~Il\.~&t. Coenraad F. M. Hendriksen. National Institute of Public Health and Environmental Protection, NL-Bilthoven. Introduction
|
|
|
- Spencer Freeman
- 5 years ago
- Views:
Transcription
1 ~Il\.~&t ~~~--- Replacement of the Toxin Neutralization Test in Mice by in vitro Serological Assay Systems for Potency Testing of Tetanus Toxoid Vaccines for Veterinary Use: Results of a European Collaborative Study Coenraad F. M. Hendriksen National Institute of Public Health and Environmental Protection, NL-Bilthoven Summary This paper describes the results af a collaborative study to the use of in vitro serological assay systems in the assessment of the potency of tetanus toxoid in single and multicomponent vaccines for veterinary use. According to the procedure described in monograph 697 of the European Pharmacopoeia (method A) these products are currently tested by toxin neutralisation tests in mice. The collaborative study was performed in seven laboratories throughout Europe. Nine commercial vaccines, representing the range of products available, and one experimental tetanus toxoid preparation were tested by immunization of groups of rabbits and guinea pigs. Individual and pooled serum samples were titrated for levels of tetanus antitoxin by indirect enzyme-linked immunosorbent assay (ELISA), toxin binding inhibition (ToB!) test, passive haemagglutination (HA) test and by the toxin neutralization (TN) test in mice. It was found that estimates of potency by in vitro tests and by the were in good agreement for the various vaccines tested and for antitoxin titres of individual serum samples. Significant intralaboratory variation occured less frequently for ELISA and ToBI than for HA. The frequency of significant interlaboratory variation was acceptable for ELISA andfor ToBI but larger variation was observedfor HA. It is concluded that ELISA and ToBI are suitable in vitro assay systems for assessing the potency of tetanus toxoid in batches of single and multicomponent vaccines for veterinary use. Rigid standardization of HA test is essential before this test can be usedfor the same quality control purpose. Zusammenfassung: Ersatrmethoden for den Toxinneutralisationstest in der Maus. Ergebnisse eines europiiischen Ringversuchs. Zur Wirksamkeitspriifung von Tierimpfstoffen gegen Tetanus wird ein Taxinbelastungsversuch an Labortieren im Europiiischen Arzneibuch verlangt. Hierzu werden zwei Methoden A und B beschrieben. Beide Tierversuche erfordern hohe Tierzahlen und sind so angelegt, daj3 ungefiihr die Hdlfte der Tiere unter erheblichem Leiden an Tetanus verendet. In den letzten lahren wurden einige immunologische Methoden zur Bestimmung von Tetanus- Antikorpern entwickelt. In einem europiiischen Ringversuch wurden drei Testsysteme (Haemagglutinationstest, Toxoid-ELISA und Toxinbindungsinhibitions-(ToB!)- Test) auf ihre Eignung als Alternativmethoden gepruft. Die Ergebnisse zeigen, dass der Toxoid-ELISA und der ToBI-Test geeignete Alternativen rum in vivo Toxin-Neutralisationstest der Methode A darstellen. Keywords: Tetanus toxoid, potency testing, serological test systems, collaborative study Introduction The year 1889 was a breakpoint in the history of tetanus. This infectious disease, already described by Hippocrates, was one of the world's greatest killers, both in human and animal populations. No therapy applied in those days, such as bleeding and aloe extract, was very effective to the state of spastic paralysis of tetanus victims. In 1889 the causative agent, Clostridium tetani, was identified by the bacteriologist Kitasato, a colleague of von Behring. It marked a milestone as from then on the development of an effective therapy and prophylaxis became possible. In 1890 von Behring and Kitasato discovered the therapeutic value of tetanus antitoxin and in 1920 Ramon succeeded in rendering tetanus toxin harmless by formaldehyde, while still remaining its antigenic power. The product was called,toxoid'. With the availability of immunological products as tetanus antiserum and tetanus toxoid the need for a quality control of these products emerged. Already in 1897 Ehrlich described an assay method for the estimation of the potency of tetanus antitoxin, based on the principle of Toxin neutralization (TN) in laboratory animals. In 1937 Prigge developed the concept of the socalled.kollektivversuch" for the potency testing of toxoid vaccines in which information on a dose-response and EDSO (Effective Dose 30
2 !It.:l".~ HENDRIKSEN --~~~ ",,,. 50%; the dose of vaccine which protects 50% of the immunized animals against the lethal or clinical effects of a challenge with the virulent agent) was obtained. Both methods; the and the.kollektivversuch" are still used. To express it even more forcibly, both methods form the back-bone for potency testing in the European Pharmacopoeia monograph on tetanus vaccines for veterinary use (Council of Europe, 1990). Potency testing of tetanus vaccines for veterinary application according to the European Pharmacopoeia For potency of tetanus vaccines for veterinary use two methods, A and B, are described in the European Pharmacopoeia (Council of Europe, 1990). Method A is an indirect protection test and is based on primary (day 0) and booster (day 28) immunization of 10 guinea pigs, followed by bleeding of the animals at day 42 and pooling of the sera. Thereafter, the potency of the pooled sera is determined in, by comparing the dose necessary to protect mice or other suitable animals against the toxic effects of a fixed dose of tetanus toxin with the quantity of a reference preparation of tetanus antitoxin, calibrated in International units (IU), necessary to give the same protection. The should be repeated at least once. When tetanus vaccine for veterinary use is presented as a mixed vaccine for use in animals other than Equidae and the potency of the other component(s) of that vaccine is normally performed in rabbits, potency testing may also be carried out in rabbits. Potencies should exceed 7.5.1U/ml in the case of tests in guinea pigs, except when the vaccine is intended for use in Eauidaa. Then the potency of the pooled sera should not be less than 30 Il.l/ml. In the case of potency tests in rabbits the potency of the pooled sera should exceed the level of 2.5 IU/ml. It should be noted that no information is given in the Mono- graph as to the rational behind the minimal levels stated. Method B in the EP Monograph is a direct protection test which strongly resembles the potency test on tetanus vaccines for human use. In Method B groups of mice are immunized only once with the vaccine under study and a reference toxoid, respectively, and challenged after 28 days with a fixed dose of tetanus toxin. Potency is calculated by parallel-line probit analysis. Method B may be used only for those preparations for which it has been shown to be suitable. In particular, the method may not be suitable for vaccines with an oily adjuvant or for multicomponent vaccines. With regard to animal welfare, both methods include serious disadvantages, being the degree of distress and suffering inflicted on the animals, and the large numbers of animals required, being about 10 guinea pigs or rabbits and at least 50 mice for Method A, half of them suffering or dying from tetanus. Therefore it is felt that replacement or refinement of the direct or indirect protection test is urgently needed, however, without jeopardizing the testing of quality of the vaccine. In vitro serological assay systems As the immunogenicity of tetanus toxoid is affected not only by its quantity, but also by its quality and by other components (e.g. adjuvant), it generally is felt that some kind of biological test system will be needed to estimate potency. Procedures which have been developed last decade are based on immunization of laboratory animals with toxoid, followed by serum titration in in vitro test systems, preferably based on a functional parameter. Unfortunately, the only biological activity of Tetanus toxin known is the inhibition of neurotransmitter release in nerve cells (Habermann und Dreyer, 1986). This rather specific activity limits the possibility of developing a functional in vitro model, such as the titration of antiserum by Toxin neutralization in cultures of nerve cells or neuroblastoma cells. So far, studies in this direction have not been very successful (Hendriksen et al., ] 988). Instead, several non-functional in vitro models have been established, based on immunological techniques, such as the haemagglutination (HA) test (Pitzurra et al., 1983), Toxoid-Enzyme Linked Immunosorbent assay (ELI- SA) (Melville-Smith