Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development
|
|
|
- Doreen King
- 5 years ago
- Views:
Transcription
1 Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development Shiroh Iwanaga, Izumi Kaneko, Tomomi Kato, Masao Yuda* Department of Medical Zoology, Mie University School of Medicine, Tsu, Mie, Japan Abstract Liver-stage malaria parasites are a promising target for drugs and vaccines against malaria infection. However, little is currently known about gene regulation in this stage. In this study, we used the rodent malaria parasite Plasmodium berghei and showed that an AP2-family transcription factor, designated AP2-L, plays a critical role in the liver-stage development of the parasite. AP2-L-depleted parasites proliferated normally in blood and in mosquitoes. However, the ability of these parasites to infect the liver was approximately 10,000 times lower than that of wild-type parasites. In vitro assays showed that the sporozoites of these parasites invaded hepatocytes normally but that their development stopped in the middle of the liver schizont stage. Expression profiling using transgenic P. berghei showed that fluorescent protein-tagged AP2-L increased rapidly during the liver schizont stage but suddenly disappeared with the formation of the mature liver schizont. DNA microarray analysis showed that the expression of several genes, including those of parasitophorous vacuole membrane proteins, was significantly decreased in the early liver stage of AP2-L-depleted parasites. Investigation of the targets of this transcription factor should greatly promote the exploration of liver-stage antigens and the elucidation of the mechanisms of hepatocyte infection by malaria parasites. Citation: Iwanaga S, Kaneko I, Kato T, Yuda M (2012) Identification of an AP2-family Protein That Is Critical for Malaria Liver Stage Development. PLoS ONE 7(11): e doi: /journal.pone Editor: Anne Charlotte Gruner, Museum National d Histoire Naturelle, France Received March 20, 2012; Accepted September 18, 2012; Published November 7, 2012 Copyright: ß 2012 Iwanaga et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This work was supported by the Ministry of Education, Science, Culture, and Sports of Japan (grant ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing Interests: The authors have declared that no competing interests exist. * m-yuda@doc.medic.mie-u.ac.jp Introduction The transmission of malaria to humans is initiated by the bite of an Anopheles mosquito, which deposits malaria sporozoites from the mosquito salivary glands into the skin of the host. The sporozoites then migrate to the liver, invade hepatocytes and transform into liver-stage (LS) parasites inside a parasitophorous vacuole (PV). The parasites undergo nuclear divisions and finally multiply into thousands of merozoites, which infect erythrocytes. The LS parasite is one of the most attractive targets for malaria vaccine development. Robust and long-term protection against malaria transmission has been induced in mice and humans by infecting them with sporozoites that are attenuated by irradiation or genetic modifications [1 3]. Development of these parasites is arrested in the LS, and cellular immunity against LS parasites is thought to have an important role in this protection [4 6]. However, few antigens of this parasite, which are specific for this stage, have been identified. To explore candidate antigens in LS parasites and to elucidate the mechanisms of liver infection, transcriptomic and proteomic studies have been carried out [7], but a comprehensive understanding of gene expression has not been achieved. The lack of progress in this area results from the difficulty of collecting sufficient quantities of LS parasites for expression analyses and genetic studies. Moreover, this difficulty is greatly increased in the early stage because of the small size of the parasite. The mechanisms of gene regulation in the LS also remain largely unknown. In particular, the transcription factors that control stage-specific gene expression have not been identified. Apetala 2 (AP2)-family proteins are transcription factors that have DNA-binding domains of,60 amino acids called AP2 domains. Recently, AP2 genes have been found in the genomes of Plasmodium parasites [8 10]. In Plasmodium falciparum, the most important human malaria parasite, 27 AP2-family genes have been identified [9]. Among these genes, 26 are conserved in the other Plasmodium species whose entire genomes have been sequenced. Each member of this family has 1 to 4 AP2 domains, and the amino acid sequences of these domains are highly conserved among Plasmodium orthologs. At present, the AP2 family is the only family of sequence-specific transcription factors whose functions have been demonstrated in Plasmodium species. The extraordinarily small number of sequence-specific transcription factors identified in the Plasmodium genome suggests that AP2-family proteins have central roles in gene regulation in these parasites and that Plasmodium parasites have a unique system of gene regulation to maintain their complex life cycle. AP2-family genes are expressed in the asexual blood stages of the Plasmodium life cycle [8]. AP2-family transcription factors play central roles in gene expression in ookinetes and sporozoites [11,12]. In this study, we report that an AP2-family transcription factor of the rodent malaria parasite P. berghei (PlasmoDB ID, PBANKA_021440, designated AP2-L) is expressed in LS parasites. We show that the disruption of AP2-L almost completely arrests LS development without affecting the proliferation of other stages in the life cycle. PLOS ONE 1 November 2012 Volume 7 Issue 11 e47557
2 Results and Discussion AP2-L is Expressed in Sporozoites and is Necessary for Parasite Infection of the Liver To investigate the involvement of AP2-family transcription factors in liver infection by P. berghei, we searched for expressed sequence tags (ESTs) encoding AP2 transcription factors in our P. berghei sporozoite EST database (available in PlasmoDB, PlasmoDB.org). We found several ESTs encoding the AP2-family gene PBANKA_ (hereafter AP2-L). AP2-L encodes a protein of 1272 amino acids with two AP2 domains (Fig. 1A). These domains are located near the C-terminus of the protein and are separated from each other by a short linker. AP2-L orthologs are present in other Plasmodium species, including P. falciparum, and the amino acid sequences of the two AP2 domains, including the linker region, are highly conserved (Fig. 1B). The comparison of the amino acid sequences of these orthologs revealed another highly conserved region of approximately 100 amino acids C- terminal to the AP2 domains (Figs. 1A and 1C). The overall amino acid sequence identity of this region between P. berghei and P falciparum proteins is 84%. Known functional motifs were not found in this region by a search using InterProScan ( ebi.ac.uk/interproscan). BLAST searches of public databases using this sequence showed that related sequences exist for some Plasmodium AP2-family proteins (Figures 1A and S1A) and in AP2- family proteins of other apicomplexan parasites (Figures 1A and S1B). These sequences are all located near the C-terminus (Fig. 1A), suggesting that they have a common function and that these proteins constitute a subfamily of apicomplexan AP2-family proteins. To investigate the function of AP2-L in the parasite life cycle, we performed targeted knockout experiments in P. berghei. The AP2-L gene was knocked out by the insertion of a targeting construct containing the pyrimethamine-resistance human dihydrofolate reductase (DHFR) gene into the AP2-L locus by double cross-over recombination (Figure 2A). Parasites that integrated the construct were separated from wild-type parasites by limiting dilution. Two parasite populations prepared by independent transfections were used in the following experiments. AP2-L(2) parasites had an intra-erythrocytic replication rate nearly identical to that of wild-type parasites (Figure 2B). All mosquito stages (oocysts, oocyst sporozoites and salivary gland sporozoites) formed in numbers similar to those of wild-type parasites (Table S1). The motility rates of salivary gland AP2-L(2) sporozoites were also normal (.90%). To examine liver infectivity, salivary gland sporozoites were injected intravenously into rats, and the prepatent time for parasitemia was monitored (Table 1). When inoculated with 30,000 sporozoites, 15 out of 16 rats were infected, and the prepatent time for parasitemia in the infected rats was significantly extended (average days) as compared with the rats inoculated with wild-type sporozoites. From the replication rate of the asexual blood stage (,6 10 fold per day) and the delay in parasitemia, the liver infection ability of AP2-L(2) parasites was estimated to be approximately 10,000 times lower than that of wild-type parasites. AP2-L(2) Parasites Migrate through Cells Normally and Productively Invade Hepatocytes The reduced ability of AP2-L(2) parasites to infect liver could be due to one of the following possibilities: (1) the cell-traversal ability of AP2-L(2) sporozoites is impaired, and thus, they do not arrive at the liver parenchyma; (2) AP2-L(2) sporozoites arrive at hepatocytes but cannot invade them productively (i.e., by forming a PV); (3) AP2-L(2) sporozoites invade hepatocytes normally but are unable to develop into merozoites. First, we examined the first and second possibilities with in vitro assays using salivary gland sporozoites. Figure 1. AP2-L and related proteins in apicomplexan parasites. A. Schematic diagrams of P. berghei AP2-L and related proteins are shown. The paralogs in P. berghei are indicated by their gene identifiers in PlasmoDB. The related proteins in other apicomplexan parasites are indicated by the species name and their GenBank accession number. AP2 domains (blue) and another conserved sequence (red) are highlighted by rectangles. Bars indicate the regions shown in B and C. B. Amino acid sequences of a region containing two A domains (highlighted with rectangles) are compared in the AP2-Ls of four Plasmodium species: P. berghei (Pb), P. falciparum (Pf), P. vivax (Pv), and P. knowlesi (Pk). The amino acids conserved across all four species are indicated by asterisks. This region is indicated in A by the left bar. C. Amino acid sequences of another region conserved among the AP2-Ls. This region is indicated in A by the right bar. The amino acids conserved across all four species are indicated by asterisks. doi: /journal.pone g001 PLOS ONE 2 November 2012 Volume 7 Issue 11 e47557
3 Figure 2. Deletion of the AP2-L gene does not affect the ability of sporozoites to invade hepatocytes. A. Schematic diagram of targeting P. berghei AP2-L by double cross-over homologous recombination. A Southern blot analysis of wild-type (WT) and two independent AP2-L knockout populations (AP2-L(2) 1 and AP2-L(2) 2) is shown on the right. B. Replication of asexual blood stages in mice. Mice were intravenously injected with blood infected with AP2-L(2) or wild-type (WT) parasites (, parasites per mouse). The parasitemia of each mouse was checked each day. The values are the mean 6 standard error of the mean (SEM) for four measurements. C. Cell-traversal assay in HepG2 cells. Sporozoites ( ) were inoculated into a culture of HepG2 cells and incubated at 37uC for 30 min. The number of injured cells that incorporated FITC-labeled dextran was counted. The values are the mean 6 SEM of four measurements. D. Double staining assay. Sporozoites ( ) were inoculated into a culture of HepG2 cells and incubated at 37uC for 1 h. The sporozoites outside (stained both red and green) and inside (stained only red) the HepG2 cells were counted independently. The results are shown as the percentage of sporozoites inside the cell out of the total. The values are the mean 6 SEM of eight measurements. E. Fluorescence images of AP2-L(2) 1 and wild-type parasites inside HepG2 cells 6 h after sporozoite inoculation. The parasites were stained with anti-csp (red) and anti-uis4 (green) antibodies. The scale bars represent 10 mm. doi: /journal.pone g002 The cell-traversal ability of sporozoites was determined by cellinjury assays in HepG2 cells, a human hepatoma-derived cell line. Sporozoites were added to a culture of HepG2 cells, and injured cells, which were marked by the incorporation of fluorescein isothiocyanate (FITC)-labeled dextran, were counted under a fluorescence microscope. Similar numbers of injured cells were PLOS ONE 3 November 2012 Volume 7 Issue 11 e47557
