CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts
|
|
|
- Margaret Atkins
- 6 years ago
- Views:
Transcription
1 Blackwell Publishing LtdOxford, UKMMIMolecular Microbiology X 2005 The Authors; Journal compilation 2005 Blackwell Publishing Ltd? Original ArticleA protein that mediates malarial transmissiont. Kariu et al. Molecular Microbiology (2006) 59(5), doi: /j x First published online 20 January 2006 CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts Tohru Kariu, 1 Tomoko Ishino, 1,2 Kazuhiko Yano, 1 Yasuo Chinzei 1,2 and Masao Yuda 1,2 * 1 Mie University, School of Medicine, Edobashi, Tsu, Mie , Japan. 2 CREST, Japan Science and Technology Agency, Kawaguchi, Saitama, Japan. Summary The malarial parasite has two hosts in its life cycle, a vertebrate and a mosquito. We report here that malarial invasion into these hosts is mediated by a protein, designated cell-traversal protein for ookinetes and sporozoites (CelTOS), which is localized to micronemes that are organelles for parasite invasive motility. Targeted disruption of the CelTOS gene in Plasmodium berghei reduced parasite infectivity in the mosquito host approximately 200-fold. The disruption also reduced the sporozoite infectivity in the liver and almost abolished its cell-passage ability. Liver infectivity was restored in Kupffer cell-depleted rats, indicating that CelTOS is necessary for sporozoite passage from the circulatory system to hepatocytes through the liver sinusoidal cell layer. Electron microscopic analysis revealed that celtos-disrupted ookinetes invade the midgut epithelial cell by rupturing the cell membrane, but then fail to cross the cell, indicating that CelTOS is necessary for migration through the cytoplasm. These results suggest that conserved cell-passage mechanisms are used by both sporozoites and ookinetes to breach host cellular barriers. Elucidation of these mechanisms might lead to novel antimalarial strategies to block parasite s transmission. Introduction The malarial parasite infects both mammalian and mosquito hosts during its complex life cycle. When the parasite changes hosts, it develops motile stages that can invade Accepted 2 December, *For correspondence. m-yuda@ doc.medic.mie-u.ac.jp; Tel. (+81) ; Fax (+81) Present addresses: Sojo University, School of Pharmacological Sciences, Ikeda, Kumamoto-shi, Kumamoto , Japan. Research Institute, International Medical Center of Japan, Toyama, Shinjuku-ku, Tokyo , Japan. These authors contributed equally to this work. the target organ in the new host, heading for the site where it can proliferate to the next motile stage. In this migration, the parasite encounters host cell barriers that hamper its advance. To circumvent these barriers and penetrate the organ, the parasite invades and traverses host cells. Cell-traversal ability is thus essential for the parasite to establish an infection in a new host. After a female mosquito ingests an infective blood meal, sexual-stage parasites are fertilized in the mosquito midgut lumen and develop into motile ookinetes. The ookinete invades the mosquito midgut epithelium and moves to the midgut basement membrane, on which it forms an oocyst and transforms into thousands of sporozoites, the salivary gland-invasive form. Malarial ookinetes traverse a single epithelial cell or additionally some neighbouring epithelial cells before they arrive at the basal lamina, indicating that cell-traversal ability is essential for the ookinete to establish infection in the mosquito vector. A recently proposed model of ookinete infection of the midgut (Zieler and Dvorak, 2000; Han and Barillas-Mury, 2002) suggests that epithelial cells invaded by ookinetes soon die and are then ejected from the midgut wall, which exposes the parasites to the danger of being removed from the epithelium with the damaged cell and requires the ookinetes to cross this layer in a limited time. Furthermore, during this midgut invasion, many ookinetes are killed by the insect defence system, and the number of malarial parasites is greatly reduced (Blandin et al., 2004). Thus, prompt passage through the epithelial cell is critical for the parasite s successful infection of the mosquito vector. Malarial transmission to the mammalian host is accomplished by sporozoite infection of the hepatocyte, in which it forms a parasitophorous vacuole and develops into thousands of erythrocyte-invasive forms. Sporozoites gathered in the mosquito salivary glands are injected into the mammalian host by a mosquito bite. The sporozoites enter the blood circulation and are transported to the liver sinusoid. There they leave the circulation by crossing the liver sinusoidal layer, the boundary between the circulation and hepatocytes. Ultrastructural studies indicate that Kupffer cells, which are hepatic macrophages and major components of the sinusoidal cell layer, are routes of this crossing, based on the observation that sporozoites remain in Kupffer cells shortly after intravenous inoculation (Meis et al., 1983). We reported that cell-passage ability is essential for the sporozoite to cross this blood- Journal compilation 2006 Blackwell Publishing Ltd
2 1370 T. Kariu et al. hepatocytic barrier and that novel microneme proteins, named sporozoite micronemal protein essential for cell traversal (SPECT) and SPECT2, are necessary for this crossing (Ishino et al., 2004; 2005; Kaiser et al., 2004). Motile stages of the malarial parasite have no locomotory organelles, such as flagella or cilia. Instead, their motility requires substrates and is achieved by highly developed cytoplasmic secretory organelles, called micronemes. The motile stages secrete the micronemal contents from an apical pore and translocate them along the cell surface (Menard, 2001). This backward movement, which is powered by an actomyosin motor of the parasite (Dobrowolski and Sibley, 1996), creates motility by gaining a foothold outside. In the sporozoite stage, thrombospondin-related anonymous protein (TRAP), a microneme protein with a single transmembrane domain, has a pivotal role in transmitting the driving force outside the plasma membrane (Sultan et al., 1997). Linked with the actomyosin motor in the cytoplasm (Kappe et al., 1999; Buscaglia et al., 2003; Jewett and Sibley, 2003), TRAP moves backwards along the parasite plasma membrane and generates surface movement. Similar driving mechanisms might be used by the ookinete to pass through the midgut epithelial cells, because a micronemal protein paralogous to TRAP has an essential role in this process (Yuda et al., 1999a; Dessens et al., 2000). It is now believed that cognate driving machinery involving TRAP-family proteins is widely used by apicomplexan parasites to invade host cells (Spano et al., 1998). To search for genes involved in parasite invasive motility, we performed a comparative analysis of expressed sequence tag (EST) databases of two motile stages, salivary gland sporozoites and ookinetes, of the rodent malarial parasite Plasmodium berghei. In this article, we report that a secretory microneme protein, named celltraversal protein of Plasmodium ookinetes and sporozoites (CelTOS), has a crucial role in the cell-traversal ability of both these stages. We show that CelTOS is necessary for both stages to break through cellular barriers and establish infection in the new host. Results Expressed sequence tags of the celtos gene are present in two different host-invasive stages To search for genes involved in parasite invasive motility, EST databases of two motile stages, salivary gland sporozoites and ookinetes, of P. berghei were used (Yuda et al., 2001; Ishino et al., 2004). EST sequences of the respective stages were separately assembled into contigs and were screened for genes encoding micronemal proteins, using sequences representing the N-terminal signal peptide as probes. A contig encoding a 25 kda protein with a secretory protein-like structure was found in both databases and named CelTOS. The numbers of celtos ESTs in the ookinete and sporozoite databases were five (of ESTs) and 10 (of 3825 ESTs) respectively. This suggests that an identical molecule acts in the malarial invasion of the two hosts, a possibility that was not anticipated. Therefore, this gene was investigated further. Study of Plasmodium genome databases (PlasmoDB) indicated that orthologous genes are present in several plasmodium species, including the medically important parasites, Plasmodium falciparum and Plasmodium vivax (Fig. 1). On the other hand, a sequence similarity search of DNA databanks showed that CelTOS has no significant similarity to other known proteins. These results indicate that the celtos gene evolved in a common ancestor of Plasmodium parasites. CelTOS is produced by the ookinete and the liver-infective sporozoite and is localized to micronemes To investigate the expression profile of the CelTOS gene P. berghei P. vivax P. falciparum P. berghei P. vivax P. falciparum P. berghei P. vivax P. falciparum P. berghei P. vivax P. falciparum Fig. 1. A comparison of amino acid sequences of P. berghei CelTOS, P. falciparum and P. vivax orthologues. The deduced amino acid sequence of P. berghei CelTOS was aligned with that of P. falciparum CelTOS (PlasmoDB identifier PFL0800c) and P. vivax CelTOS. Gaps are introduced to obtain optical matching by using GENETIX-MAC software. Conserved residues are boxed. The amino acid numbers from the first Met residue are shown on the left of each line. The predicted N-terminal signal sequence is underlined. P. berghei P. vivax P. falciparum
3 A protein that mediates malarial transmission 1371 in the malarial life cycle, a polyclonal antibody was prepared against recombinant protein produced in Escherichia coli, and indirect immunofluorescent analysis was performed in different motile stages. Positive signals were observed in the ookinete and salivary gland sporozoite (Fig. 2A). However, CelTOS was not detected in the intraerythrocytic stages by either indirect immunofluorescent analysis (Fig. 2A) or Western blot analysis (data not shown). The detected signals were mainly observed in the apical cytoplasm, suggesting localization to micronemes. CelTOS was not detected in the oocyst sporozoite, the mosquito salivary gland-infective stage. This indicates that sporozoites start to produce this protein again after salivary gland infection. The in vitro expression profile of celtos during ookinete development was investigated by Western blot analysis (Fig. 2B). CelTOS first appeared 10 h after fertilization and increased with ookinete development. In the early phase (10 h), a protein with the same molecular mass as the N-terminal signal peptide-processed form (20 kda) was detected. It was then gradually replaced by a smaller protein (17 kda). This finding suggests that processing of the N-terminal region occurred during maturation to the secreted form as reported for other microneme proteins of apicomplexan parasites (Soldati et al., 2001). Immunoelectron microscopy of mature ookinetes revealed that CelTOS is localized to the micronemes (Fig. 2C). The gold particles were localized to the micronemes of relatively high electron density and were mainly distributed within them, not at the edges. Interestingly, this localization pattern is consistent with that of the von Willebrand factor-related adhesive protein, a putative secretory microneme protein with an adhesive protein-related motif (Yuda et al., 2001), and contrasts with that of CTRP, a paralogous protein of TRAP, which is mainly detected at the edge of the microneme probably due to integration into the microneme membrane (Yuda et al., 2001). The distinct distribution patterns of CelTOS and CTRP suggest that CelTOS is secreted directly into the cytoplasm of the midgut epithelial cell, while CTRP may be first presented on the ookinete surface and then released, as TRAP is thought to be in the sporozoite stage (Kappe et al., 1999). Western blot analysis of the sporozoite stage confirmed that CelTOS is produced by the sporozoite in the salivary gland, but not by the sporozoite in the oocyst (Fig. 2D). Immunoelectron microscopy indicated that CelTOS is localized to micronemes of the salivary gland sporozoite (Fig. 2E). The size of the protein detected by Western blot analysis was smaller than that of the recombinant protein (N-terminal signal peptide-processed form) and was the same as that detected in the mature ookinete (17 kda, Fig. 2B). As mentioned above, this suggests that the N- terminal portion of this protein underwent further processing. The specific production in the liver-infective sporozoite Fig. 2. CelTOS is produced by both mosquito midgut- and mammalian liver-infective stages and localized to the micronemes. A. Expression profile of the CelTOS gene in host-invasive stages of the malarial parasite. Immunofluorescent microscopy was performed in different host invasive stages, including ookinetes, midgut sporozoites, salivary gland sporozoites and merozoites. Signals were detected using anti-celtos antibodies and secondary antibodies conjugated with FITC. Transilluminated (Trans) or 4,6-diamidino-2- phenylindole staining (DAPI) images are shown in lower panels. B. CelTOS production during ookinete development. Infected blood was cultured at 20 C in ookinete culture medium. After the time (h) indicated over each lane, the same volume was collected from the culture, and CelTOS production was studied by Western blot analysis using the same primary antibodies as in A. rceltos, recombinant signal peptide-processed form, which was produced by E. coli. C. CelTOS is localized in the ookinete to micronemes. Localization of CelTOS was examined by immunoelectron microscopy with the same primary antibodies as in A and the secondary antibodies conjugated with gold particles (10 nm in diameter). The particles were localized to micronemes of high electron density. D. CelTOS is produced by the sporozoite after salivary gland infection. Midgut sporozoites and salivary gland sporozoites were collected 24 days after an infective blood meal. The production of CelTOS was investigated in both stages ( parasites each) by Western blot analysis. E. CelTOS is localized to micronemes of the salivary gland sporozoite. Mosquito salivary glands were dissected 24 days after an infective blood meal and immunoelectron microscopy was performed as in C. Bars represent 5 µm.
4 1372 T. Kariu et al. and the localization to micronemes strongly suggest that CelTOS has a role in malarial transmission to the vertebrate host. CelTOS is secreted during sporozoite movement and left behind as a trail Malarial sporozoites exhibit a circular movement on a glass slide. This is called gliding motility, because it proceeds without changing the cell shape. While gliding on a slide, they secrete micronemal contents from the apical pole and leave them behind as a trail (Sultan et al., 1997; Stewart and Vanderberg, 1988). Because this trail contains TRAP, a major microneme protein of the sporozoite, it can be visualized on the glass slide by immunofluorescent staining with anti-trap antibodies (Fig. 3, lower right). To examine the secretion of CelTOS during sporozoite movement, immunofluorescent microscopy was performed with anti-celtos antibodies (lower left). Trails were visualized on the slide with a periodic, discontinuous appearance, indicating its secretion from individual micronemes. Notably, the trails were broader and not as sharp as those stained with anti-trap antibodies. When the parasite plasma membrane was not permeabilized after fixation with paraformaldehyde, CelTOS was not detected at the apical tip or on the parasite surface (upper Triton CelTOS TRAP Fig. 4. Targeted disruption of the CelTOS gene. A. Schematic representation of targeting procedure. The targeting vector (top) containing a selectable marker gene is integrated into the CelTOS gene locus (middle) by double crossover recombination. This recombination event resulted in the disruption of the celtos gene and conferred pyrimethamine resistance to the disruptants (bottom). B. Genomic Southern hybridization of wild-type (WT) and celtos( ) populations. Genomic DNA isolated from the respective parasite populations was digested with DraI and hybridized with the probe indicated in (A) by a solid bar. Integration of the targeting construct increased the size of the detected fragments from 0.8 to 1.3 kb. C. Immunofluorescent analysis of the celtos-disrupted ookinete. celtos-disrupted ookinetes were collected from the culture and stained with primary antibodies against CelTOS followed by FITCconjugated secondary antibodies. Compare with Fig. 1A. + Fig. 3. CelTOS is deposited as a trail during sporozoite gliding. Salivary gland sporozoites were allowed to glide in chamber slides at 37 C for 1 h and fixed with paraformaldehyde, followed (Triton +) or not followed (Triton ) by permeabilization. The slides were stained with the primary antibodies, anti-celtos (CelTOS) or anti-trap (TRAP), and secondary antibodies labelled with Cy3. Trails detected by anti-trap antibodies had a sharper appearance than those detected by anti-celtos antibodies. Parasites were not stained by anti-celtos antibodies when not permeabilized, while some of them were stained by anti-trap antibodies. Arrows indicate the apical tip of the sporozoite. left). In contrast, TRAP was capped at the apical tip in some of the sporozoites (upper right). These results indicate that CelTOS is deposited on a glass slide directly as an insoluble material, while TRAP might be first presented on the parasite surface and then released from the posterior end, leading to the formation of relatively sharp trails. This assumption correlates well with secretory protein-like (CelTOS) and membrane protein-like (TRAP) molecular structures of the respective proteins. As discussed later, the distinct secretion patterns between Cel- TOS and TRAP might reflect their functions in the parasite cell-passage machinery. Construction of CelTOS gene-disrupted parasite clones To investigate the function of CelTOS in the malarial life cycle, celtos-disrupted parasites were prepared. Figure 4A shows the knock-out vector used. It is
5 A protein that mediates malarial transmission 1373 composed of a selectable marker gene that confers resistance to the antimalarial drug, pyrimethamine and DNA fragments of the gene ligated to both ends. This construct was introduced into merozoites by electroporation, and merozoites with this construct integrated into the genome were selected using pyrimethamine. Clones were isolated by limiting dilution. Disruption of the target gene of each clone was confirmed by Southern blot (Fig. 4B) and polymerase chain reaction (PCR) analyses (data not shown). Clones were obtained from two individual experiments to confirm that the disruptants used for the following experiments were not the same clone. In the intra-erythrocytic stages, the celtos-disrupted parasites had no phenotypic differences from wild-type parasites. Growth rates in rat blood were similar for disruptants and wild types (data not shown). This indicates that CelTOS is not essential for the parasite s proliferation in the vertebrate host blood, consistent with the observation that this protein is not produced by intra-erythrocytic stages. CelTOS has a critical role in ookinete infection of the mosquito midgut Ookinetes are generated in the midgut lumen and traverse epithelial cells from the luminal to the basal side. They then attach to the basal lamina of the epithelial cell and transform to oocysts. Malarial sporozoites are released from the oocysts into the mosquito s haemolymph 2 3 weeks after an infective blood meal. The sporozoites invade the salivary gland, pass through the gland cells and gather in the lumen, where they acquire the ability to infect the mammalian liver. celtos-disrupted parasites showed normal exflagellation rates and normally developed into ookinetes in vivo and in vitro (data not shown). To assess the effect of gene disruption on parasite development in the mosquito, oocyst formation and sporozoite numbers in the midgut, haemolymph and salivary glands were examined (Table 1). celtos-disruption decreased the number of oocysts formed on the midgut by approximately 200-fold. Correspondingly, sporozoites in the midgut were decreased to approximately 1/100 of wild-type parasites. Sporozoites in the salivary gland were also decreased. This reduction can be attributed solely to the reduced sporozoite number in the midgut, because the ratio of salivary gland sporozoites to midgut sporozoites was higher in disruptants ( ) than in wild-type parasites ( ). These results indicate that CelTOS has a critical role in ookinete infection of the mosquito midgut, but does not participate in sporozoite infection of the salivary gland, consistent with the observation that CelTOS is produced by ookinetes, but not by sporozoites before salivary gland infection. CelTOS has a critical role in sporozoite infection of the liver In the mosquito salivary gland, sporozoites acquire the ability to infect a mammalian host. CelTOS is also produced in this stage. In light and electron microscopic observations of the salivary gland sporozoite, there were no obvious morphologic differences between the wildtype parasite and the disruptant. Furthermore, the ratio of motile to non-motile sporozoites was similar to that of the wild-type parasite, indicating that the secretion of micronemes was not affected by celtos-disruption. To assess the influence of the disruption on sporozoite infectivity to the liver, the salivary gland sporozoites were injected intravenously into rats, and the infection rate and the period necessary for apparent parasitaemia (prepatent days) were compared between disruptant and wild-type parasites (Table 2). After injection of 1000 wild-type sporozoites, all rats were infected, but when rats were injected with the disruptant, some rats were not infected. Furthermore, prepatent days for apparent parasitaemia of the infected rats were significantly longer with the disruptant than the wild type. By comparing prepatent days and infection rates determined by injection of various numbers Table 1. Development of celtos-disrupted parasites in Anopheles stephensi mosquitoes. Parasite population No. of oocysts/ mosquito No. of sporozoites/mosquito Midgut Haemolymph Salivary gland Wild-type 278 ± ± ± ± 1900 celtos (+) ± ± ± ± 2098 celtos (+) ± ± ± ± 2904 celtos ( ) 1 1 ± ± ± ± 126 celtos ( ) 2 2 ± ± ± ± 69 celtos ( ) 3 1 ± ± ± ± 195 Anopheles stephensi mosquitoes were fed on infected mice. On day 14 post feeding, oocysts formed in the midgut were counted under the microscope. On day 20, sporozoites in the midgut, haemolymph and salivary glands were separately collected and counted. Each value is the mean from at least three independent experiments with its standard error.
6 1374 T. Kariu et al. Table 2. Infectivity of wild-type and disruptant sporozoites to rats. Parasite population No. of injected salivary gland Sporozoites infection rate a Prepatent period of infection b (days) Wild-type / / / / /5 5.0 celtos (+) /5 3.4 celtos (+) /5 3.4 celtos ( ) / celtos ( ) / celtos ( ) / a. Number of infected rats/number of rats inoculated with salivary gland sporozoites. b. Number of days between sporozoite inoculation and detection of at least one erythrocytic stage upon a 10 min examination of a Giemsa-stained blood smear. Sporozoite infectivity in rats was assessed by infection rates and prepatent periods. Three-week-old Wistar rats were intravenously injected with 0.2 ml of sporozoite suspensions in Medium 199. Parasitaemia was checked daily by Giemsa-stained blood smears. of wild-type sporozoites (Table 2), the infectivity of the disruptant was estimated to be 1/20 to 1/50 of the wild type. celtos-knockout sporozoites are impaired in cell-passage ability Sporozoites show two types of cell invasion in vitro, infection and passage (Mota et al., 2001). Because microneme proteins have a central role in parasite motility, cellinvasion motility of disruptant sporozoites was examined using in vitro assays. Hepatocyte-infection ability of the disruptants was assessed in a human hepatoma-derived cell line, HepG2 (Hollingdale et al., 1981). Sporozoites were added to a culture of HepG2 cells and the number of exoerythrocytic forms (EEFs) produced was compared between wild-type parasites and disruptants. The numbers of EEFs were similar for both (Fig. 5A), indicating that CelTOS is not necessary for sporozoite infection of the hepatocyte. Cell-passage activity was then measured using a cell wound assay (Mota et al., 2001). Sporozoites were added to HeLa cells, and cells wounded by sporozoite penetration were identified by uptake of fluorescein isothiocyanate (FITC)-conjugated dextran from the medium. The number of wounded cells was greatly decreased in HeLa cells treated with disruptants (Fig. 5B), almost to the level of HeLa cells treated with heat-inactivated negative controls, indicating that this ability is severely damaged by the gene disruption. Taken together, these results demonstrated that CelTOS is a microneme protein specifically involved in parasite cell passage. Fig. 5. celtos-disrupted sporozoites are impaired in cell-passage activity. A. celtos-disruption does not affect sporozoite ability to infect hepatocytes. Salivary gland sporozoites (1000 or 2000 per well) were added to HepG2 cells and cultured for 48 h. Formed EEFs were counted after immunofluorescence staining with antiserum against CS protein. EEF numbers were compared between disruptants [celtos( ) 1] and wild-type (WT) parasites. Data are mean numbers of EEFs per well with standard errors from at least five independent experiments. B. celtos-disrupted sporozoites are impaired in cell-passage activity. Salivary gland sporozoites (3000 per well) were added to HeLa cells and incubated for 1 h with FITC-conjugated dextran (1 mg ml 1 ). Cellpassage activity was estimated by the number of cells wounded by sporozoite passage, which were identified by cytosolic labelling with FITC-conjugated dextran. Numbers of positive cells were compared between disruptants [celtos( ) 1] and wild-type (WT) parasites. Data are mean numbers of FITC-positive cells in one-fifth area of a well with standard errors from at least three independent experiments. C. Restoration of celtos( ) sporozoite infectivity to the liver in Kupffer cell-depleted rats. Liposome-encapsulated Cl 2 MDP (filled) or PBS (open) was injected intravenously into rats. After 48 h, 3000 sporozoites of celtos( ) 1 (circles), celtos( ) 2 (triangles), or wild-type (squares) populations were inoculated intravenously. Parasitaemia in each rat was checked by Giemsa-stained blood smears after inoculation on the days indicated. Kupffer cell depletion in rats resulted in almost equal sporozoite infectivity between disruptants and wild types. Values shown represent the mean parasitaemia (± SEM) of five rats.