et al., 1983) and Toxin binding inhibition (ToBI). test (Hendriksen et ai., 1988). These methods have shown to be very useful in the estimation of tetanus antitoxin concentration, although for Toxoid-ELISA overestimation of tetanus antitoxin has been reported (Simonsen et al., 1986) with low titre antisera. In 1991, a collaborative study in seven laboratories throughout the European Union (EU) was initiated to evaluate the suitability of the in vitro serological assay systems to provide valid and reproducible measurements of the potency of tetanus toxoid preparations for veterinary use, according to Method A of the EP. The study was financially supported by Directorate General (DG) XI of the EU. The collaborative study: design Seven laboratories (of which 6 national control laboratories) from different EU countries participated in the study (Annex I). First an informal pilot study was carried out in all participating laboratories to gain experience with the in vitro assay systems, to evaluate the study protocol and technical instructions and to pre-screen materials. For the collaborative study groups of 10 rabbits and 10 guinea pigs were immunized with each of nine commercial single and multicomponent tetanus toxoid vaccines and one experimental preparation; a monovalent tetanus toxoid heated for 36 hours at 56 C. Vaccines selected, included a wide range of products, both with regard to formulation as with regard to adjuvant products used. A specification is given in 31
3 _H_E_ND_R_I_KS_E_N ~~---- ~c~.~. Table 1. Animals were immunized according to method A of the EP at one of the coordinating laboratories. For the study, both individually rabbit serum samples (n=), mixed serum samples from pairs of two guinea pigs (n=50, 5 per vaccine) and pooled serum samples (n=20; one pool per vaccine and animal species) were used. Participants were asked to perform the HA test, the Toxoid ELISA and the ToBI test in triplicate on different days, starting each with independently prepared dilutions of freshly made solutions. Descriptions of the methods used can be found elsewhere (Hendriksen et al., 1993). Each laboratory used the same standard operating protocol for performance of test. In the organizing laboratories the potency of the 20 pooled serum samples and of 10 rabbit and 10 guinea pig serum samples were also determined in duplicate in the in vivo. Each laboratory was provided with an equal part of the code-labelled serum samples and principle materials for the in vitro assays (i.e. micro titer plates, conjugates, tetanus toxin/toxoid, sensitized red blood cells). In addition, laboratories were supplied with rabbit and guinea pig reference serum, which were produced and calibrated at the organizing laboratories. Raw data of the participants were elaborated at the organizing laboratories and intra- and interlaboratory variation in HA test, Toxoid ELISA and ToBI test was determined by one-way analysis of variance (anova). In addition in vivo data and in vitro data were used to estimate correlation. The collaborative study: results Only a selection of data will be presented here. Large intralaboratory variation was seen with the HA test, statistical significant at p < When triplicate HA tests in all participating laboratories are taken together it showed statistical significant variation in 30 out of 70 triplicate tests for Table 1: Specifications of the tetanus toxoid preparationsused in the interlaboratory collaborative study Tetanus toxoid Adjuvant Animal(s) Mono Mono (experimental) guinea pig sera and for 19 out of 68 triplicate tests for rabbit sera. Intralaboratory variation was smaller for Toxoid-ELISA (14170 and 15170, respectively) and ToB! (15170 and 10170, respectively). Large intralaboratory variation in HA test most probably is related to optical reading of the test, which, to a certain extent, is SUbjective. For interlaboratory comparison estimates of potency of each vaccine obtained in one assay performance were combined using the arithmetic mean of individual serum samples. Thus for each vaccine and for each laboratory three values were obtained per in vitro test system. Thereafter interlaboratory variation was measured by one-way anova at p < 0.05 for each vaccine, resulting in 20 estimates (10 data of tests in rabbits and 10 of tests on guinea pigs) per in vitro test system. Interlaboratory variation was seen especially with the HA test (in 18 out of 20), but variation also frequently occurred for the ELISA (10/20) and ToBI test (8/20). However, in most cases variation was due to deviating results of one or two participating laboratories. The antitoxin levels of the 40 serum samples obtained by, were in the range of 2.6 IV/ml to 266 IV/m1. The relationship between these data and those measured by the in vitro serological assay systems is presented for one of the participating laboratories in Figure 1 (guinea pigs) and Figure 2 (rabbits). Good agreement was demonstrated in our study between the Toxoid-ELISA and TN Saponin Alum.Phosphate Alum. Hydroxide Aluin Water/Oil Alum.Phosphate All species Sheep Sheep, pig, cattle Sheep, goat, cattle Sheep, goat, cattle test and between ToBI test and TN test (Table 2). Agreement between HA test and was less pronounced. However, this might be due to the large variance in test results. Conclusions and recommendations From the results of the collaborative study it was concluded that Toxoid- ELISA and ToBI test can be used as a valid alternati ve to the in vivo TN test for the estimation of potency of tetanus toxoid for veterinary use according to Method A of the EP. Further standardization of the HA test will be needed before this test can be introduced in potency testing. A major disadvantage of the Toxoid-ELISA is that marked overestimation of potency has been demonstrated at antitoxin levels < 0.16 IV/ml, indicating that the Toxoid-ELISA only poorly discriminates between neutralizing and nonneutralizing tetanus antibodies. Although this is considered to be a major drawback for clinical studies, it is not for potency testing as minimum requirements for tetanus vaccines for veterinary use are well above the critical level in which significant overestimation of potency in Toxoid- ELISA might be expected. As potency calculations of serum samples in Toxoid-ELISA and ToBI test are based on parallel-line analysis, using dose-response (optical density) relation in immunoassay, differences in avidity between reference sample and test sample may interfere with estimation of serum potency. Avidity 32 ALTEX 11. SUPPLEMENT 94
4 ~r;')~ HENDRIKSEN --h~s ~v~ r , 250 ~ 150. I- 80r ~ on avidity problems, the quality of the reference preparations should be comparable to that of test sera. Initiatives for the production of these preparations should be taken by the EP ~ 150 ui I o , o~---~---~--~ o Figure 1: Correlation of in vitro test systems with in vivo in the estimation of guinea pig samples. 80~ ~ ~ 40 w :I: , , o Figure 2: Correlation of in vitro test systems with in vivo in the estimation of rabbit serum samples. Table 2: Correlation coefficient of in vitro test systems with in vivo in the estimation of serum samples (N=40) in seven laboratories. in vitro Laboratory test system ABC D E F G HA test ELISA ToBI test Discussion A rather surprising finding in the collaborative study was the estimated high potency, both in the in vitro tests and in the, of the experimental toxoid preparation, heated for about 36h at 56 C, and therefore, expected to have an inferior quality. In an additional study, based on Method B of the EP, however, the potency was found to be low (135 IU/ml) and did not meet the minimum requirement for method B (> 150 IU/ml). This means that Method A and Method B gave conflicting results with regard to this experimental preparation. Apart from the effect of hoostering and difference of animal species, no closely reasoned explanation can be given for this conflicting finding. Similar results have been described by Lyng (1992) and Relyveld et al. e 1991) and prompts us to challenge the rational of two (qualitative) different method>: (A and B) for the estimation of potency of tetanus toxoid for veterinary use. Considering the well known effect of the strain of mice (Hardegree et al., 1972; Lyng and Nyerges, 1984) on the estimation of potency according to Method B, preference is given to Method A and to omit Method B in Monograph 697. Information, relating estimates of potency obtained by Method A or Method B to protecting antibody levels in the target species would scientifically underpin such a decision. problems are to be expected with the horse antitetanus reference serum currenly used. Therefore, two animal model homologous reference preparations; the guinea pig tetanus reference serum and the rabbit tetanus reference serum, were produced for the collaborative study. Implementa- tion of in vitro serological test systems in EP requirements for veterinary tetanus toxoid potency testing should therefore go together with the production and calibration of two new reference tetanus antitoxin preparation; a guinea pig and a rabbit standard preparation. To anticipate References Council of Europe. (1990) Vaccinum tetani ad usum veterinarium. European Pharmacopoeia, 697. Habermann, E. and Dreyer, F. (1986). Clostridial neurotoxins: handling and action at the cellular and molecular level. Curro Top. Microbial. 33