4 Table 1. The ability of AP2-L(2) parasites to infect the liver is greatly reduced. Parasite Number of injected sporozoites a Number of infected rats/ injected Prepatent time (days) b Experiment 1 Wild-type 30,000 4/4 3.0 AP2-L(2) 1 30,000 3/4 7.5 AP2-L(2) 2 30,000 4/4 7.5 Experiment 2 Wild-type 30,000 4/4 3.0 AP2-L(2) 1 30,000 4/4 7.8 AP2-L(2) 2 30,000 4/4 8.3 a. Salivary gland sporozoites were collected 24 days after an infective blood meal. b. The average prepatent time for infected rats. Parasitemia was checked at one-day intervals starting 2 days after the challenge. doi: /journal.pone t001 found upon infection with both AP2-L(2) and wild-type parasites (Figure 2C), indicating that AP2-L(2) sporozoites can rupture and traverse cells normally. Next, double staining assays were performed for examining the ability of AP2-L(2) sporozoites to productively invade hepatocytes; this ability can be indirectly estimated because the proportion of parasites inside the hepatocytes decreases significantly when the parasites cannot invade the hepatocytes [13]. Sporozoites were added to a culture of HepG2 cells, allowed to invade for 30 min, and differentially stained with anti-circumsporozoite protein (CSP) antibodies according to their location, i.e., outside or inside the HepG2 cells (Figure 2D). Approximately the same proportion of sporozoites (70 80%) was observed to be inside the HepG2 cells whether they were wild type or AP2-L(2), suggesting that the sporozoites had retained their normal ability to invade the hepatocytes productively. We further checked whether AP2-L(2) sporozoites form an intact PV membrane inside HepG2 cells by immunofluorescence staining with antibodies against the protein upregulated in infective sporozoites 4 (UIS4) [14], which is observed in the PV membrane after sporozoite invasion. Sporozoites were inoculated into a culture of HepG2 cells and immunofluorescently stained at 6 hours post inoculation (hpi) with antibodies against UIS4 and CSP. AP2-L(2) sporozoites had already started transforming into the LS, as had wild-type parasites (Figure 2E). Staining with antibodies against UIS4 showed that the majority of AP2-L(2) sporozoites (,80%) had formed a PV membrane. These results indicate that the sporozoites of AP2-L(2) parasites retain their normal ability to migrate into the liver and productively invade hepatocytes. AP2-L is Necessary for Parasite Development in Hepatocytes The delay in patency, despite normal sporozoite traversal and invasion, suggests that AP2-L(2) parasites undergo defective LS development. We next investigated the development of the AP2- L(2) LS parasites by in vitro culturing using HepG2 cells. First, cultured LS parasites were immunofluorescently stained with an anti-csp antibody at 48 hpi. At this time, the number of LS parasites formed in HepG2 cells was similar between wild-type and AP2-L(2) parasites (Figure 3A), but the AP2-L(2) LS parasites were obviously smaller than the wild-type LS parasites (data not shown, see also Figure 3C). We therefore checked the development of LS parasites at different time points (24 hpi, 36 hpi, and 48 hpi, Figure 3B and Figure 3C). At 24 hpi, the difference in size between the wild-type and AP2-L(2) parasites was still subtle, but it became clear at 36 hpi. After 36 hpi, the sizes of the AP2-L(2) LS parasites scarcely increased, whereas the wild-type parasites continued to grow until 48 hpi. In accordance with the growth of the LS parasites, arrest of nuclear division became clear at 36 hpi in the AP2-L(2) parasites. The appearance of the nuclei remained the same, and no AP2-L(2) LS parasites in the cytomere stage were formed in the later stage (Figure 3D). Furthermore, the GFP signals of the AP2-L(2) parasites started to show heterogeneous distributions at 36 hpi, suggesting that degenerative changes had occurred (Figure 3C and 3D). These observations indicate that the development of AP2-L(2) parasites almost completely arrested at about 36 hpi, i.e., in the middle of the schizont stage, which caused a severe decrease in their ability to infect the liver (Table 1). Further, we compared parasite loads in the rat liver between wild-type and AP2-L(2) parasites (Figure 3E). After intravenous inoculation of salivary gland sporozoites, rats were dissected at 24 hpi or 48 hpi, and parasite loads in the liver were determined using real time reverse-transcription PCR (RT-PCR) as the ratio of the parasite 18S rrna to rat glyceraldehyde 3-phosphate dehydrogenase control. At 24 hpi, parasite loads in the rats inoculated with AP2-L(2) parasites were the same or lower than those in rats inoculated with the wild-type parasites. Subsequently, the parasite loads decreased by approximately sixfold in rats inoculated with AP2-L(2) parasites until 48 hpi, while an approximately 100-fold increase was observed in the rats inoculated with wild-type parasites. As a result, differences between the AP2-L(2) and wild-type parasite loads increased to more than 1000-fold at 48 hpi, coinciding well with values estimated from the delay in rat parasitemia (Table 1). These results clearly demonstrate that parasite development in the liver is hindered by the disruption of AP2-L and that decreased liver infection capacity in AP2-L(2) parasites can be attributed to their impaired LS development. AP2-L is Localized in the Nucleus of Erythrocytic Trophozoites, Sporozoites and LS Parasites The expression profile of AP2-L in the blood and mosquito stages was investigated using transgenic P. berghei parasites expressing green fluorescent protein (GFP)-fused AP2-L (AP2- L::GFP parasites) (Figure 4A). The fusion of GFP did not affect parasite proliferation through the life cycle, including the LS (Table S2). During the blood stages, GFP-fused AP2-L was observed in the nuclei of trophozoites but not in the ring or schizont forms or the gametocytes (Figure S2). In the mosquito stages, GFP-fused AP2-L was first observed in the nuclei of the oocyst sporozoites (10 days after an infective blood meal, data not shown), and even more strongly in the nucleus of the salivary gland sporozoites (Figure 4B). PLOS ONE 4 November 2012 Volume 7 Issue 11 e47557
5 Figure 3. LS development is arrested during the schizont stage in AP2-L(2) parasites. A. Sporozoites ( ) were inoculated into a culture of HepG2 cells and incubated at 37uC. The number of LS parasites was counted 48 hpi after staining with anti-csp antibody. The data are the mean 6 SEM of four measurements. B. The diameters of LS AP2-L(2) 1 and wild-type parasites were compared. When the parasites were not round, the average of the longest and the shortest axis was used as the diameter. The horizontal axis indicates the number of hours post-sporozoite inoculation. The data are the mean 6 SEM of 200 measurements. C. Fluorescence images of AP2-L(2) 1 and wild-type (WT) LS parasites at 24 hpi and 48 hpi. The parasites were stained with an anti-csp antibody. The arrows indicate densely stained portions that were specifically observed in AP2- L(2) LS parasites. D. Representative image of wild-type and AP2L(2) 1 LS parasites at 53 hpi with nuclear staining by DAPI. The scale bars in C and D PLOS ONE 5 November 2012 Volume 7 Issue 11 e47557
6 represent 10 mm. E. Parasite burden in the rat liver was compared between the wild-type and AP2-L(2) parasites. Wild-type or AP2-L(2) salivary gland sporozoites were intravenously injected into rats, and the liver was dissected after perfusion at 24 hpi or 48 hpi. Parasite loads in the liver were determined by measuring P. berghei 18S rrna using RT-PCR. The rat glyceraldehyde 3-phosphate dehydrogenase gene was used as an internal control to normalize the mean cycle threshold value of the rat 18S rrna gene. Four rats were used for each condition. doi: /journal.pone g003 Expression of AP2-L in LS parasites was investigated by culturing in HepG2 cells. To identify LS parasites in the culture, we prepared parasites expressing mcherry (red fluorescent protein)-tagged AP2-L (AP2-L::mCherry parasites) from the P. berghei ANKA 507cl1 mutant line [15], which constitutively expresses GFP in the cytoplasm, essentially in the same manner as AP2- L::GFP parasites. Salivary gland sporozoites of these parasites exhibited normal infectivity in rats (Table S2). Expression profiles and the subcellular localization of AP2-L in the blood and mosquito stages of AP2-L::mCherry parasites were the same as in those of the AP2-L::GFP parasites (Figure S3). The expression of AP2-L in LS parasites was assessed at 12-h intervals after inoculation of AP2-L::mCherry sporozoites into a culture of HepG2 cells. mcherry-tagged AP2-L was visible in the nucleus beginning at 12 hpi and rapidly increased from 24 hpi to 36 hpi with the progress of nuclear division. However, fluorescent signals completely disappeared in the large sized LS parasites at 48 hpi (Figures 4B and S4), suggesting that AP2-L is rapidly eliminated from the LS parasites when parasites complete schizogony. The sudden and complete disappearance of AP2-L during this period indicates that other transcription factors begin to regulate gene expression in the LS parasites by replacing AP2-L. Sequential expression of the AP2 transcription factors may occur during LS, as has been reported for the asexual blood stages [8]. The expression of AP2-L in the blood-stage trophozoites suggests that some target genes are commonly expressed between Figure 4. The expression profile of AP2-L in P. berghei pre-erythrocytic stages. A. A schematic diagram showing the preparation of transgenic P. berghei expressing GFP-tagged AP2-L. The expression construct was introduced into the 39 portion of the endogenous AP2-L gene by double cross-over homologous recombination. The results of the Southern blot analysis of wild-type (WT) and transgenic AP2-L parasites (AP2-L ::GFP (2)) are shown on the left. B. Fluorescence images of a salivary gland sporozoite (SG; expressing GFP-tagged AP2-L, extreme left column) and LS parasites (expressing mcherry-tagged AP2-L, other columns). LS parasites were harvested at 12-h intervals following sporozoite inoculation. The LS parasites expressed GFP constitutively in the cytoplasm (upper images). The nuclei were stained with Hoechst (in sporozoites) or DAPI (in LS parasites). The scale bars represent 10 mm. doi: /journal.pone g004 PLOS ONE 6 November 2012 Volume 7 Issue 11 e47557
