ml oral solution for calves 2. Qualitative and quantitative composition Active substance Halofuginone
SUPPLEMENTARY INFORMATION
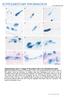 doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
NOTES. The Animal Pathogen-Like Type III Secretion System Is Required for the Intracellular Survival of Burkholderia mallei within J774.
 INFECTION AND IMMUNITY, July 2006, p. 4349 4353 Vol. 74, No. 7 0019-9567/06/$08.00 0 doi:10.1128/iai.01939-05 NOTES The Animal Pathogen-Like Type III Secretion System Is Required for the Intracellular
INFECTION AND IMMUNITY, July 2006, p. 4349 4353 Vol. 74, No. 7 0019-9567/06/$08.00 0 doi:10.1128/iai.01939-05 NOTES The Animal Pathogen-Like Type III Secretion System Is Required for the Intracellular
Antimalarial Activity of Allicin, a Biologically Active Compound from Garlic Cloves
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2006, p. 1731 1737 Vol. 50, No. 5 0066-4804/06/$08.00 0 doi:10.1128/aac.50.5.1731 1737.2006 Copyright 2006, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, May 2006, p. 1731 1737 Vol. 50, No. 5 0066-4804/06/$08.00 0 doi:10.1128/aac.50.5.1731 1737.2006 Copyright 2006, American Society for Microbiology. All Rights Reserved.
Consequences of Antimicrobial Resistant Bacteria. Antimicrobial Resistance. Molecular Genetics of Antimicrobial Resistance. Topics to be Covered
 Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
ERG on multidrug-resistant P. falciparum in the GMS
 ERG on multidrug-resistant P. falciparum in the GMS Minutes of ERG meeting Presented by D. Wirth, Chair of the ERG Geneva, 22-24 March 2017 MPAC meeting Background At the Malaria Policy Advisory Committee
ERG on multidrug-resistant P. falciparum in the GMS Minutes of ERG meeting Presented by D. Wirth, Chair of the ERG Geneva, 22-24 March 2017 MPAC meeting Background At the Malaria Policy Advisory Committee
MID 23. Antimicrobial Resistance. Consequences of Antimicrobial Resistant Bacteria. Molecular Genetics of Antimicrobial Resistance
 Antimicrobial Resistance Molecular Genetics of Antimicrobial Resistance Micro evolutionary change - point mutations Beta-lactamase mutation extends spectrum of the enzyme rpob gene (RNA polymerase) mutation
Antimicrobial Resistance Molecular Genetics of Antimicrobial Resistance Micro evolutionary change - point mutations Beta-lactamase mutation extends spectrum of the enzyme rpob gene (RNA polymerase) mutation
Applied-for scope of designation and notification of a Conformity Assessment Body Regulation (EU) 2017/746 (IVDR)
 Ref. Ares(2018)2576484-17/05/2018 NBOG s Best Practice Guide applicable for MDR IVDR NBOG F 2017-4 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article
Ref. Ares(2018)2576484-17/05/2018 NBOG s Best Practice Guide applicable for MDR IVDR NBOG F 2017-4 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article
TITLE: Anti-Inflammatory Cytokine Il-10 and Mammary Gland Development. CONTRACTING ORGANIZATION: University of Buffalo Buffalo, New York
 AD Award Number: W81XWH-06-1-0645 TITLE: Anti-Inflammatory Cytokine Il-10 and Mammary Gland Development PRINCIPAL INVESTIGATOR: Shiu-Ming Kuo CONTRACTING ORGANIZATION: University of Buffalo Buffalo, New
AD Award Number: W81XWH-06-1-0645 TITLE: Anti-Inflammatory Cytokine Il-10 and Mammary Gland Development PRINCIPAL INVESTIGATOR: Shiu-Ming Kuo CONTRACTING ORGANIZATION: University of Buffalo Buffalo, New
Udder conformation and its heritability in the Assaf (Awassi East Friesian) cross of dairy sheep in Israel
 Udder conformation and its heritability in the Assaf (Awassi East Friesian) cross of dairy sheep in Israel E. Gootwine, B. Alef, S. Gadeesh To cite this version: E. Gootwine, B. Alef, S. Gadeesh. Udder
Udder conformation and its heritability in the Assaf (Awassi East Friesian) cross of dairy sheep in Israel E. Gootwine, B. Alef, S. Gadeesh To cite this version: E. Gootwine, B. Alef, S. Gadeesh. Udder
Ultra-Fast Analysis of Contaminant Residue from Propolis by LC/MS/MS Using SPE