5 HENDRIKSEN ~~~-- ~~ "'~~ Immunol. 129, Hardegree, M. C., Pittman, M. and Maloney, C. L. (1972). Influence of mouse strain on the assayed potency (unitage) of tetanus toxoid. Applied Microbiology 24,1, Hendriksen, C. F. M., Van der Gun, J. W., Nagel, J. and Kreeftenberg, J. G. (1988). The toxin binding inhibition test as a reliable in vitro alternative to the toxin neutralization test in mice for the estimation of tetanus antitoxin in human sera. 1. Bioi. Stand. 16, Hendriksen, C. F. M., Woltjes, J., Van de Gun, J. W., Akkermans, A. M., Marsman, F., Verschure, M. H. and Veldman, K. (1993). Interlaboratory validation of in vitro serological assay systems for potency testing of tetanus toxoid vaccines for veterinary use. Biologicals, in press. Lyng, J. (1992). EP/DT study on in vitro methods for estimating antibodies to diphtheria and tetanus toxoid. An international collaborative study. WHO document, BS/ Lyng, J. and Nyerges, G. Y. (1984). The second international standard for tetanus toxoid (adsorbed). 1. Bio!. Stand. 12, Melville-Smith, M. E, Seagroatt, V. A. and Watkins, J. T. (1983). A comparison of enzyme-linked irnmunosorbent assay (ELISA) with the toxin neutralization test in mice method for the estimation of tetanus antioxin in human sera. 1. Biol. Stand. 11, Pitzurra, M., Bistoni, F., Pitzurra, L. and Marconi, P. (1983). Use of turkey red blood cells in the passive haemagglutination test for studying tetanus immunity. Bull. Wid. Hlth. Org. 61, Relyveld, E., Bengounia, A., Huet, M. and Kreeftenberg, J. G. (1991). Antibody response of pregnant women to two different adsorbed tetanus toxoids. Vaccine 19, 369. Simonsen, 0., Bentzon, M. W. and Heron, I. (1986). ELISA for routine determination of antitoxic immunity to tetanus. 1. Bioi. Stand. 14, Address Coenraad F. M. Hendriksen National Institute of Public Health and Environmental Protection P. O. Box BA Bilthoven, The Netherlands ANNEX I: LIST OF PARTICIPANTS Dr. M. Desmecht Nationaal Instituut voor Diergeneeskundig onderzoek Brussel (Ukkel) BELGIUM Dr. K. CusslerlDr. H. Gyra Paul-Ehrlich-Institut, Bundesamt fur Sera und Impfstoffe Langen GERMANY Dr. J. Woltjes Central Veterinary Institute Lelystad THE NETHERLANDS Dr. C. F. M. Hendriksen National Institute of Public Health and Environmental Protection Bilthoven THE NETHERLANDS Dr. E. D. Amore/Dr. Ciuchini/ Dr. R. Adone Instituto Superiore di Sanita Laboratorio di Medicina Veterinaria Roma ITALY Dr. F. M. A. NazarethlDr. B. Cruz Laboratorio National de Investiga Veterinaria Lisboa PORTUGAL Dr. R. Goddard Central Veterinary Laboratory MAFF New Haw Weybridge UNITED KINGDOM 34 AL TEX II, SUPPLEMENT 94
Gye and Cramer (1919) found that the ionizable salts of calcium injected together with the washed spores of Cl. tetani or of certain
 STUDIES ON TETANUS TOXOID III. ANTITOXIC RESPONSE IN GUINEA PIGS IMMUNIZED WITH TETANUS ALUM-PRECIPITATED TOXOID FOLLOWED BY TET- ANUS SPORES F. G. JONES AND W. A. JAMIESON Lilly Research Laboratories,
STUDIES ON TETANUS TOXOID III. ANTITOXIC RESPONSE IN GUINEA PIGS IMMUNIZED WITH TETANUS ALUM-PRECIPITATED TOXOID FOLLOWED BY TET- ANUS SPORES F. G. JONES AND W. A. JAMIESON Lilly Research Laboratories,
EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS WORK-PROGRAMME PROPOSAL Version 2 VISAVET. Universidad Complutense de Madrid
 EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL Directorate D Animal Health and Welfare Unit D1- Animal health and Standing Committees EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS
EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL Directorate D Animal Health and Welfare Unit D1- Animal health and Standing Committees EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 [Version 7.3.1, 11/2010] FINAL SPC, LABELLING AND PACKAGE LEAFLET ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CEVAC Clostridium Ovino suspension for injection
[Version 7.3.1, 11/2010] FINAL SPC, LABELLING AND PACKAGE LEAFLET ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CEVAC Clostridium Ovino suspension for injection
Bovine Brucellosis Control of indirect ELISA kits
 Bovine Brucellosis Control of indirect ELISA kits (Pooled milk samples) Standard Operating Procedure Control of Bovine brucellosis Milk ELISA kits SOP Page 1 / 6 02 February 2012 SAFETY PRECAUTIONS The
Bovine Brucellosis Control of indirect ELISA kits (Pooled milk samples) Standard Operating Procedure Control of Bovine brucellosis Milk ELISA kits SOP Page 1 / 6 02 February 2012 SAFETY PRECAUTIONS The
= 0.5 mg. In vitro toxin neutralisation test based on haemolysis of sheep erythrocytes. For a full list of excipients, see section 6.1.
 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 8 Suspension for injection for sheep and cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances: Potency value/quantity/ml C. perfringens
1 NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 8 Suspension for injection for sheep and cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances: Potency value/quantity/ml C. perfringens
Influence of Mouse Strain on the Assayed Potency (Unitage) of Tetanus Toxoid
 APPUED MICROBIOLOGY, July 1972, p. 120-126 Copyright 0 1972 American Society for Microbiology Vol. 24, No. 1 Printed in U.SA. Influence of Mouse Strain on the Assayed Potency (Unitage) of Tetanus Toxoid
APPUED MICROBIOLOGY, July 1972, p. 120-126 Copyright 0 1972 American Society for Microbiology Vol. 24, No. 1 Printed in U.SA. Influence of Mouse Strain on the Assayed Potency (Unitage) of Tetanus Toxoid
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 10 Suspension for injection for sheep and cattle 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Potency
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 10 Suspension for injection for sheep and cattle 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Potency
Sera from 2,500 animals from three different groups were analysed:
 FIELD TRIAL OF A BRUCELLOSIS COMPETITIVE ENZYME LINKED IMMUNOABSORBENT ASSAY (ELISA) L.E. SAMARTINO, R.J. GREGORET, G. SIGAL INTA-CICV Instituto Patobiología Area Bacteriología, Buenos Aires, Argentina
FIELD TRIAL OF A BRUCELLOSIS COMPETITIVE ENZYME LINKED IMMUNOABSORBENT ASSAY (ELISA) L.E. SAMARTINO, R.J. GREGORET, G. SIGAL INTA-CICV Instituto Patobiología Area Bacteriología, Buenos Aires, Argentina
Clostridial Vaccination Efficacy on Stimulating and Maintaining an Immune Response in Beef Cows and Calves 1,2
 Clostridial Vaccination Efficacy on Stimulating and Maintaining an Immune Response in Beef Cows and Calves 1,2 T. R. Troxel*,3, G. L. Burke*, W. T. Wallace*, L. W. Keaton*, S. R. McPeake*, D. Smith, and
Clostridial Vaccination Efficacy on Stimulating and Maintaining an Immune Response in Beef Cows and Calves 1,2 T. R. Troxel*,3, G. L. Burke*, W. T. Wallace*, L. W. Keaton*, S. R. McPeake*, D. Smith, and
Paul-Ehrlich-Institut Bundesinstitut für Impfstoffe und biomedizinische Arzneimittel Federal Institute for Vaccines and Biomedicines
 Paul-Ehrlich-Institut Bundesinstitut für Impfstoffe und biomedizinische Arzneimittel Federal Institute for Vaccines and Biomedicines PAUL-EHRLICH-INSTITUT Paul-Ehrlich-Straße 51-59 D-63225 Langen MUTUAL
Paul-Ehrlich-Institut Bundesinstitut für Impfstoffe und biomedizinische Arzneimittel Federal Institute for Vaccines and Biomedicines PAUL-EHRLICH-INSTITUT Paul-Ehrlich-Straße 51-59 D-63225 Langen MUTUAL
Summary of product characteristics As per Annex C. SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1
 Summary of product characteristics As per Annex C SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1 1. NAME OF THE MEDICINAL PRODUCT. ANNEXURE C to MODULE I Tetanus vaccine (Adsorbed) I.P. 2.
Summary of product characteristics As per Annex C SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1 1. NAME OF THE MEDICINAL PRODUCT. ANNEXURE C to MODULE I Tetanus vaccine (Adsorbed) I.P. 2.
