Mice: Basic Handling and Technique Workshop University of North Carolina, Chapel Hill
|
|
|
- Rachel Sparks
- 6 years ago
- Views:
Transcription
1 Mice: Basic Handling and Technique Workshop University of North Carolina, Chapel Hill First do no harm Greek Hippocratic Oath, Great Watch Words of Medicine Objectives: 1. Teach methods of safe, humane handling and restraint 2. Teach injection techniques: subcutaneous, intraperitoneal, intravenous and intramuscular 3. Teach blood collection techniques 4. Teach rodent identification methods 5. Teach anesthesia administration and monitoring 6. Teach proper euthanasia methods Basic Information about working with Mice: Proper Personal Protection Equipment (PPE) is a requirement for working with animals. In DLAM facilities, minimum requirements include disposable coveralls, shoe covers, head bonnet, mask, and gloves. Please review requirements before entering any animal area! Requirements may change from room to room so each door is posted with instructions. The use of a face mask reduces your risk of allergy to animals. We strongly recommend that you wear masks whenever you work with animals. Training Information: For additional training please contact the Training and Compliance Coordinators for the Institutional Animal Care and Use Committee (IACUC) at We offer training in both one on one and classroom settings. For a look at IACUC Guidelines and dates of future classes, visit our website at as well as If Bitten: DO NOT PUNISH THE MOUSE FOR ITS NATURAL RESPONSE 1. Calmly return the animal to its cage 2. Wash the wound with antibacterial soap and water 3. Bandage the wound 4. Notify your supervisor and contact the University Employee Occupational Health Clinic (UEOHC) Mouse Psychology: 1. Mice are usually mild in temperament and easy to handle. They are not usually aggressive, but can bite if frightened. There are some strains that are aggressive and can inflict painful bites. Mice groom themselves almost constantly to maintain a smooth, glossy haircoat. 2. Mice are nocturnal animals. Activities such as eating, drinking or mating are typically done at night. UNC Chapel Hill Revised 05/2014 1
2 3. Dominant mice exhibit a behavior called barbering. Barbering is the dominant mice biting or chewing on the fur of a more subordinate mouse. Barbering should not be confused with fur loss due to illness. Typically, barbering occurs around the face or back. 4. Male mice can be more aggressive and fight more often than females. Aggressive mice should be housed individually to avoid severe injury to cage mates. Generally male littermates may be housed together, but once separated, it is advisable to only house males with females. 5. Mice are creatures of habit. Everyday events do not tend to stress or excite the mice. However, handling and restraint can be stressful and result in the mouse being difficult to work with. Conditioning the mice to such handling (so they do not associate handling and restraint aversively) can make the animals much easier to work with. Handling and Restraint: When picking up adult mice, grasp them gently but firmly at the base or center of their tail. Do not pick them up by the tip of the tail. Place the animal on a surface such as the wire cage top or lid. It is best that the surface not be slick or smooth as mice will behave much more calmly if they have firm footing. While still holding the tail near the base, with your other hand firmly grasp the loose skin on the back starting near the ears using your thumb and first two fingers. The tail can then be held by the last two fingers as shown. Your grip should be firm enough to keep the mouse from struggling, but gentle enough for it to breathe comfortably. For quick handling such as cage to cage transfers, it is acceptable to use forceps. Gently grasp the loose skin on the back and quickly transfer them to the new cage. This technique is useful for fractious or aggressive animals. Be sure to clean gloves or forceps with a sterilant such as MB-10 or Spor-Klenz between cages. Gloves, either light leather, cloth or mesh greatly diminish sensitivity, increasing chance of injury to mice. They also make it more difficult to perform delicate procedures, and mice can often bite through them. UNC Chapel Hill Revised 05/2014 2
3 Examples of Commercial Restrainers: Snuggle: Lomir.com Various styles and sizes available: See Page 24 for vendors Sex Determination Gender in mice is determined by comparing anogenital distance, or the distance between the urogenital opening and the anus. Male mice typically have a larger anogenital distance when compared with the females. Be aware there are variances in ano-genital distance among strains. See diagrams below. (a) Young Rodent (b) Adult Rodent Injections: Use a fresh, sterile needle for each injection To avoid excessive leaking, keep the needle in the needle tract for a few seconds Always inject with the bevel of the needle facing up Do not reuse needles between animals UNC Chapel Hill Revised 05/2014 3
4 Injection Type Ideal Needle Size (Gauge) Recommended Volume Aspiration Required Intraperitoneal (IP) 23G 2 3mL; depending on the animal size No Subcutaneous (SQ) 23G 2 3mL; ensure the skin at the injection site is not taunt Yes; inject if no blood in the needle hub Intramuscular (IM) 27G µL per site Yes; inject slowly if no blood in the needle hub Intravenous (IV) 27G 200µL No; inject slowly 1. Intraperitoneal injections: Holding the mouse in dorsal recumbency, insert the needle in a position below the bend of the knees; left or right of the midline. Avoid the midline to prevent penetrating the bladder. Angle the needle approximately 45 to the body. 2. Subcutaneous injections: May be performed in any area of loose skin along the back or flank. Tenting the skin between the shoulder blades or over the rump creates an appropriate pocket for injection. Inserting the needle under the skin along the flank results in the outline of the needle clearly visible when correctly situated. UNC Chapel Hill Revised 05/2014 4
5 3. Intramuscular injections: Only to be used in instances where the other injections are not appropriate, since this is potentially painful. The muscle mass running along the back of the leg is used; with the needle angled parallel to the femur (avoiding the sciatic nerve). 4. Intravenous injections: Dilate the blood vessels by warming the mouse. Once warmed, place the mouse in a restrainer. An additional method to dilate the veins is rubbing alcohol on the tail. Locate one of the lateral veins. Insert the needle as low as possible towards the tip of the tail, since the vein is very superficial at the tip. As you move up toward the base of the tail the vein is located more deeply. The vein will clear from the injection site to the base of the tail if properly situated, whereas ballooning around the injection site will occur if the needle is not properly seated you will note ballooning at the injection site. Cross Section of Mouse Tail UNC Chapel Hill Revised 05/2014 5
6 Various Helpful Tips for Injections: 1. When collecting blood from laboratory animals, the largest recommended amount is 1.5% of the animal s total body weight. Collection should not occur again for two weeks. This will allow blood constituents to return to normal. If blood is needed weekly, 0.5% body weight is a safe amount. 2. When giving substances intravenously, inject slowly to avoid shock. 3. Injecting slowly when giving substances intramuscularly will cause the least amount of pain. 4. The volume of blood in an adult mouse is about ml/kg. This is approximately 10% of its body weight. Only about half of this can be recovered in a terminal blood withdrawal procedure. 5. Check rodents teeth frequently. This will insure early detection of malocclusion. If ma-occluded, teeth may become overgrown and interfere with eating. Oral Gavage: We recommend using stainless steel, ball tipped gavage needles. It is important to premeasure the needle before gavaging. The tube should measure the distance from the tip of the nose to the last rib, so that the needle will pass down the esophagus into the stomach. If the tube is too short, the injected fluid may be aspirated by the mouse causing possible pneumonia and death. If the needle is too long, it may perforate the stomach. The mouse should be firmly restrained. Insert the gavage needle into the mouth at one side. Slide the needle down the back of the throat while tilting the mouse s head back, so that the neck is in a straight line. The needle should pass easily down the esophagus; with little to no resistance. If the mouse struggles or resistance is met, stop, back up and start over. Improper gavage technique can cause tearing of the esophagus or asphyxiation. Observe the mouse carefully after the gavage is completed. No fluid should be coming from the mouth or nose and the mouse should not show signs of distress. Oral dosing should not exceed more than 10ml/kg. The following table indicates appropriate needle sizes: Mouse Wt (g) Gauge Length (inches) Ball Diameter (mm) Up to 14g g 22 1 or g 20 1, 1.5 or g 18 1, 1.5 or g 18 2 or UNC Chapel Hill Revised 05/2014 6
7 Acknowledgements: The University of North Carolina would like to thank: The AALAS Learning Library University of Minnesota, Research Animal Resources University of Texas Medical Branch at Galveston Rodent Identification There are several IACUC approved methods used to identify rodents. Each method has both advantages and disadvantages. In long-term studies, it is important to choose a method that is permanent and easily read. Long Term Methods: Ear Notching This method is frequently used in both mice and rats. There are several tools that may be purchased to achieve this. Most resemble a hole puncher and are very cheap. There are previously created maps that serve as a numbering system, or the researcher may create a map. (See diagram below for universal numbering system that may be used for identification via ear notching) A) Advantages Ear notching can be done quickly while causing very little pain or distress. The instruments are not costly and can be obtained easily. B) Disadvantages This method can not be applied until the ears are fully developed. This may be too late for those that use young rodents. This may not work with fractious strains. Rips or tears caused by fighting may leave the pattern indiscernible. Tools used to notch ears dull easily so must be replaced frequently. Ear Tagging Ear tags can be purchased with numbers and/or letters. Correct placement of the tag makes them fairly easy to read. (See images below of two commonly available ear taggers, with proper tag placement). A) Advantages Ear tags are inexpensive and are fairly easy to apply. This method does not require the use of anesthesia. Tagging can be done quickly and does not seem to cause pain and only minor distress. UNC Chapel Hill Revised 05/2014 7
8 B) Disadvantages Tags can fall out if not applied properly. They can also be lost if ears are ripped or torn in strains that fight. Different sized tags are available for different species. Tags are relatively heavy for weanlings and may cause young mice to tilt their head even when the proper sized tag is applied. Some strains are prone to scratching the tagged area which can lead to infection, hematomas, and granulomas. In a very limited number of cases, a member of the DLAM veterinary team has seen ear tags stimulate tumor growth. Microchipping Microchips, electronic transponders, are safe and reliable. A) Advantages Microchips may be applied without the use of anesthesia. Applying microchips seems to cause little or no pain. Even though the chip may migrate to a different area, they are not lost so prove to be a reliable method. Animals can be identified without handling and removing them from the cage. Some microchips are designed to provide other information such as core body temperature and heart rate. B) Disadvantages The equipment used to read the chips is fairly expensive. Microchips cost five to ten dollars each. Despite the manufacturer s recommendation, chips can be reused. In order to reuse the chips they must be sterilized by ethylene oxide. The hospital will do this as a service for fee. If not implanted properly there is a slight risk of infection. In a very limited number of cases, a member of the DLAM veterinary team has seen microchips stimulate tumor growth. Micro tattooing This method seems to be growing in popularity. It is both permanent and fairly easy to apply. A) Advantages This method works in all strains, even the more fractious. The cost is very reasonable after the initial expense. Tattoos can be applied to rodents of any age. The markings are easily read, especially when applied to the tail of light colored rodents. When placed in the proper area, it is not necessary to handle the animal to read the tattoo. Tattooing causes only minor pain and distress and does not require the use of anesthesia. B) Disadvantages The identifying marks may be a little difficult to read in young pigmented mice. This improves as the mice age. The initial cost is rather expensive. There is a small chance of inducing infection if the tattoo is not applied correctly. Toe Clipping Toe-clipping, as a method of identification of small rodents, should be used only when no other individual identification method is feasible and should be performed only on altricial neonates. 1 The IACUC allows a maximum of four toes and no more that two per foot. Do not cut the hallux ( dew-claw or little toe ) as this may decrease the rodent s grasping ability. A) Advantages Toe clipping can be done at a very early age, post natal day one. The ideal time is between post natal day five and seven when the toe is large enough to work with yet the bones are not calcified. The tissue can be used for genotyping. Toe clipping may not be performed after post natal day ten. No anesthesia is needed. This seems to cause little or no pain when performed early enough. The young react to being removed from their mother, but do not react to the clipping of the toe. B) Disadvantages The young show signs of distress when removed from their mother and siblings. This may cause a small amount of pain. The general public views this as a UNC Chapel Hill Revised 05/2014 8
9 form of brutal mutilation. There is a small possibility of infection. A reduction in the number of toes may reduce the ability to grasp objects. Short Term Methods: Hair Clipping Trim patterns into the fur. Keep a record or picture to identify rodents. A) Advantages Causes no pain. B) Disadvantages This is very temporary. The hair will grow back within ten days and the clipping must be repeated. Permanent Markers and Fur Dyes It is easy to apply marks or dyes to different body parts. A) Advantages This method is non-invasive. It causes only minor stress due to restraint. B) Disadvantages This method can be time consuming, since it must be repeated soon after the previous marking. If working with nursing pups, the mothers will groom the neonates excessively and the markings may disappear overnight. This could result in a loss of identity. If you would like to inquire about equipment used in methods discussed above, please the OACU Training/Compliance Team. You may send questions to the general IACUC account iacuc@med.unc.edu 1 Guide for the Care and Use of Laboratory Animal Care and Use. National Research Council.2011 National Academy of Sciences. UNC Chapel Hill Revised 05/2014 9
10 Ear Tagging: UNC Chapel Hill Revised 05/
11 Ear Notching: UNC Chapel Hill Revised 05/
12 ACCEPTABLE METHODS OF RODENT BLOOD WITHDRAWAL Each laboratory must designate a Laboratory Animal Coordinator (LAC) who may train research personnel in their laboratory in various animal-handling techniques, including blood collection. The LAC must be certified by the Office of Animal Care and Use (OACU) or the Division of Laboratory Animal Medicine (DLAM) and demonstrate proficiency before training others within their lab. Alternatively, laboratory personnel may register for a hands-on techniques class here or one on one session with the OACU Training and Compliance team ( ). Chronic Blood Withdrawal: For sequential blood sampling (over a period of time), the maximum survival blood withdrawal for most mammals is 1.5% of lean body weight every 14 days. Acute or Single Blood Withdrawal: The maximum survival amount of an acute blood withdrawal is 1% of the lean body weight. [e.g.; For a 20 gram adult mouse, no more than 4 X 50 ul micro capillary tubes (200 ul), may be withdrawn]. To facilitate blood collection, warm the rodent first. When using the tail veins or artery, you may dip the tail in warm water (45 C). The entire animal can be warmed with a carefully placed heat lamp for 5-10 minutes or by placing the housing cage on a circulating water pad. Alternatively, alcohol may be used initially as a vasodilator, but it should not be used on broken skin. 1) Submandibular: A relatively simple way to obtain blood from a mouse is to puncture the area behind the hinges of the jawbones. The superficial temporal vein is a large vessel positioned behind the eye, which can be traced backward to the temporal vein, the maxillary vein, and finally, the jugular vein. Scruff the mouse and pierce the skin in the relevant area. A mouse bleeding lancet is strongly recommended for use. However, an 18 gauge needle may also be used. Information on the lancets and a video of this procedure may be seen by going to the following URL: The submandibular bleed is one of the easiest methods of collecting blood from a mouse. Your LAC may train in this technique or you can contact the OACU at to arrange for training. 2) Saphenous Vein: This method of obtaining blood is often used when a series of small samples is required. Place the mouse in a conical tube and shave the caudal surface of the thigh. The saphenous vein can be seen in this area. It is advantageous to apply a lubricant to prevent wicking. Place a tourniquet above the knee and enter the vein with a 25 gauge needle. Micro-hematocrit and microvette tubes work well to collect the blood. This method of blood withdrawal does not require anesthesia, however, the method of restraint is cumbersome. For detailed instructions and pictures of this procedure please visit 3) Tail Artery / Vein (NICK): Tail veins and artery can be used for serial bleedings. Use the central tail artery or lateral tail veins. Anesthesia is not required for tail nick. Start midway up the tail and nick the artery or vein (usually with a needle or lancet). You may collect blood with micro capillary tubes, a UNC Chapel Hill Revised 05/
13 micropipette or various microtainer collection tubes. Move cranially 0.5 cm at a time applying pressure after the bleed. 4) Tail Clip Bleed and/or Tail Biopsy for Genotyping: Performed on (un)anesthetized or anesthetized animals depending on amount of tissue needed (see below): Anesthesia is optional for the removal of up to 4mm from the tail tip. It is strongly recommended that no more than 2mm be removed at a time. Anesthesia may be used as a means of animal restraint and its use must be described in the approved animal care application. Harvesting greater than 4mm requires written permission from the IACUC. Depending on the age of the animal, removal of greater than 4mm from the tail tip may involve cutting into the vertebral column. Therefore, anesthesia is always required when removing this much tail, irrespective of the age of the animal. The use of anesthesia must be described in the approved animal care application. Requests to perform tail biopsies or successive tail cuts totaling greater than 4mm without anesthesia must be scientifically justified and must receive IACUC approval prior to implementation. The IACUC has approved the tail cut method for both rats and mice to obtain blood and/or tissue. This method must be described in the animal use application and approved by the IACUC prior to use. See policy below. 1. Place animal in approved animal restrainer. (Experienced handlers may be able to perform technique in habituated rats with light or no restraint). 2. Remove any bedding material or feces from the tail. The tail tip must be disinfected with an approved disinfectant (i.e. Betadine) 3. Place the animal on a clean work surface. 4. Using a fresh scalpel blade, cut 1-2 mm of the distal tail at an angle perpendicular to the work surface. 5. Apply gentle pressure proximal to the collection site to occlude venous return and ease collection. Collect the blood in a suitable collection device. 6. Apply gentle digital pressure to the wound for seconds with a clean gauze pad to stop any hemorrhaging. For persistent bleeding, apply a silver nitrate stick, styptic powder or a cautery pen to the wound to stop bleeding. 7. Return the animal to its cage only after bleeding has stopped. 8. Serial blood samples can be obtained over a short time frame by gently removing the scab without performing an additional cut. 9. Only the fleshy portion of the tail tip should be cut. Cutting into the vertebrae is NOT permitted. As only a small portion of the tail does not contain vertebrae, the use of the tail cut procedure should be limited. 10. This procedure should be performed only by individuals trained and certified in the technique and comfortable with rodent handling. 5) Retro Orbital Bleeding: Retro-orbital or orbital sinus/plexus bleeding (permitted in rats, mice, gerbils, guinea pigs, hamsters) must be proposed to and approved by the IACUC before implementation. The IACUC UNC Chapel Hill Revised 05/
14 will permit orbital sinus bleeding when it is scientifically justified, performed with appropriate technique and anesthesia. Veterinary staff experience indicates that this method may lead to orbital damage, blindness and potentially death if not performed correctly. The IACUC encourages the primary use of the submandibular, tail artery or veins; specifically the nick or cut techniques. These methods are less likely to harm the animal and may be used repeatedly for bleeding. LACs may not train in this technique so training and certification must be obtained from OACU Training and Compliance team or DLAM veterinary services. Alternating eyes for each bleeding is mandatory, and a week must separate each bleeding. A maximum of two (2) bleedings per eye is permitted. Maximum volume withdrawn within a two week period is 1.5% body weight. Orbital sinus bleeding requires training and must be performed on anesthetized animals only with IACUC approval. Following blood collection, the eyelids should be held closed for a few seconds to allow the punctured blood vessel to clot. It is also common practice to place a small amount of ophthalmic ointment into the eye following this procedure. excerpt from Laboratory Animal Technician Training Manual 6) Cardiac Puncture: Always a terminal procedure conducted under anesthesia! Cardiac puncture as a method of blood withdrawal permitted in all species provided the following conditions are met: 1. Animal is under a surgical plane of anesthesia when procedure is conducted. 2. Animal is NOT allowed to recover from anesthesia following the puncture. 3. If the animal is euthanized prior cardiac puncture, training and certification in the technique is not required. A needle is inserted into the heart and blood is extracted until a sufficient volume is collected or the animal is exsanguinated. This procedure must be followed by a physical euthanasia method. PDF format available on the IACUC website: Click HERE UNC Chapel Hill Revised 05/
15 INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE MOUSE EUTHANASIA POLICY Performing euthanasia correctly is an ethical imperative. Proper euthanasia is quick, minimizes pain/distress and reliably causes death. Practical issues such as degree of technical difficulty, time required to perform the procedure, readily available equipment/resources to perform the procedure, as well as aesthetics and human emotion must be considered. Standardized guidelines for humane euthanasia are detailed in the June 2013 AVMA Guidelines on Euthanasia and available HERE. In addition to Division of Laboratory Animal Medicine (DLAM) personnel, only trained research personnel, listed in the IACUC approved Animal Care Application (ACAP), may euthanize animals. All animals slated for euthanasia must be housed according to UNC-Chapel Hill cage density standards, and should have access to food and water if they are being housed for more than 3 hours prior to euthanasia. Unweaned animals that are slated for euthanasia should stay with the lactating female until final preparation(s) for euthanasia are complete. Cages marked for euthanasia should not be overcrowded or stacked on top of each other, as this blocks air flow into the cage. Euthanasia must follow the method(s) described in the approved ACAP. Euthanasia must be confirmed by the physical method described in the approved ACAP. A confirmation of death by a physical method is required for all animals, irrespective of age. The DLAM staff can, for a fee, perform euthanasia of research animals. When requesting this DLAM service, research personnel must do the following: 1) Complete and submit a Request for Euthanasia in Animals form [available on the DLAM website]. Ensure all euthanasia instructions are very clear (e.g. euthanize dam and neonates or euthanize only the pre-weanling animals, not the dam ); 2) Leave the animal(s) requiring euthanasia in the cage. All unweaned animals should stay with the lactating female until the time of euthanasia. 3) Place a euthanasia card on the cage so that DLAM can readily identify the animal(s) slated for euthanasia. The investigator is responsible for ensuring proper documentation on a euthanasia request form. DLAM is not responsible for errors on the form or miscommunications that may occur during the euthanasia process. Do not make verbal arrangements with DLAM staff. Euthanasia of sick or injured animals Sick or injured animals that cannot be successfully treated or relieved of pain and distress should be euthanized promptly. Research personnel are responsible for euthanizing sick, injured or moribund animals as soon as these conditions are noted. These animals should not be held for later euthanasia by DLAM personnel. To investigate unexpected illnesses, research personnel may contact Veterinary Services to arrange for euthanasia and necropsy of the animals. DLAM veterinarians have the authority to euthanize moribund animals, as well as animals experiencing more than momentary or slight pain and/or distress. If the DLAM veterinarian is unable to contact research personnel regarding the care or treatment of a moribund animal, DLAM veterinarians or designated representatives are authorized UNC Chapel Hill Revised 05/
16 to euthanize the animal. Ensure appropriate emergency contact numbers for all research personnel are posted in the animal facility. MOUSE EUTHANASIA Section 1:Terms and Definitions Secondary physical method to ensure death in order to confirm that animals are dead, one of the following secondary physical methods must be performed on animals that have been anesthetized with approved agents: 1) cervical dislocation; 2) decapitation; 3) thoracotomy [open the chest cavity using sharp scissors or scalpel]; or 4) collection of vital organs. Note: In addition to DLAM personnel, only research personnel who have been properly trained and are listed on the approved ACAP, can perform these physical methods. Unanesthetized Physical Euthanasia Individuals who perform physical euthanasia on unanesthetized animals must first be trained and certified by IACUC approved designees. Laboratory Animal Coordinators (LAC) may not certify personnel in unanesthetized physical euthanasia. Physical euthanasia on unanesthetized animals, irrespective of age, can only be done if the procedure is described in the approved ACAP. o Cervical Dislocation cervical dislocation in unanesthetized neonatal and adult rodents is permitted only if it is performed correctly by a trained person, its use is scientifically justified, and it is described in an approved ACAP. Manual cervical dislocation is a humane method of euthanasia when limited to rodents weighing less than 200 grams. Personnel using cervical dislocation must be adequately trained, demonstrate their technical proficiency, and must consistently apply this method humanely and effectively. o Decapitation decapitation in unanesthetized neonatal and adult rodents is permitted only if it is performed correctly by a trained person, its use is scientifically justified, and it is described in an approved ACAP. When performed properly this technique is nearly instantaneous and is considered humane. Guillotines that are designed to accomplish decapitation in adult rodents in a uniformly instantaneous manner are commercially available. Sharp scissors can be used to decapitate neonatal rodents. Check guillotine and scissor blades frequently to ensure sharpness. The equipment used to perform decapitation should be maintained in good working order and serviced on a regular basis to ensure sharpness of blades. The use of plastic cones to restrain animals appears to minimize stress from handling, minimize the chance of injury to personnel, and improves positioning of the animal in the guillotine. (2013 AVMA Guidelines on Euthanasia) Note: The Physics Department s Instrument Shop, located in Phillips Hall 115A, will sharpen blades for a small fee[(919) ].. Gaseous Carbon Dioxide (CO 2 ): must be supplied using a compressed gas tank. The use of dry ice as a source of CO 2 for euthanasia is not permitted. (Refer to section 2A below.) Inhalant Anesthesia: anesthetic agent(s) delivered as a volatile gas to the respiratory tract to induce anesthesia. Personnel should minimize their exposure to these agents UNC Chapel Hill Revised 05/
17 as some are considered chemical hazards. These agents should only be used in a chemical fume hood, ducted biosafety cabinet or in a system with an active gas scavenging device. (Refer to section 2B below.) Injectable Anesthesia: chemical agent(s) administered by injection with a needle and syringe to induce anesthesia. Common routes of injection include, but are not limited to, intraperitoneal (IP), intramuscular (IM) or intravenous (IV). Injectable anesthetics are easy to administer, require minimal equipment, and avoid safety concerns associated with inhalants. (Refer to section 2CSection 2: Procedures A. Gaseous Carbon Dioxide (CO2): The 2013 AVMA Guidelines on Euthanasia recommends that the gradual displacement rate of CO2 into the euthanasia chamber should be 10-30% to minimize pain and distress. All calculations described below are for a DLAM shoe box style rat cage at 30% displacement. Note: DLAM procedure rooms have dedicated CO 2 euthanasia chambers equipped with acceptable flow meters. Investigators who wish to perform CO2 euthanasia outside of DLAM facilities must adhere to all of the following principals and must purchase the same equipment utilized by DLAM. Appropriate flow meters must be purchased from VWR and can be found through the UNC purchasing system, E-Pro, or at the following website: (part number: ). To purchase appropriately sized euthanasia chambers, contact DLAM at ( ). 1. Place the Euthanex stainless steel lid over the plastic cage. The lid should be connected to a CO 2 tank via a plastic hose. a. Make sure the two holes on the top of the lid are not blocked, as these holes allow air to be pushed out by the heavier CO 2. b. Make sure the plastic cage does not have an automatic watering opening. 2. Remove each animal from the housing chamber and place into the euthanasia chamber. Never place the housing chamber into the euthanasia chamber. Do not place different animal species in the chamber at the same time. Do not overcrowd the chamber. Each animal should have enough floor space available to lie down. 3. Turn on the valve located on top of the CO 2 tank. Next, set the flow meter by adjusting the regulator valve on the left side of the flow meter (see photo on the next page): a. Standard DLAM Shoebox style RAT cage: 8 liters per minute (lpm) b. Standard DLAM Shoebox style MOUSE cage: 1.8 liters per minute (lpm) c. Other CO2 Chambers: Use the following formula to calculate the appropriate flow rate: height x width x length = liters x.20 = flow rate/minute Continue to allow CO 2 to flow into the chamber for one minute after breathing stops (approximately 6 minutes for mice and 8 minutes for rats). Young animals, certain strains of mice, and sick animals may require more time to become deeply anesthetized. UNC Chapel Hill Revised 05/
18 5. Once animals are fully anesthetized, immediately perform a physical method of euthanasia (i.e. cervical dislocation, thoracotomy, major organ harvest, or decapitation) to confirm death. 6. Note: If a terminal procedure (i.e. cardiac puncture, tissue collection) must be performed before the secondary physical method, ensure that animals remain deeply anesthetized and that a physical method of euthanasia is performed following the terminal procedure. 7. Place dead animals into a non-pvc containing bag. DLAM provides these bags in a variety of sizes. Label the bag with the ACAP ID#. Seal the bag securely. Place the bag with dead animal(s) into the DLAM carcass freezer available in each animal facility. Please see the Policy on Rodent Carcass Disposal for more information. 8. Disinfect the euthanasia chamber bottom after each use. Step 2: Set Flow Meter to appropriate flow rate. Step 1: Turn on valve on top of the CO2 tank B. Inhalant Anesthetics (e.g. Isoflurane) Induction chambers for inhalational anesthetics must allow animals appropriate floor space without being too large. Chambers that are too large require increased volumes of the anesthetic agent and may result in slow induction time. Where applicable, induction chambers must prevent animals from coming into direct contact with an anesthetic soaked material. The lid should fit snugly and the chamber must be used in a fume hood, a ducted biosafety cabinet, or with a properly functioning active scavenging system. 1. Pre-charge the anesthetic chamber by opening the vaporizer or placing two to three pieces of absorbent material on the bottom of the chamber. Add approximately 3-5 mls of isoflurane liquid to the absorbent material (amount of isoflurane is determined UNC Chapel Hill Revised 05/
19 by the size of the chamber). Close the lid and wait 5 minutes for the liquid to form a volatile gas within the chamber. 2. Remove the lid of the chamber, quickly place the animals in the chamber, ensure the absorbent material is not in direct contact with the animal, and immediately close the lid. 3. The animals should become anesthetized in 2-5 minutes. Neonates require a longer period of time to anesthetize and should remain in the chamber for at least five (5) minutes. 4. When animals are completely recumbent and obviously deeply anesthetized, remove them from the chamber. 5. Immediately perform a physical method of euthanasia. Isoflurane is highly volatile and animals will quickly regain consciousness once removed from the chamber. Therefore, it is imperative that physical euthanasia be performed immediately. 6. Note: If a terminal procedure (i.e. cardiac puncture, tissue collection) must be performed before the secondary physical method, ensure that animals remain deeply anesthetized and that a physical method of euthanasia is performed following the terminal procedure. C. Injectable Anesthetics Injectable anesthetics can be effectively used to anesthetize animals prior to performing physical euthanasia. The agent should be an anesthetic recommended for the species, and the dosage used should be equal to or greater than the standard published reference dose for anesthesia (e.g., a common dose of pentobarbital for euthanasia is 100 mg/kg, which is approximately twice the anesthetic dose for rats and mice). Once the injectable anesthetic is administered, allow sufficient time for the animal to lose consciousness. Injectable anesthetics intended for use in adult rodents may not have the desired effect in neonates. In a pilot study conducted at UNC-Chapel Hill, few anesthetics were found to be reliably effective in neonates. The drugs that provided the most effective anesthesia are available only to veterinarians and as a result were considered impractical for use by the scientific community. Contact a DLAM veterinarian for more information about appropriate doses of injectable anesthetics. Section 3: Euthanasia of Rodent Fetuses Fetuses up to 14 days in gestation: Neural development at this stage is minimal and pain perception is considered unlikely. Euthanasia of the mother or removal of the fetus should ensure rapid death of the fetus at this stage of development. Fetuses 15 days in gestation to birth: UNC Chapel Hill Revised 05/
20 The literature on the development of pain pathways suggests the possibility of pain perception at this point in gestation. Whereas fetuses at this age are not sensitive to inhalant anesthetics, anesthesia may be induced by injection of the fetus with a chemical anesthetic, or by deep anesthesia of the mother with a chemical agent that crosses the placenta, e.g., pentobarbital. Decapitation with sharp scissors and cervical dislocation are acceptable physical methods of euthanasia when used by a trained person. The specific technique(s) employed must be described in the approved ACAP. When chemical fixation of the whole fetus is required, fetuses should be anesthetized prior to immersion in or perfusion with fixative solutions. Consult with one of the institutional veterinarians to learn more about fetal sensitivity to specific anesthetic agents. When fetuses are not required for study, the method chosen for euthanasia of a pregnant mother must ensure rapid death of the fetus. PDF format available on the IACUC website: Click HERE EUTHANASIA REFERENCES American Veterinary Medical Association (2013) AVMA Guidelines on Euthanasia. Anden NE, Magnusson T & Stock G (1974) Effect of anesthetic agents on the synthesis and disappearance of brain dopamine normally and after haloperidol, KCL or axotomy. Naunyn-Schmiederbers Archiv fur Pharm 283(4), Bergstrom DA, Bromley SD & Walters JR (1984) Dopamine agonists increase pallidal unit activity: attenuation by agonist pretreatment and anesthesia. Eur J Pharm 100(1), Bhathena SJ (1992) Comparison of effects of decapitation and anesthesia on metabolic and hormonal parameters in Sprague-Dawley rats. Life Sciences 50(21), Brown RE (1995) An Introduction to Neuroendocrinology. Cambridge. Holson RR (1992) Euthanasia by decapitation evidence that this technique produces prompt, painless unconsciousness in laboratory rodents. Neurotoxicology and Teratology 14(4), Institute of Laboratory Animal Resources Commission on Life Sciences, National Research Council (1996) Guide for the Care and Use of Laboratory Animals. National Academy Press (65-66). Malyapa RS, et al (1998) DNA damage in rat brain cells after in vivo exposure to 2450 MHz electromagnetic radiation and various methods of euthanasia. Radiation Research 149, Mantz J, Varlet C, Lecharny JB, Henzel D, Lenot P & Desmonts JM (1994) Effects of volatile anesthetics, thiopental, and ketamine on spontaneous and depolarization- UNC Chapel Hill Revised 05/
21 evoked dopamine release from striatal synaptosomes in the rat. Anesthesiology 80(2), NIH (2002) Guidelines for the Euthanasia of Rodent Feti and Neonates. (Complete document HERE) Supply and Vendor Information Isoflurane can be purchased from the UNC CH Hospital pharmacy: Call for more information or go to Ground Floor, room NG10B. A grant number and department number is required for purchase. Braintree Scientific Instruments, lab equipment, isothermal pads, tattoo paste Fisher Scientific Lab equipment, chemicals, instruments, pharmaceuticals Henry Schein Veterinary supplies, instruments, pharmaceuticals Need Vet License or Researcher DEA license JA Webster Veterinary supplies, instruments, pharmaceuticals Need Vet License or Researcher DEA license Kent Scientific Surgical equipment, telemetry equipment Med-Vet International Veterinary supplies and instruments (discounted) Need Vet License National Band and Tag ID tags, ear tags Roboz Specialize in instruments TW Medical Veterinary supply (Bill Forrester) UNC-CH Materials Management and Distribution Scientific Storeroom, General Storeroom, Chemical Storeroom Veterinary Medical Supply Veterinary Supplies out of Zebulon, NC UNC Chapel Hill Revised 05/
22 Need Vet License Southern Anesthesia This is a human source company that has a Veterinary division, will set up an account without a vet license. UNC Chapel Hill Revised 05/
23 The University of North Carolina at Chapel Hill IACUC Training Record Mice: Handling and Basic Techniques Technique needed? Technique Comments Handling and Restraint Intraperitoneal Injection Subcutaneous Injection Intramuscular Injection Intravenous Injection Oral Gavage Retro-orbital Injection Retro-orbital Bleed Tail Nick Bleed Tail Clip Bleed /Genotyping Submandibular Bleed Cardiac Puncture, terminal Ear Notch (punch) Ear Tag Anesthesia Injectables CO 2 Flow meter w/ Phys. Euthanasia Cervical Dislocation without Anesthesia Decapitation without Anesthesia Isoflurane Euthanasia / Anesthesia Drop Method Inhalational Anesthesia Training-Vaporizer Machine Training Proficiency Rating *Individual appointment during class* *Individual appointment during class* *Individual appointment during class* *Individual appointment during class* *Provided during a 1-on-1 training session. Please call the OACU to schedule an appointment Contact DLAM Vet Services at Please add address if you are a Lab Coordinator: Lab Phone #: I certify that I have received the above training: Signature: PID: Print Name: PI: Instructor Signature: Date: I II III UNC Chapel Hill Revised 05/
UNIVERSITY STANDARD. Title UNIVERSITY OF NORTH CAROLINA AT CHAPEL HILL STANDARD ON RAT AND MOUSE EUTHANASIA. Introduction
 UNIVERSITY STANDARD Title UNIVERSITY OF NORTH CAROLINA AT CHAPEL HILL STANDARD ON RAT AND MOUSE Introduction PURPOSE The standards and procedures described below provide guidance to all researchers and
UNIVERSITY STANDARD Title UNIVERSITY OF NORTH CAROLINA AT CHAPEL HILL STANDARD ON RAT AND MOUSE Introduction PURPOSE The standards and procedures described below provide guidance to all researchers and
Basic MOUSE Handling and Technique Guide UNC-IACUC First do no harm Greek Hippocratic Oath, Great Watch Words of Medicine
 Basic MOUSE Handling and Technique Guide UNC-IACUC First do no harm Greek Hippocratic Oath, Great Watch Words of Medicine Basic Information about working with Mice Proper Personal Protection Equipment
Basic MOUSE Handling and Technique Guide UNC-IACUC First do no harm Greek Hippocratic Oath, Great Watch Words of Medicine Basic Information about working with Mice Proper Personal Protection Equipment
A. BACKGROUND INFORMATION
 Institutional Animal Care and Use Committee Title: Euthanasia Guidelines Document #: 006 Version #: 01 UNTHSC Approved by IACUC Date: October 22, 2013 A. BACKGROUND INFORMATION a. Euthanasia techniques
Institutional Animal Care and Use Committee Title: Euthanasia Guidelines Document #: 006 Version #: 01 UNTHSC Approved by IACUC Date: October 22, 2013 A. BACKGROUND INFORMATION a. Euthanasia techniques
UNTHSC. Institutional Animal Care and Use Committee. Title: Euthanasia Guidelines. Document #: 006 Version #: 02
 Institutional Animal Care and Use Committee Title: Euthanasia Guidelines Document #: 006 Version #: 02 UNTHSC Approved by IACUC Date: February 28, 2017 A. BACKGROUND INFORMATION a. According to 9 CFR part
Institutional Animal Care and Use Committee Title: Euthanasia Guidelines Document #: 006 Version #: 02 UNTHSC Approved by IACUC Date: February 28, 2017 A. BACKGROUND INFORMATION a. According to 9 CFR part
The Guide for the Care and Use of Laboratory Animals, 8th Edition, November Euthanasia. pp
 Euthanasia Policy IACUP Policy Effective Date: October 2015 I. Purpose This policy establishes the standards for euthanasia of laboratory animals at UCSF. This policy has been created to ensure that euthanasia
Euthanasia Policy IACUP Policy Effective Date: October 2015 I. Purpose This policy establishes the standards for euthanasia of laboratory animals at UCSF. This policy has been created to ensure that euthanasia
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
SOP: Blood Collection in the Horse
 SOP: Blood Collection in the Horse These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
SOP: Blood Collection in the Horse These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
PROTOCOL FOR THE HUMANE CARE AND USE OF LIVE VERTEBRATE ANIMALS
 PROTOCOL FOR THE HUMANE CARE AND USE OF LIVE VERTEBRATE ANIMALS Federal animal welfare regulations require that the Institutional Animal Care and Use Committee (IACUC) must review and approve all activities
PROTOCOL FOR THE HUMANE CARE AND USE OF LIVE VERTEBRATE ANIMALS Federal animal welfare regulations require that the Institutional Animal Care and Use Committee (IACUC) must review and approve all activities
EC-AH-011v1 January 2018 Page 1 of 5. Standard Operating Procedure Equine Center Clemson University
 EC-AH-011v1 January 2018 Page 1 of 5 Standard Operating Procedure Equine Center Clemson University SOP ID: EC-AH-011v1 January 2018 Title: Injection Techniques Author(s): Julia Tagher, CU Equine Center
EC-AH-011v1 January 2018 Page 1 of 5 Standard Operating Procedure Equine Center Clemson University SOP ID: EC-AH-011v1 January 2018 Title: Injection Techniques Author(s): Julia Tagher, CU Equine Center
UNIVERSITY OF PITTSBURGH Institutional Animal Care and Use Committee
 UNIVERSITY OF PITTSBURGH Institutional Animal Care and Use Committee Policy: Surgical Guidelines EFFECTIVE ISSUE DATE: 2/21/2005 REVISION DATE(s): 2/14/15; 3/19/2018 SCOPE To describe guidelines and considerations
UNIVERSITY OF PITTSBURGH Institutional Animal Care and Use Committee Policy: Surgical Guidelines EFFECTIVE ISSUE DATE: 2/21/2005 REVISION DATE(s): 2/14/15; 3/19/2018 SCOPE To describe guidelines and considerations
SOP: Blood Collection in Swine
 SOP: Blood Collection in Swine These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol
SOP: Blood Collection in Swine These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
3. ENSURING HUMANE EUTHANASIA OF LABORATORY ANIMALS
 Page 1 of 5 1. DEFINITION Euthanasia is the act of inducing humane death in an animal by a method that induces rapid loss of consciousness and death with a minimum of pain, discomfort, or distress. 2.
Page 1 of 5 1. DEFINITION Euthanasia is the act of inducing humane death in an animal by a method that induces rapid loss of consciousness and death with a minimum of pain, discomfort, or distress. 2.
SOP #: Date Issue: Effective Date: Date Last Revision: Page 1 of 5. PPE, approved restraining devices. Disposable gloves, cap, mask, lab coat
 SOP #: Date Issue: Effective Date: Date Last Revision: Page 1 of 5 TITLE SCOPE PURPOSE EQUIPMENT Handling and Restraint of Rats Applies to all Howard University (HU) personnel working with rats in a HU
SOP #: Date Issue: Effective Date: Date Last Revision: Page 1 of 5 TITLE SCOPE PURPOSE EQUIPMENT Handling and Restraint of Rats Applies to all Howard University (HU) personnel working with rats in a HU
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
DREXEL UNIVERSITY COLLEGE OF MEDICINE ANIMAL CARE AND USE COMMITTEE POLICY FOR PREOPERATIVE AND POSTOPERATIVE CARE FOR NON-RODENT MAMMALS
 DREXEL UNIVERSITY COLLEGE OF MEDICINE ANIMAL CARE AND USE COMMITTEE POLICY FOR PREOPERATIVE AND POSTOPERATIVE CARE FOR NON-RODENT MAMMALS OBJECTIVE: This policy is to ensure that appropriate provisions
DREXEL UNIVERSITY COLLEGE OF MEDICINE ANIMAL CARE AND USE COMMITTEE POLICY FOR PREOPERATIVE AND POSTOPERATIVE CARE FOR NON-RODENT MAMMALS OBJECTIVE: This policy is to ensure that appropriate provisions
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
Animal Dairy Science Facility Handbook
 Welcome...3 Life Sciences/Veterinary Medicine Unit Staff...4 Animal Dairy Science Facility Staff... 4 Important Phone Numbers... 4 Key Cards and Facility Access... 5 Entry and Exit Procedures... 5 Facility
Welcome...3 Life Sciences/Veterinary Medicine Unit Staff...4 Animal Dairy Science Facility Staff... 4 Important Phone Numbers... 4 Key Cards and Facility Access... 5 Entry and Exit Procedures... 5 Facility
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
IN THE DAILY LIFE of a veterinarian or
 Administering Medication and Care IN THE DAILY LIFE of a veterinarian or veterinary technician, the majority of animal care involves administering medication to sick animals, giving vaccines for viruses,
Administering Medication and Care IN THE DAILY LIFE of a veterinarian or veterinary technician, the majority of animal care involves administering medication to sick animals, giving vaccines for viruses,
Psychology Animal Facility Handbook
 Welcome... 3 Life Sciences/Veterinary Medicine Unit Staff... 4 Psychology Facility Staff... 4 Important Phone Numbers... 4 Key Cards and Facility Access... 5 Entry and Exit Procedures... 5 Facility Entry...
Welcome... 3 Life Sciences/Veterinary Medicine Unit Staff... 4 Psychology Facility Staff... 4 Important Phone Numbers... 4 Key Cards and Facility Access... 5 Entry and Exit Procedures... 5 Facility Entry...
Rodent behaviour and handling
 Rodent behaviour and handling Understanding the nature of different species and the way they behave is important for your work in the animal industry. It will help you to recognise signs of stress in an
Rodent behaviour and handling Understanding the nature of different species and the way they behave is important for your work in the animal industry. It will help you to recognise signs of stress in an
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN "X" EST THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN "X" EST THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
STANDARD OPERATING PROCEDURE #111 RAT ANESTHESIA
 STANDARD OPERATING PROCEDURE #111 RAT ANESTHESIA 1. PURPOSE This Standard Operating Procedure (SOP) describes methods for anesthetizing rats. 2. RESPONSIBILITY Principal Investigators (PIs) and their research
STANDARD OPERATING PROCEDURE #111 RAT ANESTHESIA 1. PURPOSE This Standard Operating Procedure (SOP) describes methods for anesthetizing rats. 2. RESPONSIBILITY Principal Investigators (PIs) and their research
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
Policy #28: Euthanasia of Research and Teaching Animals
 1 Washington State University INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE Policy #28: Euthanasia of Research and Teaching Animals A. Definition Euthanasia is the act of inducing humane death in an animal
1 Washington State University INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE Policy #28: Euthanasia of Research and Teaching Animals A. Definition Euthanasia is the act of inducing humane death in an animal
Animal Studies Committee Policy Rodent Survival Surgery
 Animal Studies Committee Policy Rodent Survival Surgery ASC Policy: To optimize animal health and well-being, survival surgery in rodents must be performed using sterile instruments, surgical gloves, masks
Animal Studies Committee Policy Rodent Survival Surgery ASC Policy: To optimize animal health and well-being, survival surgery in rodents must be performed using sterile instruments, surgical gloves, masks
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN "X" EST THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN "X" EST THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
ANIMAL SCIENCE 140 LABORATORY ANIMAL MANAGEMENT
 Name ANIMAL SCIENCE 140 LABORATORY ANIMAL MANAGEMENT MIDTERM I Matching (20 points) Choose the most correct answer identified by the statements given. Each answer may be used more than once. There are
Name ANIMAL SCIENCE 140 LABORATORY ANIMAL MANAGEMENT MIDTERM I Matching (20 points) Choose the most correct answer identified by the statements given. Each answer may be used more than once. There are
RESEARCH AND TEACHING SURGERY GUIDELINES FOR MSU-OWNED ANIMALS
 RESEARCH AND TEACHING SURGERY GUIDELINES FOR MSU-OWNED ANIMALS I. Purpose/Scope These guidelines apply to all surgical procedures performed on animals at Mississippi State University in which the animals
RESEARCH AND TEACHING SURGERY GUIDELINES FOR MSU-OWNED ANIMALS I. Purpose/Scope These guidelines apply to all surgical procedures performed on animals at Mississippi State University in which the animals
Project Protocol Number UNIVERSITY OF HAWAII INSTITUTIONAL ANIMAL CARE &USE COMMITTEE 2002 VERTEBRATE ANIMAL USE PROTOCOL FORM
 Project Protocol Number UNIVERSITY OF HAWAII INSTITUTIONAL ANIMAL CARE &USE COMMITTEE 2002 VERTEBRATE ANIMAL USE PROTOCOL FORM The applicant is responsible for providing complete and accurate information.
Project Protocol Number UNIVERSITY OF HAWAII INSTITUTIONAL ANIMAL CARE &USE COMMITTEE 2002 VERTEBRATE ANIMAL USE PROTOCOL FORM The applicant is responsible for providing complete and accurate information.
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
Preparing for an AAALAC (and IACUC) Site Visit
 Preparing for an AAALAC (and IACUC) Site Visit Marcel Perret-Gentil, DVM, MS Neal Guentzel, PhD Updated 5/2013 About This Presentation Help you prepare for AAALAC site visit (as well as IACUC inspections)
Preparing for an AAALAC (and IACUC) Site Visit Marcel Perret-Gentil, DVM, MS Neal Guentzel, PhD Updated 5/2013 About This Presentation Help you prepare for AAALAC site visit (as well as IACUC inspections)
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
Using Animals in Research at PSU
 Using Animals in Research at PSU Contents Important Information Sources... 1 The Institutional Animal Care and Use Committee... 2 The Animal Resource Program... 2 Laboratory Animal Facilities... 2 Working
Using Animals in Research at PSU Contents Important Information Sources... 1 The Institutional Animal Care and Use Committee... 2 The Animal Resource Program... 2 Laboratory Animal Facilities... 2 Working
STANDARD OPERATING PROCEDURE #110 MOUSE ANESTHESIA
 STANDARD OPERATING PROCEDURE #110 MOUSE ANESTHESIA 1. PURPOSE This Standard Operating Procedure (SOP) describes methods for anesthetizing mice. 2. RESPONSIBILITY Principal Investigators (PIs) and their
STANDARD OPERATING PROCEDURE #110 MOUSE ANESTHESIA 1. PURPOSE This Standard Operating Procedure (SOP) describes methods for anesthetizing mice. 2. RESPONSIBILITY Principal Investigators (PIs) and their
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
AVIAN HUSBANDRY (POULTRY HATCHING AND CHICKS)
 1. PURPOSE ACEC SOP061 This Standard Operating Procedure (SOP) describes routine husbandry for housing and maintenance of laboratory poultry hatchlings and chicks up to six (6) weeks of age. 2. RESPONSIBILITY
1. PURPOSE ACEC SOP061 This Standard Operating Procedure (SOP) describes routine husbandry for housing and maintenance of laboratory poultry hatchlings and chicks up to six (6) weeks of age. 2. RESPONSIBILITY
Animal, Plant & Soil Science
 Animal, Plant & Soil Science Lesson C5-9 Veterinary Terminology Interest Approach Gather some common veterinary tools (e.g., scissors, forceps, and scalpels). Ask the students what each item is and for
Animal, Plant & Soil Science Lesson C5-9 Veterinary Terminology Interest Approach Gather some common veterinary tools (e.g., scissors, forceps, and scalpels). Ask the students what each item is and for
Disposition of Animals (Basic) Introduction. Reclamation. Adoption and Sterilization Euthanasia Carcass Disposal
 This Chapter Covers: Introduction Reclamation Adoption and Sterilization Euthanasia Carcass Disposal Introduction After an animal is impounded, there are a number of ways that the animal can leave the
This Chapter Covers: Introduction Reclamation Adoption and Sterilization Euthanasia Carcass Disposal Introduction After an animal is impounded, there are a number of ways that the animal can leave the
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
7/25/2014. Proper Injection Technique. Review Pork Quality Assurance Plus. Contact Information. Why are injections given?
 Breeding Herd Education Series 2011-12 Timely, relevant & convenient learning Thank you for participating in SowBridge 2011-12. To start this presentation, advance one slide by pressing enter or the down
Breeding Herd Education Series 2011-12 Timely, relevant & convenient learning Thank you for participating in SowBridge 2011-12. To start this presentation, advance one slide by pressing enter or the down
How To Give Your Horse An Intramuscular Injection
 ANR-1018 A L A B A M A A & M A N D A U B U R N U N I V E R S I T I E S How To Give Your Horse An Intramuscular Injection Most horse owners occasionally must give their horse an injection. Fortunately,
ANR-1018 A L A B A M A A & M A N D A U B U R N U N I V E R S I T I E S How To Give Your Horse An Intramuscular Injection Most horse owners occasionally must give their horse an injection. Fortunately,
IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee. Blood Collection Guidelines
 IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee Blood Collection Guidelines Purpose To provide Iowa State University (ISU) Institutional Animal Care and Use Committee (IACUC) guidelines
IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee Blood Collection Guidelines Purpose To provide Iowa State University (ISU) Institutional Animal Care and Use Committee (IACUC) guidelines
IACUC POLICY Rodent Survival Surgery
 BACKGROUND The University of Rhode Island s Institutional Animal Care and Use Committee (IACUC) is charged with ensuring that all surgical facilities and procedures meet the criteria set by the federal
BACKGROUND The University of Rhode Island s Institutional Animal Care and Use Committee (IACUC) is charged with ensuring that all surgical facilities and procedures meet the criteria set by the federal
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE ANIMAL PROTOCOL REVIEW QUESTIONNAIRE. Name Role on Protocol Department P. O.
 VIRGINIA STATE UNIVERSITY Petersburg, Virginia 23806 FOR IACUC USE Review Month: Protocol Number: INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE ANIMAL PROTOCOL REVIEW QUESTIONNAIRE Submission Procedures:
VIRGINIA STATE UNIVERSITY Petersburg, Virginia 23806 FOR IACUC USE Review Month: Protocol Number: INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE ANIMAL PROTOCOL REVIEW QUESTIONNAIRE Submission Procedures:
T u l a n e U n i v e r s i t y I A C U C Guidelines for Rodent & Rabbit Anesthesia, Analgesia and Tranquilization & Euthanasia Methods
 T u l a n e U n i v e r s i t y I A C U C Guidelines for Rodent & Rabbit Anesthesia, Analgesia and Tranquilization & Euthanasia Methods Abbreviations: General Considerations IV = intravenous SC = subcutaneous
T u l a n e U n i v e r s i t y I A C U C Guidelines for Rodent & Rabbit Anesthesia, Analgesia and Tranquilization & Euthanasia Methods Abbreviations: General Considerations IV = intravenous SC = subcutaneous
Anesthetic regimens for mice, rats and guinea pigs
 Comparative Medicine SOP #: 101. 01 Page: 1 of 10 Anesthetic regimens for mice, rats and guinea pigs The intent of the Standard Operating Procedure (SOP) is to describe commonly used methods to anaesthetize
Comparative Medicine SOP #: 101. 01 Page: 1 of 10 Anesthetic regimens for mice, rats and guinea pigs The intent of the Standard Operating Procedure (SOP) is to describe commonly used methods to anaesthetize
Working with Mice in Research at UAB (AU_M) Course Material
 UAB (AU_M) Course Material Introduction Welcome to the UAB (AU_M) Course Material. The goal of this course material is to introduce you to working with mice in research at UAB. However, some procedures
UAB (AU_M) Course Material Introduction Welcome to the UAB (AU_M) Course Material. The goal of this course material is to introduce you to working with mice in research at UAB. However, some procedures
MICROCHIP IMPLANTATION
 MICROCHIP IMPLANTATION A PICTORIAL Photos taken by Nick Morganelli of Winston- Salem, NC Several companies market microchips for pet identification. I use AVID microchips which stand for Animal Veterinary
MICROCHIP IMPLANTATION A PICTORIAL Photos taken by Nick Morganelli of Winston- Salem, NC Several companies market microchips for pet identification. I use AVID microchips which stand for Animal Veterinary
Veterinary Assistant Buddy Center Volunteer Training Manual
 Veterinary Assistant Buddy Center Volunteer Training Manual Thank you for volunteering as a Veterinary Assistant. This packet includes information to help familiarize you with the Veterinary Services department
Veterinary Assistant Buddy Center Volunteer Training Manual Thank you for volunteering as a Veterinary Assistant. This packet includes information to help familiarize you with the Veterinary Services department
Part I - Euthanasia as an Alternative to Death as an Endpoint in Rodents
 UNIVERSITY OF TEXAS AT ARLINGTON INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE EUTHANASIA AND HUMANE ENDPOINTS SOP Part I - Euthanasia as an Alternative to Death as an Endpoint in Rodents 1. Background Information
UNIVERSITY OF TEXAS AT ARLINGTON INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE EUTHANASIA AND HUMANE ENDPOINTS SOP Part I - Euthanasia as an Alternative to Death as an Endpoint in Rodents 1. Background Information
Administering wormers (anthelmintics) effectively
 COWS www.cattleparasites.org.uk Administering wormers (anthelmintics) effectively COWS is an industry initiative promoting sustainable control strategies for parasites in cattle Wormer administration Dec
COWS www.cattleparasites.org.uk Administering wormers (anthelmintics) effectively COWS is an industry initiative promoting sustainable control strategies for parasites in cattle Wormer administration Dec
Title: Euthanasia Procedures for the UC Davis Animal Care Program
 Policy: SC 40 102 Date: 6/7/2016 Enabled by: The Guide, APHIS, AVMA, IACUC /AV Supersedes: IACUC Policy, ALL Previous Standards of Care on Euthanasia Title: Euthanasia Procedures for the UC Davis Animal
Policy: SC 40 102 Date: 6/7/2016 Enabled by: The Guide, APHIS, AVMA, IACUC /AV Supersedes: IACUC Policy, ALL Previous Standards of Care on Euthanasia Title: Euthanasia Procedures for the UC Davis Animal
Biohazard: yes no Radioisotopes: yes no Chemical Carcinogen: yes no Agent: Agent: Agents: Project Title: Objective:
 1 Date of Submission: Biohazard: yes no Radioisotopes: yes no Chemical Carcinogen: yes no Agent: Agent: Agents: Protocol No. Species Project Title: Objective: Application to Perform Research Involving
1 Date of Submission: Biohazard: yes no Radioisotopes: yes no Chemical Carcinogen: yes no Agent: Agent: Agents: Protocol No. Species Project Title: Objective: Application to Perform Research Involving
UNIVERSITY OF TENNESSEE AT MARTIN
 UNIVERSITY OF TENNESSEE AT MARTIN Beef Teaching and Research Farm STANDARD OPERATING PROCEDURES EFFECTIVE: 5-20-05 REVISED: 5-17-10 TABLE OF CONTENTS The intent of this document is to describe the routine
UNIVERSITY OF TENNESSEE AT MARTIN Beef Teaching and Research Farm STANDARD OPERATING PROCEDURES EFFECTIVE: 5-20-05 REVISED: 5-17-10 TABLE OF CONTENTS The intent of this document is to describe the routine
Rodent Surgery (AU_RS) Course Material
 Course Material Introduction Welcome to the Course Material. You must complete this course if you perform surgical procedures on rodents (mice or rats) at UAB. The goal of this course is to make you aware
Course Material Introduction Welcome to the Course Material. You must complete this course if you perform surgical procedures on rodents (mice or rats) at UAB. The goal of this course is to make you aware
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
UiTM CARE APPLICATION FORM
 UiTM CARE APPLICATION FORM (Committee on Animal Research and Ethics) FOR UiTM CARE OFFICE USE ONLY Proposal No.:... Date of hard copy receipt:... INFORMATION FOR PRINICIPAL INVESTIGATOR Submit the duly
UiTM CARE APPLICATION FORM (Committee on Animal Research and Ethics) FOR UiTM CARE OFFICE USE ONLY Proposal No.:... Date of hard copy receipt:... INFORMATION FOR PRINICIPAL INVESTIGATOR Submit the duly
The AAALAC Site Visit. What to expect
 The AAALAC Site Visit What to expect UNC-CH will host an AAALACi site visit May 30 June 2, 2017 4-member site visit team Evaluating UNC-CH animal care program for 4 days Lead AAALACi Council Member Dr.
The AAALAC Site Visit What to expect UNC-CH will host an AAALACi site visit May 30 June 2, 2017 4-member site visit team Evaluating UNC-CH animal care program for 4 days Lead AAALACi Council Member Dr.
The AAALAC Site Visit. What to expect
 The AAALAC Site Visit What to expect UNC will host an AAALAC site visit August 4 7, 2014 Why are we accredited? AAALAC Demonstrates the University s commitment to a quality animal research program Provides
The AAALAC Site Visit What to expect UNC will host an AAALAC site visit August 4 7, 2014 Why are we accredited? AAALAC Demonstrates the University s commitment to a quality animal research program Provides
4-H CVA LEVEL I EXAM APPLICATION PLEASE PRINT. First Name: Last Name: Address: City State Postal Code: Phone:( ) Date of Application:
 OFFICE USE ONLY # 1 APPLICANT S INFORMATION 4-H CVA LEVEL I EXAM APPLICATION PLEASE PRINT Address: Phone:( ) Email: Date of Application: Applicant s Signature: 4-H SUPERVISOR S INFORMATION By affixing
OFFICE USE ONLY # 1 APPLICANT S INFORMATION 4-H CVA LEVEL I EXAM APPLICATION PLEASE PRINT Address: Phone:( ) Email: Date of Application: Applicant s Signature: 4-H SUPERVISOR S INFORMATION By affixing
Biological Sciences Animal Facility Handbook
 Welcome... 3 Life Sciences/Veterinary Medicine Unit Staff... 4 Biological Sciences Facility... 4 Important Phone Numbers... 4 Key Cards and Facility Access... 5 Entry and Exit Procedures... 5 Facility
Welcome... 3 Life Sciences/Veterinary Medicine Unit Staff... 4 Biological Sciences Facility... 4 Important Phone Numbers... 4 Key Cards and Facility Access... 5 Entry and Exit Procedures... 5 Facility
 Other vaccination recommendations will be determined on an individual basis after the risk assessment that reviews animal species, risk exposure, and personal health issues. The CMU consulting occupational
Other vaccination recommendations will be determined on an individual basis after the risk assessment that reviews animal species, risk exposure, and personal health issues. The CMU consulting occupational
GUIDELINES FOR ANESTHESIA AND FORMULARIES
 GUIDELINES FOR ANESTHESIA AND FORMULARIES Anesthesia is the act of rendering the animal senseless to pain or discomfort and is required for surgical and other procedures. Criteria for choosing an anesthetic
GUIDELINES FOR ANESTHESIA AND FORMULARIES Anesthesia is the act of rendering the animal senseless to pain or discomfort and is required for surgical and other procedures. Criteria for choosing an anesthetic
Blood Collection Healthcare
 Blood Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms
Blood Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms
Washington State University Institutional Animal Care and Use Committee
 1 Standard Operating Procedure #9 Title: Minor Medical Treatment of Rodents Washington State University Institutional Animal Care and Use Committee Purpose: Currently, the Office of the Campus Veterinarian
1 Standard Operating Procedure #9 Title: Minor Medical Treatment of Rodents Washington State University Institutional Animal Care and Use Committee Purpose: Currently, the Office of the Campus Veterinarian
SMALL ANIMAL NURSING I CLINICAL MENTORSHIP
 PURDUE UNIVERSITY COLLEGE OF VETERINARY MEDICINE Veterinary Technology Distance Learning SMALL ANIMAL NURSING I CLINICAL MENTORSHIP VM 20500 CRITERIA HANDBOOK AND LOGBOOK Purdue University is an equal
PURDUE UNIVERSITY COLLEGE OF VETERINARY MEDICINE Veterinary Technology Distance Learning SMALL ANIMAL NURSING I CLINICAL MENTORSHIP VM 20500 CRITERIA HANDBOOK AND LOGBOOK Purdue University is an equal
2015 Vet Assisting CDE Prelim Exam
 Florida FFA Association ID: A 2015 Vet Assisting CDE Prelim Exam True/False Indicate whether the statement is true or false. Mark your answer choices on your answer form. 1 Hamsters may safely be picked
Florida FFA Association ID: A 2015 Vet Assisting CDE Prelim Exam True/False Indicate whether the statement is true or false. Mark your answer choices on your answer form. 1 Hamsters may safely be picked
Clipping a Dog s Toenails
 Clipping a Dog s Toenails This information is not meant to be a substitute for veterinary care. Always follow the instructions provided by your veterinarian. In the photographs below, unless otherwise
Clipping a Dog s Toenails This information is not meant to be a substitute for veterinary care. Always follow the instructions provided by your veterinarian. In the photographs below, unless otherwise
6/10/2015. Multi Purpose Canine (MPC) Restraint and Physical Examination PFN: Terminal Learning Objective. Hours: Instructor:
 Multi Purpose Canine (MPC) Restraint and Physical Examination PFN: Hours: Instructor: Slide 1 Slide 2 Terminal Learning Objective Action: Communicate knowledge of Multi Purpose Canine (MPC) restraint and
Multi Purpose Canine (MPC) Restraint and Physical Examination PFN: Hours: Instructor: Slide 1 Slide 2 Terminal Learning Objective Action: Communicate knowledge of Multi Purpose Canine (MPC) restraint and
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site:
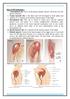 Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
PROTOCOL FOR ANIMAL USE AND CARE
 PROTOCOL FOR ANIMAL USE AND CARE Score 1: Score 2: Total: 1. Contacts Primary Investigator Alternate contact Name Sandra Weisker Name Email sweisker@ucdavis.edu Email Dept Animal Science Dept Telephone
PROTOCOL FOR ANIMAL USE AND CARE Score 1: Score 2: Total: 1. Contacts Primary Investigator Alternate contact Name Sandra Weisker Name Email sweisker@ucdavis.edu Email Dept Animal Science Dept Telephone
Institutional Biosafety Committee
 Institutional Biosafety Committee Standard Operating Procedure (SOP) - Tamoxifen Principal Investigator: Room & Building #: Department: Phone # Date: Location(s) Covered by this SOP. Building Lab # Procedure
Institutional Biosafety Committee Standard Operating Procedure (SOP) - Tamoxifen Principal Investigator: Room & Building #: Department: Phone # Date: Location(s) Covered by this SOP. Building Lab # Procedure
Administering Medication and Care
 Administering Medication and Care Unit: Animal Science and the Industry Problem Area: Animal Health and Administering Veterinary Care Student Learning Objectives. Instruction in this lesson should result
Administering Medication and Care Unit: Animal Science and the Industry Problem Area: Animal Health and Administering Veterinary Care Student Learning Objectives. Instruction in this lesson should result
Animal Care Resource Guide Veterinary Care Issue Date: August 18, 2006
 Veterinary Care Issue Date: August 18, 2006 Subject: Veterinary Care Policy #3 Expired Medical Materials Pharmaceutical-Grade Compounds in Research Surgery Pre- and Post- Procedural Care Program of Veterinary
Veterinary Care Issue Date: August 18, 2006 Subject: Veterinary Care Policy #3 Expired Medical Materials Pharmaceutical-Grade Compounds in Research Surgery Pre- and Post- Procedural Care Program of Veterinary
Some important information about the fetus and the newborn puppy
 Some important information about the fetus and the newborn puppy Dr. Harmon Rogers Veterinary Teaching Hospital Washington State University Here are a few interesting medical details about fetuses and
Some important information about the fetus and the newborn puppy Dr. Harmon Rogers Veterinary Teaching Hospital Washington State University Here are a few interesting medical details about fetuses and
Procedure # IBT IACUC Approval: December 11, 2017
 IACUC Procedure: Anesthetics and Analgesics Procedure # IBT-222.04 IACUC Approval: December 11, 2017 Purpose: The purpose is to define the anesthetics and analgesics that may be used in mice and rats.