 Ultra-Fast Analysis of Contaminant Residue from Propolis by LC/MS/MS Using SPE Matthew Trass, Philip J. Koerner and Jeff Layne Phenomenex, Inc., 411 Madrid Ave.,Torrance, CA 90501 USA PO88780811_L_2 Introduction
Ultra-Fast Analysis of Contaminant Residue from Propolis by LC/MS/MS Using SPE Matthew Trass, Philip J. Koerner and Jeff Layne Phenomenex, Inc., 411 Madrid Ave.,Torrance, CA 90501 USA PO88780811_L_2 Introduction
Sarcocystis heydorni, n. sp. (Apicomplexa: Protozoa) with cattle (Bos taurus) and human
 1 Sarcocystis heydorni, n. sp. (Apicomplexa: Protozoa) with cattle (Bos taurus) and human (Homo sapiens) cycle Jitender P. Dubey 1, Erna van Wilpe 2, Rafael Calero-Bernal 1, Shiv Kumar Verma 1, Ronald
1 Sarcocystis heydorni, n. sp. (Apicomplexa: Protozoa) with cattle (Bos taurus) and human (Homo sapiens) cycle Jitender P. Dubey 1, Erna van Wilpe 2, Rafael Calero-Bernal 1, Shiv Kumar Verma 1, Ronald
Increasing communication between a man and a dog
 Increasing communication between a man and a dog Germain Lemasson, Sylvie Pesty, Dominique Duhaut To cite this version: Germain Lemasson, Sylvie Pesty, Dominique Duhaut. Increasing communication between
Increasing communication between a man and a dog Germain Lemasson, Sylvie Pesty, Dominique Duhaut To cite this version: Germain Lemasson, Sylvie Pesty, Dominique Duhaut. Increasing communication between
Antimicrobial Resistance
 Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Acquisition of Foreign DNA
 Antimicrobial Resistance Acquisition of Foreign DNA Levy, Scientific American Horizontal gene transfer is common, even between Gram positive and negative bacteria Plasmid - transfer of single or multiple
Antimicrobial Resistance Acquisition of Foreign DNA Levy, Scientific American Horizontal gene transfer is common, even between Gram positive and negative bacteria Plasmid - transfer of single or multiple
Effects of Late-Summer Protein Supplementation and Deworming on Performance of Beef Calves Grazing Native Range
 Effects of Late-Summer Protein Supplementation and Deworming on Performance of Beef Calves Grazing Native Range D.L. Lalman, J.G. Kirkpatrick, D.E. Williams, and J.D. Steele Story in Brief The objective
Effects of Late-Summer Protein Supplementation and Deworming on Performance of Beef Calves Grazing Native Range D.L. Lalman, J.G. Kirkpatrick, D.E. Williams, and J.D. Steele Story in Brief The objective
Consuelo Pinzon-Ortiz, Jennifer Friedman, Jeffrey Esko, and Photini Sinnis
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 276, No. 29, Issue of July 20, pp. 26784 26791, 2001 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. The Binding of
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 276, No. 29, Issue of July 20, pp. 26784 26791, 2001 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. The Binding of
Effects of Cage Stocking Density on Feeding Behaviors of Group-Housed Laying Hens
 AS 651 ASL R2018 2005 Effects of Cage Stocking Density on Feeding Behaviors of Group-Housed Laying Hens R. N. Cook Iowa State University Hongwei Xin Iowa State University, hxin@iastate.edu Recommended
AS 651 ASL R2018 2005 Effects of Cage Stocking Density on Feeding Behaviors of Group-Housed Laying Hens R. N. Cook Iowa State University Hongwei Xin Iowa State University, hxin@iastate.edu Recommended
Epigenetic regulation of Plasmodium falciparum clonally. variant gene expression during development in An. gambiae
 Epigenetic regulation of Plasmodium falciparum clonally variant gene expression during development in An. gambiae Elena Gómez-Díaz, Rakiswendé S. Yerbanga, Thierry Lefèvre, Anna Cohuet, M. Jordan Rowley,
Epigenetic regulation of Plasmodium falciparum clonally variant gene expression during development in An. gambiae Elena Gómez-Díaz, Rakiswendé S. Yerbanga, Thierry Lefèvre, Anna Cohuet, M. Jordan Rowley,
Protozoa. Apicomplexa Sarcomastigophora Ciliophora. Gregarinea Coccidia Piroplasma