TOXOIDING OF SNAKE VENOM AND EVALUATION OF IMMUNOGENICITY OF THE TOXOIDS
 TOXOIDING OF SNAKE VENOM AND EVALUATION OF IMMUNOGENICITY OF THE TOXOIDS Pages with reference to book, From 9 To 13 Zahid Husain Khan ( Present Addressc Chief Research Officer, Pakistan Medical Research
TOXOIDING OF SNAKE VENOM AND EVALUATION OF IMMUNOGENICITY OF THE TOXOIDS Pages with reference to book, From 9 To 13 Zahid Husain Khan ( Present Addressc Chief Research Officer, Pakistan Medical Research
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Porcilis ColiClos suspension for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose of 2 ml
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Porcilis ColiClos suspension for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose of 2 ml
Anti4ody Response to Successive Booster Doses of Tetanus
 INFECTION AND IMMUNITY, July 1974, P. 1-5 Copyright 0 1974 American Society for Microbiology Vol. 10, No. 1 Printed in U.S.A. Anti4ody Response to Successive Booster Doses of Tetanus Toxoid in Adults JOHN
INFECTION AND IMMUNITY, July 1974, P. 1-5 Copyright 0 1974 American Society for Microbiology Vol. 10, No. 1 Printed in U.S.A. Anti4ody Response to Successive Booster Doses of Tetanus Toxoid in Adults JOHN
The Use of Homologous Antigen in the Serological Diagnosis of Brucellosis Caused by Brucella melitensis
 J. Vet. Med. B 52, 75 81 (25) Ó 25 Blackwell Verlag, Berlin ISSN 931 1793 Istituto Zooprofilattico Sperimentale dell Abruzzo e del Molise ÔG. CaporaleÕ, Campo Boario, Teramo, Italy The Use of Homologous
J. Vet. Med. B 52, 75 81 (25) Ó 25 Blackwell Verlag, Berlin ISSN 931 1793 Istituto Zooprofilattico Sperimentale dell Abruzzo e del Molise ÔG. CaporaleÕ, Campo Boario, Teramo, Italy The Use of Homologous
COMMISSION OF THE EUROPEAN COMMUNITIES REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 20.1.2005 COM(2005) 7 final. REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT FOURTH REPORT ON THE STATISTICS ON THE NUMBER OF ANIMALS
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 20.1.2005 COM(2005) 7 final. REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT FOURTH REPORT ON THE STATISTICS ON THE NUMBER OF ANIMALS
Overview and Expectations
 Overview and Expectations David M. Dusek Senior Staff Microbiologist Policy, Evaluation and Licensing Center for Veterinary Biologics Veterinary Services Animal and Plant Health Inspection Service United
Overview and Expectations David M. Dusek Senior Staff Microbiologist Policy, Evaluation and Licensing Center for Veterinary Biologics Veterinary Services Animal and Plant Health Inspection Service United
Error! Reference source not found. I. SUMMARY OF PRODUCT CHARACTERISTICS
 PRODUCTNAME NOBIVAC RABIES 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Nobivac Rabies 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active components: Rabies strain Pasteur RIV; at least 2 I.U. per dose
PRODUCTNAME NOBIVAC RABIES 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Nobivac Rabies 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active components: Rabies strain Pasteur RIV; at least 2 I.U. per dose
ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION
 ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION 2013 CONTENTS 1. Introduction 2. Summary 3. Results 3.1 Species and numbers of naive animals used in
ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION 2013 CONTENTS 1. Introduction 2. Summary 3. Results 3.1 Species and numbers of naive animals used in
Antitoxin l.evels in Botulism Patients Treated with Trivalent Equine Botulism Antitoxin to Toxin Types A, B, and E
 THE JOURNAL OF INFECTIOUS DISEASES. VOL. 150,.3. SEPTEMBER 1984 1984 by The University of Chicago. All rights reserved. Antitoxin l.evels in Botulism Patients Treated with Trivalent Equine Botulism Antitoxin
THE JOURNAL OF INFECTIOUS DISEASES. VOL. 150,.3. SEPTEMBER 1984 1984 by The University of Chicago. All rights reserved. Antitoxin l.evels in Botulism Patients Treated with Trivalent Equine Botulism Antitoxin
BIOLACTAM. Product Description. An innovative in vitro diagnostic for the rapid quantitative determination of ß-lactamase activity
 BIOLACTAM www.biolactam.eu An innovative in vitro diagnostic for the rapid quantitative determination of ß-lactamase activity 1.5-3h 20 Copyright 2014 VL-Diagnostics GmbH. All rights reserved. Product
BIOLACTAM www.biolactam.eu An innovative in vitro diagnostic for the rapid quantitative determination of ß-lactamase activity 1.5-3h 20 Copyright 2014 VL-Diagnostics GmbH. All rights reserved. Product
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections EMEA/CVMP/627/01-FINAL COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS GUIDELINE FOR THE DEMONSTRATION OF EFFICACY
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections EMEA/CVMP/627/01-FINAL COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS GUIDELINE FOR THE DEMONSTRATION OF EFFICACY
Influences on tetanus immunization in
 Archives of Emergency Medicine, 1990, 7, 163-168 Influences on tetanus immunization in accident and emergency A. MONTAGUE & E. GLUCKSMAN Accident and Emergency Department, King's College Hospital, Denmark
Archives of Emergency Medicine, 1990, 7, 163-168 Influences on tetanus immunization in accident and emergency A. MONTAGUE & E. GLUCKSMAN Accident and Emergency Department, King's College Hospital, Denmark
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2015 This report has been submitted : 2016-02-03 11:54:54 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Enzootic
OIE Reference Laboratory Reports Activities Activities in 2015 This report has been submitted : 2016-02-03 11:54:54 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Enzootic
EXPERT COMMITTEE ON BIOLOGICAL STANDARDIZATION Geneva - 8 to 12 October 2007
 WHO/BS/07.2061 ENGLISH ONLY EXPERT COMMITTEE ON BIOLOGICAL STANDARDIZATION Geneva - 8 to 12 October 2007 Calibration of Replacement International Standard of Tetanus Toxoid for use in Flocculation Test
WHO/BS/07.2061 ENGLISH ONLY EXPERT COMMITTEE ON BIOLOGICAL STANDARDIZATION Geneva - 8 to 12 October 2007 Calibration of Replacement International Standard of Tetanus Toxoid for use in Flocculation Test
WEEKLY Ag Update By Nathan Anderson 1/22/2019. First Calf Heifer Nutrition
 WEEKLY Ag Update By Nathan Anderson 1/22/2019 First Calf Heifer Nutrition A lot of the time, we treat our first calf heifers (or first calf cow) the same as the rest of the cowherd, sometimes even with
WEEKLY Ag Update By Nathan Anderson 1/22/2019 First Calf Heifer Nutrition A lot of the time, we treat our first calf heifers (or first calf cow) the same as the rest of the cowherd, sometimes even with
Tetanus. The Immunological Basis for Immunization. World Health Organization Geneva, Expanded Programme on Immunization
 The mmunological Basis for mmunization Tetanus Expanded Programme on mmunization World Health Organization Geneva, 1993 WHO/EP/GEN/93.13 Distribution: General Original: English The mmunological Basis for
The mmunological Basis for mmunization Tetanus Expanded Programme on mmunization World Health Organization Geneva, 1993 WHO/EP/GEN/93.13 Distribution: General Original: English The mmunological Basis for
Toxocariasis: serological diagnosis by enzyme
 Journal of Clinical Pathology, 1979, 32, 284-288 Toxocariasis: serological diagnosis by enzyme immunoassay D. H. DE SAVIGNY, A. VOLLER, AND A. W. WOODRUFF From the Toxocaral Reference Laboratory, Department
Journal of Clinical Pathology, 1979, 32, 284-288 Toxocariasis: serological diagnosis by enzyme immunoassay D. H. DE SAVIGNY, A. VOLLER, AND A. W. WOODRUFF From the Toxocaral Reference Laboratory, Department
zation and labor when large groups are than toxoid injections in adults, although second method is of great value, but it
 Combined Tetanus-Diphtheria Immunization of Adults: of Diphtheria Toxoid Use of Small Doses GEOFFREY EDSALL, M.D., F.A.P.H.A.; COMMANDER JAMES S. ALTMAN, MC, USNR; and LIEUTENANT (j.g.) ANDREW J. GASPAR,
Combined Tetanus-Diphtheria Immunization of Adults: of Diphtheria Toxoid Use of Small Doses GEOFFREY EDSALL, M.D., F.A.P.H.A.; COMMANDER JAMES S. ALTMAN, MC, USNR; and LIEUTENANT (j.g.) ANDREW J. GASPAR,
THE INFLUENCE OF STREPTOCOCCUS ALLER- GEN AND STREPTOLYSIN 0 ON THE FORMA- TION OF ANTITETANUS IMMUNITY DISTRIBUTED BY:
 AD-779 177 THE INFLUENCE OF STREPTOCOCCUS ALLER- GEN AND STREPTOLYSIN 0 ON THE FORMA- TION OF ANTITETANUS IMMUNITY L. A. Pozhidaeva-Sinitsina Foreign Technology Division Wright-Patterson Air Force Base,
AD-779 177 THE INFLUENCE OF STREPTOCOCCUS ALLER- GEN AND STREPTOLYSIN 0 ON THE FORMA- TION OF ANTITETANUS IMMUNITY L. A. Pozhidaeva-Sinitsina Foreign Technology Division Wright-Patterson Air Force Base,
Surveillance of Brucella Antibodies in Camels of the Eastern Region of Abu Dhabi, United Arab Emirates
 Proceedings of the Third Annual Meeting for Animal Production UnderArid Conditions, Vol. 1: 160-166 1998 United Arab Emirates University. Surveillance of Brucella Antibodies in Camels of the Eastern Region
Proceedings of the Third Annual Meeting for Animal Production UnderArid Conditions, Vol. 1: 160-166 1998 United Arab Emirates University. Surveillance of Brucella Antibodies in Camels of the Eastern Region
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BLUEVAC BTV8 suspension for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BLUEVAC BTV8 suspension for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of
VOL. XXIII NO. II THE JOURNAL OF ANTIBIOTICS 559. ANTIBIOTIC 6640.* Ill
 VOL. XXIII NO. II THE JOURNAL OF ANTIBIOTICS 559 ANTIBIOTIC 6640.* Ill BIOLOGICAL STUDIES WITH ANTIBIOTIC 6640, A NEW BROAD-SPECTRUM AMINOGLYCOSIDE ANTIBIOTIC J. Allan Waitz, Eugene L. Moss, Jr., Edwin
VOL. XXIII NO. II THE JOURNAL OF ANTIBIOTICS 559 ANTIBIOTIC 6640.* Ill BIOLOGICAL STUDIES WITH ANTIBIOTIC 6640, A NEW BROAD-SPECTRUM AMINOGLYCOSIDE ANTIBIOTIC J. Allan Waitz, Eugene L. Moss, Jr., Edwin
EU Statistical Data of all uses of animals
 Member State: Belgium Year: 2014 All uses of animals by species Animal Species Number of uses Percentage Mice 363,794 55.10% Rats 63,664 9.64% Guinea-Pigs 21,310 3.23% Hamsters (Syrian) 2,745 0.42% Hamsters
Member State: Belgium Year: 2014 All uses of animals by species Animal Species Number of uses Percentage Mice 363,794 55.10% Rats 63,664 9.64% Guinea-Pigs 21,310 3.23% Hamsters (Syrian) 2,745 0.42% Hamsters
ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION
 ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION 2015 CONTENTS 1. Introduction 2. Summary 3. Results 3.1 Species and numbers of naïve animals used in
ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION 2015 CONTENTS 1. Introduction 2. Summary 3. Results 3.1 Species and numbers of naïve animals used in
and other serological tests in experimentally infected cattle
 J. Hyg., Camb. (1982), 88, 21 21 Printed in Great Britain A comparison of the results of the brucellosis radioimmunoassay and other serological tests in experimentally infected cattle BY J. HAYES AND R.