7 the asexual intra-erythrocytic stage and the LS. Expression of AP2- L in the asexual blood stages has also been reported for P. falciparum, in which the expression of AP2-L was observed in the ring forms by DNA microarray analysis [8]. However, it remains unclear why disruption of AP2-L had no effect on parasite proliferation in the blood stages, but severe impairment of schizogony occurred in the LS. Furthermore, the expression profile in the asexual blood stages was not identical to that in the LS; peak expression of AP2-L was observed during the schizont stage in the LS but during the trophozoite stage (i.e., before the schizont) in the blood stage. Therefore, we speculate that AP2-L is expressed in the intra-erythrocytic stage, but it may not function in this stage. Further investigation is necessary to elucidate the role of this transcription factor in the blood stage. Expression of AP2-L in Salivary Gland Sporozoites is Significantly Reduced in the SLARP(2) Parasites Several genes involved in early LS development, such as UIS3 and UIS4 [2,14], are transcribed in the sporozoite stage; however, their transcripts are silently stored until the subsequent liver stage. Sporozoite and LS asparagine-rich protein (SLARP) (also known as sporozoite asparagine-rich protein 1 [SAP1]) has been suggested to be a specific regulator of these genes [16,17] because expression of these genes is significantly reduced in the SLARP-depleted (SLARP(2)) salivary gland sporozoites. Aly et al. reported that the reduction occurred because their transcripts, which are stabilized in stress granules in the cytoplasm of the salivary gland sporozoite, are routed towards degeneration pathways in the SLARP(2) sporozoites [17,18]. We examined whether expression of AP2-L is also reduced in the SLARP(2) P. berghei salivary gland sporozoites using RT-PCR (Figures S5 and S6). Expression of AP2-L in the SLARP(2) salivary gland sporozoites was approximately 10 times lower than that in the wild-type parasites. This suggests that expression of AP2-L is controlled at the translational level. In contrast, as described above, analysis with the AP2-L::GFP parasites showed that GFP-tagged AP2-L was already visible in the nuclei of the salivary gland sporozoites (Figures 4B and S3). This result indicates that some of its transcripts are translated during the sporozoite stage. Translation of AP2-L in the sporozoite stage implies that the AP2-L protein is directly carried to the LS and induces its target genes before the transcripts of AP2-L are translated in the LS. The Expression of Several Genes is Decreased in the Early LS of AP2-L(2) Parasites Next, we compared the gene expression in the LS between wildtype and AP2-L(2) parasites by DNA microarray analysis. For this study, a DNA microarray for P. berghei was newly designed based on the genome annotation published in PlasmoDB ( plasmodb.org/plasmo/). This microarray contained probes for all annotated P. berghei genes (,4800 genes). To minimize the secondary effects of AP2-L disruption on gene expression and obtain direct targets of this transcription factor, samples were obtained from LS parasites cultured for 24 hours in HepG2 cells, when only small differences in the sizes were observed between wild-type and AP2-L(2) LS parasites (see Figure 3B and 3C). Sporozoites ( ) were inoculated in each culture well of an 8- well chamber slide, and RNA was extracted from all cells in the well, that is, both uninfected and infected HepG2 cells. Because the samples used for the analysis should have contained only a small amount of parasite RNA, the obtained signals were expected to be weak. To increase the accuracy of the analysis, ten and seven biologically independent samples were used for wild-type and AP2- L(2) parasites, respectively, and genes whose expression decreased more than three-fold in AP2-L(2) parasites for all independent probes (four independent probes were attributed to each gene in this array) were selected as candidate target genes. A total of 11 genes were identified to meet these criteria (Table 2). These genes included five genes that are reportedly expressed in the LS (UIS2, UIS3, UIS4, EXP1 and liver-specific protein 1 (LISP1)). Six of the 11 genes have a putative signal sequence (PBANKA_100300, PBANKA_144170, UIS3, UIS4, EXP1 and LISP1), and four encode PV membrane proteins (UIS3, UIS4, EXP1 and LISP1). This analysis also showed that the expression of the heat shock protein 70 (HSP70) genes (PBANKA_081890, PBANKA_091440, and PBANKA_135720) scarcely changed (within 25% differences) between wild-type and AP2-L(2) parasites. We carried out RT- PCR analysis of UIS3, UIS4, and LISP1 with PBANKA_ as an internal control. As shown in Figure S7, the expression of these three genes was significantly reduced in the AP2-L(2) parasites, confirming the results of the microarray analysis. Because some of these genes (UIS2, UIS3, UIS4) are also expressed in salivary gland sporozoites, the expression of these genes may have been decreased prior to liver infection in the AP2- L(2) parasites. Therefore, we compared the gene expression between wild-type and AP2-L(2) parasites in the salivary gland sporozoites by DNA microarray analysis. The DNA microarray used in this analysis contained three independent probes for each P. berghei gene. Five biologically independent samples were prepared for each genotype, and the genes whose expression decreased more than threefold in AP2-L(2) parasites in all independent probes were selected as reduced genes. AP2-L was the only gene selected to meet these criteria among all P. berghei genes. Differences in the expression levels of five genes in Table 2 were within 50% between the two groups (UIS2, UIS3, UIS4, PBANKA_041240, and PBANKA_020830). The other six genes in Table 2 were filtered out as weakly expressed genes during the analysis process, indicating that they are not expressed in the sporozoite stage but are induced after liver infection. These results strongly suggest that AP2-L does not function in the sporozoite stage. We further examined the expression of UIS3 and UIS4 in salivary gland sporozoites by using RT-PCR. The CSP gene was used as an internal control to normalize the PCR data (the difference in the expression level of CSP in the DNA microarray analysis was less than 20% between wild-type and AP2-L(2) parasites). As shown in Figure S8, no significant differences were observed between the wild-type and AP2-L(2) salivary gland sporozoites. The normal expression of UIS3 and UIS4 in AP2-L(2) sporozoites is consistent with the finding that they were surrounded by normal PV membranes shortly after liver invasion (Figure 2E). We next searched for the binding motif of AP2-L in the upstream regions of the candidate target genes using the same statistical method used in our previous papers [11,12]. However, we could not obtain conclusive results from the analysis (data not shown). One possible reason for this failure is that the DNA binding domains of AP2-L recognize two independent sequences on the promoter simultaneously. Campbell et al. reported that neither single AP2 domain of P. falciparum AP2-L showed clear DNA binding properties [19]. This finding might indicate that these AP2 domains do not have sufficient binding affinities for DNA when separated from each other and thus a combination of two independent sequences is essential for determining binding specificity. Statistical analyses may not be suitable for the identification of such a complicated motif, and step-by-step approaches might be needed as the first step to address this issue, PLOS ONE 7 November 2012 Volume 7 Issue 11 e47557
8 Table 2. Genes whose expression is decreased in AP2-L(2) parasites. gene ID fold decrease Functional annotation signal peptide PBANKA_ sequestrin + PBANKA_ liver specific protein 1 (LISP1 ) + PBANKA_ zinc finger protein, putative 2 PBANKA_ UIS4 + PBANKA_ adenylate kinase 2 PBANKA_ conserved Plasmodium protein 2 PBANKA_ UIS2, serine/threonine protein 2 phosphatase PBANKA_ conserved Plasmodium protein + PBANKA_ EXP1 + PBANKA_ transporter, putative 2 PBANKA_ UIS3 + DNA microarray analysis was performed using LS cultures at 24 hpi. Genes whose expression was decreased at least three-fold by depletion of AP2-L are listed. Each gene is indicated by its PlasmoDB gene ID. The functional annotations are essentially in line with PlasmoDB. Signal sequences were predicted with SignalP ( cbs.dtu.dk/services/signalp/). doi: /journal.pone t002 for example, to identify the location of each binding sequence by promoter assays using various deletion constructs. Considering the results of our previous studies in other AP2- family transcription factors, AP2-L could have more target genes in LS parasites. We suspected that the 11 genes identified by the microarray analysis might cover only a portion of the target genes of AP2-L. However, the fact that half encode proteins with a signal sequence, and particularly that four encode PV membrane proteins, suggests that the target genes of AP2-L are strongly involved in host-parasite interactions in the LS. During schizont formation, the PV is rapidly enlarged because of the growth of the parasite. The PV membrane and PV membrane proteins support this growth by providing the parasite with an environment suitable for proliferation, for example, by taking up nutrients from their surroundings. Considering the importance of PV membrane proteins in this period, the reduced expression of these genes might be one of the causes of parasite growth arrest in the middle of the schizont stage. In addition to the PV membrane protein genes, the expression of two genes encoding putative secretory proteins (PBANKA_ and PBANKA_144170) was reduced in AP2- L(2) parasites. Because of their structures, these two proteins could be secreted through the plasma membrane of the parasite into the vacuolar space and then transported through the PV membrane to the cytoplasm of the hepatocyte, where they could modulate the functions of host molecules. If these proteins are transported to the host cell, they might be presented to the host immune system as antigens and serve as targets for a protective immune response. Further investigation of AP2-L target genes might provide candidate LS antigens and clues to understanding host-parasite interactions during malarial infection of hepatocytes. Materials and Methods Ethics Statement This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Mie University (Permit Number:23 29). All efforts were made to minimize animal suffering during the course of these studies. Parasite Preparations Anopheles stephensi mosquitoes were infected by feeding on mice (6- to 10-wk-old female BALB/c mice, Japan SLC, Inc., Hamamatsu, Japan) infected with the P. berghei ANKA strain and maintained at 20uC in a fumed chamber. Oocyst sporozoites and salivary gland sporozoites were harvested from the mosquitoes 14 days and 24 days after their infective blood meal, respectively. LS parasites were cultured as described previously [20]. Briefly, salivary gland sporozoites were added to HepG2 cells (Riken Cell Bank, Tsukuba, Japan) seeded on an 8-well chamber slide, incubated for 30 min at 37uC for invasion, and then cultured at 37uC in5%co 2 in medium containing 3 mg ml 21 glucose. The medium was changed twice per day. Preparation of Antibodies Against UIS4 Antibodies against UIS4 were prepared as follows. A DNA fragment encoding amino acid residues of P. berghei UIS4 was amplified by PCR by the primer pair 59-CGGGATCC- GAAGTTCGAGAAAAATTTAGAATT-39 and 59- CGGCTCGAGTCATTCGTCCTTATGTTCATCTGTAAA- 39 using P. berghei genomic DNA as a template. This fragment was digested with Bam HI and Xho I and then subcloned into the Escherichia coli expression vector pgex 6P-1 (Amersham Pharmacia Biotech, Piscataway, NJ). Recombinant protein was produced in the BL21 E. coli strain as a glutathione S-transferase (GST) fusion protein, purified with a glutathione column, and used to immunize rabbits. Antibodies against GST were removed from the antisera by incubation with GST-conjugated Sepharose. Specific antibodies were affinity-purified using an NHS-activated High Trap column (Amersham Pharmacia Biotech) linked with the GST-fused recombinant protein. Targeted Disruption of P. berghei Genes Targeted disruption of P. berghei genes was carried out in essentially the same procedure as described previously [12]. The PCR primer pairs used to prepare the targeting constructs and the Southern hybridization probes are listed in Table S3. PLOS ONE 8 November 2012 Volume 7 Issue 11 e47557
9 Preparation of Fluorescent Protein-tagged AP2-L expressing Parasites GFP-tagged AP2-L-expressing parasites were prepared as described previously [12]. To prepare the targeted insertion construct (Figure 3A), a DNA fragment containing the 39 part of the AP2-L coding region was amplified by PCR using genomic DNA as a template and inserted into the XhoI/NheI site of the construct in frame with the GFP gene. The downstream region of the AP2-L gene was also amplified by PCR and inserted into the BamHI/NotI site. The plasmids containing the construct were digested with XhoI and NotI and then used for transfection. The PCR primer pairs used to prepare the construct and the Southern hybridization probe are listed in Table S3. To monitor AP2-L expression in the LS, mcherry-tagged AP2-L-expressing parasites were prepared as described above but with the targeted insertion construct containing the mcherry gene and 507cl1 parasites, which constitutively express GFP. Sporozoite Invasion Assays For the in vitro assays, salivary gland sporozoites were harvested 24 days after an infective blood meal. Cell-traversal assays were performed essentially as described [20]. Double staining assays were performed using sporozoites per well essentially as described [13], except that the chamber slides were centrifuged at 140 6g for 2 min at RT after sporozoite inoculation. For PV formation assay, salivary gland sporozoites were inoculated into a culture of HepG2 cells seeded on an 8-well chamber slide. After incubation for 5 or 7 h at 37uC, the slide was fixed in 4% paraformaldehyde for 10 min followed by methanol for 10 min. The newly formed PVM surrounding the sporozoites was stained with a primary antibody against UIS4 (rabbit polyclonal antibody), followed by a secondary antibody conjugated to FITC. After washing with PBS and fixation in methanol for 10 min, sporozoites were stained with a primary antibody against CSP (rat polyclonal antibody), followed by a secondary antibody conjugated with Cy3. To assess liver infection ability, the harvested salivary gland sporozoites were injected into a rat tail vein, and parasitemia was examined using Giemsa staining of a blood smear. LS Parasite Culturing and Immunocytochemistry LS parasites were cultured using HepG2 cells as described above. The cultures were harvested at different time points and fixed in 4% paraformaldehyde for 10 min and then in methanol for 10 min. LS parasites were stained with a primary antibody against the CSP repeat region [20] followed by a secondary antibody conjugated with FITC. Nuclei were stained by the addition of 4,6 diamidino-2-phenylindol (DAPI, 0.02 mg ml 21 final concentration) to the secondary antibody solution. At each time point, the largest and smallest diameter of each LS parasite was measured using Aqua Cosmos software (Hamamatsu Photonics, Hamamatsu, Japan), and the average was used to compare the development of LS parasites. DNA Microarray Custom microarrays were designed by e-array, a web-based application ( based on the gene annotation of the P. berghei genome published in PlasmoDB ( An array in the format of four wells per slide, with four probes attributed to each gene, was used for the analysis of the LS parasites. An array in the format of eight wells per slide, with three probes attributed to each gene, was used for the analysis of the sporozoite stages. Total RNA extraction and the subsequent microarray analyses were performed as described previously [12]. For the analysis of gene expression in the LS parasites, ten and seven biologically independent samples were prepared from wild-type and AP2-L(2) parasites, respectively. For the analysis of the sporozoite stages, five biologically independent samples were prepared from each genotype. The obtained data were analyzed with Genespring software (Agilent Technologies). Briefly, all expression data on a chip were normalized to the 60 th percentile of the measurements taken from all values on that chip. After normalization, the expression values for each probe set were filtered for raw signal (.100). The genes satisfying this filter were further analyzed for differences between wild-type and AP2-L(2) parasites using ANOVA (analysis of variance) with Benjamini- Hochberg multiple testing correction (P#0.05). Finally, the genes whose expression decreased least threefold in all attributed probes as compared with wild-type parasites were evaluated as reduced genes. From the selected genes, those belonging to multigene families, such as the bir gene family, were excluded. All microarray data were submitted to the Gene Expression Omnibus (GEO) under Accession No. GSE RT-PCR Fifty nanograms of total RNA was treated with DNase and reverse-transcribed with random primers using a PrimeScriptH RT reagent Kit with gdna Eraser (Takara Bio Inc., Otsu, Japan) according to the manufacturer s protocol. Quantitative PCR was performed as described previously [12]. The PCR primer pairs are listed in Table S3. Computational Analysis The AP2-L binding motifs in the upstream regions of the candidate target genes were investigated with essentially the same statistical methods as reported in our previous papers [11,12]. In brief, the number of all possible five- to eight-base sequences was counted in the upstream regions of all P. berghei genes. Student s t- test was then carried out for each sequence between candidate target genes (11 genes) and all other P. berghei genes, and the sequences that appeared in the former group in significantly high frequencies were investigated. Supporting Information Figure S1 An amino acid sequence near the carboxyl-terminal end of P. berghei AP2-L is conserved in other apicomplexan AP2- family proteins. A. Sequence comparison between P. berghei AP2-L and its paralogs. B. Sequence comparison between P. berghei AP2-L and related proteins in other apicomplexan parasites. Figure S2 Expression profile of AP2-L in intra-erythrocytic stages. The expression of GFP-tagged AP2-L in AP2-L::GFP parasites was observed with fluorescence microscopy. Nuclei were stained with Hoechst stain. The scale bars represent 10 mm. Figure S3 AP2-L is localized in the nucleus of trophozoites and salivary gland sporozoites. The expression of mcherry-tagged AP2-L in AP2-L::mCherry parasites was observed with fluorescence microscopy. Nuclei were stained with Hoechst stain. A trophozoite is indicated by an arrowhead. A schizont is observed in the upper left corner. The scale bar represents 10 mm. Figure S4 AP2-L disappears in mature liver schizonts. AP2- L::mCherry LS parasites were cultured for 48 h in HepG2 cells. After fixation with 4% paraformaldehyde for 10 min and nuclear PLOS ONE 9 November 2012 Volume 7 Issue 11 e47557
10 References 1. Hoffman SL, Goh LML, Luke TC, Schneider I, Le TP, et al. (2002) Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. Journal of Infectious Diseases 185: Mueller AK, Labaied M, Kappe SH, Matuschewski K (2005) Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433: Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, et al. (2011) Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9: Tarun AS, Dumpit RF, Camargo N, Labaied M, Liu P, et al. (2007) Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J Infect Dis 196: White KL, Snyder HL, Krzych U (1996) MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol 156: Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, et al. (1987) Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330: Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, et al. (2008) A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A 105: Balaji S, Babu MM, Iyer LM, Aravind L (2005) Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res 33: Painter HJ, Campbell TL, Llinas M (2011) The Apicomplexan AP2 family: integral factors regulating Plasmodium development. Mol Biochem Parasitol 176: Iyer LM, Anantharaman V, Wolf MY, Aravind L (2008) Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int J Parasitol 38: staining with DAPI, the parasites were examined by fluorescence microscopy. AP2-L was observed only in immature, small LS parasites (indicated by arrows). The scale bar represents 10 mm. Figure S5 Schematic diagram of the targeted knockout of P. berghei SLARP by double cross-over homologous recombination. Targeted knockout of the SLARP gene was performed by essentially the same procedure as for the AP2-L gene. The result of a Southern blot analysis of wild-type (WT) and a knockout population (SLARP(2)21) is shown at the bottom of the figure. Figure S6 AP2-L expression is decreased in SLARP(2) salivary gland sporozoites. RT-PCR analysis of AP2-L in wild-type (WT) and SLARP(2) salivary gland sporozoites 24 days after an infective blood meal. The data (mean 6 SEM of four measurements) were normalized to the transcript levels of CSP. Figure S7 Expression of UIS3, UIS4 and LISP1 in AP2-L(2) LS parasites. RT-PCR analysis of UIS3, UIS4 and LISP1 was performed in cultured wild-type (WT) and SLARP(2) LS parasites at 24 hpi. The data (mean 6 SEM of three measurements) were normalized to the transcript levels of the HSP70 gene (PBANKA_091440). Figure S8 Expression of UIS3 and UIS4 in AP2-L(2) salivary gland sporozoites. RT-PCR analysis of UIS3 and UIS4 in wildtype (WT) and AP2-L(2) salivary gland sporozoites 24 days after an infective blood meal. The data (mean 6 SEM of four measurements) were normalized to the transcript levels of CSP. Table S1 AP2-L(2) parasites infect mosquitoes normally. a. Twenty mosquitoes were dissected 14 days after an infective blood meal, and the number of parasites per mosquito was calculated (standard error in parentheses). b. Twenty mosquitoes were dissected 24 days after an infective blood meal, and the number of parasites per mosquito was calculated (standard error in parentheses). (DOC) Table S2 The fusion of GFP or mcherry to AP2-L does not affect the infective ability of P. berghei parasites. a. Salivary gland sporozoites were collected 24 d after an infective blood meal. b. The average prepatent time of infected rats. Parasitemia was checked at one-day intervals starting two days after the challenge. (DOC) Table S3 (DOC) Primers used in this study. Author Contributions Conceived and designed the experiments: SI MY. Performed the experiments: SI IK TK MY. Analyzed the data: SI MY. Wrote the paper: MY. 11. Yuda M, Iwanaga S, Shigenobu S, Kato T, Kaneko I (2010) Transcription factor AP2-Sp and its target genes in malarial sporozoites. Mol Microbiol 75: Yuda M, Iwanaga S, Shigenobu S, Mair GR, Janse CJ, et al. (2009) Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol Microbiol 71: Ishino T, Chinzei Y, Yuda M (2005) Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol 58: Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, et al. (2005) Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci U S A 102: Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, et al. (2006) High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145: Silvie O, Goetz K, Matuschewski K (2008) A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog 4: e Aly AS, Mikolajczak SA, Rivera HS, Camargo N, Jacobs-Lorena V, et al. (2008) Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol Microbiol 69: Aly AS, Lindner SE, MacKellar DC, Peng X, Kappe SH (2011) SAP1 is a critical post-transcriptional regulator of infectivity in malaria parasite sporozoite stages. Mol Microbiol 79: Campbell TL, De Silva EK, Olszewski KL, Elemento O, Llinas M (2010) Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog 6: e Ishino T, Yano K, Chinzei Y, Yuda M (2004) Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol 2: E4. PLOS ONE 10 November 2012 Volume 7 Issue 11 e47557
Arrested oocyst maturation in Plasmodium parasites. lacking type II NADH:ubiquinone dehydrogenase
 Supplemental Information for: Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase Katja E. Boysen and Kai Matuschewski Contents: - Supplemental Movies 1 and
Supplemental Information for: Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase Katja E. Boysen and Kai Matuschewski Contents: - Supplemental Movies 1 and
PLASMODIUM MODULE 39.1 INTRODUCTION OBJECTIVES 39.2 MALARIAL PARASITE. Notes
 Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis
 Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis A. Reagents: 1. DMEM or RPMI DMEM (4.5g/L glucose) RPMI 1640 Cellgro #MT-10-017-CM Cellgro #MT-10-040-CM 2. Giemsa
Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis A. Reagents: 1. DMEM or RPMI DMEM (4.5g/L glucose) RPMI 1640 Cellgro #MT-10-017-CM Cellgro #MT-10-040-CM 2. Giemsa
CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts
 Blackwell Publishing LtdOxford, UKMMIMolecular Microbiology0950-382X 2005 The Authors; Journal compilation 2005 Blackwell Publishing Ltd? 200559513691379Original ArticleA protein that mediates malarial
Blackwell Publishing LtdOxford, UKMMIMolecular Microbiology0950-382X 2005 The Authors; Journal compilation 2005 Blackwell Publishing Ltd? 200559513691379Original ArticleA protein that mediates malarial
PRINCIPAL INVESTIGATOR: Dr. Jetsumon (Sattabongkot) Prachumsri
 AD (Leave blank) Award Number: W81XWH-07-2-0090 TITLE: Proteomic Study of Human Malaria Parasite Plasmodium Vivax Liver Stages for Development of Vaccines and Drugs PRINCIPAL INVESTIGATOR: Dr. Jetsumon
AD (Leave blank) Award Number: W81XWH-07-2-0090 TITLE: Proteomic Study of Human Malaria Parasite Plasmodium Vivax Liver Stages for Development of Vaccines and Drugs PRINCIPAL INVESTIGATOR: Dr. Jetsumon
Plasmodium yoelii Sporozoites with Simultaneous Deletion of P52 and P36 Are Completely Attenuated and Confer Sterile Immunity against Infection
 INFECTION AND IMMUNITY, Aug. 2007, p. 3758 3768 Vol. 75, No. 8 0019-9567/07/$08.00 0 doi:10.1128/iai.00225-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Plasmodium yoelii Sporozoites
INFECTION AND IMMUNITY, Aug. 2007, p. 3758 3768 Vol. 75, No. 8 0019-9567/07/$08.00 0 doi:10.1128/iai.00225-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Plasmodium yoelii Sporozoites
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/70169
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/70169
A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development
 A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development Christine Lehmann 1, Anna Heitmann 1, Satish Mishra 2, Paul-Christian Burda 3, Mirko Singer 4, Monica Prado
A Cysteine Protease Inhibitor of Plasmodium berghei Is Essential for Exo-erythrocytic Development Christine Lehmann 1, Anna Heitmann 1, Satish Mishra 2, Paul-Christian Burda 3, Mirko Singer 4, Monica Prado
ACCEPTED. Parasitology Unit, Max Planck Institute for Infection Biology, Berlin, Germany
 EC Accepts, published online ahead of print on 30 January 2009 Eukaryotic Cell doi:10.1128/ec.00347-08 Copyright 2009, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
EC Accepts, published online ahead of print on 30 January 2009 Eukaryotic Cell doi:10.1128/ec.00347-08 Copyright 2009, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights
Epigenetic regulation of Plasmodium falciparum clonally. variant gene expression during development in An. gambiae
 Epigenetic regulation of Plasmodium falciparum clonally variant gene expression during development in An. gambiae Elena Gómez-Díaz, Rakiswendé S. Yerbanga, Thierry Lefèvre, Anna Cohuet, M. Jordan Rowley,
Epigenetic regulation of Plasmodium falciparum clonally variant gene expression during development in An. gambiae Elena Gómez-Díaz, Rakiswendé S. Yerbanga, Thierry Lefèvre, Anna Cohuet, M. Jordan Rowley,
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign
 A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
Malaria. This sheet is from both sections recording and includes all slides and diagrams.
 Malaria This sheet is from both sections recording and includes all slides and diagrams. Malaria is caused by protozoa family called plasmodium (Genus) mainly affect blood system specially RBCs and each
Malaria This sheet is from both sections recording and includes all slides and diagrams. Malaria is caused by protozoa family called plasmodium (Genus) mainly affect blood system specially RBCs and each
Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland sporozoites
 https://doi.org/10.1186/s12936-018-2431-1 Malaria Journal RESEARCH Open Access Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland
https://doi.org/10.1186/s12936-018-2431-1 Malaria Journal RESEARCH Open Access Chimeric Plasmodium falciparum parasites expressing Plasmodium vivax circumsporozoite protein fail to produce salivary gland
Parasitology Departement Medical Faculty of USU
 Malaria Mechanism of infection Parasitology Departement Medical Faculty of USU Introduction Malaria parasites Phylum Order Suborder Family Genus Species : : Apicomplexa : Eucoccidiida : Haemosporida :
Malaria Mechanism of infection Parasitology Departement Medical Faculty of USU Introduction Malaria parasites Phylum Order Suborder Family Genus Species : : Apicomplexa : Eucoccidiida : Haemosporida :
A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S.
 VI. Malaria A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S. because they were resistant to malaria & other diseases 3. Many
VI. Malaria A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S. because they were resistant to malaria & other diseases 3. Many
A n estimated 3.3 billion people were at risk of malaria infection in There is as of yet no licensed
 OPEN SUBJECT AREAS: PARASITOLOGY MOLECULAR BIOLOGY Received 27 March 2014 Accepted 23 June 2014 Published 11 July 2014 Correspondence and requests for materials should be addressed to A.S.I.A. (aaly@tulane.
OPEN SUBJECT AREAS: PARASITOLOGY MOLECULAR BIOLOGY Received 27 March 2014 Accepted 23 June 2014 Published 11 July 2014 Correspondence and requests for materials should be addressed to A.S.I.A. (aaly@tulane.
BIO Parasitology Spring 2009
 BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 10 Malaria-Life Cycle a. Micro and macrogametocytes in mosquito stomach. b. Ookinete
BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 10 Malaria-Life Cycle a. Micro and macrogametocytes in mosquito stomach. b. Ookinete
Gliding Motility Assay for P. berghei Sporozoites
 Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding
 Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding Ashley M. Vaughan, 1 Ahmed S.I. Aly, 1 and Stefan H.I. Kappe 1,2, * 1 Seattle Biomedical Research Institute, Seattle, WA 98109, USA
Malaria Parasite Pre-Erythrocytic Stage Infection: Gliding and Hiding Ashley M. Vaughan, 1 Ahmed S.I. Aly, 1 and Stefan H.I. Kappe 1,2, * 1 Seattle Biomedical Research Institute, Seattle, WA 98109, USA
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5870/1679/dc1 Supporting Online Material for Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes Chung-hui Yang, Priyanka
www.sciencemag.org/cgi/content/full/319/5870/1679/dc1 Supporting Online Material for Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes Chung-hui Yang, Priyanka
Understanding Epidemics Section 3: Malaria & Modelling
 Understanding Epidemics Section 3: Malaria & Modelling PART B: Biology Contents: Vector and parasite Biology of the malaria parasite Biology of the anopheles mosquito life cycle Vector and parasite Malaria
Understanding Epidemics Section 3: Malaria & Modelling PART B: Biology Contents: Vector and parasite Biology of the malaria parasite Biology of the anopheles mosquito life cycle Vector and parasite Malaria
Blood protozoan: Plasmodium
 Blood protozoan: Plasmodium Dr. Hala Al Daghistani The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans: four species are associated The Plasmodium spp.
Blood protozoan: Plasmodium Dr. Hala Al Daghistani The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans: four species are associated The Plasmodium spp.
Motility precedes egress of malaria parasites from oocysts
 RESEARCH ARTICLE Motility precedes egress of malaria parasites from oocysts Dennis Klug*, Friedrich Frischknecht* Integrative Parasitology, Center for Infectious Diseases, Heidelberg University Medical
RESEARCH ARTICLE Motility precedes egress of malaria parasites from oocysts Dennis Klug*, Friedrich Frischknecht* Integrative Parasitology, Center for Infectious Diseases, Heidelberg University Medical
Quantitative Dynamics of Plasmodium yoelii Sporozoite Transmission by Infected Anopheline Mosquitoes
 INFECTION AND IMMUNITY, July 2005, p. 4363 4369 Vol. 73, No. 7 0019-9567/05/$08.00 0 doi:10.1128/iai.73.7.4363 4369.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Quantitative
INFECTION AND IMMUNITY, July 2005, p. 4363 4369 Vol. 73, No. 7 0019-9567/05/$08.00 0 doi:10.1128/iai.73.7.4363 4369.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Quantitative
Malaria parasites of rodents of the Congo (Brazzaville) :
 Annales de Parasitologie (Paris), 1976, t. 51, n 6, pp. 637 à 646 Malaria parasites of rodents of the Congo (Brazzaville) : Plasmodium cbabaudi adami subsp. nov. and Plasmodium vinckei lentum Landau, Michel,
Annales de Parasitologie (Paris), 1976, t. 51, n 6, pp. 637 à 646 Malaria parasites of rodents of the Congo (Brazzaville) : Plasmodium cbabaudi adami subsp. nov. and Plasmodium vinckei lentum Landau, Michel,
Malaria in the Mosquito Dr. Peter Billingsley
 Malaria in the Mosquito Senior Director Quality Systems and Entomology Research Sanaria Inc. Rockville MD. 1 Malaria: one of the world s foremost killers Every year 1 million children die of malaria 250
Malaria in the Mosquito Senior Director Quality Systems and Entomology Research Sanaria Inc. Rockville MD. 1 Malaria: one of the world s foremost killers Every year 1 million children die of malaria 250
Blood protozoan: Plasmodium
 Blood protozoan: Plasmodium The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans:four species are associated The Plasmodium spp. life cycle can be divided
Blood protozoan: Plasmodium The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans:four species are associated The Plasmodium spp. life cycle can be divided
alaria Parasite Bank Collection sites of P. falciparum isolates PARASITE BIOLOGY
 M alaria Parasite Bank established in 1992 is a supporting unit for research activities on different aspects of malaria. The main objective of establishing this facility is to strengthen researches at
M alaria Parasite Bank established in 1992 is a supporting unit for research activities on different aspects of malaria. The main objective of establishing this facility is to strengthen researches at
Developmental Biology of Sporozoite-Host. Malaria: Implications for Vaccine Design. Javier E. Garcia, Alvaro Puentes and Manuel E.
 Developmental Biology of Sporozoite-Host Interactions in Plasmodium falciparum Malaria: Implications for Vaccine Design Javier E. Garcia, Alvaro Puentes and Manuel E. Patarroyo Clin. Microbiol. Rev. 2006,
Developmental Biology of Sporozoite-Host Interactions in Plasmodium falciparum Malaria: Implications for Vaccine Design Javier E. Garcia, Alvaro Puentes and Manuel E. Patarroyo Clin. Microbiol. Rev. 2006,
The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands
 The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands Fabian E. Saenz 1,2, Bharath Balu 1, Jonah Smith 2, Sarita R. Mendonca 1,2, John H. Adams
The Transmembrane Isoform of Plasmodium falciparum MAEBL Is Essential for the Invasion of Anopheles Salivary Glands Fabian E. Saenz 1,2, Bharath Balu 1, Jonah Smith 2, Sarita R. Mendonca 1,2, John H. Adams
INVESTIGATING THE MOTILITY OF PLASMODIUM
 INVESTIGATING THE MOTILITY OF PLASMODIUM by Natasha Vartak A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Science Baltimore, Maryland April,
INVESTIGATING THE MOTILITY OF PLASMODIUM by Natasha Vartak A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Science Baltimore, Maryland April,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12234 Supplementary Figure 1. Embryonic naked mole-rat fibroblasts do not undergo ECI. Embryonic naked mole-rat fibroblasts ( EF) were isolated from eight mid-gestation embryos. All the
doi:10.1038/nature12234 Supplementary Figure 1. Embryonic naked mole-rat fibroblasts do not undergo ECI. Embryonic naked mole-rat fibroblasts ( EF) were isolated from eight mid-gestation embryos. All the
Downloaded from:
 Al-Khattaf, FS; Tremp, AZ; El-Houderi, A; Dessens, JT (2016) The Plasmodium alveolin IMC1a is stabilised by its terminal cysteine motifs and facilitates sporozoite morphogenesis and infectivity in a dosedependent
Al-Khattaf, FS; Tremp, AZ; El-Houderi, A; Dessens, JT (2016) The Plasmodium alveolin IMC1a is stabilised by its terminal cysteine motifs and facilitates sporozoite morphogenesis and infectivity in a dosedependent
The silent path to thousands of merozoites: the Plasmodium liver stage
 The silent path to thousands of merozoites: the Plasmodium liver stage Miguel Prudêncio*, Ana Rodriguez and Maria M. Mota* Abstract Plasmodium sporozoites are deposited in the skin of their vertebrate
The silent path to thousands of merozoites: the Plasmodium liver stage Miguel Prudêncio*, Ana Rodriguez and Maria M. Mota* Abstract Plasmodium sporozoites are deposited in the skin of their vertebrate
23 Plasmodium coatneyi Eyles, Fong, Warren, Guinn, Sandosham, and Wharton, 1962
 23 Plasmodium coatneyi Eyles, Fong, Warren, Guinn, Sandosham, and Wharton, 1962 IN the course of studies on simian malaria begun by the late Dr. Don Eyles in Malaya, he and his co-workers isolated a new
23 Plasmodium coatneyi Eyles, Fong, Warren, Guinn, Sandosham, and Wharton, 1962 IN the course of studies on simian malaria begun by the late Dr. Don Eyles in Malaya, he and his co-workers isolated a new
Malaria parasites: virulence and transmission as a basis for intervention strategies
 Malaria parasites: virulence and transmission as a basis for intervention strategies Matthias Marti Department of Immunology and Infectious Diseases Harvard School of Public Health The global malaria burden
Malaria parasites: virulence and transmission as a basis for intervention strategies Matthias Marti Department of Immunology and Infectious Diseases Harvard School of Public Health The global malaria burden
Developmentally Regulated!nfectivity of Malaria Sporozoites for Mosquito Salivary Glands and the Vertebrate Host
 Developmentally Regulated!nfectivity of Malaria Sporozoites for Mosquito Salivary Glands and the Vertebrate Host By Musa G. Touray, Alon Warburg, Andre Laughinghouse, Antoniana U. Krettli,* and Louis H.
Developmentally Regulated!nfectivity of Malaria Sporozoites for Mosquito Salivary Glands and the Vertebrate Host By Musa G. Touray, Alon Warburg, Andre Laughinghouse, Antoniana U. Krettli,* and Louis H.
Marissa Vignali*, Cate Speake* and Patrick E Duffy*
 Minireview Malaria sporozoite proteome leaves a trail Marissa Vignali*, Cate Speake* and Patrick E Duffy* Addresses: *Malaria Program, Seattle Biomedical Research Institute, Seattle, Washington 98109,
Minireview Malaria sporozoite proteome leaves a trail Marissa Vignali*, Cate Speake* and Patrick E Duffy* Addresses: *Malaria Program, Seattle Biomedical Research Institute, Seattle, Washington 98109,
Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity
 Molecular & Biochemical Parasitology 156 (2007) 32 40 Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity Kota Arun
Molecular & Biochemical Parasitology 156 (2007) 32 40 Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity Kota Arun
Novel ELISA method as exploratory tool to assess immunity induced by radiated attenuated sporozoites to decipher protective immunity
 DOI 10.1186/s12936-017-2129-9 Malaria Journal METHODOLOGY Open Access Novel ELISA method as exploratory tool to assess immunity induced by radiated attenuated sporozoites to decipher protective immunity
DOI 10.1186/s12936-017-2129-9 Malaria Journal METHODOLOGY Open Access Novel ELISA method as exploratory tool to assess immunity induced by radiated attenuated sporozoites to decipher protective immunity
Phylum:Apicomplexa Class:Sporozoa
 Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Diurnal variation in microfilaremia in cats experimentally infected with larvae of
 Hayasaki et al., Page 1 Short Communication Diurnal variation in microfilaremia in cats experimentally infected with larvae of Dirofilaria immitis M. Hayasaki a,*, J. Okajima b, K.H. Song a, K. Shiramizu
Hayasaki et al., Page 1 Short Communication Diurnal variation in microfilaremia in cats experimentally infected with larvae of Dirofilaria immitis M. Hayasaki a,*, J. Okajima b, K.H. Song a, K. Shiramizu
Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis
 Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis Brandy L. Salmon, Anna Oksman, and Daniel E. Goldberg* Howard Hughes Medical Institute, Departments
Malaria parasite exit from the host erythrocyte: A two-step process requiring extraerythrocytic proteolysis Brandy L. Salmon, Anna Oksman, and Daniel E. Goldberg* Howard Hughes Medical Institute, Departments
9 Parasitology 9 EXERCISE EQA. Objectives EXERCISE
 0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT PRAKASH SRINIVASAN
 THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT BY PRAKASH SRINIVASAN Submitted in partial fulfillment of the requirements For the degree of
THE ROLE OF RHOMBOID PROTEASES AND A OOCYST CAPSULE PROTEIN IN MALARIA PATHOGENESIS AND PARASITE DEVELOPMENT BY PRAKASH SRINIVASAN Submitted in partial fulfillment of the requirements For the degree of
Parasitology Amoebas. Sarcodina. Mastigophora
 Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
XXI. Malaria [MAL = bad; ARIA = air] (Chapter 9) 2008 A. Order Haemosporida, Family Plasmodiidae 1. Live in vertebrate tissues and blood 2.
![XXI. Malaria [MAL = bad; ARIA = air] (Chapter 9) 2008 A. Order Haemosporida, Family Plasmodiidae 1. Live in vertebrate tissues and blood 2. XXI. Malaria [MAL = bad; ARIA = air] (Chapter 9) 2008 A. Order Haemosporida, Family Plasmodiidae 1. Live in vertebrate tissues and blood 2.](/thumbs/80/81826026.jpg) XXI. Malaria [MAL = bad; ARIA = air] (Chapter 9) 2008 A. Order Haemosporida, Family Plasmodiidae 1. Live in vertebrate tissues and blood 2. SCHIZOGONY (asexual reproduction) in vertebrates 3. SPOROGONY
XXI. Malaria [MAL = bad; ARIA = air] (Chapter 9) 2008 A. Order Haemosporida, Family Plasmodiidae 1. Live in vertebrate tissues and blood 2. SCHIZOGONY (asexual reproduction) in vertebrates 3. SPOROGONY
Sporozoae: Plasmodium.
 Sporozoae: Plasmodium. Coccidian. Asexual division in Man (Schizogony), sexual division in the mosquito (sporogony). Plasmodium vivax > P. falciparum > P. malariae > P. ovale as a cause of malaria. P.
Sporozoae: Plasmodium. Coccidian. Asexual division in Man (Schizogony), sexual division in the mosquito (sporogony). Plasmodium vivax > P. falciparum > P. malariae > P. ovale as a cause of malaria. P.
Cyclic GMP Balance Is Critical for Malaria Parasite Transmission from the Mosquito to the Mammalian Host
 RESEARCH ARTICLE crossmark Cyclic GMP Balance Is Critical for Malaria Parasite Transmission from the Mosquito to the Mammalian Host Viswanathan Lakshmanan, a Matthew E. Fishbaugher, a Bob Morrison, a Michael
RESEARCH ARTICLE crossmark Cyclic GMP Balance Is Critical for Malaria Parasite Transmission from the Mosquito to the Mammalian Host Viswanathan Lakshmanan, a Matthew E. Fishbaugher, a Bob Morrison, a Michael
Evaluation of the hair growth and retention activity of two solutions on human hair explants
 activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, Massachusetts, USA, and 2
 scientific report scientificreport A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei Radha Rangarajan 1,AmyK.Bei 1, Deepa Jethwaney
scientific report scientificreport A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei Radha Rangarajan 1,AmyK.Bei 1, Deepa Jethwaney
CIRCUMSPOROZOITE PROTEINS OF HUMAN MALARIA PARASITES PLASMODIUM FALCIPARUM AND PLASMODIUM VIVA,F*
 CIRCUMSPOROZOITE PROTEINS OF HUMAN MALARIA PARASITES PLASMODIUM FALCIPARUM AND PLASMODIUM VIVA,F* BY ELIZABETH H. NARDIN, VICTOR NUSSENZWEIG, RUTH S. NUSSENZWEIG, WILLIAM E. COLLINS, K. TRANAKCHIT HARINASUTA,
CIRCUMSPOROZOITE PROTEINS OF HUMAN MALARIA PARASITES PLASMODIUM FALCIPARUM AND PLASMODIUM VIVA,F* BY ELIZABETH H. NARDIN, VICTOR NUSSENZWEIG, RUTH S. NUSSENZWEIG, WILLIAM E. COLLINS, K. TRANAKCHIT HARINASUTA,
Antibiotic Resistance in Bacteria
 Antibiotic Resistance in Bacteria Electron Micrograph of E. Coli Diseases Caused by Bacteria 1928 1 2 Fleming 3 discovers penicillin the first antibiotic. Some Clinically Important Antibiotics Antibiotic
Antibiotic Resistance in Bacteria Electron Micrograph of E. Coli Diseases Caused by Bacteria 1928 1 2 Fleming 3 discovers penicillin the first antibiotic. Some Clinically Important Antibiotics Antibiotic
PCR detection of Leptospira in. stray cat and
 PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
THE TRANSMISSION EFFICIENCY OF PLASMODIUM YOELII INFECTED MOSQUITOES
 THE TRANSMISSION EFFICIENCY OF PLASMODIUM YOELII INFECTED MOSQUITOES by Maya A. Aleshnick A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of
THE TRANSMISSION EFFICIENCY OF PLASMODIUM YOELII INFECTED MOSQUITOES by Maya A. Aleshnick A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of
Comparing DNA Sequences Cladogram Practice
 Name Period Assignment # See lecture questions 75, 122-123, 127, 137 Comparing DNA Sequences Cladogram Practice BACKGROUND Between 1990 2003, scientists working on an international research project known
Name Period Assignment # See lecture questions 75, 122-123, 127, 137 Comparing DNA Sequences Cladogram Practice BACKGROUND Between 1990 2003, scientists working on an international research project known
BioSci 110, Fall 08 Exam 2
 1. is the cell division process that results in the production of a. mitosis; 2 gametes b. meiosis; 2 gametes c. meiosis; 2 somatic (body) cells d. mitosis; 4 somatic (body) cells e. *meiosis; 4 gametes
1. is the cell division process that results in the production of a. mitosis; 2 gametes b. meiosis; 2 gametes c. meiosis; 2 somatic (body) cells d. mitosis; 4 somatic (body) cells e. *meiosis; 4 gametes
Plasmodium Pre-Erythrocytic Stages: Biology, Whole Parasite Vaccines and Transgenic Models
 American Journal of Immunology, 2012, 8 (3), 88-100 ISSN 1553-619X 2012 Science Publication doi:10.3844/ajisp.2012.88.100 Published Online 8 (3) 2012 (http://www.thescipub.com/aji.toc) Plasmodium Pre-Erythrocytic
American Journal of Immunology, 2012, 8 (3), 88-100 ISSN 1553-619X 2012 Science Publication doi:10.3844/ajisp.2012.88.100 Published Online 8 (3) 2012 (http://www.thescipub.com/aji.toc) Plasmodium Pre-Erythrocytic
Genes What are they good for? STUDENT HANDOUT. Module 4
 Genes What are they good for? Module 4 Genetics for Kids: Module 4 Genes What are they good for? Part I: Introduction Genes are sequences of DNA that contain instructions that determine the physical traits
Genes What are they good for? Module 4 Genetics for Kids: Module 4 Genes What are they good for? Part I: Introduction Genes are sequences of DNA that contain instructions that determine the physical traits
CONTRACTING ORGANIZATION: Rutgers, The State University of New Jersey Newark, NJ
 AWARD NUMBER: W81XWH-13-1-0429 TITLE: Using "Click Chemistry" to Identify Potential Drug Targets in Plasmodium PRINCIPAL INVESTIGATOR: Dr. Purnima Bhanot CONTRACTING ORGANIZATION: Rutgers, The State University
AWARD NUMBER: W81XWH-13-1-0429 TITLE: Using "Click Chemistry" to Identify Potential Drug Targets in Plasmodium PRINCIPAL INVESTIGATOR: Dr. Purnima Bhanot CONTRACTING ORGANIZATION: Rutgers, The State University
Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling
 Developmental Cell, Volume 29 Supplemental Information Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling Danyi Li, Rui
Developmental Cell, Volume 29 Supplemental Information Formation of Proximal and Anterior Limb Skeleton Requires Early Function of Irx3 and Irx5 and Is Negatively Regulated by Shh Signaling Danyi Li, Rui
Protozoan parasites of the genus Plasmodium are the causative
 Exploring the transcriptome of the malaria sporozoite stage Stefan H. I. Kappe*, Malcolm J. Gardner, Stuart M. Brown, Jessica Ross*, Kai Matuschewski*, Jose M. Ribeiro, John H. Adams, John Quackenbush,
Exploring the transcriptome of the malaria sporozoite stage Stefan H. I. Kappe*, Malcolm J. Gardner, Stuart M. Brown, Jessica Ross*, Kai Matuschewski*, Jose M. Ribeiro, John H. Adams, John Quackenbush,
TITLE: Anti-Inflammatory Cytokine Il-10 and Mammary Gland Development. CONTRACTING ORGANIZATION: University of Buffalo Buffalo, New York
 AD Award Number: W81XWH-06-1-0645 TITLE: Anti-Inflammatory Cytokine Il-10 and Mammary Gland Development PRINCIPAL INVESTIGATOR: Shiu-Ming Kuo CONTRACTING ORGANIZATION: University of Buffalo Buffalo, New
AD Award Number: W81XWH-06-1-0645 TITLE: Anti-Inflammatory Cytokine Il-10 and Mammary Gland Development PRINCIPAL INVESTIGATOR: Shiu-Ming Kuo CONTRACTING ORGANIZATION: University of Buffalo Buffalo, New
RICKETTSIA SPECIES AMONG TICKS IN AN AREA OF JAPAN ENDEMIC FOR JAPANESE SPOTTED FEVER
 RICKETTSIA SPECIES AMONG TICKS IN AN AREA OF JAPAN ENDEMIC FOR JAPANESE SPOTTED FEVER Makoto Kondo 1, Katsuhiko Ando 2, Keiichi Yamanaka 1 and Hitoshi Mizutani 1 1 Department of Dermatology, 2 Department
RICKETTSIA SPECIES AMONG TICKS IN AN AREA OF JAPAN ENDEMIC FOR JAPANESE SPOTTED FEVER Makoto Kondo 1, Katsuhiko Ando 2, Keiichi Yamanaka 1 and Hitoshi Mizutani 1 1 Department of Dermatology, 2 Department
Answer: Europeans risked death by disease when if they left the sea coast and entered the interior of the African continent.
 XXI Malaria [MAL = bad; ARIA = air] 2005 A. Order Haemosporida, Family Plasmodiidae 1. Live in vertebrate tissues and blood 2. SCHIZOGONY (asexual reproduction) in vertebrates 3. SPOROGONY (sexual reproduction)
XXI Malaria [MAL = bad; ARIA = air] 2005 A. Order Haemosporida, Family Plasmodiidae 1. Live in vertebrate tissues and blood 2. SCHIZOGONY (asexual reproduction) in vertebrates 3. SPOROGONY (sexual reproduction)
In the first half of the 20th century, Dr. Guido Fanconi published detailed clinical descriptions of several heritable human diseases.
 In the first half of the 20th century, Dr. Guido Fanconi published detailed clinical descriptions of several heritable human diseases. Two disease syndromes were named after him: Fanconi Anemia and Fanconi
In the first half of the 20th century, Dr. Guido Fanconi published detailed clinical descriptions of several heritable human diseases. Two disease syndromes were named after him: Fanconi Anemia and Fanconi
Malaria Sporozoites Traverse Host Cells within Transient Vacuoles
 Malaria Sporozoites Traverse Host Cells within Transient Vacuoles Veronica Risco-Castillo, Selma Topçu, Carine Marinach, Giulia Manzoni, Améliee. Bigorgne, Sylvie Briquet, Xavier Baudin, Maryse Lebrun,
Malaria Sporozoites Traverse Host Cells within Transient Vacuoles Veronica Risco-Castillo, Selma Topçu, Carine Marinach, Giulia Manzoni, Améliee. Bigorgne, Sylvie Briquet, Xavier Baudin, Maryse Lebrun,
THE ABUNDANCE AND INFECTION STATUS OF ANOPHELES MOSQUITOES IN LOUDOUN COUNTY, VIRGINIA
 THE ABUNDANCE AND INFECTION STATUS OF ANOPHELES MOSQUITOES IN LOUDOUN COUNTY, VIRGINIA Andrew Lima Clarke (Manassas, VA) Priya Krishnan ODU M.S. candidate (Richmond, VA) Objectives To determine: 1) the
THE ABUNDANCE AND INFECTION STATUS OF ANOPHELES MOSQUITOES IN LOUDOUN COUNTY, VIRGINIA Andrew Lima Clarke (Manassas, VA) Priya Krishnan ODU M.S. candidate (Richmond, VA) Objectives To determine: 1) the
Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST
 Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST INVESTIGATION 3 BIG IDEA 1 Lab Investigation 3: BLAST Pre-Lab Essential Question: How can bioinformatics be used as a tool to
Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST INVESTIGATION 3 BIG IDEA 1 Lab Investigation 3: BLAST Pre-Lab Essential Question: How can bioinformatics be used as a tool to
Overview. There are commonly found arrangements of bacteria based on their division. Spheres, Rods, Spirals
 Bacteria Overview Bacteria live almost everywhere. Most are microscopic ranging from 0.5 5 m in size, and unicellular. They have a variety of shapes when viewed under a microscope, most commonly: Spheres,
Bacteria Overview Bacteria live almost everywhere. Most are microscopic ranging from 0.5 5 m in size, and unicellular. They have a variety of shapes when viewed under a microscope, most commonly: Spheres,
Developmental expression of synthetic cis-regulatory systems composed of spatial control elements from two different genes
 Proc. Natl. Acad. Sci. USA Vol. 93, pp. 13849 13854, November 1996 Developmental Biology Developmental expression of synthetic cis-regulatory systems composed of spatial control elements from two different
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 13849 13854, November 1996 Developmental Biology Developmental expression of synthetic cis-regulatory systems composed of spatial control elements from two different
Plasmodium sporozoites acquire virulence and. immunogenicity during mosquito hemocoel transit
 IAI Accepts, published online ahead of print on 30 December 2013 Infect. Immun. doi:10.1128/iai.00758-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 Plasmodium sporozoites
IAI Accepts, published online ahead of print on 30 December 2013 Infect. Immun. doi:10.1128/iai.00758-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 Plasmodium sporozoites
Reverse genetics screen identifies six proteins important for malaria development in the mosquito
 Molecular Microbiology (2008) 70(1), 209220 doi:.1111/j.1365-2958.2008.06407.x First published online 27 August 2008 Reverse genetics screen identifies six proteins important for malaria development in
Molecular Microbiology (2008) 70(1), 209220 doi:.1111/j.1365-2958.2008.06407.x First published online 27 August 2008 Reverse genetics screen identifies six proteins important for malaria development in
Comparative Plasmodium gene overexpression reveals distinct perturbation of sporozoite transmission by profilin
 MBoC ARTICLE Comparative Plasmodium gene overexpression reveals distinct perturbation of sporozoite transmission by profilin Yuko Sato a,b, *, Marion Hliscs a,c, Josefine Dunst a,d, Christian Goosmann
MBoC ARTICLE Comparative Plasmodium gene overexpression reveals distinct perturbation of sporozoite transmission by profilin Yuko Sato a,b, *, Marion Hliscs a,c, Josefine Dunst a,d, Christian Goosmann
Malaria. Malaria is known to kill one child every 30 sec, 3000 children per day under the age of 5 years.
 Malaria Mal-air It is a world wide distribution disease acute or chronic characterized by fever,anemia & spleenomegaly occurs where anopheles mosquito are present & caused by genus plasmodium,which is
Malaria Mal-air It is a world wide distribution disease acute or chronic characterized by fever,anemia & spleenomegaly occurs where anopheles mosquito are present & caused by genus plasmodium,which is
EDUCATION AND PRODUCTION. Layer Performance of Four Strains of Leghorn Pullets Subjected to Various Rearing Programs
 EDUCATION AND PRODUCTION Layer Performance of Four Strains of Leghorn Pullets Subjected to Various Rearing Programs S. LEESON, L. CASTON, and J. D. SUMMERS Department of Animal and Poultry Science, University
EDUCATION AND PRODUCTION Layer Performance of Four Strains of Leghorn Pullets Subjected to Various Rearing Programs S. LEESON, L. CASTON, and J. D. SUMMERS Department of Animal and Poultry Science, University
Heartworm Disease in Dogs
 Kingsbrook Animal Hospital 5322 New Design Road, Frederick, MD, 21703 Phone: (301) 631-6900 Website: KingsbrookVet.com What causes heartworm disease? Heartworm Disease in Dogs Heartworm disease or dirofilariasis
Kingsbrook Animal Hospital 5322 New Design Road, Frederick, MD, 21703 Phone: (301) 631-6900 Website: KingsbrookVet.com What causes heartworm disease? Heartworm Disease in Dogs Heartworm disease or dirofilariasis
SUPPLEMENTARY INFORMATION
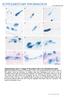 doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
Ecology of RMSF on Arizona Tribal Lands
 Ecology of RMSF on Arizona Tribal Lands Tribal Vector Borne Disease Meeting M. L. Levin Ph.D. Medical Entomology Laboratory Centers for Disease Control mlevin@cdc.gov Rocky Mountain Spotted Fever Disease
Ecology of RMSF on Arizona Tribal Lands Tribal Vector Borne Disease Meeting M. L. Levin Ph.D. Medical Entomology Laboratory Centers for Disease Control mlevin@cdc.gov Rocky Mountain Spotted Fever Disease
FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT
![FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT](/thumbs/93/113164093.jpg) FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 22 APR 2018 Biogal Galed Laboratories Acs Ltd. tel: 972-4-9898605. fax: 972-4-9898690 e-mail:info@biogal.co.il
FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 22 APR 2018 Biogal Galed Laboratories Acs Ltd. tel: 972-4-9898605. fax: 972-4-9898690 e-mail:info@biogal.co.il
Protozoan Parasites of Veterinary importance 2017
 Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Consequences of Antimicrobial Resistant Bacteria. Antimicrobial Resistance. Molecular Genetics of Antimicrobial Resistance. Topics to be Covered
 Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
MID 23. Antimicrobial Resistance. Consequences of Antimicrobial Resistant Bacteria. Molecular Genetics of Antimicrobial Resistance
 Antimicrobial Resistance Molecular Genetics of Antimicrobial Resistance Micro evolutionary change - point mutations Beta-lactamase mutation extends spectrum of the enzyme rpob gene (RNA polymerase) mutation
Antimicrobial Resistance Molecular Genetics of Antimicrobial Resistance Micro evolutionary change - point mutations Beta-lactamase mutation extends spectrum of the enzyme rpob gene (RNA polymerase) mutation
Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites
 Mediators of Inflammation, Article ID 362605, 6 pages http://dx.doi.org/10.1155/2014/362605 Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites Hong Zheng, Zhangping Tan, and
Mediators of Inflammation, Article ID 362605, 6 pages http://dx.doi.org/10.1155/2014/362605 Review Article Immune Evasion Strategies of Pre-Erythrocytic Malaria Parasites Hong Zheng, Zhangping Tan, and
Antimicrobial Resistance
 Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Acquisition of Foreign DNA
 Antimicrobial Resistance Acquisition of Foreign DNA Levy, Scientific American Horizontal gene transfer is common, even between Gram positive and negative bacteria Plasmid - transfer of single or multiple
Antimicrobial Resistance Acquisition of Foreign DNA Levy, Scientific American Horizontal gene transfer is common, even between Gram positive and negative bacteria Plasmid - transfer of single or multiple
WHY IS THIS IMPORTANT?
 CHAPTER 20 ANTIBIOTIC RESISTANCE WHY IS THIS IMPORTANT? The most important problem associated with infectious disease today is the rapid development of resistance to antibiotics It will force us to change
CHAPTER 20 ANTIBIOTIC RESISTANCE WHY IS THIS IMPORTANT? The most important problem associated with infectious disease today is the rapid development of resistance to antibiotics It will force us to change
Detection of UV-Induced Thymine Dimers in Individual Cryptosporidium parvum and Cryptosporidium hominis Oocysts by Immunofluorescence Microscopy
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Feb. 2007, p. 947 955 Vol. 73, No. 3 0099-2240/07/$08.00 0 doi:10.1128/aem.01251-06 Copyright 2007, American Society for Microbiology. All Rights Reserved. Detection
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Feb. 2007, p. 947 955 Vol. 73, No. 3 0099-2240/07/$08.00 0 doi:10.1128/aem.01251-06 Copyright 2007, American Society for Microbiology. All Rights Reserved. Detection
Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220
 Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220 Introduction Enzootic Bovine Leukosis is a transmissible disease caused by the Enzootic Bovine Leukosis Virus (BLV)
Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220 Introduction Enzootic Bovine Leukosis is a transmissible disease caused by the Enzootic Bovine Leukosis Virus (BLV)
COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST
 COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST In this laboratory investigation, you will use BLAST to compare several genes, and then use the information to construct a cladogram.
COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST In this laboratory investigation, you will use BLAST to compare several genes, and then use the information to construct a cladogram.
National Research Center
 National Research Center Update of immunodiagnosis of cystic echinococcosis cysts Global distribution of zoonotic strains of Echinococcus granulosus (Adapted from Eckert and Deplazes, 2004) Echinococcus
National Research Center Update of immunodiagnosis of cystic echinococcosis cysts Global distribution of zoonotic strains of Echinococcus granulosus (Adapted from Eckert and Deplazes, 2004) Echinococcus
Running title: Model to down-select human malaria vaccines
 CVI Accepts, published online ahead of print on 27 March 2013 Clin. Vaccine Immunol. doi:10.1128/cvi.00066-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6 7 8 9 10
CVI Accepts, published online ahead of print on 27 March 2013 Clin. Vaccine Immunol. doi:10.1128/cvi.00066-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6 7 8 9 10
EVALUATION OF THE SENSITIVITY AND SPECIFICITY OF THE EHRLICHIA CANIS DIAGNOSTIC TEST: Anigen Rapid E.canis Ab Test Kit
 EVALUATION OF THE SENSITIVITY AND SPECIFICITY OF THE EHRLICHIA CANIS DIAGNOSTIC TEST: Anigen Rapid E.canis Ab Test Kit FINAL REPORT Research contract (art. 83 of the L.O.U) between the Ehrlichiosis Diagnostic
EVALUATION OF THE SENSITIVITY AND SPECIFICITY OF THE EHRLICHIA CANIS DIAGNOSTIC TEST: Anigen Rapid E.canis Ab Test Kit FINAL REPORT Research contract (art. 83 of the L.O.U) between the Ehrlichiosis Diagnostic
Malaria remains the most important parasitic disease. Review Article
 Review Article Pre-erythrocytic Stage of Malaria Infection and the Molecular Targets Available for Interventions Dickson Adah 1,2, Yi Jun Yang 1,2, Quan Liu 1,2, Limei Qin 1, Li Qin 1, Xiaoping Chen 1
Review Article Pre-erythrocytic Stage of Malaria Infection and the Molecular Targets Available for Interventions Dickson Adah 1,2, Yi Jun Yang 1,2, Quan Liu 1,2, Limei Qin 1, Li Qin 1, Xiaoping Chen 1
15 Plasmodium ovale Stephens, 1922
 15 Plasmodium ovale Stephens, 1922 BECAUSE of the close resemblance of Plasmodium ovale to P. vivax it is impossible to tell when P. ovale was first seen. Macfie and Ingram (1917) described a parasite
15 Plasmodium ovale Stephens, 1922 BECAUSE of the close resemblance of Plasmodium ovale to P. vivax it is impossible to tell when P. ovale was first seen. Macfie and Ingram (1917) described a parasite
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology EMEA/CVMP/005/00-FINAL-Rev.1 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS GUIDELINE FOR THE TESTING
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology EMEA/CVMP/005/00-FINAL-Rev.1 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS GUIDELINE FOR THE TESTING
Malaria & Dengue Global Health Lecture Series
 Malaria & Dengue Global Health Lecture Series Julie Gutman, MD MSc Pediatric Infectious Disease 5/13/2011 What would be the most appropriate treatment for a patient presenting with malaria acquired in
Malaria & Dengue Global Health Lecture Series Julie Gutman, MD MSc Pediatric Infectious Disease 5/13/2011 What would be the most appropriate treatment for a patient presenting with malaria acquired in
Anti-tick vaccines: A potential tool for control of the blacklegged ticks and other ticks feeding on whitetailed deer
 Anti-tick vaccines: A potential tool for control of the blacklegged ticks and other ticks feeding on whitetailed deer Andrew Y. Li USDA-ARS Invasive Insect Biocontrol and Behavior Laboratory (IIBBL) Beltsville,
Anti-tick vaccines: A potential tool for control of the blacklegged ticks and other ticks feeding on whitetailed deer Andrew Y. Li USDA-ARS Invasive Insect Biocontrol and Behavior Laboratory (IIBBL) Beltsville,
Development and validation of a diagnostic test for Ridge allele copy number in Rhodesian Ridgeback dogs
 Waldo and Diaz Canine Genetics and Epidemiology (2015) 2:2 DOI 10.1186/s40575-015-0013-x RESEARCH Open Access Development and validation of a diagstic test for Ridge allele copy number in Rhodesian Ridgeback
Waldo and Diaz Canine Genetics and Epidemiology (2015) 2:2 DOI 10.1186/s40575-015-0013-x RESEARCH Open Access Development and validation of a diagstic test for Ridge allele copy number in Rhodesian Ridgeback
An Overview of Canine Babesiosis
 Page 1 of 6 C. Wyatt Cleveland, DVM; David S. Peterson, DVM, PhD; and Kenneth S. Latimer, DVM, PhD Class of 2002 (Cleveland), Department of Medical Microbiology and Parasitology (Peterson), and Department
Page 1 of 6 C. Wyatt Cleveland, DVM; David S. Peterson, DVM, PhD; and Kenneth S. Latimer, DVM, PhD Class of 2002 (Cleveland), Department of Medical Microbiology and Parasitology (Peterson), and Department