 Protozoa Apicomplexa Sarcomastigophora Ciliophora Gregarinea Coccidia Piroplasma Coccidia characterized by thick-walled oocysts excreted in feces In Humans Cryptosporidium Isospora Cyclospora Sarcocystis
Protozoa Apicomplexa Sarcomastigophora Ciliophora Gregarinea Coccidia Piroplasma Coccidia characterized by thick-walled oocysts excreted in feces In Humans Cryptosporidium Isospora Cyclospora Sarcocystis
Key words: Plasmodium, Kentropyx calcarata, Brazil, merogony, gametocytes, ultrastructure
 FOLIA PARASITOLOGICA 49: 2-8, 2002 Fine structure of erythrocytic stages of a Plasmodium tropiduri-like malaria parasite found in the lizard Kentropyx calcarata (Teiidae) from north Brazil Ilan Paperna
FOLIA PARASITOLOGICA 49: 2-8, 2002 Fine structure of erythrocytic stages of a Plasmodium tropiduri-like malaria parasite found in the lizard Kentropyx calcarata (Teiidae) from north Brazil Ilan Paperna
No-leaching. No-resistance. No-toxicity. >99.999% Introducing BIOGUARD. Best-in-class dressings for your infection control program
 Introducing BIOGUARD No-leaching. >99.999% No-resistance. No-toxicity. Just cost-efficient, broad-spectrum, rapid effectiveness you can rely on. Best-in-class dressings for your infection control program
Introducing BIOGUARD No-leaching. >99.999% No-resistance. No-toxicity. Just cost-efficient, broad-spectrum, rapid effectiveness you can rely on. Best-in-class dressings for your infection control program
Malaria parasites of rodents of the Congo (Brazzaville) :
 Annales de Parasitologie (Paris), 1976, t. 51, n 6, pp. 637 à 646 Malaria parasites of rodents of the Congo (Brazzaville) : Plasmodium cbabaudi adami subsp. nov. and Plasmodium vinckei lentum Landau, Michel,
Annales de Parasitologie (Paris), 1976, t. 51, n 6, pp. 637 à 646 Malaria parasites of rodents of the Congo (Brazzaville) : Plasmodium cbabaudi adami subsp. nov. and Plasmodium vinckei lentum Landau, Michel,
Novel ELISA method as exploratory tool to assess immunity induced by radiated attenuated sporozoites to decipher protective immunity
 DOI 10.1186/s12936-017-2129-9 Malaria Journal METHODOLOGY Open Access Novel ELISA method as exploratory tool to assess immunity induced by radiated attenuated sporozoites to decipher protective immunity
DOI 10.1186/s12936-017-2129-9 Malaria Journal METHODOLOGY Open Access Novel ELISA method as exploratory tool to assess immunity induced by radiated attenuated sporozoites to decipher protective immunity
Applied epidemiology: another tool in dairy herd health programs?
 Applied epidemiology: another tool in dairy herd health programs? K Frankena, Jp Noordhuizen, En Stassen To cite this version: K Frankena, Jp Noordhuizen, En Stassen. Applied epidemiology: another tool
Applied epidemiology: another tool in dairy herd health programs? K Frankena, Jp Noordhuizen, En Stassen To cite this version: K Frankena, Jp Noordhuizen, En Stassen. Applied epidemiology: another tool
Dual Antibiotic Delivery from Chitosan Sponges Prevents In Vivo Polymicrobial Biofilm Infections
 Dual Antibiotic Delivery from Chitosan Sponges Prevents In Vivo Polymicrobial Biofilm Infections Ashley Parker, MS 1, James Smith, MS 1, Karen Beenken, PhD 2, Jessica Amber Jennings, PhD 3, Mark Smeltzer,
Dual Antibiotic Delivery from Chitosan Sponges Prevents In Vivo Polymicrobial Biofilm Infections Ashley Parker, MS 1, James Smith, MS 1, Karen Beenken, PhD 2, Jessica Amber Jennings, PhD 3, Mark Smeltzer,
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYTOPOINT 10 mg solution for injection for dogs CYTOPOINT 20 mg solution for injection for dogs CYTOPOINT 30 mg
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYTOPOINT 10 mg solution for injection for dogs CYTOPOINT 20 mg solution for injection for dogs CYTOPOINT 30 mg
A Flexible natural gas membrane Reformer for m- CHP applications FERRET
 A Flexible natural gas membrane Reformer for m- CHP applications FERRET This project is supported by the European Union s Seventh Framework Programme (FP7/2007-2013) for the Fuel Cells and Hydrogen Joint
A Flexible natural gas membrane Reformer for m- CHP applications FERRET This project is supported by the European Union s Seventh Framework Programme (FP7/2007-2013) for the Fuel Cells and Hydrogen Joint
Principles of Anti-Microbial Therapy Assistant Professor Naza M. Ali. Lec 1
 Principles of Anti-Microbial Therapy Assistant Professor Naza M. Ali Lec 1 28 Oct 2018 References Lippincott s IIIustrated Reviews / Pharmacology 6 th Edition Katzung and Trevor s Pharmacology / Examination
Principles of Anti-Microbial Therapy Assistant Professor Naza M. Ali Lec 1 28 Oct 2018 References Lippincott s IIIustrated Reviews / Pharmacology 6 th Edition Katzung and Trevor s Pharmacology / Examination
Pierre-Louis Toutain, Ecole Nationale Vétérinaire National veterinary School of Toulouse, France Wuhan 12/10/2015
 Antimicrobial susceptibility testing for amoxicillin in pigs: the setting of the PK/PD cutoff value using population kinetic and Monte Carlo Simulation Pierre-Louis Toutain, Ecole Nationale Vétérinaire
Antimicrobial susceptibility testing for amoxicillin in pigs: the setting of the PK/PD cutoff value using population kinetic and Monte Carlo Simulation Pierre-Louis Toutain, Ecole Nationale Vétérinaire
The effects of condensed tannins against cattle nematodes
 The effects of condensed tannins against cattle nematodes Adam Novobilský 1, 2 Irene Mueller-Harvey 3 Stig Milan Thamsborg 2 1 (SLU), Department of Biomedicine and Veterinary Public Health, Uppsala, Sweden
The effects of condensed tannins against cattle nematodes Adam Novobilský 1, 2 Irene Mueller-Harvey 3 Stig Milan Thamsborg 2 1 (SLU), Department of Biomedicine and Veterinary Public Health, Uppsala, Sweden
The Evolution of Human-Biting Preference in Mosquitoes
 Got Blood? The Evolution of Human-Biting Preference in Mosquitoes by Gary H. Laverty Department of Biological Sciences University of Delaware, Newark, DE Part I A Matter of Preference So, what do we do
Got Blood? The Evolution of Human-Biting Preference in Mosquitoes by Gary H. Laverty Department of Biological Sciences University of Delaware, Newark, DE Part I A Matter of Preference So, what do we do
Differential Somatic Cell Count with the Fossomatic 7 DC - a novel parameter
 Differential Somatic Cell Count with the Fossomatic 7 DC - a novel parameter By: Dr. Daniel Schwarz, Cattle Disease Specialist, FOSS, Denmark Dedicated Analytical Solutions Somatic cell count (SCC) represents
Differential Somatic Cell Count with the Fossomatic 7 DC - a novel parameter By: Dr. Daniel Schwarz, Cattle Disease Specialist, FOSS, Denmark Dedicated Analytical Solutions Somatic cell count (SCC) represents
The effects of diet upon pupal development and cocoon formation by the cat flea (Siphonaptera: Pulicidae)
 June, 2002 Journal of Vector Ecology 39 The effects of diet upon pupal development and cocoon formation by the cat flea (Siphonaptera: Pulicidae) W. Lawrence and L. D. Foil Department of Entomology, Louisiana
June, 2002 Journal of Vector Ecology 39 The effects of diet upon pupal development and cocoon formation by the cat flea (Siphonaptera: Pulicidae) W. Lawrence and L. D. Foil Department of Entomology, Louisiana
Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling
 Developmental Cell, Volume 29 Supplemental Information Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling Danyi Li, Rui
Developmental Cell, Volume 29 Supplemental Information Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling Danyi Li, Rui
Parasitology Amoebas. Sarcodina. Mastigophora
 Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Gliding Motility Leads to Active Cellular Invasion by Cryptosporidium parvum Sporozoites
 INFECTION AND IMMUNITY, Sept. 2005, p. 5379 5387 Vol. 73, No. 9 0019-9567/05/$08.00 0 doi:10.1128/iai.73.9.5379 5387.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Gliding
INFECTION AND IMMUNITY, Sept. 2005, p. 5379 5387 Vol. 73, No. 9 0019-9567/05/$08.00 0 doi:10.1128/iai.73.9.5379 5387.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Gliding