J. Hyg., Camb. (1982), 88, 21 21 Printed in Great Britain A comparison of the results of the brucellosis radioimmunoassay and other serological tests in experimentally infected cattle BY J. HAYES AND R.
ANNEX Part 1 Model animal health certificate for imports into the Union of dogs, cats and ferrets COUNTRY:
 ANNEX Part 1 Model animal health certificate for imports into the Union of dogs, cats and COUNTRY: Veterinary certificate to EU Part I : Details of dispatched consignment I.1. I.5. Consignor I.2. Certificate
ANNEX Part 1 Model animal health certificate for imports into the Union of dogs, cats and COUNTRY: Veterinary certificate to EU Part I : Details of dispatched consignment I.1. I.5. Consignor I.2. Certificate
Seroprevalence of antibodies to Schmallenberg virus in livestock
 Seroprevalence of antibodies to Schmallenberg virus in livestock Armin R.W. Elbers Dept. Epidemiology, Crisis organisation and Diagnostics Central Veterinary Institute (CVI) part of Wageningen UR armin.elbers@wur.nl
Seroprevalence of antibodies to Schmallenberg virus in livestock Armin R.W. Elbers Dept. Epidemiology, Crisis organisation and Diagnostics Central Veterinary Institute (CVI) part of Wageningen UR armin.elbers@wur.nl
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Revised: January 2012 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Blackleg Vaccine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance(s): per ml Five strains
SUMMARY OF PRODUCT CHARACTERISTICS Revised: January 2012 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Blackleg Vaccine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance(s): per ml Five strains
EU Statistical Data of all uses of animals
 Member State: Belgium Year: 2016 All uses of animals by species Animal Species Number of uses Percentage Mice 337,027 62.90% Rats 30,337 5.66% Guinea-Pigs 16,223 3.03% Hamsters (Syrian) 1,880 0.35% Hamsters
Member State: Belgium Year: 2016 All uses of animals by species Animal Species Number of uses Percentage Mice 337,027 62.90% Rats 30,337 5.66% Guinea-Pigs 16,223 3.03% Hamsters (Syrian) 1,880 0.35% Hamsters
TABLE 1: NUMBER OF ANIMALS USED IN RELATION TO THEIR PLACE OF ORIGIN
 XI/810/04rev3 TABLE 1: NUMBER OF ANIMALS USED IN RELATION TO THEIR PLACE OF ORIGIN Origin versus species 1.1 1.a. Mice (Mus musculus) 1.b. Rats (Rattus norvegicus) 1.c. Guinea-Pigs (Cavia porcellus) 1.d.
XI/810/04rev3 TABLE 1: NUMBER OF ANIMALS USED IN RELATION TO THEIR PLACE OF ORIGIN Origin versus species 1.1 1.a. Mice (Mus musculus) 1.b. Rats (Rattus norvegicus) 1.c. Guinea-Pigs (Cavia porcellus) 1.d.
Animals used under 7 (2) of the Animal Protection Act by species
 Animals used under 7 (2) of the Animal Protection Act by species 1. Animals used by species Table 1: In total animals used by species (excludes re-use) Animal Species Number of Animals % Mice 1.350.727
Animals used under 7 (2) of the Animal Protection Act by species 1. Animals used by species Table 1: In total animals used by species (excludes re-use) Animal Species Number of Animals % Mice 1.350.727
Characterization of New Formalin-Detoxified Botulinum Neurotoxin Toxoids
 CLINICAL AND VACCINE IMMUNOLOGY, Sept. 2008, p. 1374 1379 Vol. 15, No. 9 1556-6811/08/$08.00 0 doi:10.1128/cvi.00117-08 Characterization of New Formalin-Detoxified Botulinum Neurotoxin Toxoids James E.
CLINICAL AND VACCINE IMMUNOLOGY, Sept. 2008, p. 1374 1379 Vol. 15, No. 9 1556-6811/08/$08.00 0 doi:10.1128/cvi.00117-08 Characterization of New Formalin-Detoxified Botulinum Neurotoxin Toxoids James E.
Tetanus Tetanus Clostridium tetani
 Tetanus Tetanus is an acute, often fatal, disease caused by an exotoxin produced by the bacterium Clostridium tetani. It is characterized by generalized rigidity and convulsive spasms of skeletal muscles.
Tetanus Tetanus is an acute, often fatal, disease caused by an exotoxin produced by the bacterium Clostridium tetani. It is characterized by generalized rigidity and convulsive spasms of skeletal muscles.
