List over prohibited substances and withdrawal times in Scandinavia, valid from January 1 st 2018.
|
|
|
- Sheryl Edwards
- 5 years ago
- Views:
Transcription
1 List over prohibited substances and withdrawal times in Scandinavia, valid from January 1 st This list has been developed in collaboration with the other Scandinavian countries through NEMAC (Nordic Equine Medication and Anti-doping Committee). The List of prohibited substances and withdrawal times consists of two parts: the A-list, listing substances and treatment methods that are absolutely prohibited for horses, and the B- list, listing substances that are prohibited in competition; the withdrawal times for these substances as well as treatment methods with withdrawal times. The list may be reviewed several times per year. This list is valid, starting from January 1 st, 2018, and is enforced until a new list takes effect. A valid list of withdrawal times can be found at any time on each of the Scandinavian countries official websites: - The Norwegian Trotting Association (DNT) - Swedish Trotting Association (ST) - The Danish Trotting Association (DTC) General information: Medical records The trainer is responsible for ensuring that any treatment that requires a withdrawal time is listed in the horse s medical record. The start and end dates of the treatment, the name of the treatment/medication/active substance, amount given, method of administration, withdrawal time as well as the name of the veterinarian or other person responsible for the treatment must all be listed in the medical record in accordance with DNT s Doping Regulations Passport and medical record must be available for presentation with the horse at all times. Omitting, incompletely or improperly listing treatments in the medical record constitutes a breach of the Doping Regulations. Medication control According to the Nordic countries rules and regulations, any horse registered for race can be brought in for a medication control both before the start of the race, or up to three (3) hours after the race was finished. Blood samples, urine samples and/or hair samples can be taken. One of the stable/trainers crew (aged 16 or older) must supervise the horse during the medication control. Please bring your horse s passport and medical record to the medication control. The horse is allowed to drink fresh water during the medication control, but no other supplements can be given until the medication control is finished. Contamination by pharmaceutical products Generally, great care must be taken to ensure that horses that are under medical treatment are kept in a stall of their own and are fed any medicated feed from labeled buckets or containers. 1 (13)
2 Horses undergoing drug treatment may excrete medication through urine and faeces and potentially transfer residues of the drugs to other horses through feed stuff, shavings or straw. Persons handling the horses must ensure that any medication for personal use is kept away from the horses at all times. No person may urinate in the horse s immediate environment (such as the stall, box or horse trailer) as medication excreted in urine from humans may transfer to the horse through contaminated feed or bedding. A. LIST OF PROHIBITED SUBSTANCES 1. Prohibited substances The following substances are prohibited in connection with competition: - Substances that may affect or have an effect, or both, on the following organ systems: The nervous system The cardiovascular system The respiratory system The digestive system The urinary system The reproductive system The muscular and skeletal systems Blood and blood forming organs The immune system The endocrine system - Endogenous hormones or similar synthetic substances - Substances with a masking effect - Substances which directly or indirectly manipulate the expression of genes Either a finding of the substance itself, the finding of a metabolite of the substance, or the finding of a prodrug of the substance indicates the finding of such substances. Administration of or exposure to such substances may constitute a positive finding of the substance. 2. Genetic and cellular manipulation Modification of the genome of a horse at any point in the horse s life will result in a lifetime disqualification from competing. Gene therapy or cellular manipulation applied to a race horse must not: - Positively or negatively affect the horse s performance capacity - Negatively affect the horse s welfare 3. Prohibited methods Prohibited methods include, but are not limited to: - Racing a pregnant mare after day 120 of pregnancy - Neurectomy (surgical and/or chemical) - Cryotherapy before racing 2 (13)
3 - Withholding drinking water before racing - Manipulation of blood and blood components, including administration or retransfer of homologous or heterologous blood or products of red blood cells to the circulatory system, except where such treatment is necessary to save the horse s life. - Artificially increasing oxygen uptake and/or oxygen transport in the tissue, including but not limited to the use of modified hemoglobin products - Any kind of intravascular artificial manipulation of blood or blood components 4. Absolutely prohibited substances and methods of treatment The following substances, including other substances with a similar chemical structure or similar biological effect, and their releasing factors, are prohibited to use, store, manufacture, import, export, sell, distribute, acquire, send or transfer at any time: 4.1.Non-approved substances. Substances which are not listed in any of the classes below, and which have not been approved by any national or international medicines agency, may not be administered to a race horse Anabolic substances a) Anabolic androgenic steroids b) Other anabolic substances, including but not limited to selective androgen receptor modulators (SARMs) c) Beta-2 agonists, except in cases where such substance is prescribed by a veterinary physician for use in bronchodilator treatment, and used in dosages approved for such treatment by the medicines agency Peptide hormones, growth factors, and similar substances a) Erythropoietin stimulating agents, including but not limited to erythropoietin (EPO), epoetin alfa, epoetin beta, darbepoetin alfa, methoxy polyethylene glycol-epoetin beta, peginesatide, hypoxia-inducible factor (HIF-1) stabilizers (e.g., cobalt) and activators (e.g., xenon, argon). b) Growth hormones or growth hormone inducing factors, insulin-like growth factor (IGF-1), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), mechanogrowth factor (MGF), platelet derived growth factor (PDGF) and other growth factors. c) Synthetic proteins and peptides and synthetic analogues of endogenous proteins and peptides that are not approved for use in human or veterinary medicine Hormones and metabolic modulators a) Aromatase inhibitors b) Selective estrogen receptor modulators (SERMs) and other anti-estrogen substances 3 (13)
4 c) Substances which may modify myostatin function, including but not limited to myostatin inhibitors. d) Insulin e) Peroxisome proliferator-activated receptor gamma agonists, including but not limited to GW 1516 f) AMPK activators, including but not limited to AICAR (5-aminoimidazole-4- carboxamide-1-β-d-ribofuranoside) 4.5. Other treatments and substances that are completely forbidden - Cobratoxin and other toxins with similar structure and effect - Capsaicin - Pitcher plant extracts (Sarapin, Saralgyl) - Polyacrylamide hydrogel - Cedar oil - Treatment with substances containing eg. arsenic, lead, mercury, croton oil or cedar oil. In addition, other products, preparations and substances applied in the purpose to affect circulation in the skin and underlying tissues are prohibited if this causes discomfort, pain or skin damage in the horse. - Use of radioactive implants - GnRH vaccine (Equity, Improvac) - hcg (Human chorionic gonadotropin) use in stallions - Pergolide (Prascend) - ozone - Supplying unnaturally high doses of naturally occurring substances (e.g. cobalt and nickel) - Alkalizing substances (e.g. bicarbonate and citrates) are prohibited the last 24 hours before a race - Treatment with bisphosphonates in horses younger than 4 years, or bisphosphonates administered in other ways than the drug is approved/registered for. The use of bisphosphonates without a marketing authorization for horses is also prohibited. This includes all aminobisphosphonates (e.g. zoledronate, alenodronate, pamidronate). B. LIST OF WITHDRAWAL TIMES The withdrawal times listed here should be considered minimum requirements and are counted as lasting from 1) termination of administration of medication or substance, or 2) termination of other treatment, until 12:00 (noon) on race day (Norway). The times listed below are valid unless a specific withdrawal time is given for the particular substance. When a substance or drug is detected in biological material from the horse, it is considered prohibited even if the substance or drug was administered before the times listed here. 4 (13)
5 a) No withdrawal time - Topical application on skin of substances which only have a protective, disinfecting, softening, absorbing, astringent, drying or keratolytic effect - Cooling off the horse with cold water - Use of saline- and/or lubricating laxatives (e.g. Glauber s salt). Insertion of nasoesophageal/nasogastric tube is prohibited on raceday at racetrack - Any disinfectants, e.g. chloramine, chlorhexidine, cetylpyridinium chloride b) Prohibited on the day of the race Norway: The day of the race is defined as starting at midnight, 00:00, and ends when the horse has finished its race/races. Sweden: Prohibited on raceday at the racetrack. o Nasal strips o Acupressure o Inhalation therapy o Insertion of nasoesophageal/nasogastric tube o Rectal fluid therapy o Cooling by means other than cold water, mud or topical preparations/liniments which do not have a withdrawal time o Use of electric massagers and other electrical devices c) minimum - Injection or infusion, regardless of preparation - Substances which have an effect on the nervous system - Substances which have an effect on the muscular and skeletal systems - Substances which have a muscle relaxant effect - Antimicrobial and antifungal drugs (with the exception of procaine benzylpenicillin which has a withdrawal time of ) - Pharmacy manufactured medication - Medication for human use, for which no withdrawal time is specified - Veterinary medication not indicated for use in horses - Herbal medication (for oral administration) - Homeopathic medication d) 7 days minimum - Expectorant medication, e.g. bromhexine, dembrexine (Sputolysin), acetylcysteine (Equimucin) e) minimum - Injections in the joints or bursae, joint/synovial puncture - Glucocorticoids (cortisone) with a short-term effect and rapid excretion. In the case of injections in the joints, tendon sheaths or bursae the withdrawal time is 28 days - Bronchodilator medication for inhalation (e.g. salbutamol, salmeterol, beclomethasone, budesonide) 5 (13)
6 - Anti-inflammatory medication (e.g. DMSO, NSAIDs) f) 28 days minimum - Any glucocorticoids (cortisone) with the exception of those mentioned in section d) above. Glucocorticoids such as triamcinolone acetonide, betamethasone phosphate/betamethasone acetate and methylprednisolone acetate are authorized for marketing for human use, but not for equine use. Therefore, there rests a greater responsibility upon the veterinary physician when prescribing these medications for use in horses. The recommended withdrawal times after injection of such medication in joints, bursae or tendon sheaths are based on dosages empirically established as common in clinical practice, injected in one or two joints. However, if higher doses are used or more than two joints/bursae/tendon sheaths are treated with injections, the withdrawal times should be extended significantly beyond the 28 days. In such cases, an appropriately long withdrawal time must be determined by the veterinary physician s professional judgment. Methylprednisolone acetate has a particularly long-lasting effect and is very slowly eliminated. It is therefore not recommended for use in racing horses. Triamcinolone acetonide and other depot formulations may have a very long elimination time even after intramuscular injection. g) 60 days minimum Bisphosphonates (Tildren and Osphos ) is not allowed to use in horses younger than 4 years of age. Veterinary examination and evaluation is required before bisphosphonates are administered. The products has to be administered on the indications and with the routes of administration approved by the manufacturer and the drug authorities. This implies that eg. Intra- articular injections are prohibited. Bisphosphonates without a marketing authorization for horses, including all aminobisphosphonates, are also prohibited. h) One year - Long acting hormone therapy to delay oestrus (e.g., Progesterone) Withdrawal times for drugs authorized for marketing for equine use in Scandinavian countries The withdrawal times for drugs listed in the Danish, Norwegian or Swedish Pharmaceutical Product Compendium for veterinary medicine are valid only when adhering to the manufacturer s recommendation regarding dosage, dosage intervals, administration method and duration of treatment. In the case of deviations from those, prolonging the withdrawal times may be necessary. Name of active substance and withdrawal time for drugs authorized for marketing for equine use: 6 (13)
7 Active substance Min. WT time Important info Acepromazine 7 days Acetylcysteine 7 days Adrenaline Altrenogest Only permitted for use in mares Bencylpenicillin Benzylpenicillin procaine Benzylpenicillin procaine + dihydrostreptomycin Benzylpenicillin procaine + dihydrostreptomycin + sulfadimidine Buprenorphine 6 days Buserelin acetate Butorphanol 6 days Butylscopolamine Cimetidine Clenbuterol 28 days Clodronate 28 days Not recommended in racing horses Cloprostenol Cromoglycate Dantrolene sulphate Detomidine Dexamethasone 28 days Dexamethasone sodium phosphate Dihydrostreptomycin Dinoprost Enrofloxacin Febantel Fenbendazole Firocoxib 30 days Flunixin Gentamicin Heparin Hydroxietyl salicylate Isoflurane Ivermectin Ivermectin and praziquantel Ketamine Ketanserin 0 hours Ketoprofen Levomenthol 96hours Lidocaine Lidocaine + adrenaline Luprostiol 7 (13)
8 Meloxicam Metamizole/Dipyrone 7 days 14 d. in Sweden (National legislation) Moxidectin Moxidectin/praziquantel Omeprazole Oxytetracycline Oxytetracycline + Polymyxin B Oxytocin Pergolide mesylate Prohibited Not permitted in racing horses Phenylbutazone Polysulfated glycosaminoglycan Praziquantel Prednisolone Procaine 14 d. in Sweden (National legislation) Pyrantel pamoate Ranitidine R-cloprostenol Romifidine 5 days Scopolamine Sodium hyaluronate Sucralfate Sulfadiazine Sulfadioxine Suxibuzone Tetanus vaccine Tiludronic acid 60 days Intra articular injection prohibited. Not permitted to use in horses under the age of 4 years Trimethoprim Trimethoprim + sulfadiazine Trimethoprim/sulfadoxine Vaccinations against horse flu, tetanus, herpes, rabies and ringworm Vedaprofen Vitamin A, D2 og E (for injection) Vitamin B (for injection) Vitamin E (for injection) Xylazine 8 (13)
9 Withdrawal times for medication administered through an inhaler The use of inhalation apparatus is not permitted on race day. Bronchodilator medication given through an inhaler (e.g. salbutamol, salmeterol, bechlomethasone, budesonide) Saline Other medication for use by inhalation Not permitted on race day Depending on the withdrawal time of the drug Withdrawal times for treatment methods: Acupuncture: Chiropractor treatment: Laser treatment: LED light treatment: Naprapathy treatment: Osteopathy treatment: Ultrasound treatment: Transcutaneous nerve stimulation (TNS): Shock wave or pulsed wave therapy: Intrauterine implants for delaying oestrus ( marbles ): 10 days NOTE: Treatment must only be administered by an authorized veterinary suregeon 0 hours Withdrawal times for products for external use, feed and supplements Preparations which only have a protective, disinfectant, softening, absorbing, adstringent, drying or keratolytic effect, used topically on the skin, have no withdrawal time. Ointments or liniments containing antibacterial or antifungal substances have a general withdrawal time of, with the exception of preparations containing procaine benzylpenicillin, NSAIDs, or glucocorticoids, which have a withdrawal time of. a) Withdrawal time : Substances classified as human medicines or herbal medicines, e.g.: - Benzocaine - Glucosamine - Harpagophytum procumbens (Devil s claw) (NOTE: There is a withdrawal time of for this supplement in Sweden due to National legislation) - Heparin - Caffeine (including Guarana products) (NOTE: There is a withdrawal time of 14 days for caffeine in Sweden due to National legislation) - Levomenthol, menthol 9 (13)
10 - Salicylic acid, diethylamine- hydroxyethyl- and methyl salicylate b) Withdrawal time 48 hours: Herbal medicines, e.g.: - Aesculus hippocastanum (horse chestnut) - Agnus castus (monk s pepper) - Echinacea purpurea (purple coneflower) - Hypericum perforatum L. (St John s wort) - Symphytum officinale L. (common comfrey) - Valeriana officinalis (valerian) c) No withdrawal time: Other substances and natural remedies with some documented effect - Alpha casozepine - Allium sativum (garlic) - Aloe vera (genuine aloe vera) (NOTE: There is a 96-hour withdrawal time for Aloe vera in Sweden due to National legislation) - Arnica montana - Avocado and Soybean unsaponifiable extracts (ASU) - Boswellia sp. - Bee glue (Propolis) - Calendula sp. (marigold) - Chondroitin sulfate - Curcuma longa (curcumin, turmeric) - Di-/trimethylglycine (D-/TMG) - Eucalyptus - Gamma oryzanol, rice bran oil - Ginkgo biloba - Ginseng - Green-lipped mussel extract (GLE) - Hamamelis (witch-hazel) - Hyaluronic acid (orally) - Camphor - Creatine - L-carnitine - Melaleuca alternifolia (tea tree oil) - MSM, methylsulfonylmethane - Neem oil - Octacosanol - Rosa sp. (rosehip powder) - Schisandra chinensis - Tryptophan - Yucca, Yucca schidigera - Zingiber officinale (ginger) 10 (13)
11 Threshold values for certain endogenous and/or naturally occurring substances Arsenic - 0,3 µg total arsenic per ml in urine Boldenone - 0,015 µg free and conjugated boldenone per ml i urine from male horses (other than geldings) Dimethyl - 15,0 µg Dimethyl sulphoxide per ml in urine sulphoxide (DMSO) - <1,0 µg Dimethyl sulfoxide per ml in plasma Carbon dioxide - 36 mmol/l plasma (total CO 2) Cobalt - 0,1 µg (=100 ng) total cobalt per ml in urine - 0,0025 µg (=25 ng) per ml in plasma Cortisol - 1,0 µg hydrocortisone per ml urine (hydrocortisone) Methoxytyramine - 4 µg free and conjugated 3-metoxytyramine per ml in urine Nandrolon (norteststerone) - Estranediol, mengden av fritt og konjugert 5-alfa-estrane-3β, 17-alfadiol i relasjon til mengden av fritt og konjugert 5(10)-estrene-3β, 17- alfa-diol i urin fra hingst <1 Salicylic acid µg salicylic acid per ml in urine - 6,5 µg salicylic acid per ml in plasma Theobromine - 2,0 µg theobromine per ml in urine - 0,3 µg theobromine per ml in plasma Testosterone - 0,02 µg free and conjugated testosterone per ml in urin from geldings - 0,055 µg free and conjugated testosterone per ml in urine from fillies and mares (unless in foal) pg free testosterone per ml in plasma from geldings List of substances with adopted screening limits Substances that have established screening limits are listed below. Substances for which there are screening limits in both urine and plasma are marked with an asterisk *. The actual screening limits are not public according to decisions made in the European Horserace Scientific Liaison Committee (EHSLC) and the Nordic Equine Medication and Anti-doping Committee (NEMAC). Acepromazine* Altrenogest* Atropine Betamethasone Bromhexine/ambroxol Bufotenine Butorphanol* Butylscopolamine (N-butylscopolamine) * Carprofen* Clenbuterol Dantrolene 11 (13)
12 Dembrexine* Detomidine (3 -hydroxydetomidine) * Dexamethasone Diclofenac* Dimethyltryptamine (DMT) Dipyrone (as 4-MAA) (=metamizole) * Eltenac Etamiphylline Phenylbutazone* Firocoxib* Flunixin* Furosemide* Guaifenesin Hordenine Hydroklortiazid* Ibuprofen Ipratropium Camphor Ketoprofen Cocaine Caffeine Lidocaine (3-hydroxy lidocaine) * Meclofenamic acid* Meloxicam* Menthol Mepivacaine Morphine (morphine glucuronide) Naproxen Nimesulide Omeprazole* Oxazepam Prednisolone Procaine * Romifidine* Salbutamol Scopolamine Teophylline Tiludronic acid Triamcinolone acetonide Trimethoprim* Vedaprofen* Xylazine (metabolite/s) 12 (13)
13 - NOTE: If two or more similar substances are found in a sample, the screening limits will not apply (due to the so-called cocktail rule ). This is to ascertain that multiple similar medications are not used in combination in smaller doses to avoid exceeding the screening limits. The screening limits do not apply for out-ofcompetition testing. Exceptions to the cocktail rule are findings of the following combinations: - Detomidine, romifidine or xylazine in combination with butorphanol - Atropine and scopolamine - Butylscopolamine and dipyrone/metamizole International collaboration DTC, DNT and ST are members of the Nordic Equine Medication and Anti-doping Committee (NEMAC) and joint members European Horserace Scientific Liaison Committee (EHSLC). NEMAC is responsible for developing lists of withdrawal times valid for the Nordic countries. Make sure you always use the latest version of this document you will find it on DNT s official homepage Withdrawal times listed on ST s official website, are also valid in Norway. Please note that the changes in withdrawal times for Aloe Vera, Harpagophytum procumbens (Devil s claw), and caffeine have still not been introduced in Sweden due to National legislation/regulations from the Swedish Board of Agriculture (Jordbruksverket). Therefore, the original withdrawal times of (for Aloe Vera) and (for Devil s claw and caffeine), respectively, are still to be used for any races in Sweden until further notice. See also and for more information. 13 (13)
List over prohibited substances and withdrawal times in Scandinavia, valid from January 1 st, 2019.
 List over prohibited substances and withdrawal times in Scandinavia, valid from January 1 st, 2019. This list has been developed in collaboration with the Scandinavian countries through NEMAC (Nordic Equine
List over prohibited substances and withdrawal times in Scandinavia, valid from January 1 st, 2019. This list has been developed in collaboration with the Scandinavian countries through NEMAC (Nordic Equine
List over prohibited substances and withdrawal times in Scandinavia, valid from September 3 rd 2018.
 List over prohibited substances and withdrawal times in Scandinavia, valid from September 3 rd 2018. This list has been developed in collaboration with the Scandinavian countries through NEMAC (Nordic
List over prohibited substances and withdrawal times in Scandinavia, valid from September 3 rd 2018. This list has been developed in collaboration with the Scandinavian countries through NEMAC (Nordic
DRUG REGULATIONS & WITHDRAWAL TIMES
 DRUG REGULATIONS & WITHDRAWAL TIMES UPDATED: In this document you will find information about general regulations on the use of medicinal products etc., and a list of withdrawal times for substances (drugs).
DRUG REGULATIONS & WITHDRAWAL TIMES UPDATED: In this document you will find information about general regulations on the use of medicinal products etc., and a list of withdrawal times for substances (drugs).
CHAPTER 45. PROHIBITED PRACTICES AND EQUINE TESTING
 CHAPTER 45. PROHIBITED PRACTICES AND EQUINE TESTING 325:45-1-2. Definitions In addition to the definitions provided at 3A O.S. 200.1, the following words or terms, when used in this Chapter, shall have
CHAPTER 45. PROHIBITED PRACTICES AND EQUINE TESTING 325:45-1-2. Definitions In addition to the definitions provided at 3A O.S. 200.1, the following words or terms, when used in this Chapter, shall have
ARCI Controlled Therapeutic Medication Schedule for Horses - Version 4.1 Revised January, 2019
 ARCI Schedule for Horses - Version 4.1 Revised January, 2019 Acepromazine 10 nanograms per milliliter as 2-(1- hydroxyethyl) promazine sulfoxide (HEPS) in urine Single intravenous dose of acepromazine
ARCI Schedule for Horses - Version 4.1 Revised January, 2019 Acepromazine 10 nanograms per milliliter as 2-(1- hydroxyethyl) promazine sulfoxide (HEPS) in urine Single intravenous dose of acepromazine
ARCI Controlled Therapeutic Medication Schedule for Horses - Version 3.2 Revised December 9, 2016.
 ARCI Schedule for Horses - Version 3.2 Revised December 9, 2016. Acepromazine 10 nanograms per milliliter as 2-(1- hydroxyethyl) promazine sulfoxide (HEPS) in urine Single intravenous dose of acepromazine
ARCI Schedule for Horses - Version 3.2 Revised December 9, 2016. Acepromazine 10 nanograms per milliliter as 2-(1- hydroxyethyl) promazine sulfoxide (HEPS) in urine Single intravenous dose of acepromazine
WITHDRAWAL TIME RECOMMENDATIONS ARIZONA RACE TRACKS MEET
 WITHDRAWAL TIME RECOMMENDATIONS ARIZONA RACE TRACKS 2018-19 MEET IMPORTANT WARNING: The information on drug withdrawal times does not constitute and is not a warranty, guarantee, assurance, undertaking,
WITHDRAWAL TIME RECOMMENDATIONS ARIZONA RACE TRACKS 2018-19 MEET IMPORTANT WARNING: The information on drug withdrawal times does not constitute and is not a warranty, guarantee, assurance, undertaking,
ARCI Controlled Therapeutic Medication Schedule for Horses - Version 2.2 Revised April 2015
 ARCI Schedule for Horses - Version 2.2 Revised April 2015 Acepromazine 10 nanograms per milliliter as 2-(1- hydroxyethyl) promazine sulfoxide (HEPS) in urine Single intravenous dose of acepromazine at
ARCI Schedule for Horses - Version 2.2 Revised April 2015 Acepromazine 10 nanograms per milliliter as 2-(1- hydroxyethyl) promazine sulfoxide (HEPS) in urine Single intravenous dose of acepromazine at
New Maryland Racing Medication Guidelines
 New Maryland Racing Medication Guidelines January 1, 2014 NEW MEDICATION REFORMS EFFECTIVE JANUARY 1, 2014 The Mid Atlantic racing states have joined together to implement a uniform medication and drug
New Maryland Racing Medication Guidelines January 1, 2014 NEW MEDICATION REFORMS EFFECTIVE JANUARY 1, 2014 The Mid Atlantic racing states have joined together to implement a uniform medication and drug
Maryland Racing Commission Medication Guidelines
 Maryland Racing Commission Medication Guidelines August 1, 2015 Maryland Racing Medication Guidelines The Mid Atlantic racing states have joined together to implement a uniform medication and drug testing
Maryland Racing Commission Medication Guidelines August 1, 2015 Maryland Racing Medication Guidelines The Mid Atlantic racing states have joined together to implement a uniform medication and drug testing
2016 Massachusetts Gaming Commission Manual For Practicing Veterinarians
 2016 Massachusetts Gaming Commission Manual For Practicing Veterinarians Guide to Medication and Horse Health Procedures Massachusetts Gaming Commission Veterinary Department Suffolk Downs Chief Commission
2016 Massachusetts Gaming Commission Manual For Practicing Veterinarians Guide to Medication and Horse Health Procedures Massachusetts Gaming Commission Veterinary Department Suffolk Downs Chief Commission
EQUINE DRUGS AND MEDICATIONS RULES
 EQUINE DRUGS AND MEDICATIONS RULES UNITED STATES POLO ASSOCIATION 2018 EQUINE DRUGS & MEDICATIONS RULES 1. PERMITTED MEDICATIONS The USPA will impose no penalty for the administration of the following
EQUINE DRUGS AND MEDICATIONS RULES UNITED STATES POLO ASSOCIATION 2018 EQUINE DRUGS & MEDICATIONS RULES 1. PERMITTED MEDICATIONS The USPA will impose no penalty for the administration of the following
EQUESTRIAN CANADA GUIDELINES FOR USE OF DRUGS AND MEDICATIONS
 EQUINE MEDICATION CONTROL GUIDE 2018 EQUESTRIAN CANADA GUIDELINES FOR USE OF DRUGS AND MEDICATIONS Introduction 1 Background 1-2 Permitted Medications 2-3 Guidelines for Permitted Use of NSAIDS 3-5 Prohibited
EQUINE MEDICATION CONTROL GUIDE 2018 EQUESTRIAN CANADA GUIDELINES FOR USE OF DRUGS AND MEDICATIONS Introduction 1 Background 1-2 Permitted Medications 2-3 Guidelines for Permitted Use of NSAIDS 3-5 Prohibited
2017 Massachusetts Gaming Commission
 2017 Massachusetts Gaming Commission Trainer s Reference Manual This manual provides owners, trainers, and others an overview of the rules and procedures that apply to the medication and health of horses
2017 Massachusetts Gaming Commission Trainer s Reference Manual This manual provides owners, trainers, and others an overview of the rules and procedures that apply to the medication and health of horses
edition of the Association of Racing Commissioners International (ARCI s) Uniform Classifi cation Guidelines for Foreign Substances.
 2016 STANDING RULE 35A MEDICATION AND DRUG RULES AND GUIDELINES The NCHA s Medication and Drug Rules and Guidelines ( Medication Rules ) have been put in place to protect and prolong the welfare and competitiveness
2016 STANDING RULE 35A MEDICATION AND DRUG RULES AND GUIDELINES The NCHA s Medication and Drug Rules and Guidelines ( Medication Rules ) have been put in place to protect and prolong the welfare and competitiveness
Table 2 - Medications and their ideal route of administration
 Table 2 - Medications and their ideal route of administration Pharmacologic-Therapeutic Classification Drug Route Antihistamines First-Generation Antihistamines Diphenhydramine Promethazine Anti-infective
Table 2 - Medications and their ideal route of administration Pharmacologic-Therapeutic Classification Drug Route Antihistamines First-Generation Antihistamines Diphenhydramine Promethazine Anti-infective
Fundamentals of Pharmacology for Veterinary Technicians Chapter 16
 Figure 16-1 Figure 16-2 Hypothalamus Releasing factor Releasing factor Anterior pituitary ACTH signals adrenal cortex to glucocorticoids ACTH signals adrenal cortex to glucocorticoids Glucocorticoids Glucocorticoids
Figure 16-1 Figure 16-2 Hypothalamus Releasing factor Releasing factor Anterior pituitary ACTH signals adrenal cortex to glucocorticoids ACTH signals adrenal cortex to glucocorticoids Glucocorticoids Glucocorticoids
Beef Quality Assurance Program
 Bovine Pharmacology Beef Quality Assurance Program Purpose Supply only quality beef Improve consumer perception of beef s safety Elimination of drug residues Elimination of edible tissue blemishes and
Bovine Pharmacology Beef Quality Assurance Program Purpose Supply only quality beef Improve consumer perception of beef s safety Elimination of drug residues Elimination of edible tissue blemishes and
Welcome to. Who Wants to be a Millionaire 50:50
 0:0 Welcome to Who Wants to be a Millionaire 0 $ Million $,000 $,000 $00 0 $ Million $,000 $,000 $00 What is the generic name for the drug in Ketofen? C:Ketoprofen 0:0 0 $ Million $,000 $,000 $00 A: Ketarian
0:0 Welcome to Who Wants to be a Millionaire 0 $ Million $,000 $,000 $00 0 $ Million $,000 $,000 $00 What is the generic name for the drug in Ketofen? C:Ketoprofen 0:0 0 $ Million $,000 $,000 $00 A: Ketarian
These are some brief notes on equine therapeutics, to cover broad principles and contraindications, doses are not given in most cases and should
 These are some brief notes on equine therapeutics, to cover broad principles and contraindications, doses are not given in most cases and should always be checked before administrarion... Remember that
These are some brief notes on equine therapeutics, to cover broad principles and contraindications, doses are not given in most cases and should always be checked before administrarion... Remember that
Official Journal of the European Communities
 22. 12. 1999 EN Official Journal of the European Communities L 328/23 COMMISSION REGULATION (EC) No 2728/1999 of 20 December 1999 amending Annexes I, II and III to Council Regulation (EEC) No 2377/90 laying
22. 12. 1999 EN Official Journal of the European Communities L 328/23 COMMISSION REGULATION (EC) No 2728/1999 of 20 December 1999 amending Annexes I, II and III to Council Regulation (EEC) No 2377/90 laying
Knowledge list Avian/Exotic Pharmacology and Commonly Used Drugs
 Knowledge list Avian/Exotic Pharmacology and Commonly Used Drugs For each species on the Species List, the following pharmacology topics should be mastered. Knowledge of potential drug side effects and
Knowledge list Avian/Exotic Pharmacology and Commonly Used Drugs For each species on the Species List, the following pharmacology topics should be mastered. Knowledge of potential drug side effects and
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Acute interdigital necrobacillosis, 88 92. See also acute interdigital necrobacillosis; foot rot; Infectious pododermatitis (IP) a-2adrenergic
Index Note: Page numbers of article titles are in boldface type. A Acute interdigital necrobacillosis, 88 92. See also acute interdigital necrobacillosis; foot rot; Infectious pododermatitis (IP) a-2adrenergic
Mitigating Pain in Livestock: What Options are Available
 Mitigating Pain in Livestock: What Options are Available NIAA 2014 Annual Conference Omaha, Nebraska April 2, 2014 Craig A. Lewis, DVM, MPH, DACVPM Center for Veterinary Medicine U.S. Food and Drug Administration,
Mitigating Pain in Livestock: What Options are Available NIAA 2014 Annual Conference Omaha, Nebraska April 2, 2014 Craig A. Lewis, DVM, MPH, DACVPM Center for Veterinary Medicine U.S. Food and Drug Administration,
Equine Medication Monitoring Program. Drugs and Medication Guidelines
 Equine Medication Monitoring Program Drugs and Medication Guidelines January 2016 Introduction The California Equine Medication Monitoring Program (EMMP) is an industry funded program to ensure the integrity
Equine Medication Monitoring Program Drugs and Medication Guidelines January 2016 Introduction The California Equine Medication Monitoring Program (EMMP) is an industry funded program to ensure the integrity
2016 Minnesota Racing Commission Manual For Veterinarians
 2016 Minnesota Racing Commission Manual For Veterinarians Guide to Medication and Horse Health Procedures COGGINS Under regulations from the State Board of Animal Health, no horses are allowed on the backside
2016 Minnesota Racing Commission Manual For Veterinarians Guide to Medication and Horse Health Procedures COGGINS Under regulations from the State Board of Animal Health, no horses are allowed on the backside
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Issued March 2017 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats Recicort vet 1.77 mg/ml + 17.7 mg/ml
SUMMARY OF PRODUCT CHARACTERISTICS Issued March 2017 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats Recicort vet 1.77 mg/ml + 17.7 mg/ml
GREYHOUND RACING VICTORIA PROHIBITED SUBSTANCE PENALTY GUIDELINE AND RELEVANT INFORMATION PERTAINING TO PROHIBITED SUBSTANCE OFFENCES
 GREYHOUND RACING VICTORIA PROHIBITED SUBSTANCE PENALTY GUIDELINE AND RELEVANT INFORMATION PERTAINING TO PROHIBITED SUBSTANCE OFFENCES 1. Introduction This guideline has been formulated by GRV to provide
GREYHOUND RACING VICTORIA PROHIBITED SUBSTANCE PENALTY GUIDELINE AND RELEVANT INFORMATION PERTAINING TO PROHIBITED SUBSTANCE OFFENCES 1. Introduction This guideline has been formulated by GRV to provide
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Maprelin 75 µg/ml solution for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Maprelin 75 µg/ml solution for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection
ARCI Medications and Prohibited Substances
 rule shall be considered to have committed a Prohibited Practice and is subject to a Class A Penalty. (5) The use of a nasogastric tube (a tube longer than six inches) for the administration of any substance
rule shall be considered to have committed a Prohibited Practice and is subject to a Class A Penalty. (5) The use of a nasogastric tube (a tube longer than six inches) for the administration of any substance
Mouse Formulary. The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.
 Mouse Formulary The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.): Intraperitoneal (IP) doses should not exceed 80 ml/kg
Mouse Formulary The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.): Intraperitoneal (IP) doses should not exceed 80 ml/kg
Prescription Label. Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long):
 Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
Equine Pharmaceuticals: Manufacturing/ Compounding Issues. Legal and Ethical Veterinary Compounding. Horse Industry Integrity
 Equine Chemistry Research Legal and Ethical Veterinary Compounding Scott D. Stanley, Ph.D., Professor University of California, Davis School of Veterinary Medicine Drug Detection Determination, Identification,
Equine Chemistry Research Legal and Ethical Veterinary Compounding Scott D. Stanley, Ph.D., Professor University of California, Davis School of Veterinary Medicine Drug Detection Determination, Identification,
INJECTION GMP FSC AMPICILLIN SODIUM 500mg/1 g+ SULBACTAM DRY POWDER INJECTION GMP FSC
 1 S.NO COMPOSITION DOSAGE FORM PLANT CERTIFICATION DOCUMENTS 1 AZITHROMYCIN 500mg AMOXYCILLIN 250mg/500 mg/1 g+ POTASSIUM 2 CLAVULANIC 50mg/100mg/200mg AMPICILLIN SODIUM 500mg/1 g+ SULBACTAM 3 250mg/0.5g
1 S.NO COMPOSITION DOSAGE FORM PLANT CERTIFICATION DOCUMENTS 1 AZITHROMYCIN 500mg AMOXYCILLIN 250mg/500 mg/1 g+ POTASSIUM 2 CLAVULANIC 50mg/100mg/200mg AMPICILLIN SODIUM 500mg/1 g+ SULBACTAM 3 250mg/0.5g
CHECKLIST. Owner. Veterinarian. Horse. Laminitis - understanding, cure, prevention. Name: Address: City: Phone: Postal code: Mobile phone:
 Laminitis - understanding, cure, prevention CHECKLIST Owner Name: Address: City: Phone: E-mail: Postal code: Mobile phone: Veterinarian Name: Practice: Address: City: Phone: E-mail: Postal code: Mobile
Laminitis - understanding, cure, prevention CHECKLIST Owner Name: Address: City: Phone: E-mail: Postal code: Mobile phone: Veterinarian Name: Practice: Address: City: Phone: E-mail: Postal code: Mobile
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
YOUR COMPLETE SOURCE FOR MEDICAL MISSIONS SUPPLIES.
 YOUR COMPLETE SOURCE FOR MEDICAL MISSIONS SUPPLIES. When disasters strike, Blessings International provides pharmaceuticals and medical supplies quickly, so you can bring healing to the hurting. Where
YOUR COMPLETE SOURCE FOR MEDICAL MISSIONS SUPPLIES. When disasters strike, Blessings International provides pharmaceuticals and medical supplies quickly, so you can bring healing to the hurting. Where
IMPORTANT SAFETY INFORMATION
 TECHNICAL BULLETIN The Difference is in the Details: FDA Approved Drugs vs. Compounded Products and Veterinary Medical Devices Dr. Marian G. Little, Technical Services Veterinarian, American Regent, Inc.
TECHNICAL BULLETIN The Difference is in the Details: FDA Approved Drugs vs. Compounded Products and Veterinary Medical Devices Dr. Marian G. Little, Technical Services Veterinarian, American Regent, Inc.
YOUR COMPLETE SOURCE FOR MEDICAL MISSIONS SUPPLIES.
 YOUR COMPLETE SOURCE FOR MEDICAL MISSIONS SUPPLIES. When disasters strike, Blessings International provides pharmaceuticals and medical supplies quickly, so you can bring healing to the hurting. Where
YOUR COMPLETE SOURCE FOR MEDICAL MISSIONS SUPPLIES. When disasters strike, Blessings International provides pharmaceuticals and medical supplies quickly, so you can bring healing to the hurting. Where
Mobility Issues and Arthritis
 Mobility Issues and Arthritis 1. Overview of end stage of the disease. Mobility issues are often attributed to normal aging by pet owners, and can have insidious symptoms as they may progress slowly without
Mobility Issues and Arthritis 1. Overview of end stage of the disease. Mobility issues are often attributed to normal aging by pet owners, and can have insidious symptoms as they may progress slowly without
Stimulant. Alpha adrenergic antagonist. Non-steroidal anti-inflammatory drug. Budesonide Corticosteroid (New category for 2018)
 SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Acepromazine Acetazolamide Carbonic Anhydrase Inhibitor Adrenaline Adrenocorticotropic hormone (ACTH) Albuterol (Salbutamol) Altrenogest (in males
SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Acepromazine Acetazolamide Carbonic Anhydrase Inhibitor Adrenaline Adrenocorticotropic hormone (ACTH) Albuterol (Salbutamol) Altrenogest (in males
CONCORD DRUGS LIMITED Hyderabad
 CONCORD DRUGS LIMITED Hyderabad Product List (Injectables) Tissue Bio Adhesive Product Name Packing Strength/ Each Ampoule Therapeutic use Iso Amyl 2-Cyano Acrylate Ampoule 0.25, 0.50 and 1.0 ml. Bio-Adhesive
CONCORD DRUGS LIMITED Hyderabad Product List (Injectables) Tissue Bio Adhesive Product Name Packing Strength/ Each Ampoule Therapeutic use Iso Amyl 2-Cyano Acrylate Ampoule 0.25, 0.50 and 1.0 ml. Bio-Adhesive
Cascade. Guide for veterinarians if NO authorised medicinal product is available. Federation of Veterinarians of Europe
 Cascade Guide for veterinarians if authorised medicinal product is available Federation of Veterinarians of Europe FOOD PRODUCING ANIMALS & COMPANION ANIMALS Is there an authorised product for this species
Cascade Guide for veterinarians if authorised medicinal product is available Federation of Veterinarians of Europe FOOD PRODUCING ANIMALS & COMPANION ANIMALS Is there an authorised product for this species
Prescription Label. Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long):
 Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site:
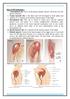 Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
BIO4 Antibiotics Expert Committee
 ANTIBIOTICS-MICROBIAL ASSAYS AMOXICILLIN INTRAMAMMARY INFUSION AMPHOTERICIN B AMPHOTERICIN B CREAM AMPHOTERICIN B FOR INJECTION AMPHOTERICIN B LOTION AMPHOTERICIN B BACITRACIN BIO4 Antibiotics Expert
ANTIBIOTICS-MICROBIAL ASSAYS AMOXICILLIN INTRAMAMMARY INFUSION AMPHOTERICIN B AMPHOTERICIN B CREAM AMPHOTERICIN B FOR INJECTION AMPHOTERICIN B LOTION AMPHOTERICIN B BACITRACIN BIO4 Antibiotics Expert
We would love to work with you so you can prescribe the best treatments for your patients.
 The following represents our main compounded medications and forms. If you do not see what you re looking for, please contact us as we may be able to help. We would love to work with you so you can prescribe
The following represents our main compounded medications and forms. If you do not see what you re looking for, please contact us as we may be able to help. We would love to work with you so you can prescribe
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 1996L0022 EN 18.12.2008 002.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COUNCIL DIRECTIVE 96/22/EC of 29 April 1996 concerning
1996L0022 EN 18.12.2008 002.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COUNCIL DIRECTIVE 96/22/EC of 29 April 1996 concerning
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 1996L0022 EN 18.12.2008 002.001 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COUNCIL DIRECTIVE 96/22/EC of 29 April 1996 concerning
1996L0022 EN 18.12.2008 002.001 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COUNCIL DIRECTIVE 96/22/EC of 29 April 1996 concerning
No July 2000 REGULATION. respecting veterinarians authorisations to prescribe drugs SECTION II
 REGULATION respecting veterinarians authorisations to prescribe drugs SECTION I Scope and definitions Article 1 This Regulation contains special provisions applying to veterinarians authorisations to prescribe
REGULATION respecting veterinarians authorisations to prescribe drugs SECTION I Scope and definitions Article 1 This Regulation contains special provisions applying to veterinarians authorisations to prescribe
Clinical Guidelines for Veterinarians Treating the Non Racing Performance Horse
 Clinical Guidelines for Veterinarians Treating the Non Racing Performance Horse American Association of Equine Practitioners 4075 Iron Works Parkway Lexington, KY 40511 (859) 233-0147 www.aaep.org Introduction
Clinical Guidelines for Veterinarians Treating the Non Racing Performance Horse American Association of Equine Practitioners 4075 Iron Works Parkway Lexington, KY 40511 (859) 233-0147 www.aaep.org Introduction
Mixtures of veterinary medicinal compounds in manured soils
 Workshop Pharmaceuticals in Soil, Sludge and Slurry Mixtures of veterinary medicinal compounds in manured soils Nadine Tauchnitz Daniela Gildemeister, Silvia Berkner Dessau-Roßlau, 18th June to 19th June
Workshop Pharmaceuticals in Soil, Sludge and Slurry Mixtures of veterinary medicinal compounds in manured soils Nadine Tauchnitz Daniela Gildemeister, Silvia Berkner Dessau-Roßlau, 18th June to 19th June
Procedure # IBT IACUC Approval: December 11, 2017
 IACUC Procedure: Anesthetics and Analgesics Procedure # IBT-222.04 IACUC Approval: December 11, 2017 Purpose: The purpose is to define the anesthetics and analgesics that may be used in mice and rats.
IACUC Procedure: Anesthetics and Analgesics Procedure # IBT-222.04 IACUC Approval: December 11, 2017 Purpose: The purpose is to define the anesthetics and analgesics that may be used in mice and rats.
N.C. A and T List of Approved Analgesics 1 of 5
 1 of 5 Note to user: This list of commonly used analgesics and sedatives is not all-inclusive. The absence of an agent does not necessarily mean it is unacceptable. For any questions, call the Clinical
1 of 5 Note to user: This list of commonly used analgesics and sedatives is not all-inclusive. The absence of an agent does not necessarily mean it is unacceptable. For any questions, call the Clinical
QUALITY HEALTH CARE YOUR PREFERRED PARTNER IN. For better health
 YOUR PREFERRED PARTNER IN QUALITY HEALTH CARE For better health Manufacturer of Pharmaceutical Products Eka Pharma 308, Samanvay Zillion, Gotri - Sevasi Road, Opp. Shaishav School, Vadodara-391 101, (Guj.)
YOUR PREFERRED PARTNER IN QUALITY HEALTH CARE For better health Manufacturer of Pharmaceutical Products Eka Pharma 308, Samanvay Zillion, Gotri - Sevasi Road, Opp. Shaishav School, Vadodara-391 101, (Guj.)
Geevet Remedies.
 +91-8048764671 Geevet Remedies https://www.geevet.com/ Geevet Remedies a leading Gujarat based company providing solutions in manufacturing, supplying and export of veterinary medicinal products and veterinary
+91-8048764671 Geevet Remedies https://www.geevet.com/ Geevet Remedies a leading Gujarat based company providing solutions in manufacturing, supplying and export of veterinary medicinal products and veterinary
Ear drops suspension. A smooth, uniform, white to off-white viscous suspension.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT OTOMAX EAR DROPS SUSPENSION 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of the veterinary medicinal product contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT OTOMAX EAR DROPS SUSPENSION 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of the veterinary medicinal product contains:
SUMMARY OF PRODUCT CHARACTERISTICS. 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Emdocam 20 mg/ml solution for injection for cattle, pigs and horses
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Emdocam 20 mg/ml solution for injection for cattle, pigs and horses 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Emdocam 20 mg/ml solution for injection for cattle, pigs and horses 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
Delegating to Auxiliaries in Food Animal & Equine Practice
 Delegating to Auxiliaries in Food Animal & Equine Practice Approved by Council: June 2004; September 2006; June 2011 Indirect definition modified June 9, 2010 Publication Date: Update September 2004, Website
Delegating to Auxiliaries in Food Animal & Equine Practice Approved by Council: June 2004; September 2006; June 2011 Indirect definition modified June 9, 2010 Publication Date: Update September 2004, Website
The Institutional Animal Care and Use Committee (IACUC) Aquatic Animals: Analgesia and Anesthesia formulary
 The Institutional Animal Care and Use Committee (IACUC) Aquatic Animals: Analgesia and Anesthesia formulary The appropriate use of pain medications (analgesics) and anesthetics is a critical aspect of
The Institutional Animal Care and Use Committee (IACUC) Aquatic Animals: Analgesia and Anesthesia formulary The appropriate use of pain medications (analgesics) and anesthetics is a critical aspect of
Knowledge list Avian/Exotic Pharmacology and Commonly Used Drugs
 Knowledge list Avian/Exotic Pharmacology and Commonly Used Drugs For each species on the Species List, the following pharmacology topics should be mastered. Knowledge of potential drug side effects and
Knowledge list Avian/Exotic Pharmacology and Commonly Used Drugs For each species on the Species List, the following pharmacology topics should be mastered. Knowledge of potential drug side effects and
Part 1 : General multiple-choice questions
 Examples of exam questions ECVPT examination Part 1 : General multiple-choice questions It possible that all answers are technically correct, but there is only one answer that fits best. Choose that answer.
Examples of exam questions ECVPT examination Part 1 : General multiple-choice questions It possible that all answers are technically correct, but there is only one answer that fits best. Choose that answer.
Beef Producers. The Judicious Use of Antimicrobials for
 The Judicious Use of Antimicrobials for Beef Producers Introduction The production of safe and wholesome animal products for human consumption is a primary goal of beef producers. To achieve that goal,
The Judicious Use of Antimicrobials for Beef Producers Introduction The production of safe and wholesome animal products for human consumption is a primary goal of beef producers. To achieve that goal,
ALPHABETICAL REFERENCE GUIDE
 ACEPROMAZINE ACETAZOLAMIDE ALBUTEROL SULFATE ALENDRONATE ALLOPURINOL ALUMINUM HYDROXIDE AMANTADINE AMINOCAPROIC ACID AMINOPHYLLINE AMITRIPTYLINE AMITRIPTYLINE/PREDNISOLONE COMBINATION AMLODIPINE AMLODIPINE/BENAZEPRIL
ACEPROMAZINE ACETAZOLAMIDE ALBUTEROL SULFATE ALENDRONATE ALLOPURINOL ALUMINUM HYDROXIDE AMANTADINE AMINOCAPROIC ACID AMINOPHYLLINE AMITRIPTYLINE AMITRIPTYLINE/PREDNISOLONE COMBINATION AMLODIPINE AMLODIPINE/BENAZEPRIL
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Xylacare 2% w/v Solution for Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Qualitative composition
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Xylacare 2% w/v Solution for Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Qualitative composition
Pain management in equine patients therapy options
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Pain management in equine patients therapy options Author : Tom Hughes Categories : Equine, Vets Date : July 13, 2015 Drugs
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Pain management in equine patients therapy options Author : Tom Hughes Categories : Equine, Vets Date : July 13, 2015 Drugs
Metacam 1.5 mg/ml oral suspension for dogs
 Metacam 1.5 mg/ml oral suspension for dogs Species:Dogs Therapeutic indication:pharmaceuticals: Neurological preparations: Analgesics, Other NSAIDs, Locomotor (including navicular and osteoarthritis) Active
Metacam 1.5 mg/ml oral suspension for dogs Species:Dogs Therapeutic indication:pharmaceuticals: Neurological preparations: Analgesics, Other NSAIDs, Locomotor (including navicular and osteoarthritis) Active
COS List. Page 1. trading & distribution excellence since Product CEP no. CoS status Country. Acamprosate OBTAINED Korea
 Page 1 trading & distribution excellence since 1986 Acamprosate 2001-160 OBTAINED Korea Acarbose* pending pending China Acetyl Salicylic Acid (Aspirin) * 2001-399 OBTAINED China Acyclovir 2001-283 OBTAINED
Page 1 trading & distribution excellence since 1986 Acamprosate 2001-160 OBTAINED Korea Acarbose* pending pending China Acetyl Salicylic Acid (Aspirin) * 2001-399 OBTAINED China Acyclovir 2001-283 OBTAINED
(Amended ) Page 1 of 34
 (Amended 01.04.2018) Page 1 of 34 This treatment Record Book belongs to _ (Trainer) of (Kennel address) The aim of this treatment book is for registered persons to meet the requirements of GAR84A - Treatment
(Amended 01.04.2018) Page 1 of 34 This treatment Record Book belongs to _ (Trainer) of (Kennel address) The aim of this treatment book is for registered persons to meet the requirements of GAR84A - Treatment
Drugs in the show ring: What you don t know can hurt your horse
 Drugs in the show ring: What you don t know can hurt your horse By Beth Minnich As introduced in last month s installment in this series on drugs in the show ring, rules regarding the use of drugs, medications,
Drugs in the show ring: What you don t know can hurt your horse By Beth Minnich As introduced in last month s installment in this series on drugs in the show ring, rules regarding the use of drugs, medications,
Pain Management in Racing Greyhounds
 Pain Management in Racing Greyhounds Pain Pain is a syndrome consisting of multiple organ system responses, and if left untreated will contribute to patient morbidity and mortality. Greyhounds incur a
Pain Management in Racing Greyhounds Pain Pain is a syndrome consisting of multiple organ system responses, and if left untreated will contribute to patient morbidity and mortality. Greyhounds incur a
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Melosolute 20 mg/ml solution for injection for cattle, pigs and horses. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Melosolute 20 mg/ml solution for injection for cattle, pigs and horses. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
CLINICAL MASTITIS PERCEPTIONS OF KANSAS DAIRY PRODUCERS. J.R. Roberson 1
 Dairy Day 2003 CLINICAL MASTITIS PERCEPTIONS OF KANSAS DAIRY PRODUCERS J.R. Roberson 1 Summary Mastitis is considered the most costly disease in the U.S. dairy industry. Treatment of clinical mastitis
Dairy Day 2003 CLINICAL MASTITIS PERCEPTIONS OF KANSAS DAIRY PRODUCERS J.R. Roberson 1 Summary Mastitis is considered the most costly disease in the U.S. dairy industry. Treatment of clinical mastitis
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, CZ, EE, ES, FR, IE, IS, IT, LT, LU, LV, NO, PL, PT, RO, SE, SI, SK, UK: Genestran 75 micrograms/ml solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, CZ, EE, ES, FR, IE, IS, IT, LT, LU, LV, NO, PL, PT, RO, SE, SI, SK, UK: Genestran 75 micrograms/ml solution for injection
T u l a n e U n i v e r s i t y I A C U C Guidelines for Rodent & Rabbit Anesthesia, Analgesia and Tranquilization & Euthanasia Methods
 T u l a n e U n i v e r s i t y I A C U C Guidelines for Rodent & Rabbit Anesthesia, Analgesia and Tranquilization & Euthanasia Methods Abbreviations: General Considerations IV = intravenous SC = subcutaneous
T u l a n e U n i v e r s i t y I A C U C Guidelines for Rodent & Rabbit Anesthesia, Analgesia and Tranquilization & Euthanasia Methods Abbreviations: General Considerations IV = intravenous SC = subcutaneous
Caledonia Classic 200 Mile Race: Rules
 Caledonia Classic 200 Mile Race: Rules 1. Mandatory Musher's Meeting: All Musher s must attend the meeting. It is recommended that all handlers attend the meeting. All entries must be paid in full prior
Caledonia Classic 200 Mile Race: Rules 1. Mandatory Musher's Meeting: All Musher s must attend the meeting. It is recommended that all handlers attend the meeting. All entries must be paid in full prior
Review of a Consequence of Highly Sensitive Drug Testing: The Need for Data on Analytical Pharmacological Relationships for Therapeutic Medications
 Review of a Consequence of Highly Sensitive Drug Testing: The Need for Data on Analytical Pharmacological Relationships for Therapeutic Medications Thomas Tobin, MRCVS, PhD; George D. Mundy, DVM; W. A.
Review of a Consequence of Highly Sensitive Drug Testing: The Need for Data on Analytical Pharmacological Relationships for Therapeutic Medications Thomas Tobin, MRCVS, PhD; George D. Mundy, DVM; W. A.
SCHEDULE P LIFE PERIOD OF DRUGS. [See rule 96] Conditions of storage
![SCHEDULE P LIFE PERIOD OF DRUGS. [See rule 96] Conditions of storage SCHEDULE P LIFE PERIOD OF DRUGS. [See rule 96] Conditions of storage](/thumbs/81/83448626.jpg) SCHEDULE P [See rule 96] LIFE PERIOD OF DRUGS No. Name of the Drug Period in months (unless otherwise specified) between date of manufacture and the date of expiry which the labelled potency period of
SCHEDULE P [See rule 96] LIFE PERIOD OF DRUGS No. Name of the Drug Period in months (unless otherwise specified) between date of manufacture and the date of expiry which the labelled potency period of
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Rycarfa 100 mg tablets for dogs (BE, DE, ES, FR, IE, IT, NL, PT, UK) Rycarfa vet 100 mg tablets for dogs (DK, FI) Carprox
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Rycarfa 100 mg tablets for dogs (BE, DE, ES, FR, IE, IT, NL, PT, UK) Rycarfa vet 100 mg tablets for dogs (DK, FI) Carprox
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Rifen 100 mg/ml solution for injection for horses, cattle and swine. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Rifen 100 mg/ml solution for injection for horses, cattle and swine. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains:
NSAIDs Are You Following the Rules?
 NSAIDs Are You Following the Rules? As equestrians, we expect a lot from our performance horses. Sometimes pain and inflammation of their joints can happen right before a show or competition. Before administering
NSAIDs Are You Following the Rules? As equestrians, we expect a lot from our performance horses. Sometimes pain and inflammation of their joints can happen right before a show or competition. Before administering
2012 guidelines for DRUGS AND MEDICATIONS
 2012 guidelines for DRUGS AND MEDICATIONS 800.633.2472 LAST REVISED NOVEMBER 1, 2011 Please direct all inquires to: United States Equestrian Federation Equine Drugs and Medications Program 956 King Avenue,
2012 guidelines for DRUGS AND MEDICATIONS 800.633.2472 LAST REVISED NOVEMBER 1, 2011 Please direct all inquires to: United States Equestrian Federation Equine Drugs and Medications Program 956 King Avenue,
GOBIERNO DE PUERTO RICO Administración de Servicios Médicos de Puerto Rico
 1 Acetylcysteine Solution 20% Vial 10ml 2 Acetylcysteine Solution 20% Vial 30ml 3 Activated Charcoal Bottle 4 fl oz or 8 fl oz 4 Adenosine 6mg/2ml Inj Solution Syringe / Vial 2ml 5 Albuterol Sulfate Inhalation
1 Acetylcysteine Solution 20% Vial 10ml 2 Acetylcysteine Solution 20% Vial 30ml 3 Activated Charcoal Bottle 4 fl oz or 8 fl oz 4 Adenosine 6mg/2ml Inj Solution Syringe / Vial 2ml 5 Albuterol Sulfate Inhalation
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Dipen 100ml Suspension for Injection for cattle, sheep and pigs 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Substance
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Dipen 100ml Suspension for Injection for cattle, sheep and pigs 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Substance
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Narketan-10 100 mg/ml Solution for Injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active substance
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Narketan-10 100 mg/ml Solution for Injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active substance
A Current Look at Navicular Syndrome. Patrick First, DVM
 A Current Look at Navicular Syndrome Patrick First, DVM Navicular syndrome is a broad term that is used to describe soreness or damage to the navicular bone and its surrounding structures in the equine
A Current Look at Navicular Syndrome Patrick First, DVM Navicular syndrome is a broad term that is used to describe soreness or damage to the navicular bone and its surrounding structures in the equine
50mg per 1000 tablets. 300mg per 1000 tablets. 100mg/ml 10ml per 100 amps. 200mg per 1000 tablets 400mg per 1000 tablets. 500mg per 1000 tablets
 info@diagimaging.com DIAGNOSTIC IMAGING LTD Elkington Lodge, Welford, Northants NN6 6HE UK T el +44 (0)845 226 0520 Fax +44 (0)845 226 0521 Acetazolamide Adrenaline injection 1mg/ml Albendazole Albendazole
info@diagimaging.com DIAGNOSTIC IMAGING LTD Elkington Lodge, Welford, Northants NN6 6HE UK T el +44 (0)845 226 0520 Fax +44 (0)845 226 0521 Acetazolamide Adrenaline injection 1mg/ml Albendazole Albendazole
POST-OPERATIVE ANALGESIA AND FORMULARIES
 POST-OPERATIVE ANALGESIA AND FORMULARIES An integral component of any animal protocol is the prevention or alleviation of pain or distress, such as that associated with surgical and other procedures. Pain
POST-OPERATIVE ANALGESIA AND FORMULARIES An integral component of any animal protocol is the prevention or alleviation of pain or distress, such as that associated with surgical and other procedures. Pain
For the treatment and prevention of infections caused by:
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYDECTIN 0.1 % W/V ORAL SOLUTION for sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Active substance Moxidectin
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYDECTIN 0.1 % W/V ORAL SOLUTION for sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Active substance Moxidectin
Drug Residue Antisera
 Drug Residue Antisera For all your assay development needs Growth promoters Antimicrobial drugs Anti-inflammatory drugs Anti-parasitic drugs Randox Life Sciences is a recognised primary manufacturer of
Drug Residue Antisera For all your assay development needs Growth promoters Antimicrobial drugs Anti-inflammatory drugs Anti-parasitic drugs Randox Life Sciences is a recognised primary manufacturer of
20mL, 50mL, 100mL. 20mL, 50mL, 100mL. 20mL, 50mL, 100mL. 20mL, 50mL, 100mL. 20mL, 50mL, 100mL. 50mL, 100mL. 50mL, 100mL.
 VETERINARY PHARMACEUTICALS PRODUCTS / ACTIVE INGREDIENTS PRESENTATION TARGET SPECIES ANTIBIOTICS ENROZOL SOLUTION FOR INJECTION ENROFLOXACIN 100 mg/ml ZOLIGEN SOLUTION FOR INJECTION GENTAMICIN SULPHATE
VETERINARY PHARMACEUTICALS PRODUCTS / ACTIVE INGREDIENTS PRESENTATION TARGET SPECIES ANTIBIOTICS ENROZOL SOLUTION FOR INJECTION ENROFLOXACIN 100 mg/ml ZOLIGEN SOLUTION FOR INJECTION GENTAMICIN SULPHATE
Residues. Mike Apley, DVM, PhD
 Residues Mike Apley, DVM, PhD Residues: It s Black and White Residues occur when detected concentrations of the marker residue are above the approved tolerance for that drug in that tissue. Residues are
Residues Mike Apley, DVM, PhD Residues: It s Black and White Residues occur when detected concentrations of the marker residue are above the approved tolerance for that drug in that tissue. Residues are
Care of the Equine Athlete
 Care of the Equine Athlete Mark T. Reilly, DVM, Dipl. ABVP (Equine) Linda J. Cimetti, DVM South Shore Equine Clinic & Diagnostic Center South Shore Equine Clinic & Diagnostic Center 151 Palmer Road Plympton,
Care of the Equine Athlete Mark T. Reilly, DVM, Dipl. ABVP (Equine) Linda J. Cimetti, DVM South Shore Equine Clinic & Diagnostic Center South Shore Equine Clinic & Diagnostic Center 151 Palmer Road Plympton,
Prohibited Substances The NCHA rules do not allow and medications that can
 A. A contestant may directly to the monitor(s) provided a liaison rer)re"en:tat is also present. other conversation will be limited to the of normal greetings a show, B. A contestant or other person will
A. A contestant may directly to the monitor(s) provided a liaison rer)re"en:tat is also present. other conversation will be limited to the of normal greetings a show, B. A contestant or other person will
Medicine / Pharmaceuticals CATALOGUE
 Medicine / Pharmaceuticals CATALOGUE YOUR COMPLETE VETERINARY SUPPLY AND SUPPORT SOLUTION Cats / 250mg x 100 tablets 500mg x 100 tablets CLAVET TABLETS E000973 E000981 Indicated for the treatment of infections
Medicine / Pharmaceuticals CATALOGUE YOUR COMPLETE VETERINARY SUPPLY AND SUPPORT SOLUTION Cats / 250mg x 100 tablets 500mg x 100 tablets CLAVET TABLETS E000973 E000981 Indicated for the treatment of infections
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Pro Penstrep Suspension for Injection for Cattle, Sheep and Pigs. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Pro Penstrep Suspension for Injection for Cattle, Sheep and Pigs. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Guidelines for Classification of Prohibited Substances
 Guidelines for Classification of Prohibited Substances Classification of Prohibited Substances in specimens collected on race day This classification document is intended as a guideline only to assist
Guidelines for Classification of Prohibited Substances Classification of Prohibited Substances in specimens collected on race day This classification document is intended as a guideline only to assist
Day 90 Labelling, PL LABELLING AND PACKAGE LEAFLET
 LABELLING AND PACKAGE LEAFLET A. LABELLING PARTICULARS TO APPEAR ON THE OUTER PACKAGE : Carton 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Alvegesic vet. 10 mg/ml Solution for injection for Horses, Dogs
LABELLING AND PACKAGE LEAFLET A. LABELLING PARTICULARS TO APPEAR ON THE OUTER PACKAGE : Carton 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Alvegesic vet. 10 mg/ml Solution for injection for Horses, Dogs
2011 guidelines for DRUGS AND MEDICATIONS
 2011 guidelines for DRUGS AND MEDICATIONS 800.633.2472 LAST REVISED MAY 15, 2011 Please direct all inquires to: United States Equestrian Federation Equine Drugs and Medications Program 956 King Avenue,
2011 guidelines for DRUGS AND MEDICATIONS 800.633.2472 LAST REVISED MAY 15, 2011 Please direct all inquires to: United States Equestrian Federation Equine Drugs and Medications Program 956 King Avenue,
A New Advancement in Anesthesia. Your clear choice for induction.
 A New Advancement in Anesthesia Your clear choice for induction. By Kirby Pasloske When using Alfaxan, patients should be continuously monitored, and facilities for maintenance of a patent airway, artificial
A New Advancement in Anesthesia Your clear choice for induction. By Kirby Pasloske When using Alfaxan, patients should be continuously monitored, and facilities for maintenance of a patent airway, artificial