7 A protein that mediates malarial transmission 1375 Depletion of Kupffer cells restored disruptant s infectivity to the rat liver We recently demonstrated that sporozoite cell-traversal motility is necessary to cross the liver sinusoidal cell layer (Ishino et al., 2004). The results of the in vitro assays therefore suggest that the liver infectivity of the disruptants was decreased at this step. To test this possibility, we studied the liver infectivity of disruptants in Kupffer cell-depleted rats (Vreden et al., 1993; Van Rooijen and Sanders, 1994), in which sporozoites can pass freely through the sinusoidal cell layer without cell passage (Ishino et al., 2004). The celtos-disrupted sporozoites were intravenously inoculated into normal rats, and parasitaemias during the exponential growth period (from 3.5 to 5.0 days after inoculation) were compared with that of the wild type (Fig. 5C). The increase of parasitaemia occurred approximately 1.5 days later with the disruptants than with the wild type. This result agrees with the data on prepatent days ( days later in disruptants, Table 2). In contrast, when inoculated into Kupffer celldepleted rats, parasitaemias were almost equal for both strains. These results demonstrate that CelTOS is necessary for cell passage required to cross the sinusoidal layer. CelTOS is involved in ookinete passage through mosquito midgut epithelial cells The sporozoite results suggest that celtos-disruption impaired ookinete cell traversal and led to a reduction in midgut infectivity. To investigate the step in which midgut infection was affected by celtos-disruption, mosquitoes were dissected 21 h after an infective blood meal, when most wild-type ookinetes were expected to have finished migrating to the basal lamina, and the location of ookinetes in the midgut epithelium was examined by transmission electron microscopy (Table 3 and Fig. 6). When mosquitoes were infected with wild-type parasites, 50 ookinetes were found in the midgut epithelium (in 254 ultrathin sections from four midguts). Among them, 28 ookinetes had already finished passage through the epithelium and attached to the basal lamina at their apical end (Fig. 6A). Furthermore, 21 ookinetes were inside epithelial cells, nine of them potentially attached to the basal lamina because they were located near the basal lamina with the apical end directed to the basal side. One ookinete was found on the luminal side of an epithelial cell with its anterior half projecting into the cell. When mosquitoes were infected with disruptants, 52 ookinetes were found in the midgut epithelium (in 302 sections from four midguts). There were no ookinetes clearly attached to the basal lamina. Only six ookinetes were potentially attached to the basal lamina, and another Table 3. Distribution of wild-type and disruptant ookinetes in the mosquito midgut epithelium. 39 in epithelial cells apparently failed to complete their migration, because they were in damaged cells nearly (or completely) detached from the epithelial cell wall (Fig. 6B) or remained intracellular far from the basal lamina (Fig. 6C). Seven ookinetes were on the luminal surface of the epithelial cell and four of them had their apical tip projecting into the epithelial cell (Fig. 6D). These results demonstrated that CelTOS is necessary for the ookinete to migrate through the cytoplasm of the midgut epithelial cell to the basal lamina. Discussion Wild-type Disruptant Cell surface a 0 3 Apical membrane b 1 4 Cytoplasm c 21 (9) d 45 (6) d Basement membrane e 28 0 Total a. Number of ookinetes in the microvilli, but did not invade the epithelium. b. Number of ookinetes penetrating the apical cell membrane. c. Number of ookinetes in the cytoplasm. d. Number of ookinetes observed in the cytoplasm, but whose arrival at the basal lamina could not be determined. e. Number of ookinetes attached to the basal lamina. Mosquitoes were fed on infected mice and dissected 21 h after blood meal. Ookinete location in the mosquito midgut epithelium was analysed by transmission electron microscopy and compared between wild types and disruptants. Mosquito midgut and vertebrate liver invasion are obligatory steps for the malarial parasite to establish infection in new hosts. In this article, we demonstrated that a microneme protein, CelTOS, is produced by parasites at these distinct host-invasive stages and has a critical role in breaking through the cellular barriers against them. In both stages, CelTOS is involved in cell-traversal ability of the parasite, suggesting that conserved mechanisms for cell traversal are used by both stages. In the ookinete stage, celtos-disrupted ookinetes stopped during passage to the basal lamina and remained in the cytoplasm of the damaged epithelial cells. This phenotype indicates that CelTOS is involved in the step after ookinetes rupture the cell membrane, i.e. migration through the cytoplasm. The number of ookinetes that invaded the midgut epithelial cells was similar for celtosdisruptants and wild type, suggesting that celtos-disrupted ookinetes could migrate to the cytoplasm of epithelial cells as well as wild type. Similarly, celtos-disrupted sporozoites are severely impaired in cell-traversal ability, although they showed normal gliding motility in vitro and infected hepatocytes in the same efficiency as wild type. These results indicate that gliding motility of the disruptants is
8 1376 T. Kariu et al. Fig. 6. celtos-disrupted ookinetes fail to migrate through the midgut epithelium. A. a wild-type ookinete that travelled through the epithelium and attached to the basal lamina at the apical end. This ookinete might have first invaded the epithelial cell at the left side, which is severely damaged and strongly stained, and then migrated to the adjacent cell that the ookinete is seen penetrating. The attachment of the parasite apex to the basement membrane (Ba) is clearly demonstrated in this section. B. celtos-disrupted ookinete (arrowhead) has been ejected from the epithelium with the damaged cell it invaded. The cell begins to collapse. Mi, microvilli. C. celtos-disrupted ookinete (arrowhead) resides in an epithelial cell. The invaded cell lost microvilli but appears relatively intact. This ookinete might have entered the epithelium from the cell at the upper right, which is strongly stained and protrudes from the epithelium, and then migrated to this cell laterally. D. celtos-disrupted ookinete (arrowhead) inserts its apical end into the epithelial cell. The invaded cell is strongly stained and protrudes over the microvilli (Mi) of neighbouring intact cells, indicating that it ruptured the epithelial cell membrane, but failed to move forward into the cytoplasm. Bars represent 5 µm. not impaired in both stages. Thus, CelTOS may be a micronemal protein solely required for cell traversal. We have previously proposed that that SPECT and SPECT2 are essential for sporozoite cell-passage activity (Ishino et al., 2004; 2005). spect- and spect2-disruptants completely lack cell-wounding activity, suggesting that these two proteins are essential for a step preceding sporozoite invasion into the cytoplasm, such as attachment to the host cell surface or rupture of the plasma membrane. Contrary to these disruptants, celtos-disruptants seem to retain weak cell-wounding activity in the sporozoite stage, which was suggested by the result that the average number of wounded cells was slightly higher with celtos-disruptants than with heat-inactivated wildtype sporozoites (Fig. 5B). In the present study, however, this observation could not be confirmed due to the very small number of disruptant sporozoites obtained from infected mosquitoes (approximately 1/100 of the wild type). To address this problem, we recently prepared a recombinant parasite with the upstream region of the CelTOS gene substituted with that of the WARP (von Willebrant factor A domain-related protein) gene, which is specifically expressed in the ookinete stage (Yuda et al., 2001). This mutant parasite expressed celtos in the ookinete stage and produced enough sporozoites for further studies. The preliminary results obtained with this parasite confirmed that a small proportion of the disruptant sporozoites can traverse culture cells. This suggests that CelTOS acts at a different step than SPECTs in cell traversal. It is possible that celtos-disrupted sporozoites proceed to the cytoplasm of invaded cells but remain intracellular as celtos-disrupted ookinetes, resulting in severe reduction of cell-passage activity. TRAP-family proteins are microneme proteins produced in both ookinete and sporozoite stages and are essential for parasite gliding motility. They are translocated to the parasite surface and move on the surface from the anterior to the posterior side, generating forward movement of parasites. If gliding motility of celtos-disrupted parasites is normal, it would implies that surface movement of microneme proteins is not impaired. The impaired cell-passage ability therefore could be due to a failure of anchoring this surface movement to the cytoplasm of the host cell. We speculate that CelTOS is a candidate protein that may play this role by interacting with molecules in the cytoplasm. It is possible that the different trail patterns
9 A protein that mediates malarial transmission 1377 between TRAP and CelTOS might represent the different functions between these molecules in the parasite mobility machinery. In summary, CelTOS is the first microneme protein that was shown to be critical for cell traversal in both ookinetes and sporozoites. Its fate after secretion from micronemes and the phenotype of the disruptants suggest that its role in cell traversal is unique among micronemal proteins. Further investigation of this protein therefore may provide new insight into the molecular mechanisms of cell traversal by malarial parasites. Experimental procedures Parasite preparations BALB/c mice infected with P. berghei ANKA strain were prepared by peritoneal injection of infected blood stored at 70 C. For ookinete culture, blood was collected by cardiac puncture from infected mice within one blood passage. Culturing was performed as described previously (Yuda et al., 1999b). Cultured ookinetes were purified by erythrocyte lysis in 0.83% NH 4 CL and used for further analysis. For construction of a cdna library, cultured ookinetes were purified by density gradient centrifugation using Nicoprep A (AXIS-SHIELD PoC AS, Oslo, Norway). For mosquito biting, infected mice were used within one blood passage. At days after the infective blood meal, mosquitoes were anaesthetized in CO 2 and sporozoites in the haemolymph, midgut and salivary glands were collected separately, as described previously (Kariu et al., 2002). Briefly, the haemolymph was collected from a pinhole in the abdomen by injection of 10 µl of Medium 199 (Invitrogen, Carlsbad, CA, USA), into the body cavity. Then the salivary glands and the midgut were dissected out, washed in saline, and collected separately in 70 µl of Medium 199 on ice. The collected tissues were gently ground in the medium to release sporozoites and centrifuged at 18 g for 3 min to remove tissue fragments. Sporozoites in the supernatant were counted using a haemocytometer and used for further analysis. Nucleic acid sequences of human Plasmodium parasites Sequence data for P. falciparum chromosome 12 were obtained from the Stanford DNA Sequencing and Technology Center website at malaria. Sequence data for P. vivax were obtained from Antibody preparation and Western blot analysis A DNA fragment encoding the signal peptide-processed form of CelTOS (amino acid residues ) was amplified by PCR by the primer pair, 5 -CGGGATCCTTGAGAGGCA AAAATGGATCAGAA-3 and 5 -CGGCTCGAGTCAATTAAA GAAATCATTATCGAAG-3, using P. berghei genomic DNA as a template. This fragment was digested with BamHI and XhoI and then subcloned into the E. coli expression vector pgex 6P-1 (Amersham Pharmacia Biotech, Piscataway, NJ). Recombinant protein was produced in an E. coli strain, BL21, as a glutathione S-transferase (GST) fusion protein, purified with a glutathione column, and used for immunization of rabbits. Antibodies against GST were removed from the antisera by incubation with GST-conjugated Sepharose. Specific antibodies were affinity-purified using an NHS-activated High Trap column (Amersham Pharmacia Biotech) linked with the GST-fused recombinant protein. For preparation of anti- P. berghei circumsporozoite (CS) protein antisera, a synthetic peptide, DPPPPNANDPAPPNANC (amino acid residues ), was conjugated to keyhole limpet haemocyanin and used for immunization of rats. For Western blot analysis, purified parasites were homogenized in SDS-PAGE sample buffer containing 1% SDS and 5% 2-mercaptoethanol and were boiled for 5 min. Recombinant CelTOS was separated from fused GST by treatment with processing protease, PreScission Protease (Amersham Pharmacia Biotech) and was boiled in the same buffer. These samples were separated by SDS-PAGE on a 10 20% gradient gel and electrophoretically transferred to a nitrocellulose membrane. The blotted membrane was blocked in phosphate-buffered saline (PBS) containing 5% skimmed milk, incubated for 60 min with purified anti-celtos antibodies diluted in the same buffer (20 µg ml 1 final concentration), washed and then incubated for 60 min with alkaline phosphatase-conjugated anti-rabbit IgG (Bio-Rad, Hercules, CA) diluted 1:1000 in the same buffer. After washing, signals were obtained using the AP Conjugate Substrate Kit (Bio-Rad). Immunofluorescence microscopy Immunofluorescence microscopy was performed as described previously (Yuda et al., 1999b). Briefly, purified parasites on glass slides were fixed in acetone for 2 min and rinsed in PBS. The slides were then blocked in PBS containing 1% bovine serum albumin (BSA), incubated for 60 min with purified anti-celtos antibodies diluted in the same buffer (20 µg ml 1 final concentration). After being rinsed five times in PBS, the slides were incubated for 60 min with FITCconjugated anti-rabbit IgGs (Zymed, South San Francisco, CA) diluted 1:40 in the same buffer, and again rinsed five times in PBS. For nuclear staining, 4,6-diamidino-2-phenylindole staining (DAPI, 0.02 µg ml 1 final concentration) was added to the secondary antibody solution. Immunofluorescence analysis of sporozoite gliding trails was performed as follows. Sporozoites were suspended in Medium199 containing 3% BSA, spotted on multi-well slides, and incubated for 1 h at 37 C. Before staining, slides were washed in PBS, fixed in 4% paraformaldehyde for 5 min, and rinsed in PBS. Samples were mounted in PermaFluor (Immunotech, Marseille, France), and micrographs were obtained with an Olympus BX60 fluorescence microscope (Plympus, Tokyo, Japan) with a C digital colour camera (Hamamatsu Photonic System, Hamamatsu, Japan). The images were processed using AquaCosmos (Hamamatsu Photonic System) and Adobe Photoshop (San Jose, CA). Immunoelectron microscopy For transmission electron microscopy analysis, mosquito
10 1378 T. Kariu et al. midguts were dissected at 21 h after an infected blood meal, fixed in 0.1 M phosphate buffer (ph 7.4) containing 1% paraformaldehyde-2.5% glutaraldehyde (TAAB, Berkshire, UK) for 90 min on ice. They were dehydrated in ethanol and embedded in LR White resin (London Resin Company, London, UK). A sequence of ultrathin sections was obtained from the posterior portion of the midgut ( 70 sections per midgut) and stained with 2% uranyl acetate and Reynold s lead citrate. Ookinetes around the epithelium were examined in each section under a transmission electron microscope. Immunoelectron microscopy was performed as described previously (Yuda et al., 2001). Briefly, purified parasites were fixed in 0.1 M phosphate buffer (ph 7.4) containing 1% paraformaldehyde-0.1% glutaraldehyde (TAAB) for 15 min on ice. They were dehydrated in ethanol and embedded in LR Gold resin. Ultrathin sections were blocked for 30 min in PBS containing 0.01% Tween 20 and 5% non-fat dry milk (blocking buffer), incubated at 4 C overnight with primary antibodies (20 µg ml 1 final concentration), and then incubated for 1 h with goat anti-rabbit IgG conjugated to gold particles (15 nm diameter; AuroProbe: Amersham Pharmacia Biotech) diluted 1:40 in blocking buffer, and washed six times. Finally, the sections were fixed with 2.5% glutaraldehyde for 10 min and stained with 2% uranyl acetate and Reynold s lead citrate. Targeted disruption of the P. berghei gene The targeting vector for the gene was constructed as follows. Two partial DNA fragments of the gene were amplified by PCR using genomic DNA as template with the primer pairs: 5 -GGCGAGCTCTATCTTTAATTTTTATTTTGTA-3 and 5 -GCCGGATCCACATAGAACATTAAAAAAGCAAAA-3, 5 - GGCCTCGAGTTGAGAGGCAAAAATGGATCAGAA-3 and 5 -GCCGGTACCTTACGATTATCACTTAATGTTGTT-3. These fragments were subcloned into either side of the selectable marker gene in pbluescript (Stratagene, La Jolla, CA) using the unique restriction sites, SacI/BamHI and XhoI/KpnI respectively. For the gene-targeting experiment, the plasmid was completely digested with the restriction enzymes, SacI and KpnI, to release the linear targeting construct. The genetargeting experiment was performed as previously described (Yuda et al., 1999a). using sporozoites collected from salivary glands 24 days after an infective blood meal. For the gliding motility assay, sporozoites were kept 2 h at 4 C in Medium 199 containing 3% BSA and then gliding on an uncoated microscope slide was observed under a phase-contrast microscope. For the infectivity assay, sporozoites were intravenously injected into 3-week-old Wistar rats. The parasitaemia was checked daily by a Giemsa-stained blood smear, and infection rates and the prepatent period of infection were determined. Sporozoite infection assay was performed out essentially as described (Cerami et al., 1992). Briefly, HepG2 cells were seeded in minimum essential medium with 10% fetal bovine serum, non-essential amino acids, 100 U ml 1 penicillin, and 100 g ml 1 streptomycin in 16-well chamber slides (Nunc, Napierville, IL) and cultured until they approached nearly confluence. Salivary gland sporozoites ( or ) were suspended in 100 µl of the same medium and added to each well. After incubation for 2 h, the medium was replaced with the same medium containing 3 mg ml 1 glucose. The cells were cultured another 48 h with changes of medium at 12-h intervals and were fixed in acetone for 2 min. EEFs formed were counted after immunofluorescent staining as described above using anti-cs protein antiserum and secondary antibodies conjugated with Cy3 (KPL, Gaithersburg, MD). At least three wells were counted in each experiment, and the results were expressed as mean values from five independent experiments. Cell traversing activity assay Cell-traversing activity of sporozoites was performed essentially as described previously (Ishino et al., 2004). Sporozoites ( ) were added to HeLa cells seeded on an 8-well chamber slide and cultured for 1 h in the presence of 1 mg ml 1 FITC-labelled dextran ( MW, lysine-fixable; Molecular Probes, Eugene, OR). The cells were incubated in complete culture medium for more than 1 h and fixed with 4% paraformaldehyde in PBS. FITC-positive cells were counted under a fluorescence microscope. Results were expressed as mean values from three independent experiments. Southern blot analysis Southern blot analysis was performed essentially as described previously (Yuda et al., 1999a). Briefly, P. berghei genomic DNA extracted from blood stage parasites (2 µg) was completely digested with the restriction enzyme, DraI. The fragments were separated on 1.2% agarose gel and transferred to nylon membranes. Partial DNA fragments of the gene were amplified by PCR using genomic DNA as the template with the primer pair, 5 -CGGGATCCTTGAGAG GCAAAAATGGATCAGAA-3 and 5 -CGGCTCGAGTCAAT TAAAGAAATCATTATCGAAG-3. The products were labelled with [ 32 P]-dCTP and used as hybridization probes. Depletion of rat Kupffer cells Depletion of Kupffer cells was performed as previously described (Van Rooijen and Sanders, 1994). Briefly, 3-weekold female Wistar rats were injected intravenously with 120 µl of liposome-encapsulated clodronate or an equal volume of PBS as control. After 48 h, sporozoites ( ) were injected into a tail vein and parasitaemia was checked by Giemsa-stained blood smear every 12 h. Elimination of Kupffer cells was confirmed by immunoperoxidase staining after liver perfusion with PBS, followed by fixation with 4% paraformaldehyde in PBS. Clodronate was a gift of Roche Diagnostics, Manheim, Germany. Assays of sporozoite gliding motility and infectivity in vitro Gliding motility and infectivity of sporozoites were assessed Acknowledgements We thank Gerard R. Wyatt for reading the manuscript. This
11 A protein that mediates malarial transmission 1379 study was supported by a grant-in-aid for Scientific Research on Priority Areas to Y.C. ( , , ) and to M.Y. ( , ), of the Ministry of Education, Science, Culture, and Sports of Japan. It was also supported by grants from the Japan Science and Technology Agency to Y.C. References Blandin, S., Shiao, S.H., Moita, L.F., Janse, C.J., Waters, A.P., Kafatos, F.C., and Levashina, E.A. (2004) Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116: Buscaglia, C.A., Coppens, I., Hol, W.G., and Nussenzweig, V. (2003) Sites of interaction between aldolase and thrombospondin-related anonymous protein in plasmodium. Mol Biol Cell 14: Cerami, C., Frevert, U., Sinnis, P., Takacs, B., Clavijo, P., Santos, M.J., and Nussenzweig, V. (1992) The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 70: Dessens, J.T., Margos, G., Rodriguez, M.C., and Sinden, R.E. (2000) Identification of differentially regulated genes of Plasmodium by suppression subtractive hybridization. Parasitol Today 16: Dobrowolski, J.M., and Sibley, L.D. (1996) Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84: Han, Y.S., and Barillas-Mury, C. (2002) Implications of Time Bomb model of ookinete invasion of midgut cells. Insect Biochem Mol Biol 32: Hollingdale, M.R., Leef, J.L., McCullough, M., and Beaudoin, R.L. (1981) In vitro cultivation of the exoerythrocytic stage of Plasmodium berghei from sporozoites. Science 213: Ishino, T., Yano, K., Chinzei, Y., and Yuda, M. (2004) Cellpassage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. Plos Biol 2: Ishino, T., Chinzei, Y., and Yuda, M. (2005) A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol 7: Jewett, T.J., and Sibley, L.D. (2003) Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol Cell 11: Kaiser, K., Camargo, N., Coppens, I., Morrisey, J.M., Vaidya, A.B., and Kappe, S.H. (2004) A member of a conserved Plasmodium protein family with membrane-attack complex/ perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol Biochem Parasitol 133: Kappe, S., Bruderer, T., Gantt, S., Fujioka, H., Nussenzweig, V., and Menard, R. (1999) Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J Cell Biol 147: Kariu, T., Yuda, M., Yano, K., and Chinzei, Y. (2002) MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J Exp Med 195: Meis, J.F., Verhave, J.P., Jap, P.H., and Meuwissen, J.H. (1983) An ultrastructural study on the role of Kupffer cells in the process of infection by Plasmodium berghei sporozoites in rats. Parasitology 86 (Pt 2): Menard, R. (2001) Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cell Microbiol 3: Mota, M.M., Pradel, G., Vanderberg, J.P., Hafalla, J.C., Frevert, U., Nussenzweig, R.S., et al. (2001) Migration of Plasmodium sporozoites through cells before infection. Science 291: Soldati, D., Dubremetz, J.F., and Lebrun, M. (2001) Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int J Parasitol 31: Spano, F., Putignani, L., Naitza, S., Puri, C., Wright, S., and Crisanti, A. (1998) Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol Biochem Parasitol 92: Stewart, M.J., and Vanderberg, J.P. (1988) Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J Protozool 35: Sultan, A.A., Thathy, V., Frevert, U., Robson, K.J., Crisanti, A., Nussenzweig, V., et al. (1997) TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90: Van Rooijen, N., and Sanders, A. (1994) Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: Vreden, S.G., Sauerwein, R.W., Verhave, J.P., Van Rooijen, N., Meuwissen, J.H., and Van Den Broek, M.F. (1993) Kupffer cell elimination enhances development of liver schizonts of Plasmodium berghei in rats. Infect Immun 61: Yuda, M., Sakaida, H., and Chinzei, Y. (1999a) Targeted disruption of the plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J Exp Med 190: Yuda, M., Sawai, T., and Chinzei, Y. (1999b) Structure and expression of an adhesive protein-like molecule of mosquito invasive-stage malarial parasite. J Exp Med 189: Yuda, M., Yano, K., Tsuboi, T., Torii, M., and Chinzei, Y. (2001) von Willebrand Factor A domain-related protein, a novel microneme protein of the malaria ookinete highly conserved throughout Plasmodium parasites. Mol Biochem Parasitol 116: Zieler, H., and Dvorak, J.A. (2000) Invasion in vitro of mosquito midgut cells by the malaria parasite proceeds by a conserved mechanism and results in death of the invaded midgut cells. Proc Natl Acad Sci USA 97:
Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development
 Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development Shiroh Iwanaga, Izumi Kaneko, Tomomi Kato, Masao Yuda* Department of Medical Zoology, Mie University School
Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development Shiroh Iwanaga, Izumi Kaneko, Tomomi Kato, Masao Yuda* Department of Medical Zoology, Mie University School
Malaria in the Mosquito Dr. Peter Billingsley
 Malaria in the Mosquito Senior Director Quality Systems and Entomology Research Sanaria Inc. Rockville MD. 1 Malaria: one of the world s foremost killers Every year 1 million children die of malaria 250
Malaria in the Mosquito Senior Director Quality Systems and Entomology Research Sanaria Inc. Rockville MD. 1 Malaria: one of the world s foremost killers Every year 1 million children die of malaria 250
PLASMODIUM MODULE 39.1 INTRODUCTION OBJECTIVES 39.2 MALARIAL PARASITE. Notes
 Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Arrested oocyst maturation in Plasmodium parasites. lacking type II NADH:ubiquinone dehydrogenase
 Supplemental Information for: Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase Katja E. Boysen and Kai Matuschewski Contents: - Supplemental Movies 1 and
Supplemental Information for: Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase Katja E. Boysen and Kai Matuschewski Contents: - Supplemental Movies 1 and
Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis
 Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis A. Reagents: 1. DMEM or RPMI DMEM (4.5g/L glucose) RPMI 1640 Cellgro #MT-10-017-CM Cellgro #MT-10-040-CM 2. Giemsa
Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis A. Reagents: 1. DMEM or RPMI DMEM (4.5g/L glucose) RPMI 1640 Cellgro #MT-10-017-CM Cellgro #MT-10-040-CM 2. Giemsa
Developmental Biology of Sporozoite-Host. Malaria: Implications for Vaccine Design. Javier E. Garcia, Alvaro Puentes and Manuel E.
 Developmental Biology of Sporozoite-Host Interactions in Plasmodium falciparum Malaria: Implications for Vaccine Design Javier E. Garcia, Alvaro Puentes and Manuel E. Patarroyo Clin. Microbiol. Rev. 2006,
Developmental Biology of Sporozoite-Host Interactions in Plasmodium falciparum Malaria: Implications for Vaccine Design Javier E. Garcia, Alvaro Puentes and Manuel E. Patarroyo Clin. Microbiol. Rev. 2006,
The silent path to thousands of merozoites: the Plasmodium liver stage
 The silent path to thousands of merozoites: the Plasmodium liver stage Miguel Prudêncio*, Ana Rodriguez and Maria M. Mota* Abstract Plasmodium sporozoites are deposited in the skin of their vertebrate
The silent path to thousands of merozoites: the Plasmodium liver stage Miguel Prudêncio*, Ana Rodriguez and Maria M. Mota* Abstract Plasmodium sporozoites are deposited in the skin of their vertebrate
Plasmodium yoelii Sporozoites with Simultaneous Deletion of P52 and P36 Are Completely Attenuated and Confer Sterile Immunity against Infection
 INFECTION AND IMMUNITY, Aug. 2007, p. 3758 3768 Vol. 75, No. 8 0019-9567/07/$08.00 0 doi:10.1128/iai.00225-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Plasmodium yoelii Sporozoites
INFECTION AND IMMUNITY, Aug. 2007, p. 3758 3768 Vol. 75, No. 8 0019-9567/07/$08.00 0 doi:10.1128/iai.00225-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Plasmodium yoelii Sporozoites
Gliding Motility Assay for P. berghei Sporozoites
 Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT PRAKASH SRINIVASAN
 THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT BY PRAKASH SRINIVASAN Submitted in partial fulfillment of the requirements For the degree of
THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT BY PRAKASH SRINIVASAN Submitted in partial fulfillment of the requirements For the degree of
Parasitology Departement Medical Faculty of USU
 Malaria Mechanism of infection Parasitology Departement Medical Faculty of USU Introduction Malaria parasites Phylum Order Suborder Family Genus Species : : Apicomplexa : Eucoccidiida : Haemosporida :
Malaria Mechanism of infection Parasitology Departement Medical Faculty of USU Introduction Malaria parasites Phylum Order Suborder Family Genus Species : : Apicomplexa : Eucoccidiida : Haemosporida :
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5870/1679/dc1 Supporting Online Material for Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes Chung-hui Yang, Priyanka
www.sciencemag.org/cgi/content/full/319/5870/1679/dc1 Supporting Online Material for Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes Chung-hui Yang, Priyanka
ACCEPTED. Parasitology Unit, Max Planck Institute for Infection Biology, Berlin, Germany
 EC Accepts, published online ahead of print on 30 January 2009 Eukaryotic Cell doi:10.1128/ec.00347-08 Copyright 2009, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
EC Accepts, published online ahead of print on 30 January 2009 Eukaryotic Cell doi:10.1128/ec.00347-08 Copyright 2009, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
PRINCIPAL INVESTIGATOR: Dr. Jetsumon (Sattabongkot) Prachumsri
 AD (Leave blank) Award Number: W81XWH-07-2-0090 TITLE: Proteomic Study of Human Malaria Parasite Plasmodium Vivax Liver Stages for Development of Vaccines and Drugs PRINCIPAL INVESTIGATOR: Dr. Jetsumon
AD (Leave blank) Award Number: W81XWH-07-2-0090 TITLE: Proteomic Study of Human Malaria Parasite Plasmodium Vivax Liver Stages for Development of Vaccines and Drugs PRINCIPAL INVESTIGATOR: Dr. Jetsumon
A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development
 A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development Christine Lehmann 1, Anna Heitmann 1, Satish Mishra 2, Paul-Christian Burda 3, Mirko Singer 4, Monica Prado
A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development Christine Lehmann 1, Anna Heitmann 1, Satish Mishra 2, Paul-Christian Burda 3, Mirko Singer 4, Monica Prado
Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity
 Molecular & Biochemical Parasitology 156 (2007) 32 40 Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity Kota Arun
Molecular & Biochemical Parasitology 156 (2007) 32 40 Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity Kota Arun
A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S.
 VI. Malaria A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S. because they were resistant to malaria & other diseases 3. Many
VI. Malaria A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S. because they were resistant to malaria & other diseases 3. Many
Diurnal variation in microfilaremia in cats experimentally infected with larvae of
 Hayasaki et al., Page 1 Short Communication Diurnal variation in microfilaremia in cats experimentally infected with larvae of Dirofilaria immitis M. Hayasaki a,*, J. Okajima b, K.H. Song a, K. Shiramizu
Hayasaki et al., Page 1 Short Communication Diurnal variation in microfilaremia in cats experimentally infected with larvae of Dirofilaria immitis M. Hayasaki a,*, J. Okajima b, K.H. Song a, K. Shiramizu
The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands
 The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands Fabian E. Saenz 1,2, Bharath Balu 1, Jonah Smith 2, Sarita R. Mendonca 1,2, John H. Adams
The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands Fabian E. Saenz 1,2, Bharath Balu 1, Jonah Smith 2, Sarita R. Mendonca 1,2, John H. Adams
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12234 Supplementary Figure 1. Embryonic naked mole-rat fibroblasts do not undergo ECI. Embryonic naked mole-rat fibroblasts ( EF) were isolated from eight mid-gestation embryos. All the
doi:10.1038/nature12234 Supplementary Figure 1. Embryonic naked mole-rat fibroblasts do not undergo ECI. Embryonic naked mole-rat fibroblasts ( EF) were isolated from eight mid-gestation embryos. All the
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign
 A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
alaria Parasite Bank Collection sites of P. falciparum isolates PARASITE BIOLOGY
 M alaria Parasite Bank established in 1992 is a supporting unit for research activities on different aspects of malaria. The main objective of establishing this facility is to strengthen researches at
M alaria Parasite Bank established in 1992 is a supporting unit for research activities on different aspects of malaria. The main objective of establishing this facility is to strengthen researches at
INVESTIGATING THE MOTILITY OF PLASMODIUM
 INVESTIGATING THE MOTILITY OF PLASMODIUM by Natasha Vartak A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Science Baltimore, Maryland April,
INVESTIGATING THE MOTILITY OF PLASMODIUM by Natasha Vartak A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Science Baltimore, Maryland April,
BIO Parasitology Spring 2009
 BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 10 Malaria-Life Cycle a. Micro and macrogametocytes in mosquito stomach. b. Ookinete
BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 10 Malaria-Life Cycle a. Micro and macrogametocytes in mosquito stomach. b. Ookinete
Quantitative Dynamics of Plasmodium yoelii Sporozoite Transmission by Infected Anopheline Mosquitoes
 INFECTION AND IMMUNITY, July 2005, p. 4363 4369 Vol. 73, No. 7 0019-9567/05/$08.00 0 doi:10.1128/iai.73.7.4363 4369.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Quantitative
INFECTION AND IMMUNITY, July 2005, p. 4363 4369 Vol. 73, No. 7 0019-9567/05/$08.00 0 doi:10.1128/iai.73.7.4363 4369.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Quantitative
Motility precedes egress of malaria parasites from oocysts
 RESEARCH ARTICLE Motility precedes egress of malaria parasites from oocysts Dennis Klug*, Friedrich Frischknecht* Integrative Parasitology, Center for Infectious Diseases, Heidelberg University Medical
RESEARCH ARTICLE Motility precedes egress of malaria parasites from oocysts Dennis Klug*, Friedrich Frischknecht* Integrative Parasitology, Center for Infectious Diseases, Heidelberg University Medical
Malaria. This sheet is from both sections recording and includes all slides and diagrams.
 Malaria This sheet is from both sections recording and includes all slides and diagrams. Malaria is caused by protozoa family called plasmodium (Genus) mainly affect blood system specially RBCs and each
Malaria This sheet is from both sections recording and includes all slides and diagrams. Malaria is caused by protozoa family called plasmodium (Genus) mainly affect blood system specially RBCs and each
Developmentally Regulated!nfectivity of Malaria Sporozoites for Mosquito Salivary Glands and the Vertebrate Host
 Developmentally Regulated!nfectivity of Malaria Sporozoites for Mosquito Salivary Glands and the Vertebrate Host By Musa G. Touray, Alon Warburg, Andre Laughinghouse, Antoniana U. Krettli,* and Louis H.
Developmentally Regulated!nfectivity of Malaria Sporozoites for Mosquito Salivary Glands and the Vertebrate Host By Musa G. Touray, Alon Warburg, Andre Laughinghouse, Antoniana U. Krettli,* and Louis H.
Understanding Epidemics Section 3: Malaria & Modelling
 Understanding Epidemics Section 3: Malaria & Modelling PART B: Biology Contents: Vector and parasite Biology of the malaria parasite Biology of the anopheles mosquito life cycle Vector and parasite Malaria
Understanding Epidemics Section 3: Malaria & Modelling PART B: Biology Contents: Vector and parasite Biology of the malaria parasite Biology of the anopheles mosquito life cycle Vector and parasite Malaria
Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland sporozoites
 https://doi.org/10.1186/s12936-018-2431-1 Malaria Journal RESEARCH Open Access Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland
https://doi.org/10.1186/s12936-018-2431-1 Malaria Journal RESEARCH Open Access Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland
A n estimated 3.3 billion people were at risk of malaria infection in There is as of yet no licensed
 OPEN SUBJECT AREAS: PARASITOLOGY MOLECULAR BIOLOGY Received 27 March 2014 Accepted 23 June 2014 Published 11 July 2014 Correspondence and requests for materials should be addressed to A.S.I.A. (aaly@tulane.
OPEN SUBJECT AREAS: PARASITOLOGY MOLECULAR BIOLOGY Received 27 March 2014 Accepted 23 June 2014 Published 11 July 2014 Correspondence and requests for materials should be addressed to A.S.I.A. (aaly@tulane.
Biotecnologicas (IIB-INTECH), Universidad Nacional de San Martin, Av. General Paz 5445, Predio INTI, edificio 24 (1650), Buenos Aires, Argentina
 [Frontiers in Bioscience 17, 726-744, January 1, 2012] Plasmodium sporozoite motility: an update Georgina N. Montagna 1, Kai Matuschewski 1, Carlos A. Buscaglia 2 1 Parasitology Unit, Max Planck Institute
[Frontiers in Bioscience 17, 726-744, January 1, 2012] Plasmodium sporozoite motility: an update Georgina N. Montagna 1, Kai Matuschewski 1, Carlos A. Buscaglia 2 1 Parasitology Unit, Max Planck Institute
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/70169
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/70169
PCR detection of Leptospira in. stray cat and
 PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding
 Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding Ashley M. Vaughan, 1 Ahmed S.I. Aly, 1 and Stefan H.I. Kappe 1,2, * 1 Seattle Biomedical Research Institute, Seattle, WA 98109, USA
Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding Ashley M. Vaughan, 1 Ahmed S.I. Aly, 1 and Stefan H.I. Kappe 1,2, * 1 Seattle Biomedical Research Institute, Seattle, WA 98109, USA
Downloaded from:
 Al-Khattaf, FS; Tremp, AZ; El-Houderi, A; Dessens, JT (2016) The Plasmodium alveolin IMC1a is stabilised by its terminal cysteine motifs and facilitates sporozoite morphogenesis and infectivity in a dosedependent
Al-Khattaf, FS; Tremp, AZ; El-Houderi, A; Dessens, JT (2016) The Plasmodium alveolin IMC1a is stabilised by its terminal cysteine motifs and facilitates sporozoite morphogenesis and infectivity in a dosedependent
Epigenetic regulation of Plasmodium falciparum clonally. variant gene expression during development in An. gambiae
 Epigenetic regulation of Plasmodium falciparum clonally variant gene expression during development in An. gambiae Elena Gómez-Díaz, Rakiswendé S. Yerbanga, Thierry Lefèvre, Anna Cohuet, M. Jordan Rowley,
Epigenetic regulation of Plasmodium falciparum clonally variant gene expression during development in An. gambiae Elena Gómez-Díaz, Rakiswendé S. Yerbanga, Thierry Lefèvre, Anna Cohuet, M. Jordan Rowley,
CERTIFIED REFERENCE MATERIAL IRMM 313
 EUROPEAN COMMISSION JOINT RESEARCH CENTRE Institute for Reference Materials and Measurements (Geel) CERTIFIED REFERENCE MATERIAL IRMM 313 CERTIFICATE OF ANALYSIS PFGE AGAROSE PLUGS Certified value 2) SmaI
EUROPEAN COMMISSION JOINT RESEARCH CENTRE Institute for Reference Materials and Measurements (Geel) CERTIFIED REFERENCE MATERIAL IRMM 313 CERTIFICATE OF ANALYSIS PFGE AGAROSE PLUGS Certified value 2) SmaI
Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220
 Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220 Introduction Enzootic Bovine Leukosis is a transmissible disease caused by the Enzootic Bovine Leukosis Virus (BLV)
Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220 Introduction Enzootic Bovine Leukosis is a transmissible disease caused by the Enzootic Bovine Leukosis Virus (BLV)
Blood protozoan: Plasmodium
 Blood protozoan: Plasmodium Dr. Hala Al Daghistani The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans: four species are associated The Plasmodium spp.
Blood protozoan: Plasmodium Dr. Hala Al Daghistani The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans: four species are associated The Plasmodium spp.
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii. Yates, Lauren A.
 A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
Blood protozoan: Plasmodium
 Blood protozoan: Plasmodium The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans:four species are associated The Plasmodium spp. life cycle can be divided
Blood protozoan: Plasmodium The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans:four species are associated The Plasmodium spp. life cycle can be divided
Giardia and Apicomplexa. G. A. Lozano UNBC
 Giardia and Apicomplexa G. A. Lozano UNBC NINE Protozoan diseases/parasites Ciliphora, Ichthyophthirius, Ick Sarcomastigophora, Giardia, giardiasis Apicomplexa: Eimeria, Toxoplasma, Sarcocystis, Cryptosporidium.
Giardia and Apicomplexa G. A. Lozano UNBC NINE Protozoan diseases/parasites Ciliphora, Ichthyophthirius, Ick Sarcomastigophora, Giardia, giardiasis Apicomplexa: Eimeria, Toxoplasma, Sarcocystis, Cryptosporidium.
Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites
 Mediators of Inflammation, Article ID 362605, 6 pages http://dx.doi.org/10.1155/2014/362605 Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites Hong Zheng, Zhangping Tan, and
Mediators of Inflammation, Article ID 362605, 6 pages http://dx.doi.org/10.1155/2014/362605 Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites Hong Zheng, Zhangping Tan, and
Antibiotic Resistance in Bacteria
 Antibiotic Resistance in Bacteria Electron Micrograph of E. Coli Diseases Caused by Bacteria 1928 1 2 Fleming 3 discovers penicillin the first antibiotic. Some Clinically Important Antibiotics Antibiotic
Antibiotic Resistance in Bacteria Electron Micrograph of E. Coli Diseases Caused by Bacteria 1928 1 2 Fleming 3 discovers penicillin the first antibiotic. Some Clinically Important Antibiotics Antibiotic
9 Parasitology 9 EXERCISE EQA. Objectives EXERCISE
 0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
Reverse genetics screen identifies six proteins important for malaria development in the mosquito
 Molecular Microbiology (2008) 70(1), 209220 doi:.1111/j.1365-2958.2008.06407.x First published online 27 August 2008 Reverse genetics screen identifies six proteins important for malaria development in
Molecular Microbiology (2008) 70(1), 209220 doi:.1111/j.1365-2958.2008.06407.x First published online 27 August 2008 Reverse genetics screen identifies six proteins important for malaria development in
Evaluation of the hair growth and retention activity of two solutions on human hair explants
 activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
Malaria remains the most important parasitic disease. Review Article
 Review Article Pre-erythrocytic Stage of Malaria Infection and the Molecular Targets Available for Interventions Dickson Adah 1,2, Yi Jun Yang 1,2, Quan Liu 1,2, Limei Qin 1, Li Qin 1, Xiaoping Chen 1
Review Article Pre-erythrocytic Stage of Malaria Infection and the Molecular Targets Available for Interventions Dickson Adah 1,2, Yi Jun Yang 1,2, Quan Liu 1,2, Limei Qin 1, Li Qin 1, Xiaoping Chen 1
Parasitology Amoebas. Sarcodina. Mastigophora
 Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Malaria parasites of rodents of the Congo (Brazzaville) :
 Annales de Parasitologie (Paris), 1976, t. 51, n 6, pp. 637 à 646 Malaria parasites of rodents of the Congo (Brazzaville) : Plasmodium cbabaudi adami subsp. nov. and Plasmodium vinckei lentum Landau, Michel,
Annales de Parasitologie (Paris), 1976, t. 51, n 6, pp. 637 à 646 Malaria parasites of rodents of the Congo (Brazzaville) : Plasmodium cbabaudi adami subsp. nov. and Plasmodium vinckei lentum Landau, Michel,
Protozoan parasites of the genus Plasmodium are the causative
 Exploring the transcriptome of the malaria sporozoite stage Stefan H. I. Kappe*, Malcolm J. Gardner, Stuart M. Brown, Jessica Ross*, Kai Matuschewski*, Jose M. Ribeiro, John H. Adams, John Quackenbush,
Exploring the transcriptome of the malaria sporozoite stage Stefan H. I. Kappe*, Malcolm J. Gardner, Stuart M. Brown, Jessica Ross*, Kai Matuschewski*, Jose M. Ribeiro, John H. Adams, John Quackenbush,
BioSci 110, Fall 08 Exam 2
 1. is the cell division process that results in the production of a. mitosis; 2 gametes b. meiosis; 2 gametes c. meiosis; 2 somatic (body) cells d. mitosis; 4 somatic (body) cells e. *meiosis; 4 gametes
1. is the cell division process that results in the production of a. mitosis; 2 gametes b. meiosis; 2 gametes c. meiosis; 2 somatic (body) cells d. mitosis; 4 somatic (body) cells e. *meiosis; 4 gametes
Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis
 Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis Brandy L. Salmon, Anna Oksman, and Daniel E. Goldberg* Howard Hughes Medical Institute, Departments
Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis Brandy L. Salmon, Anna Oksman, and Daniel E. Goldberg* Howard Hughes Medical Institute, Departments
THE TRANSMISSION EFFICIENCY OF PLASMODIUM YOELII INFECTED MOSQUITOES
 THE TRANSMISSION EFFICIENCY OF PLASMODIUM YOELII INFECTED MOSQUITOES by Maya A. Aleshnick A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of
THE TRANSMISSION EFFICIENCY OF PLASMODIUM YOELII INFECTED MOSQUITOES by Maya A. Aleshnick A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of
Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling
 Developmental Cell, Volume 29 Supplemental Information Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling Danyi Li, Rui
Developmental Cell, Volume 29 Supplemental Information Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling Danyi Li, Rui
Apicomplexans Apicomplexa Intro
 Apicomplexans Apicomplexa Intro Cryptosporidium Apicomplexan Select Characteristics Gliding motility Apical Complex organelle for invasion of host cell Life cycle alternates b/w sexual and asexual phases
Apicomplexans Apicomplexa Intro Cryptosporidium Apicomplexan Select Characteristics Gliding motility Apical Complex organelle for invasion of host cell Life cycle alternates b/w sexual and asexual phases
Malaria parasites: virulence and transmission as a basis for intervention strategies
 Malaria parasites: virulence and transmission as a basis for intervention strategies Matthias Marti Department of Immunology and Infectious Diseases Harvard School of Public Health The global malaria burden
Malaria parasites: virulence and transmission as a basis for intervention strategies Matthias Marti Department of Immunology and Infectious Diseases Harvard School of Public Health The global malaria burden
1 In 1958, scientists made a breakthrough in artificial reproductive cloning by successfully cloning a
 1 In 1958, scientists made a breakthrough in artificial reproductive cloning by successfully cloning a vertebrate species. The species cloned was the African clawed frog, Xenopus laevis. Fig. 1.1, on page
1 In 1958, scientists made a breakthrough in artificial reproductive cloning by successfully cloning a vertebrate species. The species cloned was the African clawed frog, Xenopus laevis. Fig. 1.1, on page
Phylum:Apicomplexa Class:Sporozoa
 Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Gliding Motility Leads to Active Cellular Invasion by Cryptosporidium parvum Sporozoites
 INFECTION AND IMMUNITY, Sept. 2005, p. 5379 5387 Vol. 73, No. 9 0019-9567/05/$08.00 0 doi:10.1128/iai.73.9.5379 5387.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Gliding
INFECTION AND IMMUNITY, Sept. 2005, p. 5379 5387 Vol. 73, No. 9 0019-9567/05/$08.00 0 doi:10.1128/iai.73.9.5379 5387.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Gliding
Consequences of Antimicrobial Resistant Bacteria. Antimicrobial Resistance. Molecular Genetics of Antimicrobial Resistance. Topics to be Covered
 Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
MID 23. Antimicrobial Resistance. Consequences of Antimicrobial Resistant Bacteria. Molecular Genetics of Antimicrobial Resistance
 Antimicrobial Resistance Molecular Genetics of Antimicrobial Resistance Micro evolutionary change - point mutations Beta-lactamase mutation extends spectrum of the enzyme rpob gene (RNA polymerase) mutation
Antimicrobial Resistance Molecular Genetics of Antimicrobial Resistance Micro evolutionary change - point mutations Beta-lactamase mutation extends spectrum of the enzyme rpob gene (RNA polymerase) mutation
Co-transfer of bla NDM-5 and mcr-1 by an IncX3 X4 hybrid plasmid in Escherichia coli 4
 SUPPLEMENTARY INFORMATION ARTICLE NUMBER: 16176 DOI: 10.1038/NMICROBIOL.2016.176 Co-transfer of bla NDM-5 and mcr-1 by an IncX3 X4 hybrid plasmid in Escherichia coli 4 5 6 7 8 9 10 11 12 13 14 15 16 17
SUPPLEMENTARY INFORMATION ARTICLE NUMBER: 16176 DOI: 10.1038/NMICROBIOL.2016.176 Co-transfer of bla NDM-5 and mcr-1 by an IncX3 X4 hybrid plasmid in Escherichia coli 4 5 6 7 8 9 10 11 12 13 14 15 16 17
Antimicrobial Resistance
 Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Acquisition of Foreign DNA
 Antimicrobial Resistance Acquisition of Foreign DNA Levy, Scientific American Horizontal gene transfer is common, even between Gram positive and negative bacteria Plasmid - transfer of single or multiple
Antimicrobial Resistance Acquisition of Foreign DNA Levy, Scientific American Horizontal gene transfer is common, even between Gram positive and negative bacteria Plasmid - transfer of single or multiple
Overview. There are commonly found arrangements of bacteria based on their division. Spheres, Rods, Spirals
 Bacteria Overview Bacteria live almost everywhere. Most are microscopic ranging from 0.5 5 m in size, and unicellular. They have a variety of shapes when viewed under a microscope, most commonly: Spheres,
Bacteria Overview Bacteria live almost everywhere. Most are microscopic ranging from 0.5 5 m in size, and unicellular. They have a variety of shapes when viewed under a microscope, most commonly: Spheres,
Malaria Sporozoites Traverse Host Cells within Transient Vacuoles
 Malaria Sporozoites Traverse Host Cells within Transient Vacuoles Veronica Risco-Castillo, Selma Topçu, Carine Marinach, Giulia Manzoni, Améliee. Bigorgne, Sylvie Briquet, Xavier Baudin, Maryse Lebrun,
Malaria Sporozoites Traverse Host Cells within Transient Vacuoles Veronica Risco-Castillo, Selma Topçu, Carine Marinach, Giulia Manzoni, Améliee. Bigorgne, Sylvie Briquet, Xavier Baudin, Maryse Lebrun,
Consuelo Pinzon-Ortiz, Jennifer Friedman, Jeffrey Esko, and Photini Sinnis
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 276, No. 29, Issue of July 20, pp. 26784 26791, 2001 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. The Binding of
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 276, No. 29, Issue of July 20, pp. 26784 26791, 2001 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. The Binding of
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/389/98-FINAL July 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS ENROFLOXACIN (extension to
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/389/98-FINAL July 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS ENROFLOXACIN (extension to
SCANNING electron - microscopy has
 Characteristics of the Absorptive Surface of the Small Intestine of the Chicken from 1 Day to 14 Weeks of Age 1 R. C. BAYER, C. B. CHAWAN, F. H. BIRD AND S. D. MUSGRAVE Department of Animal and Veterinary
Characteristics of the Absorptive Surface of the Small Intestine of the Chicken from 1 Day to 14 Weeks of Age 1 R. C. BAYER, C. B. CHAWAN, F. H. BIRD AND S. D. MUSGRAVE Department of Animal and Veterinary
SUPPLEMENTARY INFORMATION
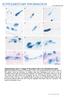 doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
A Role for Apical Membrane Antigen 1 during Invasion of Hepatocytes by Plasmodium falciparum Sporozoites*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 10, Issue of March 5, pp. 9490 9496, 2004 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. A Role for Apical
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 10, Issue of March 5, pp. 9490 9496, 2004 2004 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. A Role for Apical
INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER
 Canine VacciCheck INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER IgG ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 13 JUL 2015 Biogal Galed Laboratories Acs. Ltd., tel: 972-4-9898605.
Canine VacciCheck INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER IgG ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 13 JUL 2015 Biogal Galed Laboratories Acs. Ltd., tel: 972-4-9898605.
Automated classification of Plasmodium sporozoite movement patterns reveals a shift towards productive motility during salivary gland infection
 Automated classification of Plasmodium sporozoite movement patterns reveals a shift towards productive motility during salivary gland infection Stephan Josef Hegge, Mikhail Kudryashev, Ashley Smith, Friedrich
Automated classification of Plasmodium sporozoite movement patterns reveals a shift towards productive motility during salivary gland infection Stephan Josef Hegge, Mikhail Kudryashev, Ashley Smith, Friedrich
FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT
![FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT](/thumbs/93/113164093.jpg) FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 22 APR 2018 Biogal Galed Laboratories Acs Ltd. tel: 972-4-9898605. fax: 972-4-9898690 e-mail:info@biogal.co.il
FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 22 APR 2018 Biogal Galed Laboratories Acs Ltd. tel: 972-4-9898605. fax: 972-4-9898690 e-mail:info@biogal.co.il
Evaluation of Different Antigens in Western Blotting Technique for the Diagnosis of Sheep Haemonchosis
 Original Article Evaluation of Different Antigens in Western Blotting Technique for the Diagnosis of Sheep Haemonchosis *B Meshgi, SH Hosseini Dept. of Parasitology, Faculty of Veterinary Medicine, University
Original Article Evaluation of Different Antigens in Western Blotting Technique for the Diagnosis of Sheep Haemonchosis *B Meshgi, SH Hosseini Dept. of Parasitology, Faculty of Veterinary Medicine, University
Agarose Blenders. Code Description Size
 Agarose Blenders Code Description Size K669-100G Agarose I / TBE Blend 0.8% 100 grams K677-100G Agarose I / TBE Blend 1.5% 100 grams K678-100G Agarose I /TBE Blend 2.0% 100 grams K679-100G Agarose I /
Agarose Blenders Code Description Size K669-100G Agarose I / TBE Blend 0.8% 100 grams K677-100G Agarose I / TBE Blend 1.5% 100 grams K678-100G Agarose I /TBE Blend 2.0% 100 grams K679-100G Agarose I /
CIRCUMSPOROZOITE PROTEINS OF HUMAN MALARIA PARASITES PLASMODIUM FALCIPARUM AND PLASMODIUM VIVA,F*
 CIRCUMSPOROZOITE PROTEINS OF HUMAN MALARIA PARASITES PLASMODIUM FALCIPARUM AND PLASMODIUM VIVA,F* BY ELIZABETH H. NARDIN, VICTOR NUSSENZWEIG, RUTH S. NUSSENZWEIG, WILLIAM E. COLLINS, K. TRANAKCHIT HARINASUTA,
CIRCUMSPOROZOITE PROTEINS OF HUMAN MALARIA PARASITES PLASMODIUM FALCIPARUM AND PLASMODIUM VIVA,F* BY ELIZABETH H. NARDIN, VICTOR NUSSENZWEIG, RUTH S. NUSSENZWEIG, WILLIAM E. COLLINS, K. TRANAKCHIT HARINASUTA,
Protozoan Parasites of Veterinary importance 2017
 Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Plasmodium Pre-Erythrocytic Stages: Biology, Whole Parasite Vaccines and Transgenic Models
 American Journal of Immunology, 2012, 8 (3), 88-100 ISSN 1553-619X 2012 Science Publication doi:10.3844/ajisp.2012.88.100 Published Online 8 (3) 2012 (http://www.thescipub.com/aji.toc) Plasmodium Pre-Erythrocytic
American Journal of Immunology, 2012, 8 (3), 88-100 ISSN 1553-619X 2012 Science Publication doi:10.3844/ajisp.2012.88.100 Published Online 8 (3) 2012 (http://www.thescipub.com/aji.toc) Plasmodium Pre-Erythrocytic
HISTOPATHOLOGY. Introduction:
 Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
A role for apical membrane antigen 1 during invasion of hepatocytes
 JBC Papers in Press. Published on December 15, 2003 as Manuscript M311331200 A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. Olivier Silvie a,
JBC Papers in Press. Published on December 15, 2003 as Manuscript M311331200 A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. Olivier Silvie a,
Protocol for fabrication of microcompartments for long-term culture and imaging of small C. elegans larvae. Henrik Bringmann, March 2011.
 Protocol for fabrication of microcompartments for long-term culture and imaging of small C. elegans larvae Henrik Bringmann, March 2011. 1 Step-by-Step Protocol Step1 : Preparing a humidity dish (see illustration
Protocol for fabrication of microcompartments for long-term culture and imaging of small C. elegans larvae Henrik Bringmann, March 2011. 1 Step-by-Step Protocol Step1 : Preparing a humidity dish (see illustration
23 Plasmodium coatneyi Eyles, Fong, Warren, Guinn, Sandosham, and Wharton, 1962
 23 Plasmodium coatneyi Eyles, Fong, Warren, Guinn, Sandosham, and Wharton, 1962 IN the course of studies on simian malaria begun by the late Dr. Don Eyles in Malaya, he and his co-workers isolated a new
23 Plasmodium coatneyi Eyles, Fong, Warren, Guinn, Sandosham, and Wharton, 1962 IN the course of studies on simian malaria begun by the late Dr. Don Eyles in Malaya, he and his co-workers isolated a new
Fluoroquinolones ELISA KIT
 Fluoroquinolones ELISA KIT Cat. No.:DEIA6883 Pkg.Size:96T Intended use The Fluoroquinolones ELISA KIT is an immunoassay for the detection of Fluoroquinolones in contaminated samples including water, fish
Fluoroquinolones ELISA KIT Cat. No.:DEIA6883 Pkg.Size:96T Intended use The Fluoroquinolones ELISA KIT is an immunoassay for the detection of Fluoroquinolones in contaminated samples including water, fish
11111L A _W ' I III! MICROCOPY RESOLUTION TEST CHART NATIONAL BUREAU OF STANDARDS 1963-A 2,1
 RD-AI?2 464 CELL PNYSIOLOOY OF THE NRARIAX PRRRSITE(U) NEN VOR 1/1 UNIV NEDICRI. CENTER N V J YANOERDERO AUG 64 DADA7-73-C-3027 UNCLSSIFIED F/0 615 NL MNNE / 4r 11111L A _W '18 2.5 11111-2 2.2I 11111125
RD-AI?2 464 CELL PNYSIOLOOY OF THE NRARIAX PRRRSITE(U) NEN VOR 1/1 UNIV NEDICRI. CENTER N V J YANOERDERO AUG 64 DADA7-73-C-3027 UNCLSSIFIED F/0 615 NL MNNE / 4r 11111L A _W '18 2.5 11111-2 2.2I 11111125
THE ABUNDANCE AND INFECTION STATUS OF ANOPHELES MOSQUITOES IN LOUDOUN COUNTY, VIRGINIA
 THE ABUNDANCE AND INFECTION STATUS OF ANOPHELES MOSQUITOES IN LOUDOUN COUNTY, VIRGINIA Andrew Lima Clarke (Manassas, VA) Priya Krishnan ODU M.S. candidate (Richmond, VA) Objectives To determine: 1) the
THE ABUNDANCE AND INFECTION STATUS OF ANOPHELES MOSQUITOES IN LOUDOUN COUNTY, VIRGINIA Andrew Lima Clarke (Manassas, VA) Priya Krishnan ODU M.S. candidate (Richmond, VA) Objectives To determine: 1) the
Detection of Mastitis
 Detection of Mastitis Changes in milk composition Changes in milk composition Physical examination Signs of inflammation Empty udder Differences in firmness Unbalanced quarters Taste Test 60% of salty
Detection of Mastitis Changes in milk composition Changes in milk composition Physical examination Signs of inflammation Empty udder Differences in firmness Unbalanced quarters Taste Test 60% of salty
Marissa Vignali*, Cate Speake* and Patrick E Duffy*
 Minireview Malaria sporozoite proteome leaves a trail Marissa Vignali*, Cate Speake* and Patrick E Duffy* Addresses: *Malaria Program, Seattle Biomedical Research Institute, Seattle, Washington 98109,
Minireview Malaria sporozoite proteome leaves a trail Marissa Vignali*, Cate Speake* and Patrick E Duffy* Addresses: *Malaria Program, Seattle Biomedical Research Institute, Seattle, Washington 98109,
Transmission success of the malaria parasite Plasmodium mexicanum into its vector: role of gametocyte density and sex ratio
 Transmission success of the malaria parasite Plasmodium mexicanum into its vector: role of gametocyte density and sex ratio 575 J. J. SCHALL* Department of Biology, University of Vermont, Burlington, Vermont
Transmission success of the malaria parasite Plasmodium mexicanum into its vector: role of gametocyte density and sex ratio 575 J. J. SCHALL* Department of Biology, University of Vermont, Burlington, Vermont
A Scanning Electron Microscopic Study of Eggshell Surface Topography of Leidynema portentosae and L. appendiculatum (Nematoda: Oxyuroidea)
 The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 88, Issue 5 (December, 1988) 1988-12 A Scanning Electron Microscopic
The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 88, Issue 5 (December, 1988) 1988-12 A Scanning Electron Microscopic
Medical Genetics and Diagnosis Lab #3. Gel electrophoresis
 Medical Genetics and Diagnosis Lab #3 Gel electrophoresis Background Information Gel electrophoresis is the standard lab procedure for separating DNA by size (e.g. length in base pairs) for visualization
Medical Genetics and Diagnosis Lab #3 Gel electrophoresis Background Information Gel electrophoresis is the standard lab procedure for separating DNA by size (e.g. length in base pairs) for visualization
HYDATID CYST DISEASE
 HYDATID CYST DISEASE Hydatid disease, also called hydatidosis or echinococcosis, is a cystforming disease resulting from an infection with the metacestode, or larval form, of parasitic dog tapeworms from
HYDATID CYST DISEASE Hydatid disease, also called hydatidosis or echinococcosis, is a cystforming disease resulting from an infection with the metacestode, or larval form, of parasitic dog tapeworms from
RICKETTSIA SPECIES AMONG TICKS IN AN AREA OF JAPAN ENDEMIC FOR JAPANESE SPOTTED FEVER
 RICKETTSIA SPECIES AMONG TICKS IN AN AREA OF JAPAN ENDEMIC FOR JAPANESE SPOTTED FEVER Makoto Kondo 1, Katsuhiko Ando 2, Keiichi Yamanaka 1 and Hitoshi Mizutani 1 1 Department of Dermatology, 2 Department
RICKETTSIA SPECIES AMONG TICKS IN AN AREA OF JAPAN ENDEMIC FOR JAPANESE SPOTTED FEVER Makoto Kondo 1, Katsuhiko Ando 2, Keiichi Yamanaka 1 and Hitoshi Mizutani 1 1 Department of Dermatology, 2 Department
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12 1. NAME OF THE VETERINARY MEDICINAL PRODUCT HALOCUR 0.5 mg/ml oral solution for calves 2. Qualitative and quantitative composition Active substance Halofuginone
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12 1. NAME OF THE VETERINARY MEDICINAL PRODUCT HALOCUR 0.5 mg/ml oral solution for calves 2. Qualitative and quantitative composition Active substance Halofuginone
Protozoa. Apicomplexa Sarcomastigophora Ciliophora. Gregarinea Coccidia Piroplasma
 Protozoa Apicomplexa Sarcomastigophora Ciliophora Gregarinea Coccidia Piroplasma Coccidia characterized by thick-walled oocysts excreted in feces In Humans Cryptosporidium Isospora Cyclospora Sarcocystis
Protozoa Apicomplexa Sarcomastigophora Ciliophora Gregarinea Coccidia Piroplasma Coccidia characterized by thick-walled oocysts excreted in feces In Humans Cryptosporidium Isospora Cyclospora Sarcocystis
DLS Sample Preparation Guide
 DLS Sample Preparation Guide The Leica TCS SP8 DLS is an innovative concept to integrate the Light Sheet Microscopy technology into the confocal microscope. Due to its unique optical architecture samples
DLS Sample Preparation Guide The Leica TCS SP8 DLS is an innovative concept to integrate the Light Sheet Microscopy technology into the confocal microscope. Due to its unique optical architecture samples
Methicillin-Resistant Staphylococcus aureus
 Methicillin-Resistant Staphylococcus aureus By Karla Givens Means of Transmission and Usual Reservoirs Staphylococcus aureus is part of normal flora and can be found on the skin and in the noses of one
Methicillin-Resistant Staphylococcus aureus By Karla Givens Means of Transmission and Usual Reservoirs Staphylococcus aureus is part of normal flora and can be found on the skin and in the noses of one
Received 6 December 2000/Returned for modification 29 January 2001/Accepted 26 March 2001
 INFECTION AND IMMUNITY, June 2001, p. 3845 3852 Vol. 69, No. 6 0019-9567/01/$04.00 0 DOI: 10.1128/IAI.69.6.3845 3952.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Human Antibodies
INFECTION AND IMMUNITY, June 2001, p. 3845 3852 Vol. 69, No. 6 0019-9567/01/$04.00 0 DOI: 10.1128/IAI.69.6.3845 3952.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Human Antibodies