Monitoring methods and systems
 Monitoring methods and systems Georg von Samson-Himmelstjerna, Jürgen Krücken Institute for Parasitology and Tropical Veterinary Medicine Freie Universität Berlin What suitable and validated tools/tests
Monitoring methods and systems Georg von Samson-Himmelstjerna, Jürgen Krücken Institute for Parasitology and Tropical Veterinary Medicine Freie Universität Berlin What suitable and validated tools/tests
DP.1. Control tables
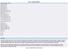 Data inclusion criteria Report year: 2015 Country: Croatia EU Submission: ALL Genetic status: ALL Animal Species: ALL Species grouping Level 1: ALL Species grouping Level 2: ALL Mammals: ALL Non-human
Data inclusion criteria Report year: 2015 Country: Croatia EU Submission: ALL Genetic status: ALL Animal Species: ALL Species grouping Level 1: ALL Species grouping Level 2: ALL Mammals: ALL Non-human
DP.1. Control tables
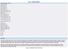 Data inclusion criteria Report year: 2014 Country: Croatia EU Submission: ALL Genetic status: ALL Animal Species: ALL Species grouping Level 1: ALL Species grouping Level 2: ALL Mammals: ALL Non-human
Data inclusion criteria Report year: 2014 Country: Croatia EU Submission: ALL Genetic status: ALL Animal Species: ALL Species grouping Level 1: ALL Species grouping Level 2: ALL Mammals: ALL Non-human
Applied-for scope of designation and notification of a Conformity Assessment Body Regulation (EU) 2017/746 (IVDR)
 Ref. Ares(2018)2576484-17/05/2018 NBOG s Best Practice Guide applicable for MDR IVDR NBOG F 2017-4 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article
Ref. Ares(2018)2576484-17/05/2018 NBOG s Best Practice Guide applicable for MDR IVDR NBOG F 2017-4 This document has been endorsed by the Medical Device Coordination Group (MDCG) established by Article
COUNCIL REGULATION (EEC) No 2377/90
 -W- -- 18. 8. 90 Official Journal of the ~uroiean Communities No L 224/P - - (Acts whose publication is obligatory) COUNCIL REGULATION (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure
-W- -- 18. 8. 90 Official Journal of the ~uroiean Communities No L 224/P - - (Acts whose publication is obligatory) COUNCIL REGULATION (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure
The OIE Relevant Standards and Guidelines for Vaccines
 The OIE Relevant Standards and Guidelines for Vaccines GALVMED/OIE STAKEHOLDER WORKSHOP ON THE HARMONISATION OF THE REGISTRATION OF VETERINARY MEDICINAL PRODUCTS, JOHANNESBURG, SOUTH AFRICA 9-11 MAY 2017
The OIE Relevant Standards and Guidelines for Vaccines GALVMED/OIE STAKEHOLDER WORKSHOP ON THE HARMONISATION OF THE REGISTRATION OF VETERINARY MEDICINAL PRODUCTS, JOHANNESBURG, SOUTH AFRICA 9-11 MAY 2017
Statistics of Scientific Procedures on Living Animals Northern Ireland 2012
 Statistics of Scientific Procedures on Living Animals Northern Ireland 2012 Statistics of Scientific Procedures on Living Animals Northern Ireland 2012 Prepared pursuant to section 21(7) of the Animals
Statistics of Scientific Procedures on Living Animals Northern Ireland 2012 Statistics of Scientific Procedures on Living Animals Northern Ireland 2012 Prepared pursuant to section 21(7) of the Animals
VMP Focal point training Casablanca 6 8 December Dr Susanne Münstermann
 VMP Focal point training Casablanca 6 8 December 2011 Dr Susanne Münstermann The OIE Specialist Commissions and their mandate The Terrestrial Manual - overview Diagnostic Tests Vaccines The Aquatic Manual
VMP Focal point training Casablanca 6 8 December 2011 Dr Susanne Münstermann The OIE Specialist Commissions and their mandate The Terrestrial Manual - overview Diagnostic Tests Vaccines The Aquatic Manual
Fluoroquinolones ELISA KIT
 Fluoroquinolones ELISA KIT Cat. No.:DEIA6883 Pkg.Size:96T Intended use The Fluoroquinolones ELISA KIT is an immunoassay for the detection of Fluoroquinolones in contaminated samples including water, fish
Fluoroquinolones ELISA KIT Cat. No.:DEIA6883 Pkg.Size:96T Intended use The Fluoroquinolones ELISA KIT is an immunoassay for the detection of Fluoroquinolones in contaminated samples including water, fish
Tetanus Tetanus Clostridium tetani
 is an acute, often fatal, disease caused by an exotoxin produced by the bacterium Clostridium tetani. It is characterized by generalized rigidity and convulsive spasms of skeletal muscles. The muscle stiffness
is an acute, often fatal, disease caused by an exotoxin produced by the bacterium Clostridium tetani. It is characterized by generalized rigidity and convulsive spasms of skeletal muscles. The muscle stiffness
The OIE Manual of Diagnostic Tests and Vaccines for Terrestrial & Aquatic Animals
 The OIE Manual of Diagnostic Tests and Vaccines for Terrestrial & Aquatic Animals Regional seminar for OIE National Focal Points for Veterinary Products, Tokyo, Japan, 3-5 December 2014 Barbara Freischem,
The OIE Manual of Diagnostic Tests and Vaccines for Terrestrial & Aquatic Animals Regional seminar for OIE National Focal Points for Veterinary Products, Tokyo, Japan, 3-5 December 2014 Barbara Freischem,
Recommended for Implementation at Step 7 of the VICH Process on 21 November 2000 by the VICH Steering Committee
 VICH GL7 (ANTHELMINTICS GENERAL) November 2000 For implementation at Step 7 EFFICACY OF ANTHELMINTICS: GENERAL REQUIREMENTS Recommended for Implementation at Step 7 of the VICH Process on 21 November 2000
VICH GL7 (ANTHELMINTICS GENERAL) November 2000 For implementation at Step 7 EFFICACY OF ANTHELMINTICS: GENERAL REQUIREMENTS Recommended for Implementation at Step 7 of the VICH Process on 21 November 2000
Combating Antibiotic Resistance: New Drugs 4 Bad Bugs (ND4BB) Subtopic 1C. Seamus O Brien and Hasan Jafri Astra Zeneca and MedImmune
 Combating Antibiotic Resistance: New Drugs 4 Bad Bugs (ND4BB) Subtopic 1C Seamus O Brien and Hasan Jafri Astra Zeneca and MedImmune Need for public-private collaboration Challenges of AB R&D: 1. Unique
Combating Antibiotic Resistance: New Drugs 4 Bad Bugs (ND4BB) Subtopic 1C Seamus O Brien and Hasan Jafri Astra Zeneca and MedImmune Need for public-private collaboration Challenges of AB R&D: 1. Unique
EU Statistical Data of all uses of animals
 Member State: Slovakia Year: 2014 All uses of animals by species Animal Species Number of uses Percentage Mice 6,774 40.29% Rats 8,067 47.98% Guinea-Pigs 903 5.37% Hamsters (Syrian) Hamsters (Chinese)
Member State: Slovakia Year: 2014 All uses of animals by species Animal Species Number of uses Percentage Mice 6,774 40.29% Rats 8,067 47.98% Guinea-Pigs 903 5.37% Hamsters (Syrian) Hamsters (Chinese)
2012 Work Programme of the
 French Agency for Food, Environmental & Occupational Health Safety Maisons-Alfort LABORATOIRE DE SANTE ANIMALE ANIMAL HEALTH LABORATORY Unité Zoonoses Bactériennes Bacterial Zoonoses Unit 5 August, 2011
French Agency for Food, Environmental & Occupational Health Safety Maisons-Alfort LABORATOIRE DE SANTE ANIMALE ANIMAL HEALTH LABORATORY Unité Zoonoses Bactériennes Bacterial Zoonoses Unit 5 August, 2011
I.3. Central competent authority. Local competent authority I.6. I.12. I.16. Entry BIP in EU. I.17. No(s) of CITES. I.22. Number of packages
 COUNTRY: I.1. Consignor I.2. Certificate reference No I.2.a. I.3. Central competent authority Veterinary certificate to EU Part I : Details of dispatched consignment I.5. Tel. Consignee Postal code Tel.
COUNTRY: I.1. Consignor I.2. Certificate reference No I.2.a. I.3. Central competent authority Veterinary certificate to EU Part I : Details of dispatched consignment I.5. Tel. Consignee Postal code Tel.