IACUC Procedure: Anesthetics and Analgesics Procedure # IBT-222.04 IACUC Approval: December 11, 2017 Purpose: The purpose is to define the anesthetics and analgesics that may be used in mice and rats.
Animal Care Resource Guide Veterinary Care Issue Date: July 17, 2007
 Policies Animal Care Resource Guide Veterinary Care Issue Date: July 17, 2007 Subject: Veterinary Care: Expired Medical Materials Pharmaceutical-Grade Compounds in Research Surgery Pre- and Post- Procedural
Policies Animal Care Resource Guide Veterinary Care Issue Date: July 17, 2007 Subject: Veterinary Care: Expired Medical Materials Pharmaceutical-Grade Compounds in Research Surgery Pre- and Post- Procedural
Mass Delivery of Nonsurgical Sterilants
 Neutersol - What Works? What Targets? What Next? Nonsurgical Sterilization Presentation By: Sean Hawkins, President November 11, 2006 ACC&D Annual Conference Mass Delivery of Nonsurgical Sterilants Fun
Neutersol - What Works? What Targets? What Next? Nonsurgical Sterilization Presentation By: Sean Hawkins, President November 11, 2006 ACC&D Annual Conference Mass Delivery of Nonsurgical Sterilants Fun
SOP: Subcutaneous Injections in Swine
 SOP: Subcutaneous Injections in Swine These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
SOP: Subcutaneous Injections in Swine These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
Euthanasia Guide for Ontario Commercial Meat Rabbit Producers
 Euthanasia Guide for Ontario Commercial Meat Rabbit Producers Published in 2016 Introduction Producers are responsible for the welfare of the rabbits in their care. Rabbits should be inspected for sickness
Euthanasia Guide for Ontario Commercial Meat Rabbit Producers Published in 2016 Introduction Producers are responsible for the welfare of the rabbits in their care. Rabbits should be inspected for sickness
Section A Definitions
 Guidelines for Surgical Procedures in Non-Rodent Mammals The University of Texas at Austin Institutional Animal Care and Use Committee These guidelines have been written to assist faculty, staff, and students
Guidelines for Surgical Procedures in Non-Rodent Mammals The University of Texas at Austin Institutional Animal Care and Use Committee These guidelines have been written to assist faculty, staff, and students
Practical Euthanasia of Cattle. Considerations for the Producer, Livestock Market Operator, Livestock Transporter, and Veterinarian
 Practical Euthanasia of Cattle Considerations for the Producer, Livestock Market Operator, Livestock Transporter, and Veterinarian Euthanasia is defined as "the intentional causing of a painless and easy
Practical Euthanasia of Cattle Considerations for the Producer, Livestock Market Operator, Livestock Transporter, and Veterinarian Euthanasia is defined as "the intentional causing of a painless and easy
Weber State University IACUC Laboratory Animal Protocol
 Weber State University IACUC Laboratory Animal Protocol 1. Name of Principal Investigator: Title: Dept./Phone: 1a. Name of Co-Investigator(s): Title: Dept./Phone: 2. Type of Project: [ ] Research [ ] Class
Weber State University IACUC Laboratory Animal Protocol 1. Name of Principal Investigator: Title: Dept./Phone: 1a. Name of Co-Investigator(s): Title: Dept./Phone: 2. Type of Project: [ ] Research [ ] Class
Mouse Formulary. The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.
 Mouse Formulary The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.): Intraperitoneal (IP) doses should not exceed 80 ml/kg
Mouse Formulary The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.): Intraperitoneal (IP) doses should not exceed 80 ml/kg
Clinical Procedures Practicum
 NATIONAL FFA CAREER AND LEADERSHIP DEVELOPMENT EVENTS HANDBOOK Clinical Procedures Practicum ADMINISTERING OPHTHALMIC MEDICATION The student wipes any discharge from the patient s eye using a gauze sponge
NATIONAL FFA CAREER AND LEADERSHIP DEVELOPMENT EVENTS HANDBOOK Clinical Procedures Practicum ADMINISTERING OPHTHALMIC MEDICATION The student wipes any discharge from the patient s eye using a gauze sponge
SURGICAL (SURVIVAL) OOCYTE COLLECTION FROM XENOUS LAEVIS
 UBC Animal Care Guidelines SOP: ACC 2013 01 Surgical Oocyte Collection from Xenopus Laevis Submitted by: Shelly McErlane Last Date Revised: Date Approved: January 28, 2013 SURGICAL (SURVIVAL) OOCYTE COLLECTION
UBC Animal Care Guidelines SOP: ACC 2013 01 Surgical Oocyte Collection from Xenopus Laevis Submitted by: Shelly McErlane Last Date Revised: Date Approved: January 28, 2013 SURGICAL (SURVIVAL) OOCYTE COLLECTION
ANIMAL USE APPLICATION FORM WILD FIELD STUDIES SUBMISSION GUIDELINES
 UCF Institutional Animal Care & Use Committee ANIMAL USE APPLICATION FORM WILD FIELD STUDIES SUBMISSION GUIDELINES The University of Central Florida Institutional Animal Care and Use Committee (IACUC)
UCF Institutional Animal Care & Use Committee ANIMAL USE APPLICATION FORM WILD FIELD STUDIES SUBMISSION GUIDELINES The University of Central Florida Institutional Animal Care and Use Committee (IACUC)
EUTHANASIA OF DOGS (Photos courtesy of KwaZulu-Natal Rabies Project and World Animal Protection)
 EUTHANASIA OF DOGS (Photos courtesy of KwaZulu-Natal Rabies Project and World Animal Protection) Euthanasia of dogs is a component of rabies control that may be necessary if suspected rabid or untreatable
EUTHANASIA OF DOGS (Photos courtesy of KwaZulu-Natal Rabies Project and World Animal Protection) Euthanasia of dogs is a component of rabies control that may be necessary if suspected rabid or untreatable
ANIMAL USE APPLICATION FORM WILDLIFE FIELD STUDIES SUBMISSION GUIDELINES
 UCF Institutional Animal Care & Use Committee ANIMAL USE APPLICATION FORM WILDLIFE FIELD STUDIES SUBMISSION GUIDELINES The University of Central Florida Institutional Animal Care and Use Committee (IACUC)
UCF Institutional Animal Care & Use Committee ANIMAL USE APPLICATION FORM WILDLIFE FIELD STUDIES SUBMISSION GUIDELINES The University of Central Florida Institutional Animal Care and Use Committee (IACUC)
UCF IACUC Breeding Addendum/Modification Form
 UCF IACUC Breeding Addendum/Modification Form Office Use Only: Date Received: Approval Date: This addendum form does NOT extend the IACUC approval period or replace the Continuing Review form for renewal
UCF IACUC Breeding Addendum/Modification Form Office Use Only: Date Received: Approval Date: This addendum form does NOT extend the IACUC approval period or replace the Continuing Review form for renewal
VETERINARY CARE PROGRAM
 VETERINARY CARE PROGRAM Institutional Animal Care and Use Committee 720 4 th Avenue South Miller Center, Room 204K St. Cloud, MN 56301-4498 320.308.5148 http://stcloudstate.edu/iacuc/ VETERINARY CARE PROGRAM
VETERINARY CARE PROGRAM Institutional Animal Care and Use Committee 720 4 th Avenue South Miller Center, Room 204K St. Cloud, MN 56301-4498 320.308.5148 http://stcloudstate.edu/iacuc/ VETERINARY CARE PROGRAM
