Ultrastructure of Spermiogenesis in the American Alligator, Alligator mississippiensis (Reptilia, Crocodylia, Alligatoridae)
|
|
|
- Theodora Phelps
- 6 years ago
- Views:
Transcription
1 JOURNAL OF MORPHOLOGY 271: (2010) Ultrastructure of Spermiogenesis in the American Alligator, Alligator mississippiensis (Reptilia, Crocodylia, Alligatoridae) Kevin M. Gribbins, 1 * Dustin S. Siegel, 2 Marla L. Anzalone, 1 Daniel P. Jackson, 1 Katherine J. Venable, 1 Justin L. Rheubert, 3 and Ruth M. Elsey 4 1 Department of Biology, Wittenberg University, Springfield, Ohio Department of Biology, Saint Louis University, St. Louis, Missouri Department of Biological Sciences, Southeastern Louisiana University, Hammond, Louisiana Louisiana Department of Wildlife and Fisheries, Rockefeller Wildlife Refuge, Grand Chenier, Louisiana ABSTRACT Testicular samples were collected to describe the ultrastructure of spermiogenisis in Alligator mississipiensis (American Alligator). Spermiogenesis commences with an acrosome vesicle forming from Golgi transport vesicles. An acrosome granule forms during vesicle contact with the nucleus, and remains posterior until mid to late elongation when it diffuses uniformly throughout the acrosomal lumen. The nucleus has uniform diffuse chromatin with small indices of heterochromatin, and the condensation of DNA is granular. The subacrosome space develops early, enlarges during elongation, and accumulates a thick layer of dark staining granules. Once the acrosome has completed its development, the nucleus of the early elongating spermatid becomes associated with thecellmembraneflatteningtheacrosomevesicleonthe apical surface of the nucleus, which aids in the migration of the acrosomal shoulders laterally. One endonuclear canal is present where the perforatorium resides. A prominent longitudinal manchette is associated with the nuclei of late elongating spermatids, and less numerous circular microtubules are observed close to the acrosome complex. The microtubule doublets of the midpiece axoneme are surrounded by a layer of dense staining granular material. The mitochondria of the midpiece abut the proximal centriole resulting in a very short neck region, and possess tubular cristae internally and concentric layers of cristae superficially. A fibrous sheath surrounds only the axoneme of the principal piece. Characters not previously described during spermiogenesis in any other amniote are observed and include (1) an endoplasmic reticulum cap during early acrosome development, (2) a concentric ring of endoplasmic reticulum around the nucleus of early to middle elongating spermatids, (3) a band of endoplasmic reticulum around the acrosome complex of late developing elongate spermatids, and (4) midpiece mitochondria that have both tubular and concentric layers of cristae. J. Morphol. 271: , Ó 2010 Wiley-Liss, Inc. KEY WORDS: spermatids; alligator; ultrastructure; spermiogenesis; acrosome INTRODUCTION Members of the Crocodylia are large, oviparous, and aquatic (fresh, brackish, or salt water) vertebrates distributed in subtropic and tropic zones (Ferguson, 1985). Males copulate with females via insertion of a single penis into the female cloaca (Gadow, 1887), and sperm is stored in the female oviduct (Gist et al., 2008; Bagwill et al., 2009) until ovulation. Eggs are deposited in a hole or mound (Thorbjarnarson, 1996) and sex is determined by nest temperature in all species of Crocodylia investigated (Lang and Andrews, 1994). Based on morphological data, extant Crocodylia can be broken up into the Alligatoroidea (alligators and caiman), Crocodylinae (crocodiles), Tomistominae (false gavial), and Gavialinae (true gavials; Gatesy et al., 2004). Mature sperm ultrastructure has been described in two Crocodylia members, Caiman crocodylus (spectacled caiman; Saita et al., 1987) and Crocodylus johnsoni (freshwater crocodile; Jamieson et al., 1997). Ultrastructural changes that occur during spermiogenesis within Crocodylia testes have been described for C. crocodylus (Saita et al., 1987) and Alligator sinensis (chinese alligator; Wang et al., 2008). No additional ultrastructural investigations exist on the sperm structure or spermiogenic stages of other Crocodylia taxa; however, Gribbins et al. (2006) evaluated the cytology of spermatogenesis in Alligator mississipiensis (American alligator) in an effort to describe the germ cell developmental strategy of this species. Also, Moore et al. (2009) illustrates the development of the testes in post-hatchling juveniles. Contract grant sponsor: Wittenberg Summer Undergraduate Research Grants. *Correspondence to: Kevin M. Gribbins, Department of Biology, Wittenberg University, PO Box 720, Springfield, OH kgribbins@wittenberg.edu Received 5 January 2010; Revised 4 March 2010; Accepted 6 March 2010 Published online 16 August 2010 in Wiley Online Library (wileyonlinelibrary.com) DOI: /jmor Ó 2010 WILEY-LISS, INC.
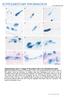
2 Interestingly, unlike their fellow Archosaurs (birds), A. mississipiensis and A. sinensis have a germ cell development strategy more similar to other members of the paraphyletic Reptilia (Gribbins and Gist, 2003; Gribbins et al., 2003, 2005, 2008; Wang et al., 2008; Rheubert et al., 2009), and thus, A. mississipiensis has a pleisiomorphiclike (temporal) germ cell development strategy. While sperm morphology is an invaluable tool for comparative and phylogenetic analyses (review: Jamieson, 1995), few studies have investigated the effects of aquatic environmental toxicants on sperm development in amniotic models. It is well established that baseline data on the ultrastructural changes during spermiogenesis are necessary to understand how potential reproductive toxins (e.g., pesticides; Russell et al., 1990) alter spermatogenesis in vertebrates. These types of studies may provide data on sperm abnormalities upon exposure to man-made toxins and may be integral in future species conservation. Considering the affinity of members of the Crocodylia to water, these reptiles may represent sentinel species for histopathological studies on how potential aquatic reproductive toxicants, such as pesticides, affect spermatogenic output in the testis of semi-aquatic reptiles. Here we describe the ultrastructure of the spermiogenic stages in Alligator mississippiensis. We then compare these data with those published for Caiman crocodylus (Saita et al., 1987) and Alligator sinensis (Wang et al., 2008). We will also note any similarities or differences between alligator spermatids and the products of spermiogenesis within the testes of the Tuatara (Sphenodon punctatus; Healy and Jamieson, 1994), snakes (Gribbins et al., 2009), lizards (Clark, 1967; Dehlawi et al., 1992; Gribbins et al., 2007; Rheubert et al., in press), chelonians (Sprando and Russell, 1988; Healy and Jamieson, 1992; Al-Dokhi and Al-Wasel, 2001a,b, 2002; Zhang et al., 2007), and birds (Lin and Jones, 1993; Soley, 1997; Aire, 2007). MATERIALS AND METHODS Animal Collection Five adult male American Alligators (Alligator mississippiensis) between 2.5 and 3 m were collected during May 2002 and May 2003 (peak month of spermiogenesis: Gribbins et al., 2006) from the Rockefeller Wildlife Refuge in Grand Chenier, LA. Alligators were captured by noose or by baited hooks that were placed in canals within the refuge. Upon capture, alligators were sacrificed and the testes were removed immediately by dissection. The testes were submerged in Trump s Fixative (EMS, Hatfield, PA) and minced thoroughly into small pieces. The testicular pieces were then placed in fresh Trump s fixative and stored under refrigeration for at least 48 h (48C). Tissue Preparation Testicular tissues cut into 2-3 mm blocks were washed twice with cacodylate buffer (ph 7.0) for 20 min each. They were then SPERMIOGENESIS IN THE ALLIGATOR 1261 post-fixed in 2% osmium tetroxide for 2 h, washed with cacodylate buffer (ph 7.0) three times for 20 min each, dehydrated in a graded series of ethanol (70%, 85%, 95% X2, 100% X2), and cleared twice with 10 min treatments of propylene oxide. Each piece of alligator testis was then gradually introduced to epoxy resin (Embed 812, EMS, Hatfield, PA) (2:1 and 1:1 solutions of propylene oxide: epoxy resin). Testicular tissues were then placed in pure Embed 812 for 24 h. Fresh resin was prepared and the tissues were embedded in small beam capsules, and subsequently cured for 48 h at 708C in a Fisher isotemperature vacuum oven (Fisher Scientific, Pittsburg, PA). Sections (90 nm) were obtained by use of a diamond knife (DDK, Wilmington, DE) on an LKB automated ultramicrotome (LKB Produkter AB, Bromma, Sweden). Sections were then placed on copper grids and stained with uranyl acetate (18 min) and lead citrate (5 min). Ultrastructural Analysis Testicular samples were viewed using a Jeol JEM-1200EX II transmission electron microscope (Jeol). Micrographs were taken of representative spermatids and structural components associated with spermiogenesis via a Gatan 785 Erlangshen digital camera (Gatan, Warrendale, PA). The micrographs were then analyzed and composite plates were assembled using Adobe Photoshop CS (Adobe Systems, San Jose, CA). RESULTS Spermatids develop within the apical portion of the seminiferous epithelium (Fig. 1) of the seminiferous tubules of Alligator mississippiensis as described previously by Gribbins et al. (2006). Once spermiogenesis is completed mature spermatozoa are shed into the lumina of the seminiferous tubules for transport to the excurrent duct system. The beginning of spermiogenesis is marked by the accumulation of round spermatids within the seminiferous epithelium of A. mississippiensis upon the completion of meiosis. During these early stages, an acrosome vesicle forms (Fig. 2A,B, Av), and a juxtapositioned Golgi apparatus (Fig. 2C, black arrowhead) dominates the spermatid cytoplasm near the apex of the nucleus. Budding from the most proximal cisternum of the Golgi are transport vesicles (Fig. 2C, white arrowhead) that merge with the developing acrosome and are responsible for its increase in size during the round spermatid stage. The acrosome vesicle is not in contact with the nuclear membrane during the vesicle s early development (Fig. 2A,) and an acrosome granule is not seen within the vesicle until contact has been made with the nucleus (Fig. 2B, white arrowhead). The cytoplasm at this time is packed with mitochondria (Fig. 2A,B, white arrows) and abundant endoplasmic reticula (Fig. 2A, SR). As the acrosome grows in size, it collapses and flattens the apical surface of the round spermatid nucleus at the climax of round spermatid development (Fig. 2D, Av) and at this point the subacrosome space can be observed (Fig. 2D, black arrowhead). The most unusual feature of the early acrosome phase of development in the A. mississippiensis is the long thin smooth endoplasmic reticulum that cov-
3 1262 K.M. GRIBBINS ET AL. Fig. 1. Light microscope views of an April seminiferous tubule in the American Alligator testis. (A) Low power view of the seminiferous tubule in transverse section. The tubule has a lumen (L) where sperm will be released after the completion of spermiogenesis. The germinal epithelium is thick (black line) and contains developing germ cells and Sertoli cells (white arrow). Bar 5 50 lm. (B) High power view of the germinal epithelium showing generations of developing germ cells. The spermatogonia and spermatocytes (M) are located near the basement membrane (black arrowhead) at the periphery of the seminiferous tubule. The round spermatids (R), which are situated between the meiotic/mitotic cells and the elongating spermatids. The elongating spermatids (E) are located near the apex of the germinal epithelium in close proximity to the lumen (L). Bar 5 20 lm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.] ers the rostral acrosome, which we term the endoplasmic reticular cap (Fig. 2A C, black arrows). Towards the termination of the round spermatid stage, the most distal part of the nucleus starts to elongate (Fig. 2D). As the spermatid nucleus stretches, it takes on a cylindrical appearance (Fig. 3A, Nu). The same organelles, mitochondria (Fig. 3A, black arrow) and smooth endoplasmic reticulum (Fig. 3A, white arrowheads; 3B, black arrows), are observed within the cytoplasm of elongating spermatids as are seen within the round spermatid stage. The acrosome begins to envelop and migrate laterally along the apex of the nucleus (Fig. 3A C). The acrosome is sandwiched between the cell membrane (Fig. 3B, black arrowhead; 3C, white arrow) and the nuclear apex. The acrosome granule is large and basally located (Fig. 3B, white arrow; 3C, black arrowhead), and it appears that dense material from the granule is diffusing out into the acrosome vesicle lumen. There is a prominent and dark staining subacrosome space between the inner acrosome membrane and the apex of the nucleus (Fig. 3C, black arrow). During the elongation phase of spermiogenesis the nucleus contains mostly diffuse euchromatin with only a few pockets of dense staining heterochromatin (Fig. 3B, white arrowhead) randomly located within the nucleoplasm. A transverse view through the early elongate spermatid nucleus reveals that the smooth endoplasmic reticulum is organized in a concentric layer located adjacent to the nuclear membrane (Fig. 3D, white arrows). As elongation continues, the acrosome vesicle begins to envelop the nucleus by moving caudally along its lateral edges, and the acrosome granule has completely diffused throughout the acrosome lumen (Fig. 4A). This results in flocculent material within the lumen of the acrosome vesicle (Fig. 4A, *). Chromatin condensation has commenced and the chromatin begins to bead in a granular style (Fig. 4A, Nu). As the acrosome shoulders (Fig. 4A white arrow; 4B, *) migrate laterally they appear to squeeze down on the apex of the nucleus resulting in a thin papilla shaped apical head that will continue to thin into the mature rostrum of the nucleus (Fig. 4B). Within the developing rostrum
4 SPERMIOGENESIS IN THE ALLIGATOR 1263 Fig. 2. Round spermatids undergoing acrosome development during the early stages of spermiogenesis within the testes of Alligator mississippiensis. Common features of the round spermatids include smooth endoplasmic reticulum (SR) and mitochondria (white arrow) in the cytoplasm, and a thin band of smooth endoplasmic reticulum (black arrow) lying apical to the acrosomal vesicle (Av). (A) The acrosomal vesicle lies apical to the nucleus (Nu), but is not yet in contact with the nucleus. Bar 5 1 lm. (B) The acrosomal vesicle is in contact with the nucleus (Nu) and the acrosomal granule (white arrowhead) has now formed. Bar 5 1 lm. (C) Transport vesicles (black arrowhead) of the Golgi apparatus (black arrow) merging with the acrosomal vesicle. Bar lm. (D) The acrosome vesicle has collapsed and flattened the apical surface of the nucleus (Nu), and the subacrosomal space (black arrowhead) can now be observed. Bar 5 1 lm. an endonuclear canal starts to form and will house the perforatorium in more mature spermatids (Fig. 4A, white arrowhead; inset, white arrowhead; 4B, black arrow). The subacrosome space becomes more prominent and is filled with a condensed dark staining granular layer (Fig. 4B, white
5 1264 K.M. GRIBBINS ET AL. Fig. 3. Germ cells immediately after the round spermatid stage when spermatid elongation begins within the testes of Alligator mississippiensis. (A) Nucleus (Nu) elongating and capped by the acrosomal vesicle stretching posteriorly over the nucleus. Mitochondria (black arrow) and smooth endoplasmic reticulum (white arrowheads) are common in the cytoplasm. Bar 5 1 lm. (B) High magnification of the acrosomal region of the elongating spermatid demonstrating the dense material of the acrosomal granule (white arrow) diffusing within the lumen of the acrosomal vesicle. At this stage the acrosomae is sandwiched between the cell membrane (black arrow) and nuclear apex, and cisternae of smooth endoplasmic reticulum are present cytoplasmicaly (black arrows). During elongation the nucleus (Nu) is euchromatic with pockets of dense staining chromatin (white arrowhead). Bar 5 1 lm. (C) Apex of the nucleus (Nu) highlighting the orientation of the nucleus, cell membrane (white arrow), acrosomal vesicle (Av), and acrosomal granule (black arrowhead) in an early elongating spermatid. Subacrsome space (black arrow). Bar lm. (D) Transverse section through an early elongating spermatid nucleus (Nu) showing the concentric layers of smooth endoplasmic reticulum (white arrow) organized adjacent to the nuclear membrane. The axoneme (black arrowhead) can also be observed abutting the cell membrane, and mitochondria (black arrow) are scattered throughout the early elongating spermatid cytoplasm. Sertoli cell process (Sp). Bar 5 1 lm.
6 SPERMIOGENESIS IN THE ALLIGATOR 1265 endonuclear canal and the enclosed developing perforatorium have increased in length and span the entire immature rostrum, and they extend all the way to the tip of the nucleus (Fig. 5, white arrowheads). During late elongation the chromatin becomes more condensed and large chromatin granules are closer together and little to no nucleoplasm is observed (Fig. 6A, NU). The microtubules of the manchette (Fig. 6B, black arrows and D, white arrows) become evident on the lateral aspects of the nucleus as the spermatids continue to thin. Fig. 4. Spermatids in the middle stages of elongation within the testes of Alligator mississippiensis. (A) The acrosomal vesicle moves caudally and envelops the nucleus (Nu) along its lateral edges, and the acrosome shoulders (white arrows) appear to squeeze the apex of the nucleus. The acrosomal granule has completely diffused throughout the acrosome lumen creating a flocculent material (*) within the acrosome vesicle. An endonuclear canal (white arrowheads) forms within the developing rostrum of the nucleus (inset is a spermatid in transverse section showing the endonuclear canal [white arrowhead] and the acrosomal space filled with flocculent material [black arrow]). Bar 5 1 lm. (B) High magnification of the nuclear apex highlighting the orientation of the nucleus (Nu), cell membrane (white arrowhead), subacrosomal space (white arrow), and acrosome shoulders (*) in sagittal section of a spermatid during middle elongation. Bar 5 1 lm. arrow). At the peak of elongation, the acrosome vesicle envelops the entire nuclear apex and the developing rostrum is triangular in shape (Fig. 5, black arrows). The nucleus has reached its final length of over 25 lm (Fig. 5, black brackets). The Fig. 5. Highly elongated spermatids during the climax of elongation within the testes of Alligator mississippiensis. The rostrum of the spermatid (black arrows) is triangular in shape and the acrosome shoulders (white arrows) have reached their most posterior position. The entire nucleus (bracket) is over 25 lm in length, and the endonuclear canal and perforatorium (white arrowheads) span the entire immature rostrum and extend to the tip of the nucleus. Bar 5 2 lm.
7 1266 K.M. GRIBBINS ET AL. Fig. 6. Spermatids in late elongation/condensation within the testes of Alligator mississippiensis. (A) The chromatin becomes more condensed in the nucleus (Nu) and forms large chromatin granules. Circular microtubules (black arrow) are located around the nucleus just caudal to the acrosome shoulders (white *). The endonuclear canal (white arrow) becomes enlarged and the microtubules of the perforatorium (white arrowhead) can be seen within the canal. The subacrosome space (black *) becomes enlarged and is filled with dense granular material. There is a thin clear lucent zone between the granular layer and the inner acrosome membrane (black arrowhead). Bar 5 1 lm. (B) Parallel microtubules of the manchette (black arrows) surrounding the entire length of the nucleus (Nu), and the proximal centriole (white arrowhead) lying in the nuclear fossa. Bar 5 1 lm. (C) The distal centriole (white arrow), posterior to the proximal centriole (white arrowhead), elongating into the neck axoneme (black arrowhead), while the annular ring (black arrows) migrates away from the nucleus. Bar 5 1 lm. (D) Trasnverse section through a spermatid in late elongation highlighting the orientation of the nucleus (white *), endonuclear lacuna (white arrowhead), manchette (white arrows), mitochondria (black arrows), and Sertoli cell process (Sp). Bar 5 1 lm. (E) Mitochondria (white arrowheads) are arranged in a single row between the nucleus and annulus (white arrows). The midpiece of the flagellum is surrounded by Sertoli cell processes forming the flagellar tunnel (black arrows) as the flagellum continues posterior to the annulus (principal piece) and is surrounded by the fibrous sheath (black arrowhead). Bar 5 1 lm. Most of the manchette is made up of parallel microtubules (Fig. 6B, black arrows) that can be found surrounding the length of the nucleus beginning just caudally to the shoulders (Fig. 6A, white *) of the acrosomal vesicle. There are also circular microtubules (though they are less numerous and organized) either observed earlier in elongation or more frequently along the region of the nucleus just under the acrosome shoulders (Fig. 6A, black arrow). The endonuclear canal (Fig. 6A, white arrow) has enlarged and the dark staining perforatorium (Fig. 6A, white arrowhead) can been seen within the canal. The nucleus is reduced into a thinner rostrum apically, which extends up into the acrosome complex (Fig. 6A). The subacrosome space is also enlarged and a dense staining granular layer occupies this space (Fig. 6A, *). However, there is a thin lucent layer within the subacrosomal space just under the inner acrosome membrane that lacks dark staining granules (Fig. 6A, black arrowhead). The proximal centriole can be seen within the nuclear fossa caudally (Fig. 6 B,C, white arrowheads). As the distal centriole (Fig. 6C, white arrow) elongates to form the neck axoneme (Fig. 6C, black arrowhead), the attached annular ring (Fig. 6C, black arrows) migrates away from
8 SPERMIOGENESIS IN THE ALLIGATOR 1267 Fig. 7. Spermatids in the final stage of spermiogenesis within the testes of Alligator mississippiensis. (A) (G) on the medial sagittal section represent the approximate location where the peripheral transverse sections were taken from. Sagittal section. Proximal centriole (black arrow). Bar 5 1 lm. (A) The tip of the acrosome demonstrating the dark staining cortex (black arrow) and lighter stating medulla (M) surrounded by the multilaminar layers of the Sertoli cell membrane (white arrowhead) and smooth endoplasmic reticulum [white arrow; labeled identically in (B) (D)]. (B) The acrosome (black arrow) rests upon a dense subacrosome space (Sa). (C) An epinuclear lucent zone (white arrowhead) rests on the tip of the nuclear rostrum within the subacrosome space (white arrowhead), lying medial to the acrosome (black arrow). (D) Medial to the acrosome (black arrow) and subacrosome space (*) lays the rostrum (white *), in which microtubules of the perforatorium reside within the endonuclear canal (white arrowhead). (E) The body of the nucleus (Nu) is surrounded by parallel microtubules of the manchette (black arrow). (F) Large mitochondria (white arrow), with tubular cristae surrounded by concentric layers of cristae, surround the axoneme and dark staining columns (black arrow). (G) A fibrous sheath (black arrow) internal to the cell membrane (black arrowhead) surrounds the axoneme (white arrow) in the principal piece of the spermatid. (H) The endpiece axoneme (black arrow) lacks a fibrous sheath and is encompassed only by the cell membrane (black arrowhead). Bars lm. the nucleus. Mitochondria also migrate (Fig. 6D, black arrow) toward the developing flagellum caudally and move in the direction of the annulus (Fig. 6E, white arrows) and away from the nucleus, and arrange themselves in a single row (Fig. 6E, white arrowhead) in sagittal section. The Sertoli cell processes enveloping late elongate spermatids extend caudally to the midpiece of the flagellum forming the flagellar tunnel (Fig. 6E, black arrows). The flagellum continues to elongate caudally past the annulus and becomes surrounded by a surplus of fibrous blocks (Fig. 6E, black arrowhead) creating a fibrous sheath around the principal piece of the flagellum. The axoneme of the midpiece, principal piece, and endpiece is made up of the typical arrangement of microtubule doublets. The final stage of spermiogenesis demonstrates many of the presumed mature structures that will be present within the American Alligator sperma-
9 1268 K.M. GRIBBINS ET AL. tozoa. This final stage represents the climax of spermiogenesis and upon completion of development the spermatids will be transferred to the lumina of the seminiferous tubules during spermiation. The acrosomal tip/body is slightly oblong in shape (Fig. 7A) and sits on the subacrosome space (Fig. 7B, Sa), and a thin nuclear rostrum extends into the subacrosome space (Fig. 7D, white *). The main body of the acrosome has a lighter staining medulla (Fig. 7A,M) and a dark staining thin cortex (Fig. 7A, black arrow). Multilaminar layers of Sertoli cell membrane (Fig. 7A, white arrowhead) are common around the acrosome complex of these late developing spermatids. In these late developing spermatids, a ring of smooth ER is common around the acrosome complex (Fig. 7B D, white arrow). A small epinuclear lucent zone rests on the tip of the nuclear rostrum (Fig. 7C, white arrowhead) within the subascrosome space (Fig. 7C, white asterisk). Within the nuclear rostrum (Fig. 7D, white asterisk) of a mature spermatid, a distinct endonuclear canal (Fig. 7D, white arrowhead) exists and has the enclosed perforatorium. The body of the nucleus is cylindrical and is surrounded by the parallel microtubules of the manchette (Fig. 7E, black arrow). The midpiece has large mitochondria that have tubular cristae internally and concentric layers of membranes externally (Fig. 7F, white arrow) surrounding the axoneme (Fig. 7F, black arrow). The midpiece axoneme lacks a fibrous sheath; however, the microtubule doublets of the midpiece are surrounded by dark staining granular columns (Fig. 7F, black arrow). The principal piece beyond the annular ring does have a fibrous sheath that is seen in cross section (Fig. 7G, black arrow), and the endpiece is easily distinguished from the principal piece, as it lacks a fibrous sheath around its axoneme (Fig. 7H, black arrow). DISCUSSION Most of the ultrastructure features of spermiogenesis within Alligator mississippiensis are similar to those described for other reptilian sauropsids. The early development of the acrosome complex within the A. mississippiensis testes is akin to what has been described for other amniote taxa. The single acrosome vesicle forms from transport vesicles delivered from the Golgi apparatus. This is comparable to what has been described for Sphenodon (Healy and Jamieson, 1994), squamates (Gribbins et al., 2007, 2009; Rheubert et al., in press), and other archosaurs including birds (Aire, 2007) and crocodilians (Saita et al., 1987; Wang et al., 2008). However, at least one species of agamid lizard was reported to have two acrosome vesicles and granules (Dehlawi et al., 1992), and within Sphenodon there are intra-acrosomal vesicles that deliver granules to the exterior of the outer acrosome membrane apically (Healy and Jamieson, 1994). We identified a single unique feature in early acrosome development in A. mississippiensis: a thin layer of endoplasmic reticulum forms a cap on top of the developing acrosome vesicle. A single large acrosome granule forms and remains in contact with the inner acrosome membrane throughout sperm development until mid to late elongation when it diffuses uniformly throughout the acrosome lumen. The nucleus of the American alligator has uniform diffuse chromatin with small indices of heterochromatin, which is much different from the intermediate to heavily heterochromatic nuclei of Sphenodon (Healy and Jamieson, 1994) and chelonians (Sprando and Russell, 1988; Healy and Jamieson, 1992; Zhang et al., 2007). Conversely, like other crocodilians (Saita et al., 1987; Wang et al., 2008), the condensation of DNA in Alligator mississippiensis packs into progressively larger granules until the nucleus contains only homogeneous dark staining DNA, resembling Sphenodon (Healy and Jamieson, 1994) and chelonians (Sprando and Russell, 1988; Healy and Jamieson, 1992; Al-Dokhi and Al-Wasel, 2001b; Zhang et al., 2007). In squamates, such as Agkistrodon piscivorus (Gribbins et al., 2009), Anolis carolinensis (Rheubert et al., in press), and Scincella laterale (Gribbins et al., 2007), the DNA condenses in a filamentous helical fashion. In A. mississippiensis, early to mid elongating spermatids have an adjacent layer of endoplasmic reticulum that surrounds the nucleus, which may be an autoapomorphic feature of spermiogenesis in A. mississippiensis as this has not been reported in any other taxa, including previously crocodilians (Saita et al., 1987; Wang et al., 2008). The subacrosome space develops early in the round spermatid stage in A. mississippiensis and continues to enlarge during elongation, and this space accumulates a thick layer of dark staining granules similar to that described in other reptilian sauropsids (Sprando and Russell, 1988; Aire, 2007; Gribbins et al., 2007, 2009). Also like other reptilian sauropsids (Clark, 1967; Sprando and Russell, 1988; Aire, 2007; Gribbins et al., 2007, 2009), once the acrosome has completed its development and growth, the nuclei of the early elongating spermatids become associated with the cell membrane. This contact with the cell membrane flattens the acrosome vesicle on the surface of the anterior nucleus, which aids in the migration of the acrosomal shoulders laterally over the apical nuclear head. In Sphenodon (Healy and Jamieson, 1994), chelonians (Sprando and Russell, 1988; Healy and Jamieson, 1992; Al-Dokhi and Al-Wasel, 2001a,b, 2002; Zhang et al., 2007), non-passarine birds (Aire, 2007) and other crocodilians (Saita et al., 1987; Wang et al., 2008), at least one endonuclear canal is present that is rod shaped and houses the perforatorium. Within crocodilians and other arch-
10 osaurs (this study; Saita et al., 1987; Aire 2007; Wang et al., 2008), these endonuclear canals are restricted to the rostral region of the nucleus, whereas in Sphenodon (Healy and Jamieson, 1994) and chelonians (Sprando and Russell, 1988; Healy and Jamieson, 1992; Al-Dokhi and Al-Wasel, 2001a; Zhang et al., 2007) the rods extend past the acrosomal region deep into the nuclear body in middle elongating spermatids. There has been some controversy on whether a perforatorium exists within the endonuclear canals of some reptilian sauropsids (Saita et al., 1987), however, in A. mississippiensis, a clearly visible perforatorium (dark staining rods) develops within the endonuclear canal, much like that observed in the Caiman (Saita et al., 1987). In contrast, all squamates studied to date have an extranuclear perforatorium (with no endonuclear canals) located in the subacrosome space within their spermatids and spermatozoa (Jameison, 1995; Gribbins et al., 2007, 2009; Rheubert et al., in press). In Alligator mississippiensis, we observed a prominent longitudinal manchette associated with the nuclei of late elongating spermatids. Also present, but less numerous, were circular microtubules associated with the nucleus close to the acrosome complex. The manchette has been implicated in the elongation process of the nucleus during spermiogenesis (Russell et al., 1990) and is considered a common structure observed during spermiogenesis in reptilian sauropsids (Ferreira and Dolder 2002). Many archosaurs have both circular and longitudinal components to their manchettes (Saita et al., 1987; Lin and Jones, 1993; Soley, 1997; Aire, 2007) as do many squamates (Da Cruz-Landim and Da Cruz-Hofling, 1977; Butler and Gabri, 1984; Dehlawi et al., 1992). However, whether both components are needed for elongation is not known and is complicated by the fact that some squamates have only the longitudinal microtubules (Gribbins et al. 2007, 2009), and at least one species of anole (Rheubert et al., in press) lacks a manchette altogether and elongation still occurs normally. There is only a single type of microtubule that makes up the circular and longitudinal manchette in A. mississippiensis, which is different from the Caiman (Saita et al., 1987) where the microtubules of the longitudinal manchette are thicker walled than those found in the circular manchette. During the late stages of elongation in some chelonians and some squamates, there are lucent zones within the distal bodies of the elongate spermatid nuclei. These clear zones have been called nuclear lacunae or intranuclear tubules (Jamieson et al., 1996; Ibarguengoytia and Cussac, 1999). It is not unusual to see these same types of lacunae within the nucleus of Alligator mississippiensis, and in many non-passerine birds (Jamieson and Tripepi, 2005). The functions of these spaces are still unknown within these developing spermatids. SPERMIOGENESIS IN THE ALLIGATOR 1269 There is also an epinuclear lucent zone present within the A. mississippiensis acrosome complex as seen in many other reptilian taxa (Jamieson, 1995; Ferreria and Dolder, 2002; Gribbins et al., 2007, 2009). The A. mississippiensis flagellum develops similarly to that described in other reptiles to date. The proximal centriole rests within the nuclear fossa of A. mississippiensis spermatids. The axoneme develops in continuity with the distal centriole. There is no prominent neck region and no peritubular dense material around this region in A. mississippiensis. The mitochondria of the midpiece abut the proximal centriole resulting in a very short neck region, which is congruent to what is observed within the Caiman (Saita et al., 1987) and chelonians (Sprando and Russell, 1988; Healy and Jamieson, 1992; Al-Dokhi and Al-Wasel, 2002). The microtubular doublets of the midpiece axoneme are surrounded by a layer of dense staining granular material that is similar to that observed within the midpiece axoneme of Caiman spermatids (Saita et al., 1987). These dense staining materials around the microtubules of the midpiece are equivalent to the striated columns found within mammalian spermatozoa (Lindemann, 1996) and most likely aid to reinforce the midpiece axoneme. The mitochondria of the midpiece, at least in the late elongating spermatids of A. mississippiensis, have tubular cristae internally and concentric layers of cristae superficially. This is slightly different from what is seen in the Caiman, which has concentric rings of cristae superficially and an electron-dense center that lacks tubular cristae. There are typically 7-9 concentric rows of mitochondria in a sagittal section of the A. mississippiensis midpiece and 7-8 concentric mitochondria within each row in cross section. These numbers seem to be consistent with what has been observed in other crocodilians (Saita et al., 1987). The concentric layers of cristae are also observed within chelonians (Hess et al., 1991; Healy and Jamieson, 1992) and Sphenodon (Healy and Jamieson, 1994). Since sperm storage is common in the female tract of turtles (Gist and Jones, 1989, Gist and Fischer, 1993, Gist and Congdon, 1998), and has recently been described in A. mississippiensis (Gist et al., 2008; Bagwill et al., 2009), it is possible that these concentric rings of cell membrane may provide energy reserves that aid the sperm s ability to survive long and short periods of time within the female reproductive tract before fertilization. The midpiece terminates at the annulus within A. mississippiensis spermatids as that described for most reptilian sauropsids. Also, like Sphenodon, chelonians, and other archosaurs the fibrous sheath of the principal piece does not penetrate into the midpiece, penetration being a synapomorphy for the squamates (Jamieson, 1995). The endpiece in American alligators is easily distinguished from the principal
11 1270 K.M. GRIBBINS ET AL. piece, as the axoneme is not surround by the fibrous sheath. Although the characteristics of spermiogenesis within Alligator mississippiensis are similar to what has been described in other amniotes, there are four ultrastructural features of the A. mississippiensis spermatids that seem to be unique and possible autoapomorphies for this species: (1) the endoplasmic reticulum cap of early acrosome development; (2) the concentric ring of endoplasmic reticulum around the nucleus of early to middle elongating spermatids; (3) the band of endoplasmic reticulum around the acrosome complex of late developing elongate spermatids; (4) composite mitochondria of the midpiece that have a tubular cristae core and concentric layers of cortical cristae superficially. Alligator mississpiensis spermatids also share two major features with Sphenodon, chelonians, and other archosaurs: (1) endonuclear canals; (2) the lack of the fibrous sheath within the midpiece. These characteristics have not been reported for Squamata. The importance of such differences and similarities in structure of the reptilian spermatids between closely and distantly related species is unknown as there are not sufficient representative data from squamates, archosaurs, and turtles to perform robust phylogenetic analyses using morphological characters from spermiogenesis. It is noteworthy to mention as do Saita et al. (1987) that the crocodilian spermatids, like that of the Caiman, seem to share far more ultrastructural features with those of chelonians and Sphenodon than they do with Squamata. Furthermore, Jamieson (2007) has shown that the sperm of paleopnath birds are so similar to those of crocodilians as to be termed crocodiloid. However, most of the similarities between the sperm of chelonians and those of Sphenodon and crocodilians have been shown in a cladistic analysis to be symplesiomorphies of these basal amniote groups (Jamieson and Healy, 1992). Until further morphological data sets are produced from spermiogenesis and spermatozoal ultrastructure within all the major groups of reptiles and birds answers to these questions will remain tentative. The ultrastructural study on A. mississippiensis spermiogenesis presented here provides baseline data that should be utilized in future evolutionary and histopathological investigations. ACKNOWLEDGMENTS The authors thank the hard working employees (Phillip Scooter L. Trosclair, III and Dwayne LeJeune) at the Rockefeller Wildlife Refuge who helped to collect and dissect the alligators used in this study. They also acknowledge Wittenberg, Saint Louis, and Southeastern Louisiana Universities for continual support of this research. LITERATURE CITED Al-Dokhi O, Al-Wasel S. 2001a. Ultrastructure of spermiogenesis in the freshwater turtle Maurymes caspica (Chelonia, Reptilia). I. The acrosomal vesicle and the endonuclear canals formation. J Egypt Ger Soc Zool 36(B): Al-Dokhi O, Al-Wasel S. 2001b. Ultrastructure of spermiogenesis in the freshwater turtle Maurymes caspica (Chelonia, Reptilia). II. The nucleus elongation and chromatin condensation. J Union Arab Biol Zool 15(A): Al-Dokhi O, Al-Wasel S Ultrastructure of spermiogenesis in the freshwater turtle Maurymes caspica (Chelonia, Reptilia). III. Sperm tail formation. J Union Arab Biol Zool 18(A): Aire TA Spermatogenesis and testicular cycles. In: Jamieson BGM, editor. Reproductive Biology and Phylogeny of Birds. New Hampshire: Science Publishers. pp Bagwill A, Sever DM, Elsey RM Seasonal variation of the oviduct of the American alligator. Alligator mississippiensis (Reptilia: Crocodylia). J Morphol 270: Butler RD, Gabri MS Structure and development of the sperm head in the lizard Podarcis (Lacerta) taurica. J Ultrastruct Res 88: Clark AQ Some aspects of spermiogenesis in a lizard. Amer J Anat 121: Da Cruz-Landim C, Da Cruz-Hofling MA Electron microscope study of lizard spermiogenesis in Tropidurus torquatus (Lacertilia). Caryologia 30: Dehlawi GY, Ismail MF, Hamdi SA, Jamjoom MB Ultrastructure of spermiogenesis of a Saudian reptile. The sperm head differentiation in Agama adramitana. Arch Androl 28: Ferguson MWJ The reproductive biology and embryology of the crocodilians. In: Gans C, Billet FS, Maderson PFS, editors. Biology of the Reptilia, Vol. 14: Development A. New York: John Wiley. pp Ferreira A, Dolder H Ultrastructural analysis of spermiogenesis of Iguana iguana (Reptilia: Sauria: Iguanidae). Euro J Morphol 40: Gadow H Remarks on the cloaca and on the copulatory organs of the Amniota. Phil Trans R Soc Lond B 178:5 37. Gatesy J, Baker RH, Hayashi C Inconsistencies in arguments for the supertree approach: Supermatrices versus supertrees of Crocodylia. Syst Biol 53: Gist DH, Bagwill AL, Lance VA, Sever DM, Elsey RM Sperm storage in the oviduct of the American alligator. J Exp Zool 309A: Gist DH, Congdon JD Oviductal sperm storage as a reproductive tactic of turtles. J Exp Zool 282: Gist DH, Fischer EN Fine structure of the sperm storage tubules in the box turtle oviduct. J Reprod Fert 97: Gist DH, Jones JM Storage of sperm in the oviducts of turtles. J Morphol 199: Gribbins K, Gist, D Cytological evaluation of the germinal epithelium and the germ cell cycle in an introduced population of European Wall Lizards, Podarcis muralis. J Morphol 256: Gribbins K, Gist D, Congdon J Cytological evaluation of spermatogenesis in the Red-Eared Slider, Trachemys scripta. J Morphol 255: Gribbins K, Happ CS, Sever DM Ultrastructure of the reproductive system of the Black Swamp Snake (Seminatrix pygaea). V. The temporal germ cell development strategy of the testis. Acta Zoologica 86: Gribbins K, Rheubert J, Collier M, Siegel D, Sever D Histological analysis of spermatogenesis and the germ cell development strategy within the testis of the male Western Cottonmouth Snake, Agkistrodon piscivorus. Annals of Anatomy 190: Gribbins KM, Elsey RM, Gist DH Cytological evaluation of the germ cell development strategy within the testis of the American alligator, Alligator mississippiensis. Acta Zool 87:
12 SPERMIOGENESIS IN THE ALLIGATOR 1271 Gribbins KM, Mills EM, Sever DM Ultrastructural examination of spermiogenesis within the testis of the ground skink. Scincella laterale (Squamata, Sauria, Scincidae). J Morphol 268: Gribbins KM, Rheubert JL, Anzalone ML, Siegel DS, Sever DM Ultrastructure of spermiogenesis in the cottonmouth, Agkistrodon piscivorus (Squamata: Viperidae: Crotalinae). J Morphol 271: Healy JM, Jamieson BGM Ultrastructure of the spermatozoa of the Tuatara (Sphenodon punctatus) and its relevance to the relationships of the Sphenodontida. Phil Trans Roy Soc London B 335: Healy JM, Jamieson BGM The ultrastructure of spermatogenesis and epididymal spermatozoa of the Tuatara Sphenodon punctatus (Sphenodontida, Amniota). Phil Trans Bio Sci 344: Hess RS, Thurston RJ, Gist DH Ultrastructure of the turtle spermatozoon. Anat Rec 229: Ibarguengoytia NR, Cussac VE Male response to low frequency of female reproduction in the viviparous lizard Liolaemus (Tropiduridae). Herpetol J 9: Jamieson BGM Evolution of tetrapod spermatozoa with particular reference to amniotes. In: Jamieson BGM, Ausio J, Justine JL, editors. Advances in Spermatozoal Phylogeny and Taxonomy, Vol Paris: Mémoires du Muséum National d Histoire Naturelle. pp Jamieson BGM Avian spermatozoa: Structure and phylogeny. In: Jamieson BGM, editor. Reproductive Biology and Phylogeny of Birds. Part A. NH: Science Publishers, Enfield. pp Jamieson BGM, Healy JM The phylogenetic position of the tuatara, Sphenodon (Sphenodontida, Amniota), as indicated by cladistic analysis of he ultrastructure of the spermatozoa. Phil Trans Roy Soc London B 335: Jamieson BGM, Tripepi R Ultratructure of the spermatozoon of Apus apus (Linnaeus 1758), the common swift (Aves: Apodiformes; Appodidae), with phylogenetic implications. Acta Zool 88: Jamieson BGM, Oliver SC, Scheltinga DM The ultastructure of spermatozoa of Squamata. I. Scincidae, Gekkonidae, and Pygonidae (Reptilia). Acta Zool 77: Jamieson BGM, Scheltinga DM, Tucker AD The ultrastructure of spermatozoa of the Australian fresh water crocodile. Crocodylus johnstoni Krefft, 1873 (Crocodylidae, Reptilia). J Submicrosc Cytol Pathol 29: Lang JW, Andrews HV Temperature-dependent sex determination in crocodilians. J Exp Zool 270: Lin M, Jones RC Spermiogenesis and spermiation in the Japanese quail (Coturnix coturnix japonica). J Anat 183: Lindemann CB Functional significance of the outer dense fibers of mammalian sperm examined by computer simulations wit the geometric clutch model. Cell Motil Cytok 34: Moore BC, Hamlin HJ, Botteri NL, Lawler AN, Mathavan KK, Guillette LJ Posthatching development of Alligator mississippiensis ovary and testis. J Morphol 271: Rheubert JL, McHugh HH, Collier MH, Sever DM, Gribbins KM Temporal germ cell development strategy during spermatogenesis within the testis of the ground skin. Scincella lateralis (Sauria: Scincidae). Therio 72: Rheubert JL, Wolf K, Wilson B, Gribbins KM. In press. Ultrastructure of spermiogenesis in the Jamaican Anole, Anolis lineatopus. Acta Zoologica (in press). Russell LD, Ettlin RA, Hikim AMP, Cleff ED Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press. pp 286. Saita A, Comazzi M, Perrotta E Electron microscope study of spermiogenesis in Caiman crocodylus L. Boll Zool 4: Soley JT Nuclear morphogenesis and the role of the manchette during spermiogenesis in the ostrich (Struthio camelus). J Anat 190: Sprando RL, Russell LD Spermiogenesis in the red-ear turtle (Pseudemys scripta) and the domestic fowl (Gallus domesticus): A of study cytoplasmic events including cell volume changes and cytoplasmic elimination. J Morphol 198: Thorbjarnarson JB Reproductive characteristics of the order Crocodylia. Herpetologica 52:8 24. Wang L, Wu X, Xu D, Wang R, Wang C Development of testis and spermatogenesis in Alligator sinensis. J Appl Anim Res 34: Zhang L, Han X, Li M, Bao H, Chen Q Spermiogenesis in soft-shelled turtle, Pelodiscus sinensis. Anat Rec 290:
Ultrastructure of Spermiognesis in the Yellow-Bellied Sea Snake, Pelamis platurus (Squamata: Elapidae: Hydrophiinae) Brenna M.
 Burkhart 1 1 2 3 4 Ultrastructure of Spermiognesis in the Yellow-Bellied Sea Snake, Pelamis platurus (Squamata: Elapidae: Hydrophiinae) Brenna M. Burkhart 5 6 7 8 9 10 11 12 Department of Biology, Wittenberg
Burkhart 1 1 2 3 4 Ultrastructure of Spermiognesis in the Yellow-Bellied Sea Snake, Pelamis platurus (Squamata: Elapidae: Hydrophiinae) Brenna M. Burkhart 5 6 7 8 9 10 11 12 Department of Biology, Wittenberg
D.M. Scheltinga, 1 * B.G.M. Jamieson, 1 R.E. Espinoza, 2 and K.S. Orrell 3
 JOURNAL OF MORPHOLOGY 247:160 171 (2001) Descriptions of the Mature Spermatozoa of the Lizards Crotaphytus bicinctores, Gambelia wislizenii (Crotaphytidae), and Anolis carolinensis (Polychrotidae) (Reptilia,
JOURNAL OF MORPHOLOGY 247:160 171 (2001) Descriptions of the Mature Spermatozoa of the Lizards Crotaphytus bicinctores, Gambelia wislizenii (Crotaphytidae), and Anolis carolinensis (Polychrotidae) (Reptilia,
Shuangli HAO 1, Liangliang PAN 2, Zhouxi FANG 2 and Yongpu ZHANG 1* 1. Introduction
 Asian Herpetological Research 2015, 6(3): 189 198 DOI: 10.16373/j.cnki.ahr.140071 ORIGINAL ARTICLE Comparative Studies on Sperm Ultrastructure of Three Gecko Species, Gekko japonicus, Gekko chinensis and
Asian Herpetological Research 2015, 6(3): 189 198 DOI: 10.16373/j.cnki.ahr.140071 ORIGINAL ARTICLE Comparative Studies on Sperm Ultrastructure of Three Gecko Species, Gekko japonicus, Gekko chinensis and
The ultrastructure of the spermatozoa of the lizard Micrablepharus maximiliani (Squamata, Gymnophthalmidae), with considerations on the use of
 Acta Zoologica (Stockholm) 80: 47±59 (January 1999) The ultrastructure of the spermatozoa of the lizard Micrablepharus maximiliani (Squamata, Gymnophthalmidae), with considerations on the use of sperm
Acta Zoologica (Stockholm) 80: 47±59 (January 1999) The ultrastructure of the spermatozoa of the lizard Micrablepharus maximiliani (Squamata, Gymnophthalmidae), with considerations on the use of sperm
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii. Yates, Lauren A.
 A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
Morphology of Shells From Viable and Nonviable Eggs of the Chinese Alligator (Alligator sinensis)
 ~ JOURNAL OF MORPHOLOGY 222:103-110 (1994) Morphology of Shells From Viable and Nonviable Eggs of the Chinese Alligator (Alligator sinensis) CAROLE S. WINK AND RUTH M. ELSEY Department of Anatomy, Louisiana
~ JOURNAL OF MORPHOLOGY 222:103-110 (1994) Morphology of Shells From Viable and Nonviable Eggs of the Chinese Alligator (Alligator sinensis) CAROLE S. WINK AND RUTH M. ELSEY Department of Anatomy, Louisiana
BY- NC-ND , 3, 2002, , (1) (2) (3) , 3, 2002, , (1) (2) A
 This Accepted Author Manuscript is copyrighted and published by Elsevier. It is posted here by agreement between Elsevier and University of Brasilia. Changes resulting from the publishing process - such
This Accepted Author Manuscript is copyrighted and published by Elsevier. It is posted here by agreement between Elsevier and University of Brasilia. Changes resulting from the publishing process - such
Supplementary Figure 1 Cartilaginous stages in non-avian amniotes. (a) Drawing of early ankle development of Alligator mississippiensis, as reported
 Supplementary Figure 1 Cartilaginous stages in non-avian amniotes. (a) Drawing of early ankle development of Alligator mississippiensis, as reported by a previous study 1. The intermedium is formed at
Supplementary Figure 1 Cartilaginous stages in non-avian amniotes. (a) Drawing of early ankle development of Alligator mississippiensis, as reported by a previous study 1. The intermedium is formed at
Texas A&M University Office of Graduate Studies Graduate Faculty Personal Record Form (Submit original with vitae only)
 Nominating Department: WFSC MaiiStop:~ 1. Name of Nominee: Dustin S. Siegel Texas A&M University Office of Graduate Studies Graduate Faculty Personal Record Form (Submit original with vitae only) 2. Department
Nominating Department: WFSC MaiiStop:~ 1. Name of Nominee: Dustin S. Siegel Texas A&M University Office of Graduate Studies Graduate Faculty Personal Record Form (Submit original with vitae only) 2. Department
Taxonomy. Chapter 20. Evolutionary Development Diagram. I. Evolution 2/24/11. Kingdom - Animalia Phylum - Chordata Class Reptilia.
 Taxonomy Chapter 20 Reptiles Kingdom - Animalia Phylum - Chordata Class Reptilia Order Testudines - turtles Order Crocodylia - crocodiles, alligators Order Sphenodontida - tuataras Order Squamata - snakes
Taxonomy Chapter 20 Reptiles Kingdom - Animalia Phylum - Chordata Class Reptilia Order Testudines - turtles Order Crocodylia - crocodiles, alligators Order Sphenodontida - tuataras Order Squamata - snakes
Key words: Plasmodium, Kentropyx calcarata, Brazil, merogony, gametocytes, ultrastructure
 FOLIA PARASITOLOGICA 49: 2-8, 2002 Fine structure of erythrocytic stages of a Plasmodium tropiduri-like malaria parasite found in the lizard Kentropyx calcarata (Teiidae) from north Brazil Ilan Paperna
FOLIA PARASITOLOGICA 49: 2-8, 2002 Fine structure of erythrocytic stages of a Plasmodium tropiduri-like malaria parasite found in the lizard Kentropyx calcarata (Teiidae) from north Brazil Ilan Paperna
GUSTAVO H. C. VIEIRA, GUARINO R. COLLI & SÔNIA N. BÁO
 Blackwell Publishing, Ltd. Phylogenetic relationships of corytophanid lizards (Iguania, Squamata, Reptilia) based on partitioned and total evidence analyses of sperm morphology, gross morphology, and DNA
Blackwell Publishing, Ltd. Phylogenetic relationships of corytophanid lizards (Iguania, Squamata, Reptilia) based on partitioned and total evidence analyses of sperm morphology, gross morphology, and DNA
Chapter 5 Male and female reproductive systems
 Chapter 5 Male and female reproductive systems This chapter begins with a description of the male and female reproductive systems followed by a section on sex determination. A good knowledge of the anatomy
Chapter 5 Male and female reproductive systems This chapter begins with a description of the male and female reproductive systems followed by a section on sex determination. A good knowledge of the anatomy
Stuart S. Sumida Biology 342. Simplified Phylogeny of Squamate Reptiles
 Stuart S. Sumida Biology 342 Simplified Phylogeny of Squamate Reptiles Amphibia Amniota Seymouriamorpha Diadectomorpha Synapsida Parareptilia Captorhinidae Diapsida Archosauromorpha Reptilia Amniota Amphibia
Stuart S. Sumida Biology 342 Simplified Phylogeny of Squamate Reptiles Amphibia Amniota Seymouriamorpha Diadectomorpha Synapsida Parareptilia Captorhinidae Diapsida Archosauromorpha Reptilia Amniota Amphibia
Reptilian Requirements Created by the North Carolina Aquarium at Fort Fisher Education Section
 Essential Question: North Carolina Aquariums Education Section Reptilian Requirements Created by the North Carolina Aquarium at Fort Fisher Education Section What physical and behavioral adaptations do
Essential Question: North Carolina Aquariums Education Section Reptilian Requirements Created by the North Carolina Aquarium at Fort Fisher Education Section What physical and behavioral adaptations do
SCANNING electron - microscopy has
 Characteristics of the Absorptive Surface of the Small Intestine of the Chicken from 1 Day to 14 Weeks of Age 1 R. C. BAYER, C. B. CHAWAN, F. H. BIRD AND S. D. MUSGRAVE Department of Animal and Veterinary
Characteristics of the Absorptive Surface of the Small Intestine of the Chicken from 1 Day to 14 Weeks of Age 1 R. C. BAYER, C. B. CHAWAN, F. H. BIRD AND S. D. MUSGRAVE Department of Animal and Veterinary
Spenn Storage in the Class Reptilia
 PENSOFT Publishers Sofia - Moscow A. Legakis, S. Sfenthourakis, R. Polymeni & M. Thessalou-Legaki (eds.) The New Panorama of Animal Evolution Pwc. 18" Int. Congr. Zoology, pp. 439-446, 2003 Spenn Storage
PENSOFT Publishers Sofia - Moscow A. Legakis, S. Sfenthourakis, R. Polymeni & M. Thessalou-Legaki (eds.) The New Panorama of Animal Evolution Pwc. 18" Int. Congr. Zoology, pp. 439-446, 2003 Spenn Storage
Lab VII. Tuatara, Lizards, and Amphisbaenids
 Lab VII Tuatara, Lizards, and Amphisbaenids Project Reminder Don t forget about your project! Written Proposals due and Presentations are given on 4/21!! Abby and Sarah will read over your written proposal
Lab VII Tuatara, Lizards, and Amphisbaenids Project Reminder Don t forget about your project! Written Proposals due and Presentations are given on 4/21!! Abby and Sarah will read over your written proposal
Development, comparative morphology and cornification of reptilian claws in relation to claws evolution in tetrapods
 Contributions to Zoology, 78 (1) 25-42 (2009) Development, comparative morphology and cornification of reptilian claws in relation to claws evolution in tetrapods Lorenzo Alibardi 1, 2 1 Dipartimento di
Contributions to Zoology, 78 (1) 25-42 (2009) Development, comparative morphology and cornification of reptilian claws in relation to claws evolution in tetrapods Lorenzo Alibardi 1, 2 1 Dipartimento di
Class Reptilia Testudines Squamata Crocodilia Sphenodontia
 Class Reptilia Testudines (around 300 species Tortoises and Turtles) Squamata (around 7,900 species Snakes, Lizards and amphisbaenids) Crocodilia (around 23 species Alligators, Crocodiles, Caimans and
Class Reptilia Testudines (around 300 species Tortoises and Turtles) Squamata (around 7,900 species Snakes, Lizards and amphisbaenids) Crocodilia (around 23 species Alligators, Crocodiles, Caimans and
REPRODUCTIVE BIOLOGY OF THE MALE AUSTRALIAN PARROT INTRODUCTION
 REPRODUCTIVE BIOLOGY OF THE MALE AUSTRALIAN PARROT Erica Lovas School of Veterinary Science St Lucia, Australia 4072. and: School of Animal Studies Gatton, Australia 4343 Steve Johnston School of Animal
REPRODUCTIVE BIOLOGY OF THE MALE AUSTRALIAN PARROT Erica Lovas School of Veterinary Science St Lucia, Australia 4072. and: School of Animal Studies Gatton, Australia 4343 Steve Johnston School of Animal
Sec KEY CONCEPT Reptiles, birds, and mammals are amniotes.
 Thu 4/27 Learning Target Class Activities *attached below (scroll down)* Website: my.hrw.com Username: bio678 Password:a4s5s Activities Students will describe the evolutionary significance of amniotic
Thu 4/27 Learning Target Class Activities *attached below (scroll down)* Website: my.hrw.com Username: bio678 Password:a4s5s Activities Students will describe the evolutionary significance of amniotic
Criconemoides similis 1 G. W. BIRD ~
 Somatic Musculature of Trichodorus porosus and Criconemoides similis 1 G. W. BIRD ~ Abstract: The somatic musculature of Trichodorus porosus is transversely striated, and that of Criconemoides similis
Somatic Musculature of Trichodorus porosus and Criconemoides similis 1 G. W. BIRD ~ Abstract: The somatic musculature of Trichodorus porosus is transversely striated, and that of Criconemoides similis
CHELONIAN CONSERVATION AND BIOLOGY International Journal of Turtle and Tortoise Research
 CHELONIAN CONSERVATION AND BIOLOGY International Journal of Turtle and Tortoise Research Growth in Kyphotic Ringed Sawbacks, Graptemys oculifera (Testudines: Emydidae) WILL SELMAN 1,2 AND ROBERT L. JONES
CHELONIAN CONSERVATION AND BIOLOGY International Journal of Turtle and Tortoise Research Growth in Kyphotic Ringed Sawbacks, Graptemys oculifera (Testudines: Emydidae) WILL SELMAN 1,2 AND ROBERT L. JONES
8/19/2013. Topic 5: The Origin of Amniotes. What are some stem Amniotes? What are some stem Amniotes? The Amniotic Egg. What is an Amniote?
 Topic 5: The Origin of Amniotes Where do amniotes fall out on the vertebrate phylogeny? What are some stem Amniotes? What is an Amniote? What changes were involved with the transition to dry habitats?
Topic 5: The Origin of Amniotes Where do amniotes fall out on the vertebrate phylogeny? What are some stem Amniotes? What is an Amniote? What changes were involved with the transition to dry habitats?
Key words: Coccidia, Choleoeimeria rochalimai, fine structure, gall bladder epithelium, Hemidactylus mabouia, Brazil
 FOLIA PARASITOLOGICA 47: 91-96, 2000 Ultrastructural study of meronts and gamonts of Choleoeimeria rochalimai (Apicomplexa: Eimeriidae) developing in the gall bladder of the gecko Hemidactylus mabouia
FOLIA PARASITOLOGICA 47: 91-96, 2000 Ultrastructural study of meronts and gamonts of Choleoeimeria rochalimai (Apicomplexa: Eimeriidae) developing in the gall bladder of the gecko Hemidactylus mabouia
BEAK AND FEATHER DYSTROPHY IN WILD SULPHUR-CRESTED COCKATOOS (CACATUA GALERITA)
 BEAK AND FEATHER DYSTROPHY IN WILD SULPHUR-CRESTED COCKATOOS (CACATUA GALERITA) Author(s): Steven McOrist, Douglas G. Black, David A. Pass, Peter C. Scott, and John Marshall Source: Journal of Wildlife
BEAK AND FEATHER DYSTROPHY IN WILD SULPHUR-CRESTED COCKATOOS (CACATUA GALERITA) Author(s): Steven McOrist, Douglas G. Black, David A. Pass, Peter C. Scott, and John Marshall Source: Journal of Wildlife
Plestiodon (=Eumeces) fasciatus Family Scincidae
 Plestiodon (=Eumeces) fasciatus Family Scincidae Living specimens: - Five distinct longitudinal light lines on dorsum - Juveniles have bright blue tail - Head of male reddish during breeding season - Old
Plestiodon (=Eumeces) fasciatus Family Scincidae Living specimens: - Five distinct longitudinal light lines on dorsum - Juveniles have bright blue tail - Head of male reddish during breeding season - Old
Reproductive activity of Lacerta agilis and Zootoca vivipara (Reptilia: Sauria: Lacertidae) in western Siberia
 M. Vences, J. Köhler, T. Ziegler, W. Böhme (eds): Herpetologia Bonnensis II. Proceedings of the 13th Congress of the Societas Europaea Herpetologica. pp. 133-137 (2006) Reproductive activity of Lacerta
M. Vences, J. Köhler, T. Ziegler, W. Böhme (eds): Herpetologia Bonnensis II. Proceedings of the 13th Congress of the Societas Europaea Herpetologica. pp. 133-137 (2006) Reproductive activity of Lacerta
,,, THE MORPHOLOGY AND MORPHOMETRY OF THE PECTEN OCULI IN DIURNAL AND NOCTURNAL BIRDS: A
 ,,, THE MORPHOLOGY AND MORPHOMETRY OF THE PECTEN OCULI IN DIURNAL AND NOCTURNAL BIRDS: A COMPARATIVE STUDY" BY llijama, S.G., B. V. M. (NBI), Department of Veteri nary Anatomy, University of I\Jairobi.
,,, THE MORPHOLOGY AND MORPHOMETRY OF THE PECTEN OCULI IN DIURNAL AND NOCTURNAL BIRDS: A COMPARATIVE STUDY" BY llijama, S.G., B. V. M. (NBI), Department of Veteri nary Anatomy, University of I\Jairobi.
Liver and Gallbladder Morphology of the juvenile Nile crocodile, Crocodylus niloticus (Laurenti, 1768)
 Liver and Gallbladder Morphology of the juvenile Nile crocodile, Crocodylus niloticus (Laurenti, 1768) by ERNA VAN WILPE Submitted in partial fulfilment of the requirements for the degree MSc DEPARTMENT
Liver and Gallbladder Morphology of the juvenile Nile crocodile, Crocodylus niloticus (Laurenti, 1768) by ERNA VAN WILPE Submitted in partial fulfilment of the requirements for the degree MSc DEPARTMENT
Class Reptilia. Lecture 19: Animal Classification. Adaptations for life on land
 Lecture 19: Animal Classification Class Reptilia Adaptations for life on land بيض جنيني egg. Amniotic Water-tight scales. One occipital condyle one point of attachement of the skull with the vertebral
Lecture 19: Animal Classification Class Reptilia Adaptations for life on land بيض جنيني egg. Amniotic Water-tight scales. One occipital condyle one point of attachement of the skull with the vertebral
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
The 1st studies on the blood of reptiles
 Zoological Studies 42(1): 173-178 (2003) Erythrocyte Size and Morphology of Some Tortoises and Turtles from Turkey. I smail HakkI Uǧurta *, Murat Sevinç and Hikmet Sami YIldIrImhan Science and Art Faculty,
Zoological Studies 42(1): 173-178 (2003) Erythrocyte Size and Morphology of Some Tortoises and Turtles from Turkey. I smail HakkI Uǧurta *, Murat Sevinç and Hikmet Sami YIldIrImhan Science and Art Faculty,
Herpetology Biol 119. Herpetology Introduction. Philip Bergmann. Philip Bergmann - Research. TA: Allegra Mitchell. Philip Bergmann - Personal
 Herpetology Biol 119 Clark University Fall 2011 Lecture: Tuesday, Thursday 9:00-10:15 in Lasry 124 Lab: Tuesday 13:25-16:10 in Lasry 150 Office hours: T 10:15-11:15 in Lasry 331 Contact: pbergmann@clarku.edu
Herpetology Biol 119 Clark University Fall 2011 Lecture: Tuesday, Thursday 9:00-10:15 in Lasry 124 Lab: Tuesday 13:25-16:10 in Lasry 150 Office hours: T 10:15-11:15 in Lasry 331 Contact: pbergmann@clarku.edu
Importance of Electron Microscopy to reveal species-specific characteristics of gland secretion
 mportance of Electron Microscopy to reveal species-specific characteristics of gland secretion Gabriella Chieffi Baccari 1, Alessandra Santillo 1, and Sergio Minucci 2 1 Department of Life Sciences, Second
mportance of Electron Microscopy to reveal species-specific characteristics of gland secretion Gabriella Chieffi Baccari 1, Alessandra Santillo 1, and Sergio Minucci 2 1 Department of Life Sciences, Second
Light, Scanning and Transmission Electron Microscopical Study on the Oviduct of the Ostrich (Struthio
 Light, Scanning and Transmission Electron Microscopical Study on the Oviduct of the Ostrich (Struthio camelus) A.S.Saber*, S.A.M.Emara*, O.M.M.AboSaeda** * Faculty of Veterinary Medicine, Sadat City Branch,
Light, Scanning and Transmission Electron Microscopical Study on the Oviduct of the Ostrich (Struthio camelus) A.S.Saber*, S.A.M.Emara*, O.M.M.AboSaeda** * Faculty of Veterinary Medicine, Sadat City Branch,
Reproductive cycle of the common rough-scaled lizard, Ichnotropis squamulosa (Squamata: Lacertidae) from southern Africa.
 Reproductive cycle of the common rough-scaled lizard, Ichnotropis squamulosa (Squamata: Lacertidae) from southern Africa. Print Author: Goldberg, Stephen R. Article Type: Report Geographic Code: 6SOUT
Reproductive cycle of the common rough-scaled lizard, Ichnotropis squamulosa (Squamata: Lacertidae) from southern Africa. Print Author: Goldberg, Stephen R. Article Type: Report Geographic Code: 6SOUT
Title: Phylogenetic Methods and Vertebrate Phylogeny
 Title: Phylogenetic Methods and Vertebrate Phylogeny Central Question: How can evolutionary relationships be determined objectively? Sub-questions: 1. What affect does the selection of the outgroup have
Title: Phylogenetic Methods and Vertebrate Phylogeny Central Question: How can evolutionary relationships be determined objectively? Sub-questions: 1. What affect does the selection of the outgroup have
Title. CitationJapanese Journal of Veterinary Research, 24(1-2): 37. Issue Date DOI. Doc URL. Type. File Information
 Title DISTRIBUTION OF LYMPHATIC TISSUES IN DUCK CAECA Author(s)KITAMURA, Hirokazu; SUGIMURA, Makoto; HASHIMOTO, Yos CitationJapanese Journal of Veterinary Research, 24(1-2): 37 Issue Date 1976-05 DOI 10.14943/jjvr.24.1-2.37
Title DISTRIBUTION OF LYMPHATIC TISSUES IN DUCK CAECA Author(s)KITAMURA, Hirokazu; SUGIMURA, Makoto; HASHIMOTO, Yos CitationJapanese Journal of Veterinary Research, 24(1-2): 37 Issue Date 1976-05 DOI 10.14943/jjvr.24.1-2.37
Biology Slide 1 of 50
 Biology 1 of 50 2 of 50 What Is a Reptile? What are the characteristics of reptiles? 3 of 50 What Is a Reptile? What Is a Reptile? A reptile is a vertebrate that has dry, scaly skin, lungs, and terrestrial
Biology 1 of 50 2 of 50 What Is a Reptile? What are the characteristics of reptiles? 3 of 50 What Is a Reptile? What Is a Reptile? A reptile is a vertebrate that has dry, scaly skin, lungs, and terrestrial
Alligator Production: Breeding, Egg Collection, Incubation, and Hatching
 Southern Regional Aquaculture Center SRAC Publication No. 0231 April 2018 Revision PR VI Alligator Production: Breeding, Egg Collection, Incubation, and Hatching Mark G. Shirley 1 and Ruth M. Elsey 2 The
Southern Regional Aquaculture Center SRAC Publication No. 0231 April 2018 Revision PR VI Alligator Production: Breeding, Egg Collection, Incubation, and Hatching Mark G. Shirley 1 and Ruth M. Elsey 2 The
HISTOPATHOLOGY. Introduction:
 Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
Temperature-Dependent Sex Determination in Crocodilians
 THE JOURNAL OF EXPERIMENTAL ZOOLOGY 270:28-44 (1994) Temperature-Dependent Sex Determination in Crocodilians JEFFREY W. LANG AND HARRY V. ANDREWS Department of BioZogy, University of North Dakota, Grand
THE JOURNAL OF EXPERIMENTAL ZOOLOGY 270:28-44 (1994) Temperature-Dependent Sex Determination in Crocodilians JEFFREY W. LANG AND HARRY V. ANDREWS Department of BioZogy, University of North Dakota, Grand
WITH THE TABLE OF THE MORPHOLOGICAL FEATURES OF TAPEWORMS IN VAMPIROLEPIS. (Received: December 22nd, 1965)
 Japan. J. Med. Sci. Biol. 19, 51-57, 1966 *ON A NEW TAPEWORM, VAMPIROLEPIS ISENSIS, FOUND IN BATS WITH THE TABLE OF THE MORPHOLOGICAL FEATURES OF TAPEWORMS IN VAMPIROLEPIS ISAMU SAWADA Biological Laboratory,
Japan. J. Med. Sci. Biol. 19, 51-57, 1966 *ON A NEW TAPEWORM, VAMPIROLEPIS ISENSIS, FOUND IN BATS WITH THE TABLE OF THE MORPHOLOGICAL FEATURES OF TAPEWORMS IN VAMPIROLEPIS ISAMU SAWADA Biological Laboratory,
Introduction to Herpetology
 Introduction to Herpetology Lesson Aims Discuss the nature and scope of reptiles. Identify credible resources, and begin to develop networking with organisations and individuals involved with the study
Introduction to Herpetology Lesson Aims Discuss the nature and scope of reptiles. Identify credible resources, and begin to develop networking with organisations and individuals involved with the study
Renal Sexual Segment of the Cottonmouth Snake, Agkistrodon piscivorous (Reptilia, Squamata, Viperidae)
 JOURNAL OF MORPHOLOGY 000:000 000 (2007) Renal Sexual Segment of the Cottonmouth Snake, Agkistrodon piscivorous (Reptilia, Squamata, Viperidae) David M. Sever,* Dustin S. Siegel, April Bagwill, Mallory
JOURNAL OF MORPHOLOGY 000:000 000 (2007) Renal Sexual Segment of the Cottonmouth Snake, Agkistrodon piscivorous (Reptilia, Squamata, Viperidae) David M. Sever,* Dustin S. Siegel, April Bagwill, Mallory
Anatomy. Name Section. The Vertebrate Skeleton
 Name Section Anatomy The Vertebrate Skeleton Vertebrate paleontologists get most of their knowledge about past organisms from skeletal remains. Skeletons are useful for gleaning information about an organism
Name Section Anatomy The Vertebrate Skeleton Vertebrate paleontologists get most of their knowledge about past organisms from skeletal remains. Skeletons are useful for gleaning information about an organism
Characteristics of a Reptile. Vertebrate animals Lungs Scaly skin Amniotic egg
 Reptiles Characteristics of a Reptile Vertebrate animals Lungs Scaly skin Amniotic egg Characteristics of Reptiles Adaptations to life on land More efficient lungs and a better circulator system were develope
Reptiles Characteristics of a Reptile Vertebrate animals Lungs Scaly skin Amniotic egg Characteristics of Reptiles Adaptations to life on land More efficient lungs and a better circulator system were develope
*Using the 2018 List. Use the image below to answer question 6.
 Herpetology Test 1. Hearts in all herps other than consists of atria and one ventricle somewhat divided by a septum. (2 pts) a. snakes; two b. crocodiles; two c. turtles; three d. frogs; four 2. The food
Herpetology Test 1. Hearts in all herps other than consists of atria and one ventricle somewhat divided by a septum. (2 pts) a. snakes; two b. crocodiles; two c. turtles; three d. frogs; four 2. The food
A Scanning Electron Microscopic Study of Eggshell Surface Topography of Leidynema portentosae and L. appendiculatum (Nematoda: Oxyuroidea)
 The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 88, Issue 5 (December, 1988) 1988-12 A Scanning Electron Microscopic
The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 88, Issue 5 (December, 1988) 1988-12 A Scanning Electron Microscopic
Amniote Relationships. Reptilian Ancestor. Reptilia. Mesosuarus freshwater dwelling reptile
 Amniote Relationships mammals Synapsida turtles lizards,? Anapsida snakes, birds, crocs Diapsida Reptilia Amniota Reptilian Ancestor Mesosuarus freshwater dwelling reptile Reptilia General characteristics
Amniote Relationships mammals Synapsida turtles lizards,? Anapsida snakes, birds, crocs Diapsida Reptilia Amniota Reptilian Ancestor Mesosuarus freshwater dwelling reptile Reptilia General characteristics
THE ROLE OF WATER IN THE EVOLUTION OF THE TERRESTRIAL VERTEBRATES
 26 THE ROLE OF WATER IN THE EVOLUTION OF THE TERRESTRIAL VERTEBRATES BY J. GRAY, M.A., King's College, Cambridge. (From the Zoological Laboratory, Cambridge.) (Received igth January 1928.) (With Three
26 THE ROLE OF WATER IN THE EVOLUTION OF THE TERRESTRIAL VERTEBRATES BY J. GRAY, M.A., King's College, Cambridge. (From the Zoological Laboratory, Cambridge.) (Received igth January 1928.) (With Three
Biology. Slide 1of 50. End Show. Copyright Pearson Prentice Hall
 Biology 1of 50 2of 50 Phylogeny of Chordates Nonvertebrate chordates Jawless fishes Sharks & their relatives Bony fishes Reptiles Amphibians Birds Mammals Invertebrate ancestor 3of 50 A vertebrate dry,
Biology 1of 50 2of 50 Phylogeny of Chordates Nonvertebrate chordates Jawless fishes Sharks & their relatives Bony fishes Reptiles Amphibians Birds Mammals Invertebrate ancestor 3of 50 A vertebrate dry,
DEVELOPMENT OF THE HEAD AND NECK PLACODES
 DEVELOPMENT OF THE HEAD AND NECK Placodes and the development of organs of special sense L. Moss-Salentijn PLACODES Localized thickened areas of specialized ectoderm, lateral to the neural crest, at the
DEVELOPMENT OF THE HEAD AND NECK Placodes and the development of organs of special sense L. Moss-Salentijn PLACODES Localized thickened areas of specialized ectoderm, lateral to the neural crest, at the
REPTILES. Scientific Classification of Reptiles To creep. Kingdom: Animalia Phylum: Chordata Subphylum: Vertebrata Class: Reptilia
 Scientific Classification of Reptiles To creep Kingdom: Animalia Phylum: Chordata Subphylum: Vertebrata Class: Reptilia REPTILES tetrapods - 4 legs adapted for land, hip/girdle Amniotes - animals whose
Scientific Classification of Reptiles To creep Kingdom: Animalia Phylum: Chordata Subphylum: Vertebrata Class: Reptilia REPTILES tetrapods - 4 legs adapted for land, hip/girdle Amniotes - animals whose
COMPARATIVE VERTEBRATE HISTOLOGY ZOO 4756c Syllabus for Fall 2018
 COMPARATIVE VERTEBRATE HISTOLOGY ZOO 4756c Syllabus for Fall 2018 Instructor: Frank T. Logiudice Office: Biology Building, Room 202c Office Phone Number: (407) - 823-2495 Email Address: Frank.Logiudice@ucf.edu
COMPARATIVE VERTEBRATE HISTOLOGY ZOO 4756c Syllabus for Fall 2018 Instructor: Frank T. Logiudice Office: Biology Building, Room 202c Office Phone Number: (407) - 823-2495 Email Address: Frank.Logiudice@ucf.edu
Lacerta viridis. Functional anatomy of the lungs of the green lizard, (Accepted 18 February 1977)
 J. Anat. (1978), 125, 2, pp. 421-431 421 With 9 figures Printed in Great Britain Functional anatomy of the lungs of the green lizard, Lacerta viridis C. MEBAN Department of Anatomy, The Queen's University
J. Anat. (1978), 125, 2, pp. 421-431 421 With 9 figures Printed in Great Britain Functional anatomy of the lungs of the green lizard, Lacerta viridis C. MEBAN Department of Anatomy, The Queen's University
DRAFT TANZANIA STANDARD
 Hatching eggs Specification DRAFT TANZANIA STANDARD TANZANIA BUREAU OF STANDARDS 1 Hatching eggs Specification TBS/AFDC 22 (5271) P3 0 FOREWORD This Tanzania standard was developed due to rapid increase
Hatching eggs Specification DRAFT TANZANIA STANDARD TANZANIA BUREAU OF STANDARDS 1 Hatching eggs Specification TBS/AFDC 22 (5271) P3 0 FOREWORD This Tanzania standard was developed due to rapid increase
1 Describe the anatomy and function of the turtle shell. 2 Describe respiration in turtles. How does the shell affect respiration?
 GVZ 2017 Practice Questions Set 1 Test 3 1 Describe the anatomy and function of the turtle shell. 2 Describe respiration in turtles. How does the shell affect respiration? 3 According to the most recent
GVZ 2017 Practice Questions Set 1 Test 3 1 Describe the anatomy and function of the turtle shell. 2 Describe respiration in turtles. How does the shell affect respiration? 3 According to the most recent
Prof. Neil. J.L. Heideman
 Prof. Neil. J.L. Heideman Position Office Mailing address E-mail : Vice-dean (Professor of Zoology) : No. 10, Biology Building : P.O. Box 339 (Internal Box 44), Bloemfontein 9300, South Africa : heidemannj.sci@mail.uovs.ac.za
Prof. Neil. J.L. Heideman Position Office Mailing address E-mail : Vice-dean (Professor of Zoology) : No. 10, Biology Building : P.O. Box 339 (Internal Box 44), Bloemfontein 9300, South Africa : heidemannj.sci@mail.uovs.ac.za
Writing: Lesson 31. Today the students will be learning how to write more advanced middle paragraphs using a variety of elaborative techniques.
 Top Score Writing Grade 4 Lesson 31 Writing: Lesson 31 Today the students will be learning how to write more advanced middle paragraphs using a variety of elaborative techniques. The following passages
Top Score Writing Grade 4 Lesson 31 Writing: Lesson 31 Today the students will be learning how to write more advanced middle paragraphs using a variety of elaborative techniques. The following passages
Development of the Intestinal Villi Associated
 Development of the Intestinal Villi Associated with the Increased Epithelial Cell Mitosis in Chickens Koh-en YAMAUCHI, Eiji NAKAMURA and Yutaka ISSHIKI Laboratory of Animal Science, Faculty of Agriculture,
Development of the Intestinal Villi Associated with the Increased Epithelial Cell Mitosis in Chickens Koh-en YAMAUCHI, Eiji NAKAMURA and Yutaka ISSHIKI Laboratory of Animal Science, Faculty of Agriculture,
Ectoparasites Myobia musculi Radfordia affinis Radfordia ensifera
 Ectoparasites Fleas, ticks, and lice are uncommon in modern laboratory facilities, but may be seen on wild or feral rodents. Most ectoparasite infestations seen in rats and mice used for research are various
Ectoparasites Fleas, ticks, and lice are uncommon in modern laboratory facilities, but may be seen on wild or feral rodents. Most ectoparasite infestations seen in rats and mice used for research are various
Today there are approximately 250 species of turtles and tortoises.
 I WHAT IS A TURTLE OR TORTOISE? Over 200 million years ago chelonians with fully formed shells appeared in the fossil record. Unlike modern species, they had teeth and could not withdraw into their shells.
I WHAT IS A TURTLE OR TORTOISE? Over 200 million years ago chelonians with fully formed shells appeared in the fossil record. Unlike modern species, they had teeth and could not withdraw into their shells.
Question Set 1: Animal EVOLUTIONARY BIODIVERSITY
 Biology 162 LAB EXAM 2, AM Version Thursday 24 April 2003 page 1 Question Set 1: Animal EVOLUTIONARY BIODIVERSITY (a). We have mentioned several times in class that the concepts of Developed and Evolved
Biology 162 LAB EXAM 2, AM Version Thursday 24 April 2003 page 1 Question Set 1: Animal EVOLUTIONARY BIODIVERSITY (a). We have mentioned several times in class that the concepts of Developed and Evolved
EXOTIC CLINICAL PATHOLOGY
 Brittney Exarhos, LVT, RVT Toledo Zoo and Aquarium 2700 Broadway St. Toledo OH 43609 EXOTIC CLINICAL PATHOLOGY Veterinary technicians in a zoo setting often spend a lot of time in the lab. They must have
Brittney Exarhos, LVT, RVT Toledo Zoo and Aquarium 2700 Broadway St. Toledo OH 43609 EXOTIC CLINICAL PATHOLOGY Veterinary technicians in a zoo setting often spend a lot of time in the lab. They must have
What are taxonomy, classification, and systematics?
 Topic 2: Comparative Method o Taxonomy, classification, systematics o Importance of phylogenies o A closer look at systematics o Some key concepts o Parts of a cladogram o Groups and characters o Homology
Topic 2: Comparative Method o Taxonomy, classification, systematics o Importance of phylogenies o A closer look at systematics o Some key concepts o Parts of a cladogram o Groups and characters o Homology
4 Many species of mammals, birds, reptiles, amphibians and fish 940L. Source 1 Habitats
 Source 1 Habitats 1 American Alligators can be found in fresh water environments like rivers, lakes, ponds, swamps and marshes. They also like to live in areas that are brackish, which means the water
Source 1 Habitats 1 American Alligators can be found in fresh water environments like rivers, lakes, ponds, swamps and marshes. They also like to live in areas that are brackish, which means the water
Reptiles. Ectothermic vertebrates Very successful Have scales and toenails Amniotes (lay eggs with yolk on land) Made up of 4 orders:
 Reptiles of Florida Reptiles Ectothermic vertebrates Very successful Have scales and toenails Amniotes (lay eggs with yolk on land) Made up of 4 orders: Crocodylia (alligators & crocodiles) Squamata (amphisbaenids
Reptiles of Florida Reptiles Ectothermic vertebrates Very successful Have scales and toenails Amniotes (lay eggs with yolk on land) Made up of 4 orders: Crocodylia (alligators & crocodiles) Squamata (amphisbaenids
Phylum Platyhelminthes Flatworms
 Phylum Platyhelminthes Flatworms The Acoelomates The acoelomates are animals that lack a coelom. Acoelomates lack a body cavity, and instead the space between the body wall and the digestive tract is filled
Phylum Platyhelminthes Flatworms The Acoelomates The acoelomates are animals that lack a coelom. Acoelomates lack a body cavity, and instead the space between the body wall and the digestive tract is filled
Characteristics of Tetrapods
 Marine Tetrapods Characteristics of Tetrapods Tetrapod = four-footed Reptiles, Birds, & Mammals No marine species of amphibian Air-breathing lungs Class Reptilia Saltwater Crocodiles, Sea turtles, sea
Marine Tetrapods Characteristics of Tetrapods Tetrapod = four-footed Reptiles, Birds, & Mammals No marine species of amphibian Air-breathing lungs Class Reptilia Saltwater Crocodiles, Sea turtles, sea
Reproductive physiology and eggs
 Reproductive physiology and eggs Class Business Reading for this lecture Required. Gill: Chapter 14 1. Reproductive physiology In lecture I will only have time to go over reproductive physiology briefly,
Reproductive physiology and eggs Class Business Reading for this lecture Required. Gill: Chapter 14 1. Reproductive physiology In lecture I will only have time to go over reproductive physiology briefly,
Video Assignments. Microraptor PBS The Four-winged Dinosaur Mark Davis SUNY Cortland Library Online
 Video Assignments Microraptor PBS The Four-winged Dinosaur Mark Davis SUNY Cortland Library Online Radiolab Apocalyptical http://www.youtube.com/watch?v=k52vd4wbdlw&feature=youtu.be Minute 13 through minute
Video Assignments Microraptor PBS The Four-winged Dinosaur Mark Davis SUNY Cortland Library Online Radiolab Apocalyptical http://www.youtube.com/watch?v=k52vd4wbdlw&feature=youtu.be Minute 13 through minute
Phylum Chordata. Fish, Amphibians, Reptiles
 Phylum Chordata Fish, Amphibians, Reptiles Chordates Three different groups Vertebrates Lancelets Tunicates At some point in their lives, they all have four special body parts Notocord Hollow nerve cord
Phylum Chordata Fish, Amphibians, Reptiles Chordates Three different groups Vertebrates Lancelets Tunicates At some point in their lives, they all have four special body parts Notocord Hollow nerve cord
13. Swim bladder function: A. What happens to the density of a fish if the volume of its swim bladder increases?
 Ch 11 Review - Use this worksheet as practice and as an addition to your Chapter 11 Study Guide. Test will only be over Ch 11.1-11.4. (Ch 11.5 Fossil and Paleontology section will not be on your test)
Ch 11 Review - Use this worksheet as practice and as an addition to your Chapter 11 Study Guide. Test will only be over Ch 11.1-11.4. (Ch 11.5 Fossil and Paleontology section will not be on your test)
Fischthal and Kuntz (1964) reported the
 Zoological Studies 41(3): 283-287 (2002) Meristocotyle provitellaria sp. nov. (Digenea: Meristocotylidae) from Varanus salvator in China Wei Liu 1, Qing-Kui Li 2, Hsiu-Hui Shih 3 and Zhao-Zhi Qiu 1, *
Zoological Studies 41(3): 283-287 (2002) Meristocotyle provitellaria sp. nov. (Digenea: Meristocotylidae) from Varanus salvator in China Wei Liu 1, Qing-Kui Li 2, Hsiu-Hui Shih 3 and Zhao-Zhi Qiu 1, *
VARIATION IN MONIEZIA EXPANSA RUDOLPHI
 VARIATION IN MONIEZIA EXPANSA RUDOLPHI STEPHEN R. WILLIAMS, Miami University, Oxford, Ohio In making a number of preparations of proglottids for class study at the stage when sex organs are mature and
VARIATION IN MONIEZIA EXPANSA RUDOLPHI STEPHEN R. WILLIAMS, Miami University, Oxford, Ohio In making a number of preparations of proglottids for class study at the stage when sex organs are mature and
Field Herpetology Final Guide
 Field Herpetology Final Guide Questions with more complexity will be worth more points Incorrect spelling is OK as long as the name is recognizable ( by the instructor s discretion ) Common names will
Field Herpetology Final Guide Questions with more complexity will be worth more points Incorrect spelling is OK as long as the name is recognizable ( by the instructor s discretion ) Common names will
PLASMODIUM MODULE 39.1 INTRODUCTION OBJECTIVES 39.2 MALARIAL PARASITE. Notes
 Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 95(1): 43-47, Jan./Feb. 2000 The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 95(1): 43-47, Jan./Feb. 2000 The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
HERPETOLOGY. Name: School:
 HERPETOLOGY November 4 th Scrimmage Name: School: Directions: DO NOT open the packet until prompted to. You will have 50 minutes for the test. Please answer each question to the best of your ability. Spelling
HERPETOLOGY November 4 th Scrimmage Name: School: Directions: DO NOT open the packet until prompted to. You will have 50 minutes for the test. Please answer each question to the best of your ability. Spelling
17.2 Classification Based on Evolutionary Relationships Organization of all that speciation!
 Organization of all that speciation! Patterns of evolution.. Taxonomy gets an over haul! Using more than morphology! 3 domains, 6 kingdoms KEY CONCEPT Modern classification is based on evolutionary relationships.
Organization of all that speciation! Patterns of evolution.. Taxonomy gets an over haul! Using more than morphology! 3 domains, 6 kingdoms KEY CONCEPT Modern classification is based on evolutionary relationships.
Transformed centrioles In adult and aged cat pinealocytes
 Transformed centrioles In adult and aged cat pinealocytes J. L. Calvo. J. Boya*. J. E. Garcia-Mauriño and D. Rancaño Department of Histology. Faculty of Medicine. University Complutense, 28040 Madrid.
Transformed centrioles In adult and aged cat pinealocytes J. L. Calvo. J. Boya*. J. E. Garcia-Mauriño and D. Rancaño Department of Histology. Faculty of Medicine. University Complutense, 28040 Madrid.
Long-distance Movement by American Alligators in Southwest Louisiana
 2011 SOUTHEASTERN NATURALIST 10(3):389 398 Long-distance Movement by American Alligators in Southwest Louisiana Valentine A. Lance 1,*, Ruth M. Elsey 2, Phillip L. Trosclair III 2, and Leisa A. Nunez 2
2011 SOUTHEASTERN NATURALIST 10(3):389 398 Long-distance Movement by American Alligators in Southwest Louisiana Valentine A. Lance 1,*, Ruth M. Elsey 2, Phillip L. Trosclair III 2, and Leisa A. Nunez 2
These small issues are easily addressed by small changes in wording, and should in no way delay publication of this first- rate paper.
 Reviewers' comments: Reviewer #1 (Remarks to the Author): This paper reports on a highly significant discovery and associated analysis that are likely to be of broad interest to the scientific community.
Reviewers' comments: Reviewer #1 (Remarks to the Author): This paper reports on a highly significant discovery and associated analysis that are likely to be of broad interest to the scientific community.
TWO NEW SPECIES OF WATER MITES FROM OHIO 1-2
 TWO NEW SPECIES OF WATER MITES FROM OHIO 1-2 DAVID R. COOK Wayne State University, Detroit, Michigan ABSTRACT Two new species of Hydracarina, Tiphys weaveri (Acarina: Pionidae) and Axonopsis ohioensis
TWO NEW SPECIES OF WATER MITES FROM OHIO 1-2 DAVID R. COOK Wayne State University, Detroit, Michigan ABSTRACT Two new species of Hydracarina, Tiphys weaveri (Acarina: Pionidae) and Axonopsis ohioensis
SEMESTER ONE 2007 INFECTION and IMMUNITY GRADUATE ENTRY PROGRAMME PARASITOLOGY PRACTICAL 9 Dr TW Jones NEMATODES
 SEMESTER ONE 2007 INFECTION and IMMUNITY GRADUATE ENTRY PROGRAMME PARASITOLOGY PRACTICAL 9 Dr TW Jones NEMATODES Objectives After this class I expect you to be able to: 1. Describe and recognise the range
SEMESTER ONE 2007 INFECTION and IMMUNITY GRADUATE ENTRY PROGRAMME PARASITOLOGY PRACTICAL 9 Dr TW Jones NEMATODES Objectives After this class I expect you to be able to: 1. Describe and recognise the range
HISTOLOGY OF MAMMARY GLAND DURING LACTATING AND NON-LACTATING PHASES OF MADRAS RED SHEEP WITH SPECIAL REFERENCE TO INVOLUTION
 International Journal of Science, Environment and Technology, Vol. 5, No 3, 2016, 991 996 ISSN 2278-3687 (O) 2277-663X (P) HISTOLOGY OF MAMMARY GLAND DURING LACTATING AND NON-LACTATING PHASES OF MADRAS
International Journal of Science, Environment and Technology, Vol. 5, No 3, 2016, 991 996 ISSN 2278-3687 (O) 2277-663X (P) HISTOLOGY OF MAMMARY GLAND DURING LACTATING AND NON-LACTATING PHASES OF MADRAS
Animals & Reptiles (PA) LD P KER CHIPS. *** Variations
 Animals & Reptiles (PA) LD P KER CHIPS 1 PA-AB thru PA-CW PA-AB Beaver PA-AF Bear *** PA-AJ Dancing Bears Embossed / v:e PA-AP Buffalo Head PA-AS Buffalo Head PA-AV Old Tom *** PA-BC House Cat PA-BG House
Animals & Reptiles (PA) LD P KER CHIPS 1 PA-AB thru PA-CW PA-AB Beaver PA-AF Bear *** PA-AJ Dancing Bears Embossed / v:e PA-AP Buffalo Head PA-AS Buffalo Head PA-AV Old Tom *** PA-BC House Cat PA-BG House
Short-term Water Potential Fluctuations and Eggs of the Red-eared Slider Turtle (Trachemys scripta elegans)
 Zoology and Genetics Publications Zoology and Genetics 2001 Short-term Water Potential Fluctuations and Eggs of the Red-eared Slider Turtle (Trachemys scripta elegans) John K. Tucker Illinois Natural History
Zoology and Genetics Publications Zoology and Genetics 2001 Short-term Water Potential Fluctuations and Eggs of the Red-eared Slider Turtle (Trachemys scripta elegans) John K. Tucker Illinois Natural History
Both Rod and Cone Disc Shedding ore Related to Light Onset in the Cat
 Both Rod and Cone Disc Shedding ore Related to Light Onset in the Cat Sreven K. Fisher,* Bruce A. Pfeffer.f and Don H. Anderson* Nineteen domestic cats were entrained to a 12-hr light/12-hr dark lighting
Both Rod and Cone Disc Shedding ore Related to Light Onset in the Cat Sreven K. Fisher,* Bruce A. Pfeffer.f and Don H. Anderson* Nineteen domestic cats were entrained to a 12-hr light/12-hr dark lighting
Exotic Hematology Lab Leigh-Ann Horne, LVT, CWR Wildlife Center of Virginia
 Exotic Hematology Lab Leigh-Ann Horne, LVT, CWR Wildlife Center of Virginia lhorne@wildlifecenter.org Anne Lynch, LVT Cedarcrest Animal Clinic amllvt9@gmail.com Introduction While the general set-up for
Exotic Hematology Lab Leigh-Ann Horne, LVT, CWR Wildlife Center of Virginia lhorne@wildlifecenter.org Anne Lynch, LVT Cedarcrest Animal Clinic amllvt9@gmail.com Introduction While the general set-up for
Geo 302D: Age of Dinosaurs LAB 4: Systematics Part 1
 Geo 302D: Age of Dinosaurs LAB 4: Systematics Part 1 Systematics is the comparative study of biological diversity with the intent of determining the relationships between organisms. Humankind has always
Geo 302D: Age of Dinosaurs LAB 4: Systematics Part 1 Systematics is the comparative study of biological diversity with the intent of determining the relationships between organisms. Humankind has always
Vol. XIV, No. 1, March, The Larva and Pupa of Brontispa namorikia Maulik (Coleoptera: Chrysomelidae: Hispinae) By S.
 Vol. XIV, No. 1, March, 1950 167 The Larva and Pupa of Brontispa namorikia Maulik (Coleoptera: Chrysomelidae: Hispinae) By S. MAULIK BRITISH MUSEUM (NATURAL HISTORY) (Presented by Mr. Van Zwaluwenburg
Vol. XIV, No. 1, March, 1950 167 The Larva and Pupa of Brontispa namorikia Maulik (Coleoptera: Chrysomelidae: Hispinae) By S. MAULIK BRITISH MUSEUM (NATURAL HISTORY) (Presented by Mr. Van Zwaluwenburg
Animal Diversity wrap-up Lecture 9 Winter 2014
 Animal Diversity wrap-up Lecture 9 Winter 2014 1 Animal phylogeny based on morphology & development Fig. 32.10 2 Animal phylogeny based on molecular data Fig. 32.11 New Clades 3 Lophotrochozoa Lophophore:
Animal Diversity wrap-up Lecture 9 Winter 2014 1 Animal phylogeny based on morphology & development Fig. 32.10 2 Animal phylogeny based on molecular data Fig. 32.11 New Clades 3 Lophotrochozoa Lophophore:
Mesosomes are a definite event in antibiotic-treated Staphylococcus aureus ATCC 25923
 Tropical Biomedicine 24(1): 105 109 (2007) Mesosomes are a definite event in antibiotic-treated Staphylococcus aureus ATCC 25923 Santhana Raj, L. 1*, Hing, H.L. 2, Baharudin Omar 2, Teh Hamidah, Z. 1,
Tropical Biomedicine 24(1): 105 109 (2007) Mesosomes are a definite event in antibiotic-treated Staphylococcus aureus ATCC 25923 Santhana Raj, L. 1*, Hing, H.L. 2, Baharudin Omar 2, Teh Hamidah, Z. 1,
REPTILES OF JAMAICA. Peter Vogel Department of Life Sciences Mona Campus University of the West Indies
 REPTILES OF JAMAICA Peter Vogel Department of Life Sciences Mona Campus University of the West Indies Order Testudines: Turtles Jamaican Slider Turtle (freshwater) Marine Turtles Jamaican Slider Turtle
REPTILES OF JAMAICA Peter Vogel Department of Life Sciences Mona Campus University of the West Indies Order Testudines: Turtles Jamaican Slider Turtle (freshwater) Marine Turtles Jamaican Slider Turtle
Recommended Resources: The following resources may be useful in teaching this
 Unit B: Anatomy and Physiology of Poultry Lesson1: Internal Anatomy of Poultry Student Learning Objectives: Instruction in this lesson should result in students achieving the following objectives: 1. Identify
Unit B: Anatomy and Physiology of Poultry Lesson1: Internal Anatomy of Poultry Student Learning Objectives: Instruction in this lesson should result in students achieving the following objectives: 1. Identify
Anat. Labor. of Prof. H. SETO, Tohoku University, On the Sensory Terminations Formed along the Ductus
 Anat. Labor. of Prof. H. SETO, Tohoku University, Sendai. On the Sensory Terminations Formed along the Ductus Pancreaticus in Cat. The existence of PACINIan bodies in the pancreas of mammals, especially
Anat. Labor. of Prof. H. SETO, Tohoku University, Sendai. On the Sensory Terminations Formed along the Ductus Pancreaticus in Cat. The existence of PACINIan bodies in the pancreas of mammals, especially