European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes *
 European Treaty Series - No. 123 European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes * Strasbourg, 18.III.1986 Appendix B Statistical tables
European Treaty Series - No. 123 European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes * Strasbourg, 18.III.1986 Appendix B Statistical tables
STATISTICAL REPORT. Preliminary Analysis of the Second Collaborative Study of the Hard Surface Carrier Test
 STATISTICAL REPORT To: From: Subject: Diane Boesenberg, Reckitt Benckiser Emily Mitchell, Product Science Branch, Antimicrobials Division/Office of Pesticide Programs/US EPA Martin Hamilton, Statistician
STATISTICAL REPORT To: From: Subject: Diane Boesenberg, Reckitt Benckiser Emily Mitchell, Product Science Branch, Antimicrobials Division/Office of Pesticide Programs/US EPA Martin Hamilton, Statistician
The use of serology to monitor Trichinella infection in wildlife
 The use of serology to monitor Trichinella infection in wildlife Edoardo Pozio Community Reference Laboratory for Parasites Istituto Superiore di Sanità, Rome, Italy The usefulness of serological tests
The use of serology to monitor Trichinella infection in wildlife Edoardo Pozio Community Reference Laboratory for Parasites Istituto Superiore di Sanità, Rome, Italy The usefulness of serological tests
The OIE Relevant Standards and Guidelines for Veterinary Medicinal Products
 The OIE Relevant Standards and Guidelines for Veterinary Medicinal Products REGIONAL SEMINAR OIE NATIONAL FOCAL POINTS FOR VETERINARY PRODUCTS EZULWINI, SWAZILAND, 6-8 DECEMBER 2017 Dr Mária Szabó OIE
The OIE Relevant Standards and Guidelines for Veterinary Medicinal Products REGIONAL SEMINAR OIE NATIONAL FOCAL POINTS FOR VETERINARY PRODUCTS EZULWINI, SWAZILAND, 6-8 DECEMBER 2017 Dr Mária Szabó OIE
Tetanus Toxoid For Booster Use Only
 275 3107598 AHFS Category 80:08 Tetanus Toxoid For Booster Use Only (t recommended for primary immunization) Page 1 of 5 DESCRIPTION Tetanus Toxoid, for intramuscular or subcutaneous use, is a sterile
275 3107598 AHFS Category 80:08 Tetanus Toxoid For Booster Use Only (t recommended for primary immunization) Page 1 of 5 DESCRIPTION Tetanus Toxoid, for intramuscular or subcutaneous use, is a sterile
SPREADSHEET MODELS FOR FOCUSING RESEARCH ON HIGH YIELD PREVENTION AND CONTROL STRATEGIES
 Department of Epidemiology Course EPI 415 School of Public Health University of California, Los Angeles Session 18 SPREADSHEET MODELS FOR FOCUSING RESEARCH ON HIGH YIELD PREVENTION AND CONTROL STRATEGIES
Department of Epidemiology Course EPI 415 School of Public Health University of California, Los Angeles Session 18 SPREADSHEET MODELS FOR FOCUSING RESEARCH ON HIGH YIELD PREVENTION AND CONTROL STRATEGIES
European public MRL assessment report (EPMAR)
 15 January 2013 EMA/CVMP/914694/2011 Committee for Medicinal Products for Veterinary Use (CVMP) European public MRL assessment report (EPMAR) Fenbendazole (extension to chicken and extrapolation to all
15 January 2013 EMA/CVMP/914694/2011 Committee for Medicinal Products for Veterinary Use (CVMP) European public MRL assessment report (EPMAR) Fenbendazole (extension to chicken and extrapolation to all
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology EMEA/MRL/728/00-FINAL April 2000 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS STREPTOMYCIN AND
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology EMEA/MRL/728/00-FINAL April 2000 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS STREPTOMYCIN AND
COMMISSION IMPLEMENTING REGULATION (EU)
 L 34/4 Official Journal of the European Union 5.2.2013 COMMISSION IMPLEMENTING REGULATION (EU) No 102/2013 of 4 February 2013 amending Regulation (EU) No 206/2010 as regards the entry for the United States
L 34/4 Official Journal of the European Union 5.2.2013 COMMISSION IMPLEMENTING REGULATION (EU) No 102/2013 of 4 February 2013 amending Regulation (EU) No 206/2010 as regards the entry for the United States
REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT
 EUROPEAN COMMISSION Brussels, 5.12.2013 COM(2013) 859 final REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT Seventh Report on the Statistics on the Number of Animals used for Experimental
EUROPEAN COMMISSION Brussels, 5.12.2013 COM(2013) 859 final REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT Seventh Report on the Statistics on the Number of Animals used for Experimental
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY
 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Brussels, 27 February 2018 NOTICE TO STAKEHOLDERS WITHDRAWAL OF THE UNITED KINGDOM AND EU RULES ON ANIMAL HEALTH AND WELFARE AND PUBLIC
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Brussels, 27 February 2018 NOTICE TO STAKEHOLDERS WITHDRAWAL OF THE UNITED KINGDOM AND EU RULES ON ANIMAL HEALTH AND WELFARE AND PUBLIC
Antibody Test Kit for Feline Calici, Herpes and Panleukopenia Viruses (2011)
 Sensitivity-specificity and accuracy of the ImmunoComb Feline VacciCheck Antibody Test Kit for Feline Calici, Herpes and Panleukopenia Viruses (2011) Mazar S 1, DiGangi B 2, Levy J 2 and Dubovi E 3 1 Biogal,
Sensitivity-specificity and accuracy of the ImmunoComb Feline VacciCheck Antibody Test Kit for Feline Calici, Herpes and Panleukopenia Viruses (2011) Mazar S 1, DiGangi B 2, Levy J 2 and Dubovi E 3 1 Biogal,
Comparison of Immune Responses Following the Administration of Enterotoxaemia Vaccine in Sheep and Goats ABSTRACT
 Comparison of Immune Responses Following the Administration of Enterotoxaemia Vaccine in Sheep and Goats S. Naz, M. A. Ghuman, A. A. Anjum * A. W. Manzoor, W. Rana and R. Akhter * Veterinary Research Institute,
Comparison of Immune Responses Following the Administration of Enterotoxaemia Vaccine in Sheep and Goats S. Naz, M. A. Ghuman, A. A. Anjum * A. W. Manzoor, W. Rana and R. Akhter * Veterinary Research Institute,
European Public MRL assessment report (EPMAR)
 18 March 2016 EMA/CVMP/619817/2015 Committee for Medicinal Products for Veterinary Use European Public MRL assessment report (EPMAR) Gentamicin (all mammalian food producing species and fin fish) On 3
18 March 2016 EMA/CVMP/619817/2015 Committee for Medicinal Products for Veterinary Use European Public MRL assessment report (EPMAR) Gentamicin (all mammalian food producing species and fin fish) On 3
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT VEPURED suspension for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose of 1 ml contains:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT VEPURED suspension for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose of 1 ml contains:
LIVE ANIMAL TRANSPORT
 KEY RECCOMENDATIONS LIVE ANIMAL TRANSPORT A growing number of animals is transported alive across and from the European Union (EU). Despite scientific bodies and institutions have stressed on the detrimental
KEY RECCOMENDATIONS LIVE ANIMAL TRANSPORT A growing number of animals is transported alive across and from the European Union (EU). Despite scientific bodies and institutions have stressed on the detrimental
NOTES IMMUNOGENICITY IN MONKEYS OF A COMBINED TOXOID FROM THE MAIN TOXIC PRINCIPLES SEPARATED FROM HABU SNAKE VENOM
 Japan. J. Med. Sci. Biol., 23, 413-418, 1970 NOTES IMMUNOGENICITY IN MONKEYS OF A COMBINED TOXOID FROM THE MAIN TOXIC PRINCIPLES SEPARATED FROM HABU SNAKE VENOM Antivenine has been proved useful as a treatment
Japan. J. Med. Sci. Biol., 23, 413-418, 1970 NOTES IMMUNOGENICITY IN MONKEYS OF A COMBINED TOXOID FROM THE MAIN TOXIC PRINCIPLES SEPARATED FROM HABU SNAKE VENOM Antivenine has been proved useful as a treatment
Histomoniasis. Diagnosis, Prophylaxis, Treatment - Research developments. Koen De Gussem, DVM Parma, 22/01/2013
 Histomoniasis Diagnosis, Prophylaxis, Treatment - Research developments Koen De Gussem, DVM Parma, 22/01/2013 Agenda Diagnosis and monitoring Profylaxis and control General control measures Specific approaches
Histomoniasis Diagnosis, Prophylaxis, Treatment - Research developments Koen De Gussem, DVM Parma, 22/01/2013 Agenda Diagnosis and monitoring Profylaxis and control General control measures Specific approaches
An experimental study on triclabendazole resistance of Fasciola hepatica in sheep
 Veterinary Parasitology 95 (2001) 37 43 An experimental study on triclabendazole resistance of Fasciola hepatica in sheep C.P.H. Gaasenbeek a,, L. Moll b, J.B.W.J. Cornelissen a, P. Vellema b, F.H.M. Borgsteede
Veterinary Parasitology 95 (2001) 37 43 An experimental study on triclabendazole resistance of Fasciola hepatica in sheep C.P.H. Gaasenbeek a,, L. Moll b, J.B.W.J. Cornelissen a, P. Vellema b, F.H.M. Borgsteede
Clinical Practice Guidelines
 Community Health Services Home 1 of 15 Population and Public Health Nov 2, Family Med/Primary Mental Health 1.0 PURPOSE 1.1 To provide timely public health investigation of individuals who have experienced
Community Health Services Home 1 of 15 Population and Public Health Nov 2, Family Med/Primary Mental Health 1.0 PURPOSE 1.1 To provide timely public health investigation of individuals who have experienced
OIE international standards on Rabies:
 Regional cooperation towards eradicating the oldest known zoonotic disease in Europe Antalya, Turkey 4-5 December 2008 OIE international standards on Rabies: Dr. Lea Knopf Scientific and Technical Department
Regional cooperation towards eradicating the oldest known zoonotic disease in Europe Antalya, Turkey 4-5 December 2008 OIE international standards on Rabies: Dr. Lea Knopf Scientific and Technical Department
Amoxicillin trihydrate. Amoxicillin trihydrate. Amoxicillin trihydrate. Amoxicillin trihydrate. Amoxicillin trihydrate. Amoxicillin trihydrate
 Annex I List of the names, pharmaceutical form, strength of the veterinary medicinal product, animal species, route of administration, applicant in the Member States Member State EU/EEA Applicant Name
Annex I List of the names, pharmaceutical form, strength of the veterinary medicinal product, animal species, route of administration, applicant in the Member States Member State EU/EEA Applicant Name
Materials and Methods: Anti-snake venom activities of Asparagus racernosus
 Sunil Prashar. et al.: Asian Journal of Pharmacology and Toxicology, 04(16), 2016,Ol-08. RESEARCH ARTICLE Received on: 201 1212016 Published on:29/ 12120 16 Corresponding Author Sunil Prashar, Department
Sunil Prashar. et al.: Asian Journal of Pharmacology and Toxicology, 04(16), 2016,Ol-08. RESEARCH ARTICLE Received on: 201 1212016 Published on:29/ 12120 16 Corresponding Author Sunil Prashar, Department
LIFE.2.B EUROPEAN UNION. Brussels, 14 November 2018 (OR. en) 2014/0255 (COD) PE-CONS 43/18 AGRILEG 102 VETER 52 CODEC 1149
 EUROPEAN UNION THE EUROPEAN PARLIAMT THE COUNCIL Brussels, 14 November 2018 (OR. en) 2014/0255 (COD) PE-CONS 43/18 AGRILEG 102 VETER 52 CODEC 1149 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: REGULATION
EUROPEAN UNION THE EUROPEAN PARLIAMT THE COUNCIL Brussels, 14 November 2018 (OR. en) 2014/0255 (COD) PE-CONS 43/18 AGRILEG 102 VETER 52 CODEC 1149 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: REGULATION
Presentation Outline. Commercial RVF vaccines. RVF Clone 13 performance in the field. Candidate RVF vaccines in the pipeline
 Presentation Outline Commercial RVF vaccines Old Smithburn, inactivated New Clone 13 RVF Clone 13 performance in the field Candidate RVF vaccines in the pipeline 2 Onderstepoort Biological Products November
Presentation Outline Commercial RVF vaccines Old Smithburn, inactivated New Clone 13 RVF Clone 13 performance in the field Candidate RVF vaccines in the pipeline 2 Onderstepoort Biological Products November
ELlSA Seropositivity for Toxocara canis Antibodies in Malaysia,
 ELlSA Seropositivity for Toxocara canis Antibodies in Malaysia, 1989.. 1991 S. L. Hakim, MSc ].w. Mak, MRCPath P.L.W. Lam, MSc Institute for Medical Research, Jalan Pahang, 50588 Kuala Lumpur Introduction
ELlSA Seropositivity for Toxocara canis Antibodies in Malaysia, 1989.. 1991 S. L. Hakim, MSc ].w. Mak, MRCPath P.L.W. Lam, MSc Institute for Medical Research, Jalan Pahang, 50588 Kuala Lumpur Introduction
Updated assessment of the health risks posed by longer-term consumption of foods contaminated with fipronil
 Updated assessment of the health risks posed by longer-term consumption of foods contaminated with fipronil Updated BfR Communication No. 023/2017 of 21 August 2017 1 Based on currently available information,
Updated assessment of the health risks posed by longer-term consumption of foods contaminated with fipronil Updated BfR Communication No. 023/2017 of 21 August 2017 1 Based on currently available information,
Contact Immunization. is now being done to improve methods. and technics of control. in the past in most countries, and if
 July, 939 Mass Immunization Against with Sordelli's Toxoid and ~~~~0 ID *** Contact Immunization Diphtheria ALBERTO P. LEON, M.D., M.P.H., R. HERNANDEZ VALLADOS, AND F. ESCARZA Chief; and Epidemiologists,
July, 939 Mass Immunization Against with Sordelli's Toxoid and ~~~~0 ID *** Contact Immunization Diphtheria ALBERTO P. LEON, M.D., M.P.H., R. HERNANDEZ VALLADOS, AND F. ESCARZA Chief; and Epidemiologists,
Terrestrial and Aquatic Manuals and the mechanism of standard adoption
 Dr Patrick Bastiaensen Programme Officer OIE Sub-Regional Representation for Eastern Africa Terrestrial and Aquatic Manuals and the mechanism of standard adoption Presented during the Regional Workshop
Dr Patrick Bastiaensen Programme Officer OIE Sub-Regional Representation for Eastern Africa Terrestrial and Aquatic Manuals and the mechanism of standard adoption Presented during the Regional Workshop
National experience of application of the requirements for marketing authorisations and other ways of making vaccines available - small MS perspective
 National experience of application of the requirements for marketing authorisations and other ways of making vaccines available - small MS perspective J.Bureš ÚSKVBL, Czech Republic 25 March 2015 CR introduction
National experience of application of the requirements for marketing authorisations and other ways of making vaccines available - small MS perspective J.Bureš ÚSKVBL, Czech Republic 25 March 2015 CR introduction
Neutralization of Micrurus distans distans venom by antivenin (Micrurus fulvius)
 Journal of Wilderness Medicine 3,377-381 (1992) ORIGINAL ARTICLE Neutralization of Micrurus distans distans venom by antivenin (Micrurus fulvius) R.e. DART, MD, PhD l, 2, P.e. O'BRIEN, Pharm D2, R.A. GARCIA,
Journal of Wilderness Medicine 3,377-381 (1992) ORIGINAL ARTICLE Neutralization of Micrurus distans distans venom by antivenin (Micrurus fulvius) R.e. DART, MD, PhD l, 2, P.e. O'BRIEN, Pharm D2, R.A. GARCIA,
COMMISSION OF THE EUROPEAN COMMUNITIES REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 8.10.2007 COM(2007) 578 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL in connection with Article 23 of Regulation (EC) No
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 8.10.2007 COM(2007) 578 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL in connection with Article 23 of Regulation (EC) No
COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE
 European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/211249/2005-FINAL July 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE DIHYDROSTREPTOMYCIN (Extrapolation to all ruminants)
European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/211249/2005-FINAL July 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE DIHYDROSTREPTOMYCIN (Extrapolation to all ruminants)
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2017 This report has been submitted : 2018-01-24 10:31:11 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Classical
OIE Reference Laboratory Reports Activities Activities in 2017 This report has been submitted : 2018-01-24 10:31:11 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Classical
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2016 This report h been submitted : 2017-01-11 18:55:37 Name of disee (or topic) for which you are a designated OIE Reference Laboratory: Brucellosis
OIE Reference Laboratory Reports Activities Activities in 2016 This report h been submitted : 2017-01-11 18:55:37 Name of disee (or topic) for which you are a designated OIE Reference Laboratory: Brucellosis
Laws and Regulations
 Laws and Regulations Historical background Government oversight USDA NIH/PHS AAALAC Other Historical Use of Animals 1600s: Blood transfusion developed in dogs Need for oxygen discovered using rats 1700s
Laws and Regulations Historical background Government oversight USDA NIH/PHS AAALAC Other Historical Use of Animals 1600s: Blood transfusion developed in dogs Need for oxygen discovered using rats 1700s
EFSA s activities on Antimicrobial Resistance
 EFSA s activities on Antimicrobial Resistance CRL-AR, Copenhagen 23 April 2009 Annual Workshop of CRL - AR 1 Efsa s Role and Activities on AMR Scientific advices Analyses of data on AR submitted by MSs
EFSA s activities on Antimicrobial Resistance CRL-AR, Copenhagen 23 April 2009 Annual Workshop of CRL - AR 1 Efsa s Role and Activities on AMR Scientific advices Analyses of data on AR submitted by MSs
PREVALENCE OF BORDER DISEASE VIRUS ANTIBODIES AMONG NATIVE AND IMPORTED SHEEP HERDS IN ZABOL. Sari-Iran.
 PREVALENCE OF BORDER DISEASE VIRUS ANTIBODIES AMONG NATIVE AND IMPORTED SHEEP HERDS IN ZABOL B. Shohreh 1, M.R. Hajinejad 2, S. Yousefi 1 1 Department of Animal Sciences Sari University of Agricultural
PREVALENCE OF BORDER DISEASE VIRUS ANTIBODIES AMONG NATIVE AND IMPORTED SHEEP HERDS IN ZABOL B. Shohreh 1, M.R. Hajinejad 2, S. Yousefi 1 1 Department of Animal Sciences Sari University of Agricultural
Bovine Viral Diarrhea (BVD)
 Bovine Viral Diarrhea (BVD) Why should you test your herd, or additions to your herd? Answer: BVD has been shown to cause lower pregnancy rates, increased abortions, higher calf morbidity and mortality;
Bovine Viral Diarrhea (BVD) Why should you test your herd, or additions to your herd? Answer: BVD has been shown to cause lower pregnancy rates, increased abortions, higher calf morbidity and mortality;
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Medicinal product no longer authorised
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BTVPUR AlSap 1 suspension for injection for sheep and cattle. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BTVPUR AlSap 1 suspension for injection for sheep and cattle. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose
