VARIATION IN THE ENDOCRINE AND IMMUNE SYSTEM OF JUVENILE ALLIGATORS: ENVIRONMENTAL INFLUENCE ON PHYSIOLOGY
|
|
|
- Janel Hunt
- 5 years ago
- Views:
Transcription
1 VARIATION IN THE ENDOCRINE AND IMMUNE SYSTEM OF JUVENILE ALLIGATORS: ENVIRONMENTAL INFLUENCE ON PHYSIOLOGY By ANDREW A. ROONEY A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1998
2 to Sam
3 ACKNOWLEDGMENTS My dissertation has proven to me that research is not done alone and certainly not without the help and support of others. I could not have done my work without the support of an active lab in the form of physical, ideological, practical, emotional and argumentative support. I would principally like to thank Lou Guillette for his grand mentoring. I would like to thank all of my committee members for the free and open access to their labs. Dave Evans I would like to thank for freely and rapidly giving opinions, comments, and recommendations. Larry McEdward, I would like to thank for his insight, comments, and efforts in organizing the concepts in this dissertation. Eric Sobel for his expertise, flexibility, and access to his lab. Earl Gray for his willingness to provide greatly appreciated additional points of view, even when he wasn t on my committee. I would also like to thank: Michel Fournier for opening the door to my future; Denis Flipo for speaking English better than I will ever speak French; Colette St. Mary for access to her lab even though I work on reptiles; Craig Osenberg for being defensive about his computers but not defensive in my defense; the Santa Fe Teaching Zoo for a wonderful collaborative attitude; Laurie Walz for continuing last minute help; Frank Nordlie and Jane Brockmann for a surprising amount of support including critical assistance in going to meetings; Drew Crain, John Matter, Dan Pickford and Cathy Cox for their pivotal roles in my graduate development; Andrea Galle for strength; Ed Orlando iii
4 for countless arguments and more help then he could have imagined; Matt Milnes for years of help; Mary Beth Evans for not coming to grad school in 1998; Mark Gunderson for not needing anything from me; Gerry Binczik for sarcasm; and special thanks to Karen Scharlette, Kevin Carter, Michael McLean, Adrian Ariyayagan, Meyur Dev, Abe Kim, Michelle Kirby, Elizabeth Fout, Kimberly Eschbach, Bobby Lippelman, Patricia Penha, Hillary Warren, Veronica Lye, Debbie Bolt, Colleen Kelley, Satish Degala and Jenny Freeman for doing my work. I would also like to take time to acknowledge the research achievements of Meredith Tsue, Dieldrich Bermudez, Leah Garcés, Kristine Owen, Hilary Hullard, Shiera Gilbert, Marc Inglese, and Kimara March. I feel immensely fortunate to have been a small part of their accomplishments. iv
5 TABLE OF CONTENTS page ACKNOWLEDGMENTS...iii LIST OF TABLES...viii LIST OF FIGURES...ix ABSTRACT...xii CHAPTER 1 BIOTIC AND ABIOTIC FACTORS IN ENDOCRINE AND IMMUNE FUNCTION: THE CHALLENGE OF A MODERN ENVIRONMENT...1 Overview...1 Biotic and Abiotic Factors and the Reptilian Endocrine System...2 The Reptilian Endocrine System Reproduction and Stress...2 Anthropogenic Factors: Endocrine Disrupting Contaminants (EDCs)...4 Biotic an Abiotic Factors and Reptilian Immunity...6 The Reptilian Immune system...6 Anthropogenic Factors: Immunotoxins...7 Endocrine Effects on the Immune Systems...8 Introduction to Alligator Biology: Why Alligators?...9 The System...11 Rationale and Questions...12 Outline...14 CHAPTER 2 SEASONAL VARIATION IN PLASMA SEX STEROID CONCENTRATIONS IN JUVENILE AMERICAN ALLIGATORS...16 Introduction...16 Methods...21 Animals...21 Radioimmunoassay...23 Analysis...23 Results...24 Juvenile Alligators from Lake Woodruff NWR...24 Females...24 Males...24 Males and Females...25 v
6 Juvenile Alligators from Lake Apopka...26 Females...26 Males...27 Males and Females...28 Comparisons Between Lakes Apopka and Woodruff...28 Female Alligators from Lakes Apopka and Woodruff...28 Male Alligators from Lakes Apopka and Woodruff...28 Discussion...29 CHAPTER 3 THE EFFECT OF CAPTURE STRESS ON PLASMA CORTICOSTERONE AND TESTOSTERONE IN JUVENILE ALLIGATORS FROM TWO LAKES IN FLORIDA...46 Introduction...46 Methods...49 Animals...49 Radioimmunoassay and Analysis...51 Results...52 Wild Population...52 Captive Population...53 Comparison Between Wild and Captive Alligators...54 Discussion...55 CHAPTER 4 POPULATION VARIATION IN IMMUNITY THYMUS AND SPLEEN OF JUVENILE AMERICAN ALLIGATORS...64 Introduction...64 Methods...71 Animals...71 Wild Alligators Used in Histological Comparison...71 Captive Alligators Used in Histological Comparison...72 Captive Alligators Used in Mitogen-induced Blastic Transformation Assay...73 Captive Alligators Used in Phagocytic Assay...73 Histology...74 Analysis...74 Thymus...74 Spleen...75 Radioimmunoassays...77 Mitogen-induced Blastic Transformation of Lymphocytes...78 Phagocytosis Assay...79 Data Analysis...79 Results...80 Size and Sex-steroid Effects on Immune Parameters...81 Thymus...82 Spleen...82 vi
7 Mitogen-induced Blastic Transformation of Lymphocytes...83 Phagocytosis Assay...84 Discussion...84 CHAPTER 5 DEVELOPMENTAL EFFECTS OF CONTAMINANTS ON IMMUNE TISSUE: THYMUS AND SPLEEN OF AMERICAN ALLIGATORS Introduction Methods Histology Analysis Thymus Spleen Data Analysis Results Dose, Allometric, Sex and Clutch Effects Thymus Spleen Discussion CHAPTER 6 SUMMARY AND CONCLUSIONS Suggestions LIST OF REFERENCES BIOGRAPHICAL SKETCH vii
8 LIST OF TABLES Table page 4.1: Plasma testosterone concentration in juvenile alligators from each study lake : Treatment regime for contaminant exposure of alligator eggs viii
9 LIST OF FIGURES Figure page 2.1: Air and water temperature (August, 1997 July, 1998) for Lake Woodruff and Lake Apopka : Plasma estradiol-17β concentrations in female juvenile alligators from Lake Woodruff during the months of the study : Plasma testosterone concentrations in female juvenile alligators from Lake Woodruff during the months of the study : Plasma estradiol-17β concentrations in male juvenile alligators from Lake Woodruff during the months of the study : Plasma testosterone (T) concentrations for individual male alligators from Lake Woodruff. Plasma T is plotted relative to snout-vent length : Plasma testosterone concentrations in male juvenile alligators from Lake Woodruff during the months of the study : Plasma estradiol-17β concentrations in male and female juvenile alligators from Lake Woodruff during the months of the study : Plasma testosterone concentrations in male and female juvenile alligators from Lake Woodruff during the months of the study. Asterisk indicates a difference between sexes : Plasma estradiol-17β concentrations in female juvenile alligators from Lake Woodruff and Lake Apopka : Plasma testosterone (T) concentrations in female juvenile alligators from Lake Woodruff and Lake Apopka : Plasma estradiol-17β (E2 ) in male juvenile alligators from Lake Woodruff and Lake Apopka : Plasma testosterone (T) in male juvenile alligators from Lake Woodruff and Lake Apopka...45 ix
10 3.1: Corticosterone (B) during capture stress in wild juvenile alligators from Lake Apopka and Lake Woodruff : Basal testosterone concentration in captive and the wild juvenile alligators : Testosterone (T) during capture stress in wild juvenile alligators from Lake Apopka and Lake Woodruff : Body temperature during capture stress in wild juvenile alligators from Lake Apopka and Lake Woodruff : Corticosterone (B) during capture stress in captive juvenile alligators : Testosterone (T) during capture stress in captive juvenile alligators : Corticosterone (B) during capture stress in captive and wild juvenile alligators Alligator populations in north central Florida : Cross section of the thymus from a juvenile alligator : Red and white pulp in the spleen of a juvenile alligator : A lymphocyte sheath in the spleen of a juvenile alligator : Malpighian bodies in the spleen of a juvenile alligator : The relative ratio of thymic areas in wild juvenile alligators from Lakes Apopka, Woodruff and Orange : Thymic cortex and medullary areas for wild juvenile alligators from Lakes Apopka, Woodruff and Orange : Lymphocyte sheath width in the spleen of captive-raised juvenile female alligators : Malpighian body area in the spleen of wild juvenile alligators from Lakes Apopka, Woodruff and Orange : The relative ratio of thymic areas in captive-raised juvenile alligators : Lymphocyte sheath width in the spleen of captive female alligators : Malpighian body area for captive female alligators : Stimulation of peripheral blood lymphocytes in juvenile alligators with various doses of Con A...99 x
11 4.15: Stimulation of peripheral blood lymphocytes in juvenile alligators with various doses of PHA : Phagocytic response of peripheral leukocytes in juvenile alligators : Cross section of the thymus from a hatchling alligator : Cross section of the spleen from a hatchling alligator : Treatment-induced reversal in alligator development : The relative ratio of thymic areas in hatchling alligators : Temperature effect on thymus development in alligators : Lymphocyte sheath width in the spleen of hatchling alligators xi
12 Abstract of Dissertation Presented to the Graduate School of the University of Florida in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy VARIATION IN THE ENDOCRINE AND IMMUNE SYSTEM OF JUVENILE ALLIGATORS: ENVIRONMENTAL INFLUENCE ON PHYSIOLOGY Chairman: Dr. Louis J. Guillette, Jr. Major Department: Zoology By Andrew A. Rooney December 1998 The influence of the environment on the endocrine and immune systems has become an extreme example of the extent of human influence and human potential to disrupt biological systems. Many natural aspects of the environment (i.e., temperature, food availability, and predator interactions) modify both the endocrine and immune systems. Exposure to xenobiotic chemicals can also modify endocrine and immune parameters, especially when exposure occurs during embryonic development. Furthermore, the endocrine system is an important regulator of the immune system. Therefore, the interactions between exposure to xenobiotics in wild populations of alligators and the following variables were considered: sex hormones and seasonal variation in sex hormones, stress response, morphology of the spleen and thymus, mitogenic response, and phagocytic response. xii
13 Seasonal variation in sex steroids was found in juvenile alligators. Plasma testosterone concentrations differed in contaminant-associated alligators relative to alligators from reference lake and the difference was influenced by the season. Contaminant-associated alligators also had a modified plasma corticosterone profile during capture stress; and, this modified response appears to be from an activational influence rather than a developmental effect. Several differences were also noted in immune parameters associated with the contaminated alligator population: an increase in the cortical region of the thymus, a reduction in lymphocyte presence in the spleen, and a greater proliferation response of lymphocytes. Alligators from the reference lake had sexually dimorphic characteristics in the spleen; no such difference was present in contaminant-associated alligators. Additionally, a morphological effect on the spleen and thymus was found in eggs treated with trans-nonachlor, a contaminant present in higher concentrations at the contaminated study site. In conclusion, all organisms, including the American alligator live in a dynamic environment. Changing biotic and abiotic factors including anthropogenic changes such as the presence of contaminants must be transduced by the endocrine and immune systems of an organism to enable homeostatic mechanisms. This research provides evidence of additional possible effects of contaminant exposure to the immune system and the endocrine-immune systems of wild, juvenile alligators. The data are particularly important because they suggest seasonality in reproductive steroidogenesis of wild, juvenile alligators. xiii
14 CHAPTER 1 BIOTIC AND ABIOTIC FACTORS IN ENDOCRINE AND IMMUNE FUNCTION: THE CHALLENGE OF A MODERN ENVIRONMENT Overview All animals integrate with their surroundings by responding to fluctuating natural environmental conditions via physiological and behavioral adaptations. In vertebrates, this integration involves both the endocrine and immune systems mediating environmental flux with homeostatic set points. In addition to natural environmental conditions, the modern environment also includes anthropogenic compounds that have been shown to alter the development and regulation of the endocrine system (reviewed in Gray et al., 1996; Guillette et al., 1996a; Golden et al., 1998). This dissertation focuses on evidence that anthropogenic compounds can alter the endocrine and immune systems. Important consideration is given to natural variation in the endocrine system including evidence of a potential seasonal cycle in sex steroids in juvenile alligators. It also discusses the modification of the stress response as regulated by the endocrine system, and the morphological alterations of important immune system tissue in alligators exposed to a polluted environment. Furthermore, it suggests research questions regarding the potential disruption of the known cross talk between the endocrine and immune systems. 1
15 2 Biotic and Abiotic Factors and the Reptilian Endocrine System The Reptilian Endocrine System Reproduction and Stress Normal development and activity of the reproductive system in reptiles, as in other vertebrates, is controlled by the neuroendocrine system (Licht, 1984). The nervous system receives input from biotic and abiotic environmental factors. These inputs are translated into changes in hypothalamic secretion of gonadotropin releasing hormone (GnRH). In reptiles, GnRH stimulates secretion of the gonadotropins: follicle stimulating hormone (FSH) and luteinizing hormone (LH). Although squamates appear to possess a single FSH-like gonadotropin, all other reptiles possess both gonadotropins (Ishii, 1991). Reptilian gonads are stimulated to undergo gametogenesis by FSH and LH induces steroidogenesis. The principal steroid secreted by the testes of most reptiles is testosterone, with dihydrotestosterone being secreted in lesser amounts (Bradshaw et al., 1988). The ovary synthesizes both estradiol-17β (E 2 ) and progesterone, with secretory rates changing in relation to reproductive status. These gonadal hormones are essential for maturation and seasonal maintenance of the reproductive tracts, as well as secondary sexual characteristics and reproductive behavior in reptiles (Whittier & Crews, 1987; Jones & Baxter, 1991; Moore & Lindzey, 1992). The endocrine system of reptiles responds to both biotic (e.g., nutrition, age, sex, and social interactions) and abiotic (e.g., temperature, photoperiod, rainfall, and ph) factors. To illustrate, female reptiles often reproduce in a biennial or triennial reproductive cycle, and somatic condition (a measure of health based on body mass) has been shown to be a primary regulator of that cycle (Whittier, 1994). Other important biotic factors include age and sex. For example, mean plasma testosterone of adult male
16 3 alligators in Louisiana is between 35,000 and 50,000 pg/ml in April and May (data estimated from graph in Lance, 1989), whereas it is between 20 and 3000 pg/ml in juvenile males (pers. obs.). Plasma testosterone in adult female alligators was between 300 and 1500 pg/ml over the same months (Lance, 1989), a concentration similar to that of juvenile males. Abiotic factors also effect the endocrine system. Sex steroids in adult animals fluctuate in relation to the reproductive cycle, and that cycle can be affected by a variety of abiotic environmental factors. The hormonal changes in seasonally breeding reptiles are generally coupled to changes in temperature or photoperiod (reviewed in Licht, 1984; Whittier & Crews, 1987). Seasonal rainfall patterns have also been linked to changes in the endocrine system of several reptiles, including crocodilians (Grahme et al., 1977). In addition to responding to environmental variables that control reproductive events, reptiles must maintain the appropriate physiological state in response to a changing environment. An understanding of the stress response is essential to the appreciation of the complex endocrine mechanisms maintaining that physiological state. Extreme environmental stimuli, or stressors, reaching the nervous system are translated into changes in hypothalamic secretion of corticotropin-releasing hormone (CRH). CRH elicits the release of adrenocorticotropin (ACTH) from the pituitary. ACTH then stimulates the synthesis and release of corticosteroids from the adrenal gland. Increases in plasma corticosteroids in the acute stress response can be detected in minutes, suggesting a CRH response within seconds. Stressors have been shown to stimulate the secretion of corticosterone from the adrenal gland in all reptiles studied to date (Guillette et al., 1995b), suggesting that corticosterone secretion is essential for the appropriate
17 4 response to extreme environmental stimuli in reptiles. Corticosterone, in turn, is correlated with decreased growth, reduced or delayed reproduction, and impaired immunity (Guillette et al., 1995b). For example, captive alligators maintained at high stocking density exhibit reduced growth and reduced fecundity (Elsey et al., 1990a; Elsey et al., 1990b). Anthropogenic Factors: Endocrine Disrupting Contaminants (EDCs) The enhanced vulnerability during embryonic development can be explained by a fundamental difference in the potential effects of EDCs depending on the developmental stage at which they are encountered (Guillette et al., 1995a). As originally defined, organizational effects are permanent changes to structure or function that occur early in development and activational effects are temporary alteration of the adult (Phoenix et al., 1959). Organizational changes can be induced by exposure to a small amount of EDC and this exposure has permanent effects on the developing neuroendocrine or immune tissue (e.g., embryonic exposure to DES decreased GnRH induced LH secretion in adult rats Faber et al., 1991). Development of the embryo involves organogenesis and other events where the timing of gene expression and the strength of endocrine signals are critically important. Therefore, organizational effects are usually initiated in the developing embryo. In contrast, activational effects are transitory, appear to require larger quantities of EDC, and generally apply to juvenile and adult animals. Although such activational effects are important and in extreme cases can lead to death, organizational effects are on a different scale. Organizational effects of exposure to xenobiotic compounds include abnormalities of the endocrine system with teratogenic effects (e.g., from PCB exposure as seen in turtles, Bergeron et al., 1994), potential for
18 5 increased incidence of reproductive cancer as adults (e.g., from DES exposure in humans and rodents, Bern, 1992) and potential sex-reversal caused by exposure to EDCs that bind to the estrogen receptor (Guillette et al., 1995a; Crain & Guillette, 1997; Matter et al., 1998). The major research focus on the effects of EDCs involves two principal factors: steroids and steroid receptors. It should be noted that these are not the only possible EDC mechanisms (see Crain & Guillette, 1997 for discussion of additional factors). Steroids are one major group of endocrine system messengers. These hydrophobic molecules pass through the cell and nuclear membranes, bind to nuclear steroid receptors, and exert effects by initiating transcription through steroid-response elements on the DNA. EDCs modify normal steroid action in several ways: 1) by modifying steroid levels through changes in steroidogenesis or steroid degradation; 2) by direct binding to steroid receptors to either inappropriately activate or prevent activation of the receptor; and 3) by modifying accessory factors (e.g., heat shock proteins, etc.) that would indirectly modify steroid-receptor interactions. Inappropriately large endocrine signals such as exposure of an embryo to adult-like levels of estrogens, such as diethylstilbestrol (DES), can have long-term consequences. These consequences include increased incidence of reproductive tract cancers in females and increased testicular abnormalities and cancer in males (reviewed in McLachlan & Arnold, 1996; Toppari et al., 1996). Although most EDCs have much lower affinity for receptors than natural steroids, they are resistant to degradation. Thus, they can remain associated with steroid receptors for considerably longer than natural steroids, a factor that could contribute to a large effect from small quantities of these EDC compounds (Guillette et al., 1996a). Alternatively, exposure to
19 6 EDCs can occupy a receptor and block normal steroid-receptor interactions, and thereby prevent the normal timing of steroid-receptor interactions. The most obvious effects of exposure to xenobiotics include death, but the subtle effects include permanent modification of the reproductive system. The range of altered reproductive events includes modified steroid production, altered gonad anatomy and ultimately decreased reproductive output (Brouwer et al., 1995; Crain & Guillette, 1997). Biotic an Abiotic Factors and Reptilian Immunity The Reptilian Immune system The immune system of all vertebrates shares some features including the possession of lymphocytes and the ability to synthesize immunoglobulins (Saad, 1988). The major immune organs of reptiles include the thymus, spleen, mucus-associated lymphoid tissue, and bone marrow (Zapata et al., 1992). Reptilian lymphocytes can be separated in to B- and T-lymphocyte populations (El Ridi et al., 1988). Humoral immune response is well developed (Saad et al., 1992b) and reptiles secrete at least two classes of immunoglobulin (Ig). The high molecular weight Ig is related to mammalian IgM, and a second lower molecular weight Ig is more distantly related, and therefore called IgY (Zapata et al., 1992). Reptiles also posses a major histocompatability complex (MHC), based on functional studies such as allograft rejection and mixed lymphocyte reaction (Farag & El Ridi, 1990). Few specific cell-surface antigens have been detected in reptiles that would allow the study of specific populations of lymphocytes. However, mitogens that are B- and T-lymphocyte specific in mammals and birds have been used successfully in reptilian mitogen-induced blastic transformation assays (El Deeb & Saad, 1987; Saad
20 7 & Shoukrey, 1988). Evidence of lymphokines is also limited in reptiles, although a snake interleukin 2 has been demonstrated (El Ridi et al., 1987). Like the endocrine system, the immune system of reptiles responds to changes in both biotic and abiotic environmental factors. Seasonal involution of the thymus, and depletion of lymphoid cells in the spleen, exhibit well established patterns in a variety of reptiles (reviewed in Zapata et al., 1992). The trend is for transient regression in lymphoid tissue during two separate times of the year the breeding period and winter. Typically, lymphoid regression is accompanied by impaired functional immune response (reviewed in Zapata et al., 1992; Guillette et al., 1995b). For example, during winter, the immune system of the lizard, Chalcides ocellatus, is characterized by involution of the thymus and spleen, impaired skin allograft rejection, impaired mixed leukocyte reaction, and impaired mitogen-induced blastic transformation (Saad & El Ridi, 1984; Saad et al., 1984a). Anthropogenic Factors: Immunotoxins Many drugs and chemicals significantly decrease the ability of an animal to resist infectious disease by suppressing some aspect of the immune system (Dean et al., 1982). Additionally, a number of environmentally-relevant chemicals have been implicated in immune suppression in wildlife and humans (Luster et al., 1992). The mechanisms of chemical-induced immune suppression appear to be varied, and it is probable that compounds have multiple immunosuppressive effects due to multiple target sites or methods of action (Luster et al., 1992). Preliminary evaluation of chemical-induced immunotoxicity should begin with an evaluation of immunopathology, followed by investigation of functional changes in humoral, cellular, or nonspecific immunity
21 8 (Krzystyniak et al., 1995). Consideration of indirect immunosuppression through modification of the gonadal or adrenal function should also be considered. This indirect suppression of the immune system is especially well documented in cases of increased adrenal secretion of glucocorticoids and subsequent lymphoid depletion (Pruett et al., 1993). Endocrine Effects on the Immune Systems The integration of the neuroendocrine and immune systems has been well established in all vertebrates and creates additional complexity in the study of either system (reviewed in Selye, 1973; Zapata et al., 1992; Besedovsky & del Rey, 1996; Balm, 1997). In reptiles, as in other vertebrates, testosterone is a well-established immunosuppressant. An inverse correlation between endogenous testosterone concentrations and robustness of the plaque-forming and rosette-forming cell responses was found in C. ocellatus (Saad et al., 1992a). Female Psammophis sibilans, a snake, demonstrated elevated immune responsiveness compared to males in several functional measures including higher proliferative responses to several mitogens and increased rosette-forming cell production, suggesting a role for sex steroids in either immune suppression or enhancement (Saad & Shoukrey, 1988). There is also evidence of glucocorticoid-induced depression in a variety of immune measures and in several reptilian species (reviewed in Saad, 1988). For example, injections of hydrocortisone acetate caused thymus involution and spleen suppression in the lizard Chalcides ocellatus (Saad et al., 1984a; Saad et al., 1984b; Saad et al., 1986). Endogenous corticosteroid concentrations have also been linked to immunity in C. ocellatus. High endogenous concentrations of corticosterone were coincident with periods of depleted lymphoid
22 9 elements and reduced immune reactivity. Furthermore, inhibition of corticosteroid synthesis with metyrapone simultaneously decreased circulating corticosterone concentrations and increased morphological and functional immune parameters (Saad & El Ridi, 1988). Introduction to Alligator Biology: Why Alligators? Alligators are profoundly influenced by their surroundings, by virtue of their reliance on environmental factors for both temperature regulation and sex determination. The most critical period of environmental influence is certainly the embryonic period when conditions experienced by the egg have ramifications as permanent and profound as sex determination. In alligators, the sex of developing embryos is not predetermined genetically; instead, sex is determined by the incubation temperature experienced during a critical developmental window or temperature-sensitive period (Pieau et al., 1994). Both low and high incubation temperatures generate female crocodilians, whereas intermediate temperatures produce males (Lang & Andrews, 1994). Temperatures experienced during early development also effect growth, onset of reproductive activity, and clutch-size (Allsteadt & Lang, 1995; Lang, 1998). A common feature of animals with temperaturedependent sex determination (TSD) is the vulnerability of developing embryos to sexreversal (male to female) by exposure to natural steroids or steroid mimics (Guillette & Crain, 1996). For example, alligators incubated at a male-inducing temperature become female when exposed to estrogenic substances such as estradiol-17β (Bull et al., 1988), tamoxifen, etc. (Crain et al., 1997; Matter et al., 1998). The ecology of alligators as top predators in aquatic environments increases their likelihood of exposure to EDCs. Many EDCs are lipid soluble and environmentally
23 10 persistent; therefore, they accumulate in fat and are biomagnified in animals at higher trophic levels (Oehme et al., 1996). Furthermore, the tropical and semi-tropical wetlands that alligators inhabit are watersheds that often receive primary drainage from cities and intensive agricultural activity (e.g., Domagalski, 1996; Miles & Pfeuffer, 1997; Johnson et al., 1998). The effect of exogenous compounds on alligators is particularly significant due to this prevalence of EDCs in aquatic ecosystems and the number of EDCs that interact with steroid receptors (reviewed in Rooney & Guillette, 1999). Therefore, the combined influence of biomagnification and the vulnerability of embryos to chemical sex-reversal suggest that alligators can function as monitors of environmental contaminants in areas where they occur. In addition to their potential value as an indicator species, alligator populations show several characteristics beneficial to research on the effects of EDCs. First, some alligator populations have already been shown to be highly contaminated with EDCs (Woodward et al., 1993; Guillette et al., 1994; Guillette et al., 1995c; Guillette et al., 1996b; Cobb et al., 1997; Crain & Guillette, 1997; Jagoe et al., 1998). Second, oviparous animals present an excellent system in which to study organizational effects of EDCs due to the ease of treating individual eggs during development. Alligators that have been exposed to EDCs in ovo show organizational endocrine effects and sex reversal (e.g., Crain et al., 1997). Third, alligators have large clutch sizes (37-44 eggs depending on age of female; Ferguson, 1985) allowing within and between clutch comparisons to control for the effects of genetic variation in a genetically diverse population. Fourth, TSD itself allows the sex of offspring to be controlled experimentally. Lastly, the ability
24 11 to study alligators in both wild and captive settings enhances the breadth and relevance of these studies. The System It is essential to understand normal variation in hormones to evaluate potential adverse consequences of altered hormone concentrations associated with exposure to EDCs. Steroid hormones exhibit ontogenetic, sex, and seasonal variation. For example, E 2 concentration in alligators increases from pg/ml in a hatchling to pg/ml in adult females (Crain et al., 1997; Guillette et al., 1997d). Sex steroid concentrations have major implications for reproduction, behavior, and immune function in adults. The increase in plasma E 2 in adult female alligators from 200 pg/ml in February to pg/ml during March-April has both a seasonal component and a functional association with vitellogenesis (Guillette et al., 1997d). Male lizards implanted with testosterone experience higher levels of aggression and parasitic load as well as a decreased number of circulating white blood cells (Salvador et al., 1996). There are also critical growth-associated changes in sex steroids necessary for proper reproductive development and the development and regulation of the immune system. Juvenile alligators from Lake Apopka, Florida, display altered hormone concentrations, particularly the sex steroids estradiol and testosterone (Guillette et al., 1996b; Guillette et al., 1997c; Crain et al., 1998a). The modified sex steroid concentrations of juvenile alligators from Lake Apopka have been linked to a major pesticide spill and increased concentrations of EDCs in alligators from Lake Apopka compared to alligators from Lake
25 12 Woodruff National Wildlife Refuge, a healthy population of alligators (Woodward et al., 1993; Guillette et al., 1999a). Rationale and Questions The potential for sex, season, and stress effects on sex steroids suggested a series of interesting questions that could be addressed using the contaminant-associated alligators and a reference population of alligators. Each of the following questions is designed to explore some aspect of the hypothesis that sex steroids are altered in the juvenile alligators from Lake Apopka and that the difference is associated with exposure to EDCs. Studies demonstrating contaminant-associated differences in testosterone concentrations in wild, juvenile alligators from Lake Apopka, were performed on animals collected in the spring (April and May; Guillette et al., 1996b; Guillette et al., 1997c). There is significant variation in the magnitude of the difference in testosterone concentration between the contaminant-associated alligators and alligators from a reference lake among previous studies (Guillette et al., 1999b). A further understanding of the temporal variation in sex steroid concentrations of juvenile alligators is required to fully interpret the effect of contaminants on sex steroids in juveniles. Although many species of adult vertebrates have demonstrated seasonal cycles in sex steroids (van Tienhoven, 1983; Licht, 1984), no study has examined seasonal sex steroids patterns in a non-mammalian juvenile. Therefore, my question was: 1) do juvenile alligators exhibit a seasonal pattern in sex steroid concentrations; and, if so 2) what is the pattern?
26 13 Furthermore, if this pattern were present, 3) does the seasonal pattern affect the magnitude of contaminant-associated differences in sex steroid concentrations? Several lines of evidence suggest that the altered sex steroid concentrations in juvenile alligators from Lake Apopka are the result of an organizational modification of the gonad or gonadal regulation. One such line of evidence is the ability to produce similar hormonal abnormalities in hatchlings by treating normal eggs with contaminants present at elevated concentrations in alligators living in Lake Apopka (Crain et al., 1997). The persistence of abnormalities in captive alligators raised without EDC exposure from eggs collected on Lake Apopka presents additional evidence of an organizational cause of the altered steroid concentrations (Guillette et al., 1994; Guillette et al., 1995c). Given the apparent organizational modifications in the gonad of alligators from Lake Apopka, the similarity in the organization and regulation of the gonad and adrenal suggests potential organizational modification of the adrenal gland. Therefore, my next questions were: 3) do alligators from the contaminated lake exhibit an altered response to extended capture and confinement stress; and if the stress response is abnormal 4) is the difference a result of organizational changes? Additional endpoints to measure EDC exposure are suggested by the ability of sex steroids (especially testosterone) to regulate immune tissue and immune function. Previous studies of alligators from Lake Apopka have revealed abnormal testosterone concentrations (Guillette et al., 1994; Guillette et al., 1995c; Guillette et al., 1996b; Guillette et al., 1997c; Crain et al., 1998a). My specific question, based on the importance of testosterone to immune function, was: 5) is the lymphoid tissue altered in Lake Apopka alligators?
27 14 Finally, the exposure of the immune system to EDCs can cause organizational or activational changes. My final question relates to the potential organizational effects of chemical exposure: 6) given the endocrine disrupting potential of contaminants present at elevated concentrations on Lake Apopka, could these compounds modify the development of lymphoid tissue in alligator eggs from a unpolluted lake? Outline To more fully understand the ability of an alligator s endocrine and immune systems to interact with the environment, Chapter 2: Seasonal Variation in Plasma Sex Steroid Concentrations in Juvenile American Alligators examined seasonal variation in plasma sex steroid concentrations in juvenile alligators from a reference population. The relevance of seasonal variation in sex steroid concentrations to previous contaminantassociated sex steroid abnormalities was also examined. Comparisons of plasma sex steroid concentrations during the spring and summer were then made between animals from a contaminated lake, Lake Apopka, and the reference lake. To examine potential developmental effects of contaminants on the hypothalamicpituitary-adrenal axis, Chapter 3: The Effect of Capture and Confinement Stress on Plasma Corticosterone and Testosterone in Juvenile Alligators from Two Lakes in Florida examines plasma corticosterone (B) and testosterone (T) during 20 hours of capture and confinement stress. First, capture and confinement stress was examined in wild alligators from Lake Apopka and Lake Woodruff. Then, to examine the possibility of an organizational modification of the stress response, eggs were obtained from both lakes, incubated to hatching, and raised to the juvenile stage in captivity. The identical
28 15 capture and confinement stress experiment was performed on the captive-raised alligators. The potential effect of abnormal sex steroid concentrations on immune tissue in wild juvenile alligators was investigated in Chapter 4: Population Variation in Immunity Thymus and Spleen of Juvenile American Alligators. Lymphoid tissue of alligators from Lake Apopka and two reference lakes were examined. To further examine the functional significance of these potential differences, a blastogenic-stimulation assay and a phagocytic test were performed. To understand the potential developmental effects of contaminants present in elevated concentrations in Lake Apopka, Chapter 5: Developmental Effects on Immune Tissue Spleen and Thymus of American Alligators examines the effect of embryonic exposure to various contaminants on the development of the spleen and thymus. Finally, Chapter 6 Summary and Conclusions presents general conclusions of this work along with the relationships of the various chapters. Future research suggestions designed to answer questions resulting from this research are then made. Each chapter was written for publication and will be submitted to the following journals: Chapter 2 General and Comparative Endocrinology; Chapter 3 Comparative Biochemistry and Physiology A: Comparative physiology; Chapter 4 Developmental and Comparative Immunology; Chapter 5 Environmental Health Perspectives. Although the chapters are integrated pieces of the dissertation, there is some repetition in areas of the methods because they are also designed to stand alone for publication.
29 CHAPTER 2 SEASONAL VARIATION IN PLASMA SEX STEROID CONCENTRATIONS IN JUVENILE AMERICAN ALLIGATORS Introduction Seasonal patterns in sex steroids have been demonstrated for a wide variety of non-mammalian species including, fish (Callard et al., 1991; Snelson et al., 1997), amphibians, and reptiles (Licht, 1984). Among the reptiles, clear annual cycles have been reported for squamates (Whittier, 1994), turtles (Callard et al., 1978), tuatara (Cree et al., 1992), and crocodilians (Lance, 1989; Guillette et al., 1997d). Sex steroid periodicity has been principally linked to seasonal breeding events in sexually mature individuals. Most reptiles, and all crocodilians, experience annual periods of reproductive activity punctuated by quiescence or reproductive inactivity (Licht, 1984). Cyclical patterns in reproductive activity and reproductive hormones has been assumed to imply sexual maturity. However, in species exhibiting long lives, individuals could require several years to reach sexual maturity, as occurs in many primates (Adams & Steiner, 1988; Plant, 1988). During this period, gonadal steroidogenesis increases with concomitant increases in plasma sex steroid concentrations. Elevations in sex steroids are involved in many aspects of homeostasis that are only peripherally related to reproduction, including: growth, stress, and immune regulation (Norris, 1997). Among crocodilians, seasonal cycles in sex steroids have been demonstrated in alligators and caimans. The difficulty in maintaining breeding crocodilians in captivity 16
30 17 and the deleterious effects of restraint on sex steroid concentrations and breeding success has limited our knowledge of most species (Lance, 1989). The reproductive cycle for the American alligator (Alligator mississippiensis) was first published in late 1980s (Lance & Vliet, 1987; Lance, 1989). Lance presented extensive anatomical and histological data on adult male and female alligators from southern Louisiana. The hormonal data on males presented was limited to plasma testosterone (T) concentrations from March through November, with a single sample taken in February. Measurements of plasma T and estrogens were restricted to five months (March through July) in adult females. These data indicated that reproductive activity was maximal during the late spring, a period when elevated steroid concentrations were observed. Recently, Guillette et al. (Guillette et al., 1997d) examined the reproductive cycle of adult female alligators in central Florida. This detailed endocrinological study demonstrated that females exhibit elevated ovarian steroidogenesis in September and October with associated hepatic vitellogenin synthesis. Ovarian and hepatic activity is suppressed during the winter months but resumes rapidly with significant increases in plasma estradiol-17β (E 2 ) and vitellogenin in spring prior to ovulation in May or June. For the months in common, the two published hormonal cycles for female plasma E 2 and testosterone patterns are similar. No studies have systematically examined seasonal variation in plasma sex steroids in juvenile alligators or other crocodilians to our knowledge. However, data from several different studies examining plasma T concentrations in juvenile alligators of similar size from several lakes in central Florida suggest that such a seasonal pattern could exist (Guillette et al., 1999b).
31 18 The documentation of season patterns in sex steroids is important for reasons other than reproduction, as noted above. For example, there are significant seasonal patterns of disease and death among many animal populations (Nelson et al., 1995). Generally, wild animals exhibit compromised immune function and decreased immune tissue size in fall and winter (Nelson & Demas, 1997). Gonadal steroids have been implicated in the well documented sexual dimorphism in immune function and incidence of autoimmune diseases in rodents and humans (Olsen & Kovacs, 1996). Both E 2 and T have immune suppressive effects and cause thymic involution in mammals (Olsen & Kovacs, 1996). Testosterone has also been implicated in thymic involution and lymphopenia in the spleen of reptiles (Saad et al., 1990; Saad et al., 1991). Receptormediated testosterone-induced immune suppression of circulating leukocytes has been reported in fish (Slater & Schreck, 1997). Not all effects of sex steroids on immune function are detrimental. Testosterone also has been linked to the beneficial effect of enhanced cytotoxic/suppressor T cell action; and, estradiol increases helper T cell populations in mammals (Olsen & Kovacs, 1996). Furthermore, temperate reptiles exhibit seasonal changes that can be quite dramatic, especially in response to varying ambient temperature. Given the observations reported above, sex steroids were examined to determine if seasonal variations in sex steroids occurred in juveniles. The study of sex steroid concentrations in juveniles is complicated by the potential interaction between a seasonal pattern and an ontogenetic pattern in steroid production. Hatchling alligators have plasma T concentrations of pg/ml and E 2 concentrations of pg/ml (Guillette, unpub. data). Adult male alligators have plasma T concentrations of 2,000 50,000 ng/ml (Lance, 1989). Adult female alligators
32 19 have plasma E 2 concentrations of pg/ml (Guillette et al., 1997d). As juvenile alligators grow and mature, it is not known how the plasma steroid concentrations increase to adult levels. Sex steroid concentrations could increase dramatically in a brief period of puberty. Alternatively, sex steroid concentrations could increase slowly during a prolonged pubescent period. Any seasonal pattern in sex steroid concentrations would be superimposed on these potential patterns of hormonal increase, making the determination of a seasonal pattern very difficult. The ideal data to answer these questions would be data that tracked hormone concentrations of individual juvenile alligators from month to month over the entire juvenile period (10 15 years). It is unrealistic to expect to obtain this data in wild alligators, but observations can be made that begin to address the question of seasonal variation in sex steroids in juvenile alligators. Animals in the following study were sampled over a 12 month period, and mean concentrations of sex steroids from each sampling period were compared to investigate seasonal variations in sex steroids in juveniles. Furthermore, if a seasonal pattern exists, are plasma sex steroid concentrations changing in juvenile alligators in a pattern that is similar to the reproductive cycle observed in adults? Previous research has shown that neonatal and juvenile alligators from several lakes in central Florida have abnormal sex hormone concentrations when compared in well-controlled sampling studies. Examples include reduced testosterone in captiveraised and wild juvenile male alligators from Lake Apopka relative to males from Lake Woodruff, a National Wildlife Refuge (Guillette et al., 1994; Guillette et al., 1996b; Crain et al., 1997; Guillette et al., 1999a) and Lake Okeechobee (Crain et al., 1998a).
33 20 Plasma E 2 and dehydrotestosterone concentrations were higher in juvenile females from Lake Apopka compared to female alligators of similar size from Lake Woodruff in some studies (Guillette et al., 1994; Pickford, 1995). However, a different study failed to observe a difference in plasma E 2 concentrations in larger juveniles from the two lakes (Guillette et al., 1997c). Lake Apopka is associated with several sources of contamination, including direct agricultural runoff, city effluent and this lake received contaminants following a major pesticide spill of dicofol (contaminated with DDT and its metabolites DDD, DDE and chloro-ddt) and sulfuric acid in 1980 (EPA: Unpublished report). The alligators in this lake have serum contaminant concentrations that are 3 to 4 times those observed in alligators from Lake Woodruff (Guillette et al., 1999a). Many of the contaminants found in higher concentrations in the eggs and serum of Lake Apopka alligators show an affinity for the alligator estrogen receptor (Vonier et al., 1996). Several have been shown to have endocrine disrupting ability (Crain & Guillette, 1997; Matter et al., 1998). The published endocrine abnormalities associated with Lake Apopka alligators principally rely on data from hatchlings and juveniles. In fact, all of the wild alligators in previous studies that compare animals from Lakes Apopka and Woodruff (Guillette et al., 1997c; Guillette et al., 1999a) were juveniles collected in early spring (March and April). In spring, adult alligators from Florida and Louisiana have elevated plasma concentrations of estrogens and testosterone (Lance, 1989; Guillette et al., 1997d). To determine if juvenile alligators exhibited seasonality in sex-hormones, we examined male and female alligators from Lake Woodruff National Wildlife Refuge. Previous data demonstrated a consistent sexual dimorphism during the spring in
34 21 testosterone and estradiol concentrations in juvenile alligators from Lake Woodruff National Wildlife Refuge (Guillette et al., 1996b; Guillette et al., 1997c). We hypothesized that the sexual dimorphism in the sex-hormones would be maintained throughout the year. Finally, juvenile alligators from Lake Woodruff NWR were examined relative to animals from Lake Apopka during the spring and summer months (April through July). As discussed above, we have found EDC associated endocrine abnormalities in Lake Apopka alligators relative to the animals from Lake Woodruff. We hypothesized that lake differences would be maintained in the sex steroid concentrations during the months sampled. Conversely, differences between the lakes could be due to one lake showing seasonality while the other does not. Methods Animals Juvenile American alligators (Alligator mississippiensis) were collected from June, 1997 through July, All animals were captured by hand or noose from an airboat at night. The alligators ranged in size from 56 cm to 172 cm in total length. Air and surface water temperature was recorded each night (Figure 2.1). Animals were collected from Lake Woodruff National Wildlife Refuge, throughout the study. Lake Woodruff was selected as the best site to test for normal seasonal differences in juvenile circulating sex steroids due to previous data that has established Lake Woodruff as a reference site and relatively unpolluted lake (Guillette et al., 1999a). Animals of similar size were also collected from Lake Apopka, a population of alligators with known reproductive problems and endocrine abnormalities (Woodward et al., 1993; Guillette et
35 22 al., 1994 and above), during April, May, June, and July of These samples were used to compare sex steroids between lakes during several spring and summer (an expected time of hormonal change and a time period during which many previous field studies have been performed). Abnormal endocrine parameters have been observed in juvenile alligators from Lake Apopka and have been hypothesized to be due to embryonic or neonatal exposure to endocrine disrupting contaminants (EDCs): sources of EDCs include city effluent, agricultural run off, and a major pesticide spill in Recent data suggests that phallus size exhibits regional variation on Lake Apopka (Guillette et al., 1996b) as does egg DDT/DDE concentrations (Giroux, 1998). The south end of Lake Apopka, Gourd Neck Spring (GNS), is the area of the lake with the greatest proximity to the site of the 1980 pesticide spill. The GNS area of the lake is also the area with the smallest phallus size in males (Guillette et al., 1996b). Phallus growth is an endocrine-dependent phenomenon, depending on embryonic as well as neonatal and juvenile concentrations of androgens. Thus, this study will consider regional differences within the lake. Following capture, animals were secured and tagged with a unique toe tag. Animals were either placed in cloth bags or immediately bled from the post cranial sinus depending on other experimental constraints. Animals that were not immediately bled were removed from the cloth bags and bled within two hours of capture. We have previously established that sex steroids do not change significantly within two hours of capture using these methodologies (Guillette et al., 1997c). Blood samples were taken with sterile syringes and stored on ice for 2 to 18 hours in Vacutainer tubes containing sodium heparin. Sex, snout-vent length, and total length were recorded from each animal
36 23 after blood was drawn. Sex was determined by manual palpation and extrusion of the phallus (Guillette et al., 1996b). The blood samples were centrifuged at 1800g in a refrigerated centrifuge immediately upon removal from ice; and the resulting plasma was stored at 80 C until radioimmunoassays were performed. Radioimmunoassay Plasma was assayed for estradiol-17β (E 2 ) and testosterone (T) using assays previously validated for juvenile alligators (Crain et al., 1997; Guillette et al., 1997d). All samples were analyzed in duplicate in a single assay. Plasma samples (150 µl for both E 2 and T) were extracted twice with 5 ml of ethyl ether to remove the lipophilic steroids. Extraction efficiency averaged 96 % for T and 94 % for E 2. Interassay and intraassay variances were 19.1 and 3.5 % respectively for the estradiol-17β assay and 14.5 and 3.6 % for the testosterone assay. Transformation of cpm to hormone values was done with a log-linear cubic spline standard curve generated by Microplate Manager 4.0 (Bio- Rad Laboratories Inc., Hercules, CA). Analysis All analyses were performed using Statview 5.0 (SAS Institute Inc., Cary, NC). Homoscedastic data were analyzed with one-factor analysis of variance (ANOVA). Heteroscedastic data was log transformed to achieve homoscedasticity. Data that remained heteroscedastic were analyzed with Kruskal-Wallis non parametric analyses or the Mann Whitney U comparison for paired variables such as sex or a direct comparison between two months.
37 24 Results Juvenile Alligators from Lake Woodruff NWR Juvenile female and male alligators were collected every month except November and February. We were prevented from collecting animals during those months because of weather or elevated water levels on the lake that prevented access to juvenile alligators. The females displayed a mean snout vent length (SVL) of 46 ± 1.1 cm whereas males had a mean SVL of 47 ± 1.5 cm. Females were smaller than males in the month of May (p = ) but there were no differences in the snout-vent-length of alligators by sex for any other months. Within each sex, there was no difference in SVL of alligators by month (females p = ; males p = ). Females Plasma estradiol-17β (E 2 ) or testosterone (T) exhibited no relationship with SVL in juvenile female alligators (r 2 = 0.002, r 2 = 0.001, respectively). Plasma E 2 concentration of female alligators differed by month (p = ; Figure 2.2). Female alligators in December and January had lower plasma E 2 than females from all other months except October and March. Plasma T concentrations differed by month (P = ) with higher plasma T in the fall (August-October; Figure 2.3). Females in April exhibited a large variation in plasma T with a mean concentration of 32 ± 7.7 pg/ml compared to 23 ± 1.7 pg/ml for females in March and May. Males There was no relationship between SVL and plasma E 2 concentrations in male alligators from Lake Woodruff (r 2 = 0.023). Male alligators in July had higher plasma E 2
38 25 than males in January (p = ) and December (p = ; Figure 2.4). Plasma E 2 concentrations did not differ among any other months in male alligators. Previous studies have demonstrated a relationship between SVL and plasma testosterone concentrations, such that animals smaller than 40 cm SVL had very low concentrations. We observed a similar phenomenon, as only males greater than or equal to 38 cm SVL had plasma T concentrations over 50 pg/ml (Figure 2.5). Thus, only the larger juvenile males were used in the monthly comparison of plasma T concentrations because animals smaller than 38 cm had consistently low, basal plasma T concentrations. Males greater than 38 cm SVL exhibited no correlation between SVL and plasma T when animals from all months were examined (r 2 = 0.027). Juvenile male alligators from Lake Woodruff exhibit a pronounced seasonal pattern in plasma T concentrations when examined by monthly sampling (p = ; Figure 2.6). Males from December and January had the lowest concentrations with males from March exhibiting the greatest mean concentration (Figure 2.6). The March sample showed great variation as did samples from August and April, periods of apparent transition in the seasonal pattern. Males and Females Female alligators had higher plasma E 2 concentration than males during the months of August (p = ) and April (p = ; Figure 2.7). In addition, females had significantly higher plasma E 2 concentrations than male alligators (p = ) when comparing all samples, irrespective of month. Due to the size-specific elevation in plasma T in male alligators, only female alligators greater or equal to 38 cm SVL were used to examine the relationship between plasma T in males and females. The huge variation in plasma T had a large effect on the
39 26 statistical determination of sexual dimorphism in this hormone (Figure 2.8). Plasma T concentrations in males were greater than those in females during April (p =0.0104) and July (p = ). However, it is apparent in Figure 2.8 that many males had greatly elevated concentrations of Plasma T compared to females in other months as well. When all months were averaged, males had higher plasma T than females (p = ). Juvenile Alligators from Lake Apopka Females Plasma E 2 concentrations had no relationship to the SVL of female alligators from the north end of Lake Apopka (r 2 = 0.095) whereas females from the Gourd Neck Spring (GNS) area in the south exhibited a positive correlation with SVL (r 2 = 0.321; p = ). Plasma T was positively correlated to SVL of female alligators from the north end of Lake Apopka (r 2 = 0.165; p = ). However, females from GNS had no relationship between plasma T and SVL (r 2 = 0.061). The data from each end of Lake Apopka could not be compared with analysis of co-variance due to the unequal relationship between size and plasma sex steroids for the two regions. However, no difference was found in mean SVL of female alligators by lake region when compared monthly. Therefore, we analyzed the data using an ANOVA. Females from the north end of Lake Apopka had a higher plasma E 2 concentration in May than females in April (p = ) and July (p =0.0003; Figure 4.9). North end females also had a higher plasma concentration of E 2 in June than females in July (p = ). Females from GNS had a higher plasma E 2 concentration in April and May than females in June (p = ; p = ) and July (p =0.0257; p = ). Plasma
40 27 E 2 concentrations were greater in females from GNS than in the north end of Lake Apopka in April (p = ) and July (p = ) with no regional differences observed in May or June (Figure 4.9). No difference among months was found in plasma T concentrations for female alligators by lake region (Figure 4.10). Therefore, all animals were grouped for monthly comparisons; plasma T did not differ by month among juvenile female alligators from Lake Apopka. Males Plasma E 2 concentrations showed no relationship with SVL of male alligators from the north end of Lake Apopka (r 2 = 0.019) whereas males from GNS exhibited a significant positive correlation (r 2 = 0.198; p = ). No difference was found between the SVL of male alligators by lake region when compared monthly. No difference was found in plasma E 2 concentration by area of lake in any month (Figure 4.11). Male alligators (grouped from both ends of Lake Apopka) had smaller plasma E 2 concentrations in July than males in May (p = ). No other month-associated difference in plasma E 2 was found in male alligators. Plasma T was analyzed in male alligators greater than 38 cm SVL. Plasma T concentrations exhibited no relationship to the SVL of male alligators from either end of Lake Apopka (north: r 2 = 0.011; GNS: r 2 = 0.049). No significant variation in monthly concentrations of plasma T were observed (Figure 4.12).
41 28 Males and Females No difference was observed in any month in plasma E 2 or T concentrations when male and female alligators from Lake Apopka were compared. Comparisons Between Lakes Apopka and Woodruff Female Alligators from Lakes Apopka and Woodruff The mean size of female alligators from Lake Woodruff did not differ from that of females from either end of Lake Apopka. No difference in plasma E 2 concentrations was found between female alligators from the two lakes during any month. Plasma T was higher in female alligators from Lake Apopka compared to females from Lake Woodruff during May (p = ), June (p = ) and July (p = ) when alligators from both ends of Lake Apopka were grouped. No difference was found between female alligators from Lakes Apopka and Woodruff during April (p = ). Male Alligators from Lakes Apopka and Woodruff The mean size of male alligators from Lake Woodruff was larger than that of male alligators from Lake Apopka. Therefore, only male alligators cm in SVL from the two lakes were used in the lake comparison. Male alligators from Lake Woodruff had higher plasma T in July than males from Lake Apopka (p = ). No other month displayed a lake associated difference in plasma T of male alligators. However, examination of Figure 4.12 shows that plasma T concentrations in May in both lakes were similar, whereas plasma T in many males from Lake Woodruff in April and from GNS in May were elevated; no significance in the comparisons were noted due to the large degree of variation in the recorded values. Comparison of plasma E 2 between males from Lake
42 29 Woodruff and Lake Apopka demonstrated higher plasma E 2 in males from Lake Apopka during May (p = ; Figure 4.11). Plasma E 2 did not differ among males of the two lakes during any other month. Discussion Male and female juvenile alligators from Lake Woodruff NWR displayed temporal patterns in plasma estradiol-17β (E 2 ) and testosterone (T) concentrations that appear to be seasonal. In contrast to animals observed on Lake Woodruff, a smaller data set suggested that many of the juvenile alligators from Lake Apopka exhibited no spring to summer variation or sexual dimorphism in plasma sex steroids as observed for animals obtained from Lake Woodruff NWR. Further, females from Lake Apopka exhibited elevated plasma T concentrations compared to animals of a similar size from Lake Woodruff. We also observed that males from Lake Woodruff have higher plasma T concentrations than those observed in males from Lake Apopka for several, but not all, of the months examined. From these data, juvenile male alligators appear to fit into 2 categories: 1) neonatal alligators below 38 cm SVL these animals exhibit basal plasma T (below 50 pg/ml; and 2) peripubescent alligators above 38 cm SVL these animals exhibit elevated plasma T. Our data indicate that male alligators begin to exhibit increasing gonadal androgen synthesis upon reaching 38 cm SVL. Not all males of this size have elevated plasma testosterone, but it is not uncommon for individuals of a given population to begin puberty at differing ages or body sizes (van Tienhoven, 1983; Coutinho et al., 1998). Sexual maturity in the American alligator is reported to be based
43 30 principally on body size because growth rates can vary tremendously based on diet and ambient temperature. Sexual maturity in wild populations occurs when the animals are approximately cm SVL or years of age (Joanen & McNease, 1980; Ferguson, 1985), although the age of sexual maturity can apparently be shortened dramatically by accelerating growth under captive conditions that provide optimal temperatures and almost unlimited food (Joanen & McNease, 1989). Our data suggest that testicular activity in males begins years before the animals would be considered sexually mature. Further, it should be noted, that even after attaining sexual maturity at approximately 100 cm SVL, the majority of males are not believed to reproduce until they are 140 cm SVL or more, due to the aggression and territoriality associated with courtship and mating in this species (Joanen & McNease, 1980). No other studies have been identified that have examined seasonal variation in plasma sex steroids in wild, non-mammalian, juvenile animals. The majority of species used in reproductive studies (e.g., rodents, salmon, chickens, sheep) have relatively short lives and thus, puberty is an event that occurs within a few days or months and seldom stretches over a year (van Tienhoven, 1983; Foster, 1988; Ojeda & Urbanski, 1988). Studies of primates indicate that puberty is an event that can be stretched over months or years, but little data are available on seasonal cyclicity of sex steroids in pubescent individuals living under wild conditions (Plant, 1988). Although seasonal activity appears in some species, it has become clear that those species showing a prolonged pubescent period exhibit plasma steroid profiles that reflect gradually increasing gonadal steroidogenesis (Plant, 1988; Lincoln, 1998).
44 31 A non-seasonal explanation of the data must also be considered. The variation in sex steroid concentrations may relate to the interaction of juvenile alligators with environmental variables unrelated to season. For example, the animals could have responded to a change in density of conspecifics, a factor known to influence growth and corticosterone concentration in juvenile alligators (Elsey et al., 1990b). Aggressive behavior in juvenile crocodilians under captive conditions has also been correlated to density as well as to diet (Morpurgo et al., 1993). However, we support a seasonal interpretation of the pattern of sex steroid concentrations observed in juvenile alligators in this study as the most logical interpretation based on comparison of adult patterns. The effect of size on the pattern of testosterone concentrations in juvenile males is almost identical to that reported previously for mature animals (Lance, 1989). Lance (1989) reported work from southwestern Louisiana, USA, and males from that region displayed a significant rise in plasma T concentrations during April and May, with another elevation occurring in the fall months. We have observed that juvenile alligators from Lake Woodruff NWR in central Florida, USA have elevated plasma T concentrations during March and April with a second elevation occurring in late summer. Given the strong photoperiodic and temperature influences on gonadal steroidogenesis reported for reptiles (Licht, 1984), it is not surprising that males from Florida might show a slight advance on the pattern compared to those at the more northern locality in Louisiana. Although the pattern of androgen production is similar, the plasma concentrations reported in the two studies vary greatly juveniles display 1/10 to 1/100 adult concentrations -- as would be expected. Why these juveniles are apparently responding to the same environmental signals as the
45 32 adults is unknown. Furthermore, what role these low, but non-basal levels of testosterone play in juvenile males is not known at this time. It is clear, that larger males have elevated plasma T concentrations and larger phallus size (this study and previous studies from our laboratory: Guillette et al., 1996b; Guillette et al., 1997c). Developmental influences on other reproductive organs as well as augmentation of somatic growth are likely effects. Whether pg/ml concentrations of plasma T, as seen in juvenile males, influence immune function cannot be ruled out. Elevated sex steroid concentrations (ng/ml) clearly alter immune function in adult reptiles (reviewed in Guillette et al., 1995b). Sexual dimorphism in at least one morphological feature of the immune system has been observed in juvenile alligator, with females from Lake Woodruff having larger periarterial lymphoid sheaths compared to males (Chapter 4). Sheath diameter is T-cell dependent, but any possible role of plasma T in this phenomenon is unknown at this time. Low, but detectable concentrations of E 2 also were recorded in males, but little seasonal variation was noted. Our laboratory has previously shown that hatchling male alligators have detectable gonadal aromatase activity and plasma E 2 concentrations, as do juveniles (Crain et al., 1997), an observation not unlike that reported for another crocodilian (Smith & Joss, 1994) and male birds and mammals (Kwon et al., 1995; Hess et al., 1997). Recent studies in mammals and birds suggest that estrogens are essential for normal gonadal and reproductive tract functioning, supporting the observation that males of all ages synthesize estrogens and have detectable estrogen receptors (Lubahn et al., 1993; Hess et al., 1997). Like males, juvenile female alligators from Lake Woodruff NWR displayed a clear temporal pattern; however, in females the major pattern was in plasma
46 33 concentrations of E 2. Unlike males, there appeared to be no obvious size influence on plasma estrogen concentrations. However, it should also be noted, that plasma concentrations of E 2 never were higher than 100 pg/ml, approximately 1/7 to 1/15 the concentration of E 2 found in adult females prior to ovulation (Guillette et al., 1997d). Reproductively active female alligators exhibit a plasma estradiol cycle that begins in fall and culminates with ovulation in early June (Guillette et al., 1997d). Like the pattern presented by the juvenile females, plasma E 2 concentrations in adult females increase dramatically in the spring, with increasing water and air temperatures. With ovulation, plasma E 2 declines sharply in adults, but in juvenile females no decline in plasma concentrations occur until the onset of cooler weather in the fall. The function of plasma E 2 concentrations above basal levels in juvenile females is still poorly understood. As with other studies of pubescent animals, rising levels of plasma sex steroids would initiate maturation and growth of reproductive organs. In adult females, the reproductive tract is partitioned into distinct regions associated with its function in fertilization, albumen, and egg shell secretion (Palmer & Guillette, 1992). At hatching, the oviduct is a thin thread of material, and this structure shows little differentiation. Exogenous administration of E 2 will stimulate an increases in weight of the oviduct (Rooney and Guillette, unpubl. data), but it is not until juvenile females are greater than 80 cm SVL that we have observed oviductal differentiation similar to that observed in non reproductive sexually mature female (Guillette, pers. comm.). This differentiation could occur at smaller sizes, but no data are currently available to clarify this phenomenon. However, differentiation and growth of the oviduct in alligators is clearly estrogen dependent (Forbes, 1940), and seasonal elevations in plasma E 2 are likely to have an
47 34 important role in the normal development of this structure as it does during puberty in other species (Adams & Steiner, 1988). As noted above, the periarterial lymphoid sheaths in juvenile female alligators from Lake Woodruff are larger than those observed in males. Whether this difference is due to androgen suppression or estrogen augmentation is unknown and needs to be examined in further detail. Previous studies examining plasma sex steroid concentrations in alligators have noted a difference in plasma T concentrations when males from Lakes Apopka and Woodruff NWR were compared (Guillette et al., 1996b; Crain et al., 1997; Guillette et al., 1997c). The original research reported that yearling alligators hatched from eggs collected on these two lakes, and raised under identical conditions, displayed different plasma sex steroid concentrations, as well as altered gonadal steroidogenesis in vitro (Guillette et al., 1994; Guillette et al., 1995c). These studies have been the basis for the hypothesis that the endocrine and reproductive abnormalities observed in the alligator population at Lake Apopka are due to embryonic exposure to endocrine disrupting contaminants (Guillette, 1995). A recent re-evaluation of several juvenile studies, has suggested that collection date could influence the magnitude of the differences reported between the lakes (Guillette et al., 1999b). Unfortunately, we could not obtain monthly data for juvenile alligators for Lake Apopka, due in part to the depleted juvenile population on this lake relative to that on Lake Woodruff. However, the data that are available confirm the hypothesis that seasonal variation in plasma sex steroids does influence the magnitude of the difference observed. For example, we noted that as reported previously, plasma T concentrations were statistically higher in males from Lake
48 35 Woodruff NWR for some months, but were not elevated for other months. Interestingly, the lack of a difference is due, in part, to the large variation in plasma T concentrations. Importantly, males from Lake Apopka exhibited no monthly variation in plasma T during the period when samples were obtained, whereas males from Lake Woodruff did. This analysis was performed examining only males larger that 38 cm SVL, or those showing elevated plasma T concentrations in the Lake Woodruff NWR population. One explanation for these observations suggests that animals from Lake Apopka are developmentally delayed and have not begun to exhibit the peripubital rise in plasma T concentrations as observed in animals from Lake Woodruff NWR. Embryonic and neonatal exposure to endocrine disrupting contaminants have been shown to alter the timing of puberty in rodents, and anti-androgenic contaminants have been shown to delay the onset of testicular steroidogenesis and puberty (Gray et al., 1989; Kelce et al., 1995; Gray et al., 1996). For example, the persistent metabolite of DDT, p,p'-dde is a potent anti-androgen in rodents (Kelce et al., 1995). The juvenile alligators from Lake Apopka have ng/ml serum concentrations of p,p'-dde that are 4 to 5 times that observed in juveniles from Lake Woodruff NWR (Guillette et al., 1999a). Alternately, seasonal patterns in sex steroids in reptiles have been shown to be influenced by photoperiod, temperature, or nutrition (Licht, 1984), suggesting that the differences observed here are due to non-synchronous patterns in the animals from the two lakes being compared. As we do not have complete data sets for both lakes, this hypothesis must remain a viable alternative. However, we detected no differences in air or lake water temperatures, and the lakes lie within 30 miles of one another, suggesting that photoperiod is unlikely to be a causal agent in the differences noted. Nutritional differences could influence growth
49 36 and puberty as has been reported for numerous species (van Tienhoven, 1983; Foster, 1988; Ojeda & Urbanski, 1988; Plant, 1988). However, we again have noted no difference in the weight to length parameter of the animals used in the lake comparison. This does not mean that differences in food quality might not occur that influence the onset of puberty, and this possibility must be examined. In addition to reduced plasma T concentrations in males for some months, we also observed that plasma T concentrations were elevated in females from Lake Apopka three out of the four months examined, when compared to females from Lake Woodruff. No difference was seen in plasma E 2 concentrations when juveniles females from the two lakes were compared. Reproductively active female alligators show a dramatic increase in plasma T concentrations during the late vitellogenic phase of the reproductive cycle, associated with a period of intense courtship and mating (Lance, 1989; Guillette et al., 1997d). This elevation in plasma T is coincident with elevated plasma E 2 concentrations in adult females. We did not see this pattern with juveniles and elevated plasma T was only observed in juvenile females from Lake Apopka. Previously, Pickford (Pickford, 1995) reported that juvenile female alligators from Lake Apopka had elevated plasma concentrations of dihydrotestosterone, but not testosterone, in females collected in late April and early May, We found no difference between females from the two lakes in April but a elevated testosterone concentrations in females from Lake Apopka in May, June and July. These data suggest that the females from Lake Apopka are masculinized. The original report on the endocrine abnormalities in yearling alligators from Lake Apopka noted a number of females with enlarged clitero-phalli (Guillette et al., 1994). This has been confirmed in a number of juvenile female alligators from Lake Apopka
50 37 (Guillette, Rooney and Woodward, unpubl. data). Recent examination of hepatic androgen metabolism also supports the hypothesis that females from Lake Apopka are masculinized (LeBlanc and Guillette, unpubl. data). The mechanisms underlying this masculinization are unknown. The presence of contaminants that can act as mixed function steroid receptor agonists/antagonists has been reported to masculinize or feminize vertebrates (Gray et al., 1996). A significant number of the persistent contaminants identified in the serum of alligators show some degree of affinity for the alligator estrogen receptor (Vonier et al., 1996), suggesting that antiestrogenic mechanisms are possible. In fact, recent egg treatment studies suggest that various contaminants can not only alter the sex of developing embryos, but they can also masculinize or feminize gonadal enzymes systems associated with steroidogenesis (Crain et al., 1997; Matter et al., 1998; Guillette et al., in preparation). In conclusion, juvenile alligators of both sexes display seasonal patterns in plasma T and E concentrations. These patterns appear to change with seasonal temperature, rising in spring and declining during the autumn months. Puberty appears to be a multiyear phenomenon in alligators, as in other species with long life spans, such as the great apes. Further studies need to examine other long-lived wildlife species in natural settings. The roles of seasonally changing sex steroids in peripubital juveniles need to be examined in more detail to clarify this potentially important developmental phenomenon. Additionally, we have documented differences in sex steroid concentrations in individuals living in a reference and contaminated lakes. These observations confirm previous studies, but for the first time, we have shown that the magnitude of the difference between the lakes is due, in part, to seasonal cyclicity in plasma sex steroid
51 38 concentrations. Previous studies have been designed to minimize seasonal variation as all samples would be collected on sequential nights or within a week of one another. Our current data suggest that this design is essential but further indicates that the month of sampling will also affect the outcome of studies. We cannot conclude that the animals from Lake Apopka are developmentally delayed, but we saw no evidence of elevated plasma T concentrations in males. Further studies on the responsiveness of the reproductive system in males and females from Lake Apopka to endogenous and exogenous stimulators are needed. Likewise, studies determining the mechanisms by which the alligators on this lake are masculinized or feminized are needed.
52 39 35 Woodruff Air Apopka Air Woodruff Water Apopka Water 30 Temperature ( C) Aug Sep Oct Nov Dec Jan Feb Mar April May Jun Jul Figure 2.1: Air and water temperature (August, 1997 July, 1998) for Lake Woodruff and Lake Apopka. Air and surface water temperature at the time animals were collected and for each month in the study a a Estradiol-17β (pg/ml) a a a, b b b a, b a a 0 Aug 97 Sep 97 Oct 97 Nov 97 Dec 97 Jan 98 Feb 98 Mar 98 April 98 May 98 Jun 98 Jul 98 Figure 2.2: Plasma estradiol-17β concentrations in female juvenile alligators from Lake Woodruff during the months of the study. Shared superscript indicates lack of statistical difference. Each point represents the mean (±1 SE) of 5 to 11 animals.
53 a a,b,d,e Testosterone (pg/ml) b,e a,b,c c,d,e d,e d,e a,b,d,e e d,e 0 Aug 97 Sep 97 Oct 97 Nov 97 Dec 97 Jan 98 Feb 98 Mar 98 April 98 May 98 Jun 98 Jul 98 Figure 2.3: Plasma testosterone concentrations in female juvenile alligators from Lake Woodruff during the months of the study. Shared superscript indicates lack of statistical difference. Each point represents the mean (±1 SE) of 5 to 11 animals Estradiol-17β (pg/ml) a,b a,b a,b a a a,b a,b a,b a,b b 0 Aug 97 Sep 97 Oct 97 Nov 97 Dec 97 Jan 98 Feb 98 Mar 98 April 98 May 98 Jun 98 Jul 98 Figure 2.4: Plasma estradiol-17β concentrations in male juvenile alligators from Lake Woodruff during the months of the study. Shared superscript indicates lack of statistical difference. Each point represents the mean (±1 SE) of 4 to 21 animals.
54 Testosterone (pg/ml Snout Vent Length 50 pg/ml Figure 2.5: Plasma testosterone (T) concentrations for individual male alligators from Lake Woodruff. Plasma T is plotted relative to snout-vent length. The indicated line is at 50 pg/ml a plasma T concentration not achieved by any male below 38 cm in snout-vent length. Some symbols represent more than one animal due to similar plasma concentrations.
55 42 Estradiol-17β (pg/ml) Testosterone (pg/ml) Figure 2.6: Plasma testosterone concentrations in male juvenile alligators from Lake Woodruff during the months of the study. Note the log scale. Shared superscript indicates lack of statistical difference. Each point represents the mean (±1 SE) of 4 to 21 animals Aug 97 a,c Aug 97 Sep 97 a,d Sep 97 Oct 97 a,d Oct 97 Nov 97 Nov 97 Dec 97 b Dec 97 Figure 2.7: Plasma estradiol-17β concentrations in male and female juvenile alligators from Lake Woodruff during the months of the study. Asterisk indicates a difference between sexes. See Figures 2.2 and 2.4 for monthly variation within each sex. Each bar represents the mean (±1 SE) of 4 to 21 animals. b Jan 98 Female Jan 98 Feb 98 Feb 98 Male c,e Mar 98 * * Mar 98 c,e April 98 April 98 b,c,d c,d,e May 98 May 98 Jun 98 Jun 98 e Jul 98 Jul 98
56 43 Female Male Estradiol-17β (pg/ml) Testosterone (pg/ml) Aug 97 Sep 97 Oct 97 Nov 97 Dec 97 Figure 2.8: Plasma testosterone concentrations in male and female juvenile alligators from Lake Woodruff during the months of the study. Asterisk indicates a difference between sexes. Note the log scale. See Figures 2.3 and 2.6 for monthly variation within each sex. Each bar represents the mean (±1 SE) of 4 to 21 animals acg April May June July Figure 2.9: Plasma estradiol-17β concentrations in female juvenile alligators from Lake Woodruff and Lake Apopka. Gourd Neck Spring (GNS) and North Apopka are plotted separately. Each bar represents the mean (±1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses. Jan 98 Feb 98 Mar 98 * * April 98 May 98 Jun 98 GNS Apopka North Apopka Woodruff deh ghjkl ai afj ghj bdeh (5) (6) (7) (4) (3) (4) (6) (8) (3) (9) (5) (7) cdfj ghij bl em Jul 98 hjklm
57 44 Apopka (GNS and North) Woodruff Estradiol-17β (pg/ml) Testosterone (pg/ml) a (11) (7) (7) (5) (14) (3) (16) (9) April May June July Figure 2.10: Plasma testosterone (T) concentrations in female juvenile alligators from Lake Woodruff and Lake Apopka. Animals from Gourd Neck Spring (GNS) and North Apopka are grouped because no regional difference was found in plasma T. Each bar represents the mean (±1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses a,b a,b a,b Apopka (GNS and North) a,b a b b b b April May June July Figure 2.11: Plasma estradiol-17β (E 2 ) in male juvenile alligators from Lake Woodruff and Lake Apopka. Animals from Gourd Neck Spring (GNS) and North Apopka are grouped because no regional difference was found in plasma E 2. Each bar represents the mean (±1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses. a a Woodruff (16) (6) (12) (6) (12) (4) (10) (5) a,b a,b b a,b
58 45 Apopka (GNS and North) Woodruff a,b Testosterone (pg/ml) a a,b a,b (17) (6) (12) (6) (14) (4) (13) (7) April May June July a a,b a b Figure 2.12: Plasma testosterone (T) in male juvenile alligators from Lake Woodruff and Lake Apopka. Animals from Gourd Neck Spring (GNS) and North Apopka are grouped because no regional difference was found in plasma T. Each bar represents the mean (±1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses.
59 CHAPTER 3 THE EFFECT OF CAPTURE STRESS ON PLASMA CORTICOSTERONE AND TESTOSTERONE IN JUVENILE ALLIGATORS FROM TWO LAKES IN FLORIDA Introduction The survival of animals in a constantly changing environment is dependent on an appropriate balance of physiological responses. Reptiles are subjected to a multitude of environmental factors that stimulate hormonal responses. If the environmental conditions exceed an animal s tolerance, they elicit a stress response, defined here as a physiological response to a perceived threat that includes increased adrenal steroid hormone secretion. The stress response is a mechanism essential for the maintenance of proper endocrine and immune responses under severe conditions. Reptiles exhibit a typical vertebrate stress response, largely dependent on a increase in plasma corticosterone concentrations. Corticosterone has extensive effects on metabolism, concentrations of other hormones, and immunocompetence in reptiles (reviewed in Guillette et al., 1995b). In captive juvenile alligators, basal corticosterone was correlated with stocking density and negatively correlated with growth (Elsey et al., 1990b). Stocking density also increased basal corticosterone concentrations in adult alligators with a concomitant reduction in nesting success (Elsey et al., 1990a). Wild alligators appear to exhibit a normal vertebrate acute stress response initiated by capture with a rapid increase in circulating corticosterone and a sex-specific decrease in sex steroids (Lance & Lauren, 1984; Lance & Elsey, 1986). Capture stress in adult wild 46
60 47 alligators is associated with a dramatic increase in circulating corticosterone, with females showing a decrease in plasma estradiol-17β concentrations (Elsey et al., 1991) and males exhibiting a dramatic decrease in plasma testosterone concentrations (Lance & Elsey, 1986). A stress response has been demonstrated following stimulation by a wide variety of factors. These factors can be biotic, such as crowding that increases basal corticosterone concentrations in alligators coincident with decreased growth, or abiotic, such as alterations in temperature or salinity or exposure to toxic chemicals (Guillette et al., 1995b). We are particularly interested in the possibility that the stress response could also be altered by xenobiotic chemical exposure. The original description of the stress response included exposure to noxious chemicals (Selye, 1936). Environmental contaminants can act as stressors, altering circulating concentrations of glucocorticoids (Hontela et al., 1992; McMaster et al., 1994). However, previous research from this laboratory has demonstrated that there was no difference in basal corticosterone concentrations between alligators from a reference or contaminated site (Guillette et al., 1997c). Additionally, there was no effect of the lake of origin on the concentration of plasma corticosterone after two hours of capture stress (Guillette et al., 1997c). In contrast to the very acute (2 hr) stress response, other endocrine parameters have been reported to be altered for alligators living in contaminated lakes (Guillette et al., 1994; Guillette et al., 1996b; Crain et al., 1997). The endocrine and reproductive alterations in alligators from Lake Apopka have been hypothesized to be due to embryonic exposure to endocrine disrupting xenobiotic compounds found at elevated levels in the Lake Apopka environment (Guillette, 1994; Guillette et al., 1996a). Altered
61 48 steroidogenesis and endocrine modifications similar to those observed in Lake Apopka alligators, have been produced in laboratory experiments by treating eggs from a reference lake with known endocrine-disrupting contaminants (EDCs) (e.g., Crain et al., 1997; Matter et al., 1998). These data suggest that the endocrine system of alligators from Lake Apopka could be organizationally modified during development by exposure to EDCs (Guillette et al., 1995a). However, the possibility remains that living in a contaminated environment, such as Lake Apopka, could continually alter the endocrine system to its apparent modified state. Such temporary modification would be considered an activational influence (Guillette et al., 1995a). Given that gonadal steroidogenesis can be modified during development, the potential for altered adrenal gland steroidogenesis was considered. Therefore, we examined the stress response in more detail. A previously reported change in sex steroids in adult alligators took place 24 to 48 hours after the onset of restraint stress (Lance & Elsey, 1986; Elsey et al., 1991). The prevalence of sex steroid differences between Lake Apopka and Woodruff suggested returning to the lakes and repeating the 2 hr study (Guillette et al., 1997c) over a longer time period. Therefore, we examined plasma corticosterone and testosterone concentrations immediately upon capture and after 3, 6, 12, and 20 hours of restraint in male and female juvenile alligators living in either a contaminated lake, Lake Apopka, or a relatively pristine lake, Lake Woodruff National Wildlife Refuge. Additionally, we examined captive-raised alligators to evaluate organizational rather than activational causes for any differences we might observe. The captive alligators were collected as eggs from Lake Apopka and Lake Woodruff and
62 49 incubated under similar conditions in the same incubator. The resulting hatchlings were then raised under identical conditions and tested. Methods Animals Juvenile American alligators (Alligator mississippiensis) were hand captured from an air-boat on two Florida lakes in June, Lake Woodruff was selected as the best site to test for normal stress-induced endocrine changes in juvenile alligators due to previous data that established this lake as a reference site (Guillette et al., 1994; Crain et al., 1998a; Guillette et al., 1999a). Animals were also collected from Lake Apopka, a population of alligators with known endocrine abnormalities as discussed above. Animals were collected from Lake Woodruff, a National Wildlife Refuge, between 8 and 10 PM on June 4, Additional animals were collected from Lake Woodruff between 8 and 10 PM on June 5, 1997, to determine if the hormonal changes observed were associated with capture restraint not with a difference between the 24 hours of the experiment. Alligators were also collected from the south end of Lake Apopka (Gourd Neck Spring) on June 6, 1997 and the north end of Lake Apopka on June 7, 1997, using a protocol identical to the Lake Woodruff collections. The capture and sampling protocol involved the following steps. Immediately following capture (within 30 sec), the alligator s mouth was bound, blood was taken from the post cranial sinus (within 2 minutes of capture), and the time of this initial sampling was recorded. Body temperature was obtained with a cloacal thermometer immediately after each blood sample throughout the experiment. The alligators were then tagged with
63 50 a unique toe tag, placed in a cloth sack and transported to a secure room. At 3, 6, 12, and 20 hours after the initial blood sample was taken, alligators were individually removed from the cloth bags, bled and then returned to their sack. Weight, snout-vent length, total length, sex, and phallus measurements were recorded after the final blood sample was taken. Alligators were sexed by extrusion of the penis by manual palpation and measurements were taken using methods described previously (Guillette et al., 1996b). Blood samples were taken with sterile syringes and stored on ice for 2 to 12 hours in Vacutainer tubes containing sodium heparin. Samples were then centrifuged at 1800g, and the resulting plasma was stored at 80 C until radioimmunoassays were performed. Captive juvenile alligators were also studied under the above protocol. The captive alligators were collected as eggs from both Lake Woodruff and Lake Apopka, and incubated in the laboratory. After hatching, the captive animals were housed in a 20 x 40 foot enclosure with several hiding boxes and a single swimming area under densities much higher than that generally encountered in the wild. The alligator enclosure was cleaned daily, and the captive animals were caught and handled at least twice per week to verify health and check identification tag integrity. The captive animals were fed ad lib commercially available alligator food (Burris Feed, New Orleans, LA). The following differences are identified between the captive and field studies. Animal collection for the captive experiment required walking into the enclosure to grab animals, a fact which could have disturbed animals up to 40 minutes prior to their capture for the experiment. The captive alligators were all of known age, 36 months, whereas the wild alligators were of unknown age (estimated to be between 3 and 6 years; Guillette, pers. com.). The captive alligators were smaller on average, but within the size range of the wild alligators;
64 51 wild alligators ranged from 67.5 to 134 cm in total length whereas the captive-raised alligators were 67 to 95.5 cm in total length. Radioimmunoassay and Analysis Plasma was assayed for corticosterone (B) and testosterone (T) using assays previously validated for juvenile alligators (Crain et al., 1997; Guillette et al., 1997d). All samples were analyzed in duplicate in a single assay. Plasma samples (100 µl for B and 150 µl for T) were extracted twice with 5 ml of ethyl ether to remove the lipophilic steroids. Extraction efficiency averaged 96 % for T and 92 % for B. Interassay and intraassay variation were 13.5 and 6.4 % respectively for the corticosterone assay and 15.0 and 7.9 % for the testosterone assay. Transformation of cpm to hormone values was done with a log-linear cubic spline standard curve generated by Microplate Manager 4.0 (Bio-Rad Laboratories Inc., Hercules, CA). Analyses were performed on Statview 5.0 (SAS Institute Inc., Cary, NC). Homoscedasticity in the data allowed ANOVA for comparisons of individual time point data between lakes, sexes, or captive-raised to wild animals. Homoscedasticity of compacted data allowed ANOVA of repeated measures for comparisons of data over the individual restraint periods. Heteroscedasticity in some data required the performance of nonparametric tests. For those data, comparison of two factors such as sex, lake, or captive raised to wild populations were done with the Mann Whitney U test. Comparisons among three or more factors were performed with the Kruskal-Wallis test.
65 52 Results Wild Population Plasma corticosterone (B) data obtained for the wild alligators from each lake were homoscedastic. No relationship was found between snout-vent length (SVL) and initial plasma B by lake (r 2 = Woodruff and r 2 = Apopka). Plasma B was not correlated with SVL during any time period for either lake (0.125 < r 2 <0.007). No difference was found in initial plasma B concentrations between lakes (p = ); neither were there differences in plasma B concentrations between lakes after 3 (p = ) or 20 (p = ) hours of restraint. There was a sharp rise in plasma B concentrations following capture for both lakes, but the profile of increased plasma B differed between lakes (see Figure 3.1). Repeated measures ANOVA yielded no lake difference in plasma B by lake (p = ), a strong difference by restraint time (p < ) and a strong difference for the interaction of lake and restraint time (p < ). Plasma B concentrations in animals from the reference lake peaked after 6 hours of restraint and rapidly declined thereafter. In contrast, plasma B concentrations in animals from Lake Apopka peaked after just 3 hours of restraint and declined steadily thereafter. These plasma B profiles differed, and the difference was evident in comparison of plasma B values at 6 (p = ) and 12 (p = ) hours of restraint. There was no difference in plasma B concentrations between sexes within a lake over the course of the study or at any given sampling period. Repeated measures ANOVA revealed no difference by sex (p = AP, p = WO) or the interaction of sex X restraint time (p = AP, p = WO) for alligators from either lake.
66 53 Initial plasma testosterone (T) concentrations did not differ between by sex (Figure 3.2). Heteroscedasticity of plasma T data prevented repeated measures ANOVA analysis over restraint period of the experiment. Graphical presentation of the data in Figure 3.3 show that there is high variation and little change in plasma T in juvenile alligators over the period of the study. Furthermore, males and females did not differ in plasma T within lakes. The data in Figure 3.2 display the high variation in male plasma T, variation that prevents a statistically significant sexual dimorphism in plasma T concentration. Initial body temperature differed substantially between the two lakes with Lake Apopka animals captured at a mean temperature of 28.3 ±0.2 C and Lake Woodruff animals captured at 25.8 ±0.1 C (p < ). Body temperature declined from that of initial capture by three hours of capture and remained low over the rest of the experiment in animals from each lake (Figure 3.4). Therefore, the effect of body temperature on plasma B during the stress response was examined. Analysis of plasma B for each time period using ANCOVA with respect to body temperature showed no differences in lake, body temperature, and lake X body temperature (p > 0.05). Captive Population Plasma B concentrations in the captive alligators were heteroscedastic; therefore, repeated measures ANOVA was not valid and individual comparisons were completed with nonparametric analyses. There was a significant increase in plasma B concentrations within 3 hours of capture (p < ; Figure 3.5). No lake difference was found in plasma B at the time of capture (p = ). There was no difference in plasma B between lakes after any restraint time (3 hrs p = ; 6 hrs p = ; 12 hrs
67 54 p = ; or 20 hrs p = ). Plasma T was also compared during individual restraint time periods with nonparametric analyses. No lake difference in plasma T concentrations were evident during the first 12 hours of restraint (initial p = ; 3 hrs p = ; 6 hrs p = ; 12 hrs p = ; Figure 3.6). After 20 hours of restraint, plasma T concentrations in alligators from Lake Woodruff were elevated compared to those in the alligators from Lake Apopka (p = ). The body temperature data displayed homoscedasticity of variance; and therefore, ANOVA and repeated measures ANOVA was completed for analysis of body temperature effects on the captive alligators. The initial body temperature did not differ between captive female alligators from the two lakes (26 ± 0.05 C for both lakes; p = ). In fact, although body temperature changed during the capture restraint, there was no lake associated difference in body temperature at any time during the experiment. Repeated measures ANOVA found no body temperature difference by lake (p = ), a difference in body temperature associated with restraint time (p < ), but no difference by lake X restraint time (p = ). Comparison Between Wild and Captive Alligators Comparing the two studies graphically in Figure 3.7, the initial plasma B concentration of captive alligators was 3.0 ± 0.8 ng/ml whereas the initial plasma B concentration of wild alligators was 0.4 ng/ml. The pattern of the stress response is very similar between the wild and captive studies. Further comparisons between the two studies should be limited due to potential effect of the many environmental differences between the captive and wild envrionment.
68 55 Discussion Juvenile alligators in both lakes had very low initial plasma corticosterone (B) concentrations that rose dramatically with capture. Although similar maximal plasma concentrations were obtained from wild populations of males and females from the two study lakes, the profile of the increase was different. These data suggested the hypothesis that a developmentally-altered (organizational) stress response, as measured by plasma B concentrations, was present in animals from Lake Apopka. To begin to test this hypothesis, animals obtained from both lakes as eggs and hatched and raised in captivity were examined. These animals provided no evidence that an organizational abnormality was present as there was no difference in the stress response, as indicated by plasma B concentrations. These data suggest that the altered profile could be due to environmental factors present in the juvenile's environment. Finally, unlike our previous studies (Guillette et al., 1996b; Guillette et al., 1997c), there was no sexually dimorphic pattern in plasma T concentrations nor a difference between males from the two lakes. Plasma concentrations of B increased by an average of 30 fold. Maximal plasma B concentrations were observed three hours after capture in alligators of both sexes on Lake Apopka whereas a peak in plasma concentrations of B were found at 6 hours on lake Woodruff. Peak values of circulating B observed in the juveniles studied here (10-15 ng/ml) and capture in early June 1997 were slightly greater than half of those reported for juveniles caught on the same lakes during late May, Those animals exhibited an almost 40 fold increase in plasma B and peak values of ng/ml were recorded 2 hr after capture - the total length of the experiment (Guillette et al., 1997). However, like
69 56 the present study, no differences were note in basal or peak values between sexes or animals from different lakes. Moreover, no difference in the basal levels of plasma B was evident when the two studies are compared. In the present study, animals were sampled during a different month and were not sampled animals at 2 hr. The animals could have peaked before the first sample was obtained at 3 hr. However, we do not believe that this occurred as plasma B concentrations were still rising in animals from Lake Woodruff at 3 hr and did not peak until 6 hr. A study with shorter sampling periods during several months could resolve these questions. Juvenile animals can display a different response to stress than adults. Sex does influence circulating B concentrations in a number of adult reptiles (Guillette et al., 1995b), including alligators (Elsey et al., 1990a). This study examined sexually immature alligators and thus, if sex steroids influence a sexually dimorphic stress response in adults then no difference should exist - as was observed. In addition, no significant variation in plasma sex steroid concentrations was found between the sexes in animals from either lake in this study. However, previous studies of juvenile alligators have shown that such a dimorphism exists (Guillette et al., 1994; Guillette et al., 1996b; Guillette et al., 1997c; Crain et al., 1998a; Chapter 2). Interestingly, the month during which animals are captured and sampled strongly influences the magnitude of the sexually dimorphic pattern in plasma sex steroid concentrations (Chapter 2). We have recently shown that animals obtained during most of the summer months displayed no sexual dimorphism in plasma T or E 2 concentrations. In part, this is due to a large degree of variation among individuals in plasma sex steroid concentrations during this period. Stress can alter sex steroid concentrations in the
70 57 plasma of vertebrates by modifying gonadotropin release, gonadal steroidogenesis, and hepatic degradation (Wingfield et al., 1998). The basal sex steroid concentrations we observed are indicative of a non stressed organism. Thus, it is unlikely that the low plasma T concentrations found in the animals sampled for this study are due to stressinduced suppression of gonadal steroidogenesis. It also should be noted that the variation in plasma B concentrations found in many adults is hypothesized and demonstrated in some species, to be due to their behavior as sexually mature organisms. For example, many male reptiles show intense territorial and aggressive behavior during the seasonal reproductive activity period (reviewed in Moore & Lindzey, 1992). Alligators exhibit intense aggressive displays, with males more active than females (Joanen & McNease, 1989; Vliet, 1989). These behaviors are not observed in juveniles, although in high density environments aggressive behaviors can be observed. The observations from the study of captive animals, suggest that the observed difference in the stress response profile of wild animals was due to the local environment. No differences in the basal or maximal plasma B concentrations were evident in the captive, juvenile alligators between animals from the two lakes. Additionally, the pattern of the stress response profile was also identical in the captive animals obtained from both lakes. We have observed that gonadal steroidogenesis is altered in alligators from Lake Apopka. Further, we had hypothesized that the adrenal, another steroidogenic organ could also be developmentally altered. Thus, the adrenal secretion of corticosterone in the stress response could be altered. However, these data suggest that this is not occurring. A number of the developmental abnormalities found in the alligators of Lake Apopka, as well as those described in other wildlife and laboratory species, can be
71 58 attributed to embryonic exposure to contaminants acting as estrogen or androgen agonists or antagonists (Gray et al., 1996; Crain & Guillette, 1997). No data are currently available, to our knowledge, that indicate that any contaminant interacts with the glucocorticoid receptors either as an agonist or antagonist. Finally, it is important to note that the juvenile alligators caught on Lake Apopka displayed an apparently normal response to acute capture stress although they responded more rapidly than animals of similar size obtained from Lake Woodruff. Although the juvenile alligators on Lake Apopka had a warmer body temperature at the time of capture compared to those on Lake Woodruff, their temperatures were similar at 12 hr. Our statistical analysis indicated that temperature had little role in the stress response pattern. However, it is still plausible that the initial body temperature of the Lake Apopka animals was a causal agent for the more rapid rise in plasma B concentrations. In ectotherms, enzymatic activity, including those associated with steroidogenesis, occurs over a wide spectrum of temperatures, and efficiency is strongly influenced by body temperature, although not to the degree that is seen in endothermic organisms (Norris, 1997). Of the few studies that have examined the stress response of wildlife living in contaminated environments, most have reported a depressed stress response with a suppression of glucocorticoid release. Hontela et al. (Hontela et al., 1992) observed that fish chronically exposed to polycyclic aromatic hydrocarbons, polychlorinated biphenyls (PCBs), and mercury exhibited little or no elevation in plasma cortisol levels in response to capture stress. Similar observations have been reported for fish exposed to pulp mill effluent (McMaster et al., 1994) and mining effluents (Norris, 1998). These fish are chronically exposed to industrial pollutants, whereas the alligators studied on Lake
72 59 Apopka, are exposed to agricultural and municipal waste chemicals. We have observed metal contaminants and PCBs in the alligators of Lake Apopka, but the concentrations of these pollutants are relatively low and not different from concentrations observed in animals obtained from Lake Woodruff (Guillette et al., 1999a; Burger et al., submitted). Future research should examine the underlying mechanism that influences the stress response of these species, as well as examine other species living in chronically polluted environments.
73 60 Apopka Woodruff Corticosterone (ng/ml) a b,c b b,d b,d,e b f,e f f,e a Restraint Time (hrs) Figure 3.1: Corticosterone (B) during capture stress in wild juvenile alligators from Lake Apopka and Lake Woodruff. Mean (± 1 SE) plasma B concentration in juvenile alligators during 20 hours of capture restraint. Each point represents animals. Shared superscript indicates lack of statistical difference. 300 Testosterone (pg/ml) (21) (6) (13) (10) (6) (17) Wild Male Woodruff Wild Female Woodruff Captive Female Woodruff Wild Male Apopka Wild Female Apopka Captive Female Apopka Figure 3.2: Basal testosterone concentration in captive and the wild juvenile alligators. Mean (± 1 SE) plasma testosterone concentration in juvenile alligators at time of capture. Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses.
74 61 Female Apopka Female Woodruff Male Apopka Male Woodruff Testosterone (pg/ml) Restraint Time (hrs) Figure 3.3: Testosterone (T) during capture stress in wild juvenile alligators from Lake Apopka and Lake Woodruff. Mean (± 1 SE) plasma T concentration in juvenile alligators during 20 hours of capture restraint. Each point represents 6-19 animals. No difference in plasma T was found. Apopka Woodruff 29 a Temperature ( C) b b,c b,c d d d d d d Restraint Time (hrs) Figure 3.4: Body temperature during capture stress in wild juvenile alligators from Lake Apopka and Lake Woodruff. Mean (± 1 SE) body temperature in juvenile alligators during 20 hours of capture restraint. Each point represents animals. Shared superscript indicates lack of statistical difference
75 62 Captive Apopka Captive Woodruff Corticosterone (ng/ml) a a b b b,c b,c c c c c Restraint Time (hrs) Figure 3.5: Corticosterone (B) during capture stress in captive juvenile alligators. Mean (± 1 SE) plasma B concentration in captive, female juvenile alligators during 20 hours of capture restraint. Each point represents animals. Shared superscript indicates lack of statistical difference Captive Woodruff Captive Apopka Testosterone (pg/ml) a,b b a,b a,b a,b a,b a,b a,b a a,b Restraint Time (hrs) Figure 3.6: Testosterone (T) during capture stress in captive juvenile alligators. Mean (± 1 SE) plasma T concentration in captive, female juvenile alligators during 20 hours of capture restraint. Each point represents animals. Shared superscript indicates lack of statistical difference
76 63 Wild Apopka Captive Apopka Wild Woodruff Captive Woodruff 20 Corticosterone (ng/ml) Restraint Time (hrs) Figure 3.7: Corticosterone (B) during capture stress in captive and wild juvenile alligators. Mean (± 1 SE) plasma B concentration in juvenile alligators during 20 hours of capture restraint. Each point represents animals. Data are provided for restricted comparisons only due to the dramatic differences in environmental variables between the two studies.
77 CHAPTER 4 POPULATION VARIATION IN IMMUNITY THYMUS AND SPLEEN OF JUVENILE AMERICAN ALLIGATORS Introduction The immune system provides an animal with an immediate response and protection from harmful organisms. The most important immune organs of vertebrates are the thymus and spleen. Although the immune system of reptiles remains largely uninvestigated, much of the work that has been done has concentrated on the thymus and spleen. The reptilian spleen is similar to that of other vertebrates; it is the site of hemopoiesis, antigen trapping, and initiation of cellular immune response (Kroese, 1983; Kroese et al., 1985). In fact, due to the lack of organized lymph organs like the mammalian Peyers patches and lymph nodes, or the Bursa of Fabricius in birds, the spleen in reptiles is most likely a more important functional lymphoid organ in reptiles than it is in mammals. The organization of splenic parenchyma of alligators is similar to that of other vertebrates. It is composed of two types of tissue: white and red pulp (Tanaka & Elsey, 1997). The majority of splenic tissue is red pulp, an area with a lower concentration of lymphocytes compared to the white pulp. In mammals, the red pulp is an important site for the removal of senescent blood cells, serum proteins, and foreign particles from the blood. Phagocytic cells aid in this removal and play an important role in initiation of immune responses in mammals (Bona & Bonilla, 1990) and birds (Askew 64
78 65 et al., 1995). The red pulp in reptiles is similar to that of mammals, with a dense network of reticular fibers (e.g., snakes Kroese et al., 1985). White pulp in reptiles is in the form of lymphocyte sheaths surrounding and following the splenic arteries. In some areas of the lymphocyte sheath, splenic nodules or Malpighian bodies extend from the artery such that the associated artery remains in an eccentric position. Within the lymphocyte sheaths, there is a morphological segregation of T and B lymphocytes in mammals and birds (Jeurissen, 1993; Yoshida et al., 1993). T lymphocytes are located on the interior of the general lymphocyte sheath, whereas B lymphocytes are concentrated in the Malpighian bodies and along the edge of the lymphocyte sheath. This T lymphocyte rich boundary between red and white pulp is a marginal zone of diffuse lymphocyte tissue containing numerous macrophages. This marginal zone participates in antigen trapping and the initiation of an immune response. Although no conclusive evidence of this T and B lymphocyte organization exists in reptiles, evidence of antigen trapping and plasma cell development suggest reptiles share this organization (Borysenko, 1976; Muthukkaruppan et al., 1983). The reptilian thymus consists of lymphoid cells within an epithelial framework (Saad & Zapata, 1992). The thymus is separated into many smaller lobules that are incompletely divided due to connections between the central or medullary regions of adjacent lobules. The medullary region is histologically distinguishable from the peripheral cortex in most reptiles (Saad & Zapata, 1992). The cortex appears darker in standard hematoxylin staining due to the closely packed lymphocytes in the cortical region. The medulla is pale in comparison due to preponderance of non-lymphoid cells.
79 66 Hormonal regulation of the immune system has been demonstrated in splenic and thymic tissue. Principle immune studies on reptiles have involved the immunosuppressive effects of corticosterone and testosterone. For example, exogenous doses of testosterone have induced reduced immune function and contributed to the involution of the thymus and depletion of the white pulp from the spleen in the lizard Chalcides ocellatus (Saad et al., 1990; Saad et al., 1991; El Deeb et al., 1993). Endogenous concentrations of corticosterone and testosterone have also been reciprocally correlated with the robustness of immune tissue and function in reptiles (Leceta & Zapata, 1985). Estrogen induced immune regulation in mammals and birds appears to be regulated through the thymus (Stimson & Hunter, 1980; Luster et al., 1984b; Selvaraj & Pitchappan, 1985). Morphological differences in spleen and thymus are closely related to functional immune differences. Involution of the thymus and depletion of splenic white pulp is typically seen as a degenerative state corresponding to reduced immune function in reptiles (El Ridi et al., 1988). For example, the seasonal involution of the thymus corresponds to reduced functional immunity in lizards (Hussein et al., 1979), snakes (El Ridi et al., 1981; Hussein et al., 1982) and turtles (Leceta & Zapata, 1985; Leceta & Zapata, 1986; Leceta et al., 1989). Reduced splenic white pulp also corresponds to reduced functional immunity in lizards (Hussein et al., 1979), snakes (Farag & El Ridi, 1986) and turtles (Leceta & Zapata, 1985). However, there is evidence from the pregnancy-associated thymic involution in humans that a reduction in the thymic cortex might not correspond to reduced functional ability (Clarke & Kendall, 1994). The authors suggest that pregnancy is associated with a unique T cell populations with
80 67 suppressive functions. This suppression is part of a highly active thymus rather than the inactive thymus portrayed in traditional explanations of thymic involution associated with pregnancy. The alligators in north central Florida have been extensively investigated for endocrine parameters. The investigations have been largely driven by the population crash and dramatically low hatching rates of alligators in Lake Apopka. Lake Apopka is the fourth largest lake in Florida and historically had a large population of alligators (Figure 4.1). The juvenile alligator population of Lake Apopka plunged to 10% of recently recorded levels coincident with declines in hatching rates and unexplained juvenile and adult mortality (Woodward et al., 1993). Lake Apopka is associated with several sources of contaminants. Historically, it received a direct supply of city effluent and direct agricultural runoff through 1998 (after 1998 all farms adjacent to Lake Apopka will be closed). Additionally, Lake Apopka is directly connected to the site of a major pesticide spill of dicofol (contaminated with DDT and its metabolites DDD, DDE, and chloro-ddt) and sulfuric acid in 1980 (EPA: Unpublished report). Endocrine abnormalities associated with Lake Apopka alligators have been detected in hatchlings and juveniles. The gonads of hatchling alligators from Lake Apopka displayed histological abnormalities (Guillette et al., 1994) and abnormal steroidogenic ability (Guillette et al., 1995c). Sex steroid and thyroid hormone concentrations are also abnormal in Apopka alligators (Crain et al., 1998b). In addition, morphological alterations have been observed in the size of the phallus of male Apopka alligators (Pickford, 1995; Guillette et al., 1996b). The phallus was examined as an endocrine-
81 68 dependent tissue that was specifically dependent on androgen concentrations. The thymus is also an endocrine-dependent tissue that is androgen-dependent. The differences between alligators from Lake Apopka and other populations strongly suggest that endocrine abnormalities observed are the result the increased presence of xenobiotic compounds in Lake Apopka acting as endocrine disruptors (Guillette, 1994; Guillette et al., 1996a). Altered steroidogenesis and endocrine modifications similar to those observed in Lake Apopka alligators, have been produced in laboratory experiments by treating eggs from other lakes with known endocrinedisrupting contaminants (EDCs) (e.g., Crain et al., 1997; Matter et al., 1998). These data suggest that the endocrine system of Lake Apopka alligators is organizationally modified during development by exposure to endocrine disrupting contaminants (EDCs). However, the possibility remains that constant exposure to a contaminated environment, such as Lake Apopka, could continually push the endocrine system into its modified state. Such, temporary modification would be considered an activational influence (Guillette et al., 1995a). Therefore, both activational and organizational mechanisms should be consideration when examining contaminant-associated abnormalities. The population crash on Lake Apopka and the subsequent observations of abnormal endocrine measures in alligators from Lake Apopka have been described relative to other alligator populations in north central Florida. Two lakes were used to provide normal immune measures in juvenile alligators: Lakes Orange and Woodruff (Figure 4.1). Immune or endocrine values unaffected by contaminants are impossible in today s environment due to the ubiquitous presence of EDCs. Instead, a best case or reference population is used for comparison. The alligator population closest to normal
82 69 in north-central Florida is the population on Lake Woodruff, a National Wildlife Refuge. The alligators from Lake Woodruff have consistently displayed high concentrations of testosterone relative to other lakes, and this is coincident with high hatching rates. Additionally, Lake Woodruff does not receive agricultural runoff or city effluent two prominent sources of EDCs. The alligators in Orange Lake were selected as another reference population. Orange lake has similar low levels of pollutants to Lake Woodruff; and, the alligators on Orange Lake display similar hatching rates compared to hatching rates of alligators from Lake Woodruff (Guillette et al., 1999a). The wild population of alligators living in Lake Apopka is endocrinologically challenged. The EDC-related abnormalities in Lake Apopka alligators, suggest the existence of additional symptoms. Direct contaminant effects on the immune system can be expected and they can be compounded by the presence of hormone-mimicking, xenobiotic chemicals. The strong endocrine-immune connections (Besedovsky & Sorkin, 1977; Ahmed et al., 1985; Besedovsky & del Rey, 1996) also suggest that the morphology of immune tissue and immune function could be compromised when the endocrine system is altered. The endocrine-immune connection is particularly strong in the thymus (Spangelo, 1995). Therefore, histological examination of the spleen and thymus was undertaken to evaluate the immune system of alligators in relatively pristine reference lakes and a highly contaminated lake. Additionally, this study was undertaken to evaluate the morphology of the spleen and thymus in a quantitative manner, and thereby develop criteria for rigorous evaluation of histological preparations of these lymphatic organs. Previous histological studies of the thymus and spleen in reptiles have been done using qualitative evaluation or limited quantitative analysis such as the use of
83 70 an ocular lattice and a point-counting method (e.g., Leceta & Zapata, 1985). Quantitative analysis of the histology in this experiment was accomplished using video capture of histological images and a digital analysis system. This allowed accurate comparison of the area of lymphoid elements in the spleen and thymus. Rigorous quantitative analysis will hopefully aid in linking spleen and thymus histology to functional immune measures as these are developed for a wider variety of species. The evaluation of immune tissue from wild alligators populations was done for juvenile animals of both sexes from Lake Apopka, Lake Woodruff, and Orange Lake. Population differences in histological measures of the thymus and spleen were expected with the largest differences occurring between Lake Apopka animals and animals from both of the other lakes. That is because Lakes Woodruff and Orange have reduced contaminant loads when compared to Lake Apopka. Within each lake, immune tissue was examined for possible sexually dimorphic structures due to the importance of sexsteroids in immune modulation. Furthermore, the altered sex-steroid concentrations within Lake Apopka, suggest that a sexual dimorphism in immune tissue could be abrogated if it is testosterone dependent (as Lake Apopka males have previously shown reduced testosterone concentrations relative to the other lakes). Exposure to steroidmimicking or -inhibiting contaminants, as the Lake Apopka animals are, could alter normal immunomodulating effects of sex steroids. Therefore, the hypothesized sexual dimorphism could differ by lake and thus provide another biomarker of EDC exposure. Many of the endocrine abnormalities observed in the alligators from Lake Apopka are believed to be of developmental origin. Therefore, an additional examination of captive raised alligators was undertaken to evaluate organizational or activational causes of
84 71 potential immune differences. The captive alligators were collected as eggs from Lake Apopka and Lake Woodruff and the resulting hatchlings were then raised under identical conditions. Captive-raised alligators were also tested in two in vitro assays for functional immune measures to compare alligators from Lake Apopka and Woodruff. Both tests performed were preliminary examinations in an effort to begin to link the histological differences in immune tissue to the immune ability of the juvenile alligators. In addition, the in vitro mitogen-induced blastic transformation assay was designed to test the function of T-lymphocytes for a specific functional measure of one cell population observed in the histological assay. Mitogen-induced blastic transformation measures the ability of lymphocytes to respond to polyclonal stimulation rather than a specific response to natural antigens. However, the in vivo response to antigen can be accurately predicted by the in vitro response to polyclonal stimulation (Kristensen et al., 1982). The second functional test was a phagocytic assay of a population of peripheral leukocytes.. Methods Animals Wild Alligators Used in Histological Comparison Juvenile American alligators (Alligator mississippiensis) were collected from May 5 through July 1, All animals were captured by hand or noose. The alligators ranged in size from 62 cm to cm in total length. Animals were collected from three lakes: 1) Lake Woodruff; 2) Orange Lake; and 3) Lake Apopka, FL. Lake Woodruff and Orange Lake were selected as relatively unpolluted lakes with healthy alligator
85 72 populations (Guillette et al., 1999a); animals from Lake Woodruff were considered to represent normal immune conditions in juvenile alligators (for explanation see above). Finally, Lake Apopka was used as a test lake with the expectation that animals from this lake would exhibit altered thymus and spleen histology due to previous data demonstrating increased prevalence of EDCs in the animals and the environment along with data showing a variety of endocrine abnormalities in the alligator population from Lake Apopka (see above and Matter et al., 1998). Following capture, animals were secured, tagged with a unique toe tag, and placed in a cloth sack. Within two hours of capture, the animals were removed from the sack, measured, and bled from the post-cranial sinus. Alligators were sexed by extrusion of the penis by manual palpation. Physical measurements consisted of total and snout-vent length. Blood samples were taken with sterile syringes and stored on ice for 8 to 12 hours in Vacutainer tubes containing sodium heparin. Samples were then centrifuged at 1800g, and the resulting plasma was stored at 80 C until radioimmunoassays were performed. The animals were brought back to the lab and killed by lethal injection of sodium pentobarbital (Sigma P-3761) within 18 hours of capture. Relevant portions of the thymus and the spleen were removed and placed in Bouin s fixative. Captive Alligators Used in Histological Comparison Captive juvenile female alligators were also studied using the above protocol. The following differences existed between the captive and field studies. The captive alligators were all of known age, 3 years old, whereas the wild alligators were assumed to be 3-5 years of age (Guillette, pers. comm.). Wild alligators ranged from 67.5 to 134 cm in total length and the captive-raised alligators were 67 to 95.5 cm in total length. The
86 73 captive alligators were from eggs laid on either Lake Woodruff or Lake Apopka, but incubated in the laboratory. After hatching, the captive animals were housed in a 20 x 40 foot enclosure with several hiding boxes and a single swimming area under densities much higher than that generally encountered in the wild. The alligator enclosure was cleaned daily, and the animals were handled at least twice per week to verify health and check tag integrity. The captive animals were fed ad lib with commercially available alligator food from Burris Feed, New Orleans, LA. The captive animals were killed and tissues taken in October of Captive Alligators Used in Mitogen-induced Blastic Transformation Assay Captive alligators raised under the conditions described above were also used to study mitogenic stimulation. Four month old alligators raised from eggs collected on Lake Apopka and Woodruff were bled from the post cranial sinus in December of Blood samples were taken with sterile syringes and stored on ice in Vacutainer tubes containing sodium heparin. Then, the blood was flown from Florida to the University of Quebec, Montreal Canada. Ten alligators were used from each lake. Captive Alligators Used in Phagocytic Assay Captive alligators raised under the conditions described above were also used to study the phagocytic response of a suspected neutrophil population in whole alligator blood. Two year old alligators raised from eggs collected on Lake Apopka and Woodruff were bled from the post cranial sinus in August of Blood samples were taken with sterile syringes and stored on ice in Vacutainer tubes containing sodium heparin. Three alligators were used from each lake.
87 74 Histology The alligator spleen is a bean shaped organ lying caudal to the stomach with a clear cranial-caudal axis. The caudal end of the spleen was removed (approximated 0.5 to 1.0 cm 3 of tissue), fixed in Bouin s fixative, and processed for standard light microscopy. Similarly, the anterior end of the right thymus also was prepared for light microscopy. The alligator thymus is a paired, elongate organ with an enlarged, body ventral-lateral to the heart and a long, thin cranial extension reaching to the base of the skull. Tissue was fixed for 48 to 72 hours and transferred through several changes of 70% ethanol for storage. Tissues were than dehydrated through a graded ethanol series, cleared in two changes of Hemo-De, and infiltrated with paraffin (Fisher-555) under increasing vacuum (12, 15, 20 and 22 lbs./in. 2 ). The resulting paraffin-tissue blocks were sectioned at 7µm and stained with a modified trichrome of Harris (Humason, 1997). Analysis All histological measurements were taken by a single researcher using single blind procedures to prevent researcher bias. Prior to data collection, the microscope was calibrated with a stage micrometer. Lengths were converted into µm and area measurements were converted to µm 2. Thymus The cortex is the morphologically and functionally distinct peripheral zone of the thymus; the interior of the thymus is the medulla (Figure 4.2). Medullary and cortical boundaries and areas change with immunological ability as well as season in reptiles. Therefore, measurements of the cortical and medullary areas were obtained as a
88 75 histological assessment of the immunological status of the thymus. Measurements of the areas of the cortex and medulla were performed with the aid of the Optimas 6.0 imaging system and an Olympus microscope using 1.5x and 2x objective lenses. The lens that provided the maximum magnification while maintaining the entire cross section of the thymus in the field of view was selected for each section. The procedure consisted of obtaining a video capture of a cross section of the thymus, then tracing the perimeter of the medullary portion, as well as the perimeter of cortical area, on a given cross section with a computer mouse. Lobules were measured individually; then area measurements were added for the total medullary and total thymic area of a given cross section. Cortical area was found by subtracting the area of the medulla from the total area of the thymus. Three individual cross sections were digitized to obtain three separate measures of the cortex and medulla of each animal. The first cross section digitized was selected at random from a region of the spleen where area was near maximum. The second and third sections digitized were selected at a fixed distance from the first each section was 175 ± 20 µm from the previous section digitized to avoid re-measuring the same structure and to obtain a better overall description of the thymus. Regional variation was not detected in previous studies utilizing this method; however, the chain-like cranial extension of the thymus was not included in the analysis. Spleen The organization of splenic parenchyma of alligators is similar to that of other vertebrates (i.e., it is composed of two types of tissue: white and red pulp). Two different lymphocyte-associated white pulp structures were measured: T-cell associated lymphocyte sheaths (periarteriolar lymphocyte sheaths-pals and periellipsoidal
89 76 lymphocyte sheaths-pels) and B-cell associated Malpighian bodies (Figures 4.3 and 4.5). Lymphocyte sheaths are the structures created by the ring of lymphocytes surrounding arteries in the spleen. Cross sections of these lymphocyte sheaths were as likely to be longitudinal with respect to the artery as they were to be perpendicular and additional complications were posed by the complexity of the arterial branching patterns. It was hypothesized that area measurements would overestimate measurements of ellipsoidal lymphocyte sheaths; therefore, the thickness or width of the lymphocyte sheath was measured rather than the area. Efforts were also made to measure the representative width of the lymphocyte ring by avoiding unusually thick or unusually thin areas of a given lymphocyte sheath regions that could be created by branches or turns in the affiliated artery. The thickness of lymphocyte sheaths was measured from the boundary of the consanguine artery and the lymphocyte sheath to the outer edge of the lymphocyte sheath (Figure 4.4). Measurements of the lymphocyte sheaths (PALS and PELS) were taken with a Nikon microscope using the 40x objective lens and an ocular micrometer calibrated to µm using a stage micrometer. The width of five PALS were compared with five PELS within the spleen of ten animals to determine if the two classes of lymphocyte sheaths were equivalent using these morphometric analyses. No difference was found between lymphocyte sheath measurements of PALS or PELS (see results below). Therefore, all additional measurements of lymphocyte sheaths were taken by grouping measurements of both PALS and PELS. Ten lymphocyte sheaths were measured per spleen. Measurements were taken from a minimum of two sections separated by least 100 µm to provide a general measure of lymphoid elements in the spleen. Previous data demonstrated a lack of regional variation along the length of the alligator spleen (see
90 77 chapter 5). Within each section, lymphocyte sheaths were chosen at random and measured. Area measurements were taken of the Malpighian bodies using the 20x objective lens with the aid of the Optimas 6.0 imaging system and an Olympus microscope. The procedure involved random selection of a splenic cross section, and location of a Malpighian body. Several consecutive sections were examined to ensure selection of the maximum cross section of the Malpighian body, and then the appropriate cross section was captured for analysis. Area calculations were done by the Optimas 6.0 imaging system after the perimeter of Malpighian bodies were manually traced using a computer mouse. Nine additional Malpighian bodies were also digitized using the same protocol. Radioimmunoassays Plasma was assayed for testosterone (T) as part of additional studies and will be published elsewhere (Guillette et al., in preparation). The data are used here for analysis of possible relationships between plasma T and immunological data. The T radioimmunoassay was previously validated for juvenile alligators (Crain et al., 1997; Guillette et al., 1997c). All samples were analyzed in duplicate in a single assay. Plasma samples (150 µl) were extracted twice with 5 ml of ethyl ether to remove the lipophilic steroids. Extraction efficiency averaged 96%. The extracted samples were dried under forced air and incubated overnight with tritiated testosterone in borate buffer at 4 C. Separation of the bound steroid from free steroid was achieved with the addition of charcoal-dextran and immediate centrifugation. Supernatant was then removed and added to scintillation cocktail for analysis. Transformation of cpm to hormone values was done using a log-linear to cubic spline standard curve generated by Microplate
91 78 Manager 4.0, Bio-Rad Laboratories Inc., Hercules, CA. Interassay and intraassay variation were 8.9 and 4.5% respectively. Mitogen-induced Blastic Transformation of Lymphocytes After blood collection, the techniques of Cuchens and Clem (Cuchens & Clem, 1979) were used with the several modifications. Twelve to eighteen hours after collecting the blood, the blood was layered drop-wise on Ficoll-Paque ( ;Pharmacia) and centrifuged at 300g for 30 minutes and the lymphocyte rich layer was removed. The cells were then washed twice with additional sterile medium. Cells were washed and cultured in sterile RPMI 1640 medium ( ; Gibco) supplemented with 10% fetal bovine serum, Penicillin/Streptomycin ( ; Gibco) and adjusted to M NaCl. All samples were assayed in triplicate in cultures incubated at 32 ºC in a humidified atmosphere of 5% CO 2 plus 95% air for 5 days. Lymphocytes were counted and adjusted to 1 X 10 6 viable cells/ml culture medium. Cells were plated in sterile 96 well flat-bottomed microplates (3072; Falcon). The mitogens used were concanavalin A (Con A; 0412; Sigma) and phytohemagglutinin-p (PHA; L-9132; Sigma). Final concentration of each mitogen was as follows: Con A (0, 5, 10, and 20 µg/ml) and PHA (0, 10, 20, and 40 µg/ml). Cultures were pulsed with 0.5 µci of [ 3 H]thymidine (24066; ICN) during the final 18 hours of incubation. The cells were harvested on a Titerteck cell harvester, and the amount of [ 3 H]thymidine incorporated was determined in a Beckman liquid scintillation counter (model LS1801). The raw data were expressed in disintegrations per minute (DPM), triplicates averaged, and the results expressed as the stimulation index (the ratio of mitogen stimulated cells to cells cultured in the absence of mitogen).
92 79 Phagocytosis Assay A volume of 100 µl of whole alligator blood was incubated with constant agitation at 4 ºC (negative control) and 32 ºC in the presence of yellow-green latex FluoSpheres (F-8823; Molecular Probes Inc.) at the ratio of approximately 100:1 (beads: leukocytes). After 0, 5, 10, 15, 30, 60, and 120 minutes, red blood cells were lysed with a ammonium chloride solution and centrifuged. The leukocytes were then removed and layered over a 3% bovine serum albumin (BSA) gradient and centrifuged at 150g for 5 minutes at room temperature to remove free beads. The free beads and BSA were then aspirated and the cell pellets were resuspended in 0.5 ml of 0.5% formalin in hematall. Samples were then analyzed using a FACScan (Becton Dickinson) with an air cooled argon laser providing an excitation at 488 nm. Fluorescence emission was collected at 520 nm. A gate was established to acquire the population of cells suspected to be neutrophils. Comparison of the forward and right angle scatter properties to that seen for neutrophils from other species was used to establish the correct cell population. The suspected neutrophils did phagocytose latex beads, lending additional evidence that neutrophils were selected. A total of 10,000 events were acquired for each sample and stored. Analysis for total fluorescence was performed using WinMDI 2.4 (1996 Joseph Trotter) for each sample. Data Analysis All statistical analyses were performed on Statview 5.0 (SAS Institute Inc., Cary, NC). Size data were analyzed with ANOVA by lake and by sex within each lake with post hoc analysis using Fisher s PLSD after confirming homoscedasticity of size data. The following tests refer to the wild population unless specified. Thymus data were
93 80 primarily in ratio format of medulla/cortex and were therefore, arcsin transformed in an attempt to achieve equality of variances. Continued heteroscedasticity of transformed data required thymus data to be tested with the following non parametric analyses (Sokal & Rohlf, 1995): the Kruskal-Wallis test was used to examine lake difference in thymus ratios and the Mann-Whitney U test was used to examine sex differences and paired lake comparisons of thymic ratios. Correlation analyses were performed to look for a correlation between snout-vent length of the alligator and each of the following immunological criteria: medullary/cortical ratio, average lymphocyte sheath width and average Malpighian body area. Correlation between sex steroid concentrations and immune data was also tested using correlation analyses. Paired t-tests were completed for comparison of PALS and PELS. Homoscedasticity in spleen lymphocyte sheath widths and Malpighian body areas was found for lake comparisons and sex comparisons within and between lakes; therefore, ANOVA s were completed to analyze spleen data with post hoc analysis using Fisher s PLSD. Within the captive-raised alligators, spleen data were also homoscedastic. A probability of less than 0.05 was considered significant in all tests. Results The organization of the alligator thymus is similar to that of other vertebrates examined (Bockman, 1967), with morphologically distinct lobules and a functional separation into cortical and medullary tissues (Figure 4.2). Connective tissue septa, containing large blood vessels, divide the thymus into lobules (Bona & Bonilla, 1990). The cortex is comprised of densely packed lymphocytes arranged in the peripheral zone
94 81 of the thymus. The medulla contains fewer, less densely organized lymphocytes and an increased concentration of epithelial cells on the interior of the thymus. Size and Sex-steroid Effects on Immune Parameters Lake and sex differences in alligator size were examined first, to address possible age and size differences. There was no difference in the snout-vent length between lakes Apopka (AP), Woodruff (WO), Orange (OR) for females (p = AP/WO; p = AP/OR; p = WO/OR); however, males from Lake Apopka were smaller than males from the other two lakes (p = AP/WO; p = AP/OR; p = WO/OR). Additionally, the males were smaller than the females from Lake Apopka (p = ). No difference between the sexes in snout-vent length for either Lake Woodruff (p = ) or Orange (p = ) was observed. Although there was no difference in snout-vent length among lakes or between sexes other than the small Apopka males, regression analyses were completed for possible relationships between size and immune data. Regression analyses indicates very weak, non significant relationships between snout-vent length and spleen or thymus parameters (lymphocyte sheath width r 2 = 0.016; Malpighian body area r 2 = 0.028; thymus medullary/cortical ratio r 2 = 0.021) therefore, size was excluded in further analysis. Captive alligators also displayed no significant relationship between snout-vent length and the above immune parameters. Plasma T concentration did not differ by lake or sex within any lake (see Table 4.1). Relationships between immune parameters and testosterone (T) was then examined with regression analysis. Plasma T concentration had no correlation with any immune
95 82 parameters (lymphocyte sheath width r 2 = 0.036; Malpighian body area r 2 = 0.025; and thymus medullary/cortical ratio r 2 = 0.004). Thymus No sex difference was found in medulla/cortex ratios for any lake (p = AP; p = ; p = ). Lake differences in thymic ratios, however, were found (Figure 4.6; p = ). Paired comparison by lake found that Lake Apopka animals had smaller thymic ratios than animals from either reference lake (AP < WO = OR; p = AP/WO; p = AP/OR; p = WO/OR). Although the relative ratio of cortex to medulla was smaller for Lake Apopka alligators, additional tests were necessary to discover which area of the thymus drove the difference (Figure 4.7). Kruskal-Wallis analysis indicated that no lake differences existed in medullary areas (p = ); however, a lake difference in cortical areas (p = ) was found. Paired Mann- Whitney U comparisons by lake demonstrated that Lake Apopka animals had larger cortical areas than animals from either reference lake (AP > WO = OR; p = AP/WO; p = AP/OR; p = WO/OR). No lake difference was found in the thymic ratio of captive-raised female alligators (p = ; Figure 4.11). Spleen No difference was found when the two types of lymphocyte sheaths were compared within each of the ten animals used to compare PALS and PELS by a paired t-test (p = ). Therefore, both types of lymphocyte sheaths (PALS and/or PELS) were analyzed under the single, inclusive variable of lymphocyte sheaths. No sex difference was found in lymphocyte sheath width for Lakes Apopka and Orange (p = AP; p = ); however, males had significantly smaller lymphocytes
96 83 sheaths than females on Lake Woodruff (Figure 4.8; p = ). Lymphocyte sheath width differed by lake for females with AP < WO < OR (p = AP/WO; p < AP/OR; p = WO/OR). Males from Orange Lake had larger lymphocyte sheaths with AP = WO < OR (p = AP/WO; p = AP/OR; p = ). Captiveraised female alligators from Lake Apopka and Lake Woodruff also displayed lake associated differences in lymphocyte sheath width; Lake Apopka alligators had smaller lymphocyte sheath width than Woodruff alligators (Figure 4.9 and Figure 4.12; p = ). No difference was found between sexes in the mean area of Malpighian bodies within any lake (p = AP; p = WO; p = OR). Therefore, sexes were combined for the analysis of lake differences in Malpighian bodies. Malpighian body area of differed among alligators from all lakes, with animals from Orange Lake having the largest Malpighian bodies, Lake Woodruff intermediate Malpighian bodies and Lake Apopka having the smallest Malpighian bodies (Figure 4.10; p < AP/WO; p < AP/OR; p = OR/WO). Malpighian body areas did not differ by lake in the captive-raised female alligators (p = ; Figure 4.13). Mitogen-induced Blastic Transformation of Lymphocytes The stimulation index for concanavalin A (Con A) exhibited no difference between animals from Lake Apopka and Lake Woodruff (concentration Con A X lake p = ; Figure 4.14) when analyzed with a repeated measures ANOVA. However, when each concentration was examined individually, the stimulation index for Con A was greater for animals from Lake Apopka for the highest Con A concentration tested (5 µg/ml p = ; 10 µg/ml p = ; 20 µg/ml p = ). The stimulation index
97 84 for phytohemagglutinin-p (PHA) did not differ between animals from Lake Apopka and Lake Woodruff (concentration PHA X lake p = ; Figure 4.15). Phagocytosis Assay The uptake of florescent beads is expressed as an increase in mean florescence of the gated population of cells. The phagocytosis of beads first appears after 60 minutes of incubation with a slight rise in florescence (Figure 4.16). A dramatic increase in phagocytosis occurred by 120 minutes of incubation for animals from both lakes (p < ). No difference in the phagocytic ability of cells was seen between animals from Lake Apopka and Lake Woodruff when analyzed with a repeated measures ANOVA (incubation time X lake p = ). Discussion Wild alligators from Lake Apopka, the contaminated lake, showed a variety of immune tissue differences when compared to the two reference lakes. The Lake Apopka alligators had a smaller medullary to cortical thymic ratio than alligators from the reference lakes. The lake difference in thymic ratios was a result of alterations in the cortical area of the thymus as no lake difference in medullary areas was detected. The thymic cortex of Lake Apopka alligators was much larger than that observed in animals from Lake Woodruff or Orange Lake. Hypothetically this enlarged cortex represents a change in the T-lymphocyte maturation within the thymus of alligators from Lake Apopka. The following parallels are drawn from mammals and birds. The thymic cortex is an area with a high concentration of dividing T-lymphocytes. Although there is evidence of T-lymphocyte maturation outside of the thymus in mice, the vast majority of
98 85 development and maturation of these cells occur in the thymic cortex. The outer layer of the cortex is the area of highest cell division as well as the area where prethymic T- lymphocytes enter the thymus. As T-lymphocytes decrease in size, and relative rates of cell division, they migrate toward the medulla. Prior to reaching the boundary between the cortical and medullary areas, the developing T-lymphocytes undergo positive selection for cells able to recognize MHC molecules associated with the recognition of self. Those cells that fail to bind to MHC molecules undergo apoptosis. T-lymphocytes that develop the ability to recognize MHC markers reach the corticomedullary boundary and undergo an additional round of selection. During this process, the developing T- lymphocytes are negatively selected for the ability to bind to self antigens such as surface proteins found on muscle, or blood cells. T-lymphocytes that recognize self antigens could produce harmful autoimmune reactions and therefore these cells also undergo apoptosis. Mature T-lymphocytes represent only 1-5% of the prethymic lymphocytes that entered the thymus. The increased cortical thymic area in the Lake Apopka alligators indicates a shift in the number of developing T-lymphocytes in Lake Apopka alligators relative to animals from the other lakes. However, the data do not indicate whether the increase in cortical area is from a more rapidly dividing T-lymphocyte population, or through longer retention times within the cortex. Functional links are also difficult to make. At this point, we have demonstrated a difference in the cortical region of the thymus that is associated with specific habitats or lakes. Alligators from Lake Apopka have a higher concentration of some EDCs in fat, liver and serum (Guillette et al., 1999a). There was
99 86 no size or sex correlation with any thymic variable on any lake, suggesting that sex steroid and size considerations were not important in juvenile alligators. Observations of the disparate spleen morphology could be related to the observed thymic differences. First, the thymus serves as a primary immune organ and is responsible for the maturation of functional T-lymphocytes. The spleen, on the other hand, is a major peripheral lymphoid organ containing a large number of lymphocytes that are separated morphologically into T and B-lymphocyte populations. In the lizard, Calotes versicolor, adult thymectomy results in severe depletion of the white pulp of the spleen; although specific reference to T and B-lymphocyte specific areas was not made (Pitchappan & Muthukkaruppan, 1977). T-lymphocytes reside primarily in the lymphocyte sheaths, and B-lymphocytes comprise most of the Malpighian bodies. With the observed increase in size of the cortical thymic area in alligators from Lake Apopka, one would hypothesize two possible alterations in the morphology of the spleen. If the increase in thymic cortical area represents increased production of mature T-lymphocytes, the alligators from Lake Apopka should have larger lymphocyte sheaths than those observed in animals from the reference lakes. Alternatively, if the increase in cortical area represents a thymic response designed to increase circulating mature T-lymphocytes, or a backlog of immature T-lymphocytes then the alligators from Lake Apopka should have smaller lymphocyte sheaths. The alligators from Lake Apopka have the smallest lymphocytes sheaths (with the exception of the male alligators from Lake Woodruff) suggesting that animals from Lake Apopka have a highly active thymus to counter a reduced T-lymphocyte presence in the spleen. An additional possibility exists, whereby the increased cortical width of the thymus in alligators from Lake Apopka is not in
100 87 response to the spleen, but rather a symptom of lymphocyte production difficulty in the thymus. Alligators from Lake Apopka could have an ineffective thymus that produces an inordinately high percentage of cells that undergo apoptosis. Antigenic stimulation has been shown to stimulate expansion of the while pulp regions of the spleen resulting in increased lymphocyte sheath width in snapping turtles (Borysenko, 1976). The preliminary functional data on mitogen-induced blastic transformation is particularly relevant to the developmental modification of T-lymphocytes apparent in the alligators from Lake Apopka. Both mitogens tested were T-lymphocyte mitogens based on mammalian studies. Lymphocytes from both lakes responded identically to PHA stimulation. However, PHA stimulation did not achieve a dose response curve and the concentrations tested did not differ in their stimulatory ability. Greater concentrations of both mitogens should be tested in the future to see if a lake difference exists at the point in the stimulation curve where lymphocytes from normal alligators begin to exhibit a cytotoxic effect of increasing mitogen. The Con A stimulation at the highest dose (20 µg/ml) begins to reduce the stimulation of lymphocytes form alligators from Lake Woodruff. The stimulation of lymphocytes form alligators from Lake Apopka was not effected by increasing concentrations of Con A indicating an apparently over stimulation or lack of regulation in these animals. The phagocytic assay data demonstrate that juvenile alligators do have a population of phagocytic cells in plasma. Although histological analysis of the population of cells responsible for the phagocytosis was not completed, the forward and right angle scatter properties suggest that the population of phagocytic cells were neutrophils. The time for phagocytosis of the first beads was very close to 60 minutes
101 88 with a large number of cells engulfing beads after 120 minutes. Although the data were presented as total florescence, cells with 1, 2, 3, and greater than 3 beads were evident (personal observation). The preliminary data from the phagocytic assay needs to be tested with a larger sample size to examine lake-associated differences. In addition, the assay should be further optimized to examined for the effect of a range of bead concentrations. The males from Lake Woodruff have a lymphocyte sheath width that does not differ from the lymphocyte sheath of male or female alligators from Lake Apopka, but was smaller than the lymphocyte sheath of male and female alligators from Orange Lake. In fact, the males from Lake Woodruff have a smaller lymphocyte sheath width than females from the same lake. This indicates a sexual dimorphism not present on the other lakes and indicates a possible inhibiting effect of testosterone on the male lymphocyte sheath width within animals from Lake Woodruff. However, there was no difference in the concentration of testosterone between sex in any lake during this sampling period. The lack of sexual difference in plasma testosterone is due in large part to the variation in testosterone concentration (between 20 and 1494 pg/ml) in the male alligators. There was also no difference in testosterone concentrations between lakes. The wild alligators in this study were captured between May 5 and July 1, a period of reduced plasma T in juvenile male alligators (see Chapter 2) that can result in abrogation of the sexual dimorphism in found in plasma T in March and April. No correlation was found between testosterone concentration and lymphocyte sheath width of alligators from any lake. Thus, circulating testosterone concentrations, at the time the animal was killed were apparently not the causal agent for the observed sexual dimorphism in lymphocyte sheath width. Additionally, the Malpighian bodies showed no correlation with testosterone and
102 89 sexual dimorphism was lacking in the morphology of these structures. Here again, the animals from Lake Apopka had the smallest Malpighian bodies with Lake Woodruff having significantly larger but intermediately sized Malpighian bodies compared to those from animals obtained from Orange Lake. Although corticosterone was not tested as part of this study, previous data suggests that there is no basal corticosterone difference between lakes or sexes; therefore, immune modulation by plasma corticosterone could not explain the lake or sex differences in immune tissues (Guillette et al., 1997c, see also chapter 5). The observed populational variation in spleen and thymus morphometrics indicate several possible associations including: 1) genetic differences unrelated to function or conditions; 2) functional differences related to current environmental variables; or 3) developmental differences related to alterations in the nesting environment (i.e., embryonic exposure to xenobiotic compounds). Therefore, an additional comparison of captive-raised alligators was performed examining juvenile females from Lakes Apopka and Woodruff. The alligators used in this study were collected as eggs from the two lakes and raised identically. Therefore, any residual difference among the animals from the two lakes was of embryonic origin. That is, the eggs could have differences in genotype or in the components of the egg increased contaminant loads have previously been shown in the eggs from Lake Apopka (Heinz et al., 1991). The captive-raised female alligators displayed no difference in thymic properties or Malpighian body areas in the spleen when compared by lake. However, captive-raised female alligators from Lake Apopka had smaller lymphocyte sheaths than those from Lake Woodruff identical to the pattern observed in the wild animals. The two studies
103 90 cannot be directly compared due to the differences in age, season, sex, and an abundance of environmental variables. The animals from the captive study were all three year old females. The wild animals were both male and females. The captive animals were killed in October, over two months after the last wild animal was killed. In addition, there are many differences between food and habitat structure experienced by the wild alligators and the alligator feed provided ad lib to the captive animals within their cage. However, the captive alligators are useful as an test of organizational differences between alligators from Lake Apopka and Lake Woodruff. Although wild alligators from Lake Apopka had smaller thymic ratios, smaller Malpighian bodies in the spleen and smaller lymphocyte sheath widths in the spleen, captive-raised alligators only differed in lymphocyte sheath width. The lack of a lake difference in thymic ratios and Malpighian body areas in captive raised alligators suggests that the environment experienced by the wild alligators creates lake differences in these histological features. The maintenance of a smaller lymphocyte sheath width in wild and captive-raised alligators from Lake Apopka when compared to similar alligators from Lake Woodruff suggests that a developmental difference is responsible for the smaller lymphocyte sheath width in the alligators from Lake Apopka. Clearly, there are lake differences in the histology of the thymus and spleen of wild alligators. Some differences exist between the two reference lakes and more differences exist between the reference and contaminated lake. Additionally, the lake difference in captive alligator splenic lymphocyte sheath width demonstrate possible developmental modification of alligator immune tissue. At this time, we have limited functional data, but the difference in the thymic cortex combined with the alteration in
104 91 lymphocyte sheath widths suggest a T-lymphocyte-associated difference. Furthermore, the increased mitogenic response to high doses of Con A seen in alligators from Lake Apopka relative to alligators from Lake Woodruff suggests that there is an inappropriate proliferative response in these alligators. The strong Malpighian body differences in the wild animals suggest a change in the B-lymphocyte population. Therefore, additional functional tests for both T and B-lymphocytes would be expected to show lake associated differences and need to be performed. In addition, functional tests should examine the differences between the plasma, thymic, and splenic lymphocyte populations. Table 4.1: Plasma testosterone concentration in juvenile alligators from each study lake. Mean testosterone concentrations, standard error, and samples size are listed. Lake Woodruff Lake Orange Lake Apopka Male Female Male Female Male Female T (pg/ml) SE n
105 92 Figure 4.1 Alligator populations in north central Florida. Lake Woodruff is a National Wildlife Refuge and both Lake Woodruff and Orange Lake are considered relatively clean sites. Lake Apopka has been associated with agriculture, city effluent and a major pesticide spill; it was used as the test site for EDC effects on immune tissue.
106 93 Figure 4.2: Cross section of the thymus from a juvenile alligator. Note septa (S), cortex (C) and medulla (M). Bar equals 500 µm.
107 94 Figure 4.3: Red and white pulp in the spleen of a juvenile alligator. Note white pulp (WP), red pulp (RP), periarteriolar lymphoid sheath (PALS) and periellipsoidal lymphoid sheath (PELS). Bar equals 100 µm. Figure 4.4: A lymphocyte sheath in the spleen of a juvenile alligator. The yellow bracket indicates the width of a lymphocyte sheath. Bar equals 50 µm.
108 95 Figure 4.5: Malpighian bodies in the spleen of a juvenile alligator. One of the Malpighian bodies is circled to indicate a representative measurement of Malpighian body area. Bar equals 100 µm.. Ratio of Medullary Area to Cortical Area b b a (20 (20 (10 Apopka Woodruff Orange Figure 4.6: The relative ratio of thymic areas in wild juvenile alligators from Lakes Apopka, Woodruff and Orange. Data represent mean (± 1 SE) ratio of thymic medulla to cortex. Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses.
109 b Thymus Area (µm 2 ) a a a (20 (20 (10 (20 (20 (10 Apopka Medulla Woodruff Medulla Orange Medulla Apopka Cortex c Woodruff Cortex c Orange Cortex Figure 4.7: Thymic cortex and medullary areas for wild juvenile alligators from Lakes Apopka, Woodruff and Orange. Data represent the mean (± 1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses. Lymphocyte Sheath Width (µm) a Apopka Female b Woodruff Female c (10 (9) (5) (10 (11 (5) Orange Female a,b Apopka Male a Woodruff Male c Orange Male Figure 4.8 Lymphocyte sheath width in the spleen of wild juvenile alligators from Lakes Apopka, Woodruff and Orange. Mean (± 1 SE) values for each sex are plotted separately due to the sexual dimorphism exhibited by alligators from Lake Woodruff. Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses.
110 97 Lymphocyte Sheath Width (µm) a (25 (14 Apopka Woodruff Figure 4.9: Lymphocyte sheath width in the spleen of captive-raised juvenile female alligators. Data represent the mean (± 1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses. b Malpighian Body Area (µm 2 ) a b (20 (20 (10 Apopka Woodruff Orange c Figure 4.10: Malpighian body area in the spleen of wild juvenile alligators from Lakes Apopka, Woodruff and Orange. Data represent the mean (± 1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses.
111 98 Figure 4.11: The relative ratio of thymic areas in captive-raised juvenile alligators. Mean (± 1 SE) ratio of thymic medulla to cortex for alligators from Lakes Apopka and Woodruff. The thymic ratio of wild alligators from Lakes Apopka and Woodruff are provided for comparison. Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses. Lymphocyte Sheath Width (µm) Ratio of Medullary Area to Cortical Area a Captive Apopka a a (25 (14 (20 (20 Captive Woodruff b Wild Apopka Wild Woodruff (25 (14 (20 (20 Captive Captive Wild Wild Apopka Woodruff Apopka Woodruff Figure 4.12: Lymphocyte sheath width in the spleen of captive female alligators. Data represent the mean (± 1 SE). Lymphocyte sheath width of wild caught alligators are provided for comparison. Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses.
112 99 Malpighian Body Area (µm 2 ) a Captive Apopka a (25) (14) (20) (20) Captive Woodruff Wild Apopka Wild Woodruff Figure 4.13: Malpighian body area for captive female alligators. Data represent the mean (± 1 SE). Malpighian body area of wild alligators are provided for comparison. Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses. Woodruff Apopka Stimulation Index b b a,b a,b a,b a (10) (10) Con A 5 Con A 10 Con A 20 Figure 4.14: Stimulation of peripheral blood lymphocytes in juvenile alligators with various doses of Con A. Data represent the mean (± 1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses.
113 100 Woodruff Apopka Stimulation Index a a (10) (9) PHA 5 PHA 10 PHA 40 Figure 4.15: Stimulation of peripheral blood lymphocytes in juvenile alligators with various doses of PHA. Data represent the mean (± 1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses. a a a a Woodruff Apopka Mean Florescence Figure 4.16: Phagocytic response of peripheral leukocytes in juvenile alligators. Data represent the mean (± 1 SE). Shared superscript indicates lack of statistical difference. Sample size is indicated in parentheses. (3) (3)
114 CHAPTER 5 DEVELOPMENTAL EFFECTS OF CONTAMINANTS ON IMMUNE TISSUE: THYMUS AND SPLEEN OF AMERICAN ALLIGATORS Introduction The critical role of the thymus in the development of effective immune function is a well established principle of immunology (Bockman, 1997). Thymectomy early in development has severe negative consequences on adult immunity in amphibians (Robert et al., 1997), birds (Sreter et al., 1996), reptiles, and mammals (Bonomo et al., 1995). The spleen is the largest lymphatic immune organ in most vertebrates, including reptiles (Cooper et al., 1985). In reptiles, the spleen plays a critical function in hemopoiesis, blood cell recycling and initiation of immune responses. The role of the spleen in cellmediated and humoral immune functions in reptiles clearly makes the spleen the most important peripheral lymphoid organ (Muthukkaruppan et al., 1983). Endogenous (e.g., hormones) and exogenous (e.g., immunotoxins) compounds exert effects on the structure and function of the thymus and spleen. Sex steroids have a host of effects on the immune system. The first indication of immunomodulatory effects of sex steroid came from sex-associated differences in immune cancers and immune function (Grossman, 1984; Grossman et al., 1991). Sex differences in immune function also have been found in reptiles (Saad & Shoukrey, 1988; Saad, 1989a). Many estrogenic effects on the mammalian immune system have been shown to be regulated by the thymus (Luster et al., 1984a). 101
115 102 Comparative research is particularly important in light of phylogenetic differences in the effectiveness of sex steroids in immune suppression. For example, estrogen treatment of adult chickens had little effect on the proliferation of lymphocytes from the spleen or thymus (Novotny et al., 1983). However, in a parallel experiment, the same estrogen treatment decreased the percentage of proliferating cells in the mouse thymus and increased the percentage of proliferating cells in the spleen (Novotny et al., 1983). Examples of immune suppression from sex steroids in reptiles are restricted to testosterone. Testosterone injection or implantation induces involution of the thymus and depletion of lymphoid elements of the spleen in turtles (Saad et al., 1991) and has both morphological and functional consequences in the lizard Chalcides ocellatus (Saad et al., 1990). In addition, pregnancy-associated involution of the thymus in C. ocellatus could represent immunosuppression due to increased circulating progesterone (Saad, 1989b). The organization of the splenic parenchyma of alligators is similar to that of other vertebrates (as it is composed of two types of tissue: white and red pulp, see Chapter 4). The majority of splenic tissue is red pulp, an area with a lower concentration of lymphocytes compared to white pulp. Two types of white pulp are present: arteriolar lymphocyte sheaths and Malpighian bodies. In mammals, the red pulp is an important site for the removal of senescent blood cells, serum proteins, and foreign particles from the blood. Phagocytic cells aid in this housekeeping function and play an important role in initiation of immune responses in mammals (Bona & Bonilla, 1990). The red pulp in reptiles is similar to that of mammals with a dense network of reticular fibers (Zapata et al., 1981; Cooper et al., 1985).
116 103 The organization of the alligator thymus is similar to that of other vertebrates examined (Bockman, 1967). Connective tissue septa, containing large blood vessels, divide the thymus into lobules (Bona & Bonilla, 1990). The peripheral zone of the thymus, comprised of densely packed lymphocytes, is the cortex. The interior region, comprised of less densely organized lymphocytes and an increased concentration of epithelial cells, is the medulla. Medullary and cortical boundaries and the volume of each region change with ability to respond immunologically as well as season in reptiles (El Ridi et al., 1988). Alligators provide an excellent animal to examine various aspects of immune system development, including the possible role of endocrine disrupting contaminant (EDC) exposure. First, alligators have temperature dependent sex determination and therefore allow the researcher to control the sex of developing eggs and to look at possible sex linked developmental effects of the endocrine system on the immune system. Second, there is abundant evidence that alligator populations in north-central Florida are under severe pressure from EDCs. Much of the evidence comes from the alligators in Lake Apopka, a population that has demonstrated several endocrine abnormalities including alterations in gonadal morphology (Guillette et al., 1994), gonadal steroidogenesis (Guillette et al., 1995c), and reduced phallus size in males (an endocrine dependent tissue Guillette et al., 1996b). The effects of EDCs on wildlife and humans has received a great deal of attention (Guillette et al., 1996a; Guillette & Crain, 1996; McLachlan & Arnold, 1996; Guillette et al., 1997a). Most of this attention has focused on reproductive events, although there is data suggesting the immune system can be affected as well (Holladay & Luster, 1996; Luebke et al., 1997).
117 104 The existence of immune or endocrine values in individuals unaffected by contaminants is unlikely in today s environment due to the ubiquitous presence of contaminants. Instead, a best case or reference wildlife population is used for comparison. The closest alligator population to normal in north-central Florida is the population on Lake Woodruff, a National Wildlife Refuge. The alligators from Lake Woodruff have historically displayed high concentrations of testosterone relative to other lakes; this and other healthy endocrine parameters is coincident with high hatching rates. Additionally, Lake Woodruff receives no direct sources of agricultural runoff or city effluent two prominent sources of EDCs. Therefore, eggs were collected from Lake Woodruff for the investigation of immunomodulatory effects of contaminants in alligators. The following treatment study was undertaken to evaluate the effect of embryonic exposure to contaminants on the development of the thymus and spleen in alligators. The temperature-dependent nature of sex determination allowed us to control the sex of developing alligators and to alter normal development by exposing embryos to natural hormones as well as endocrine disrupting chemicals exogenously. Methods Alligator eggs were collected from nests at Lake Woodruff National Wildlife Refuge, Florida, in June of Lake Woodruff has been used as a reference site by our lab because of several lines of data that indicate a healthy alligator population coincident with a relatively low level of contaminants in both the environment and alligators from Lake Woodruff (Guillette et al., 1999a, see above). A total of 8 clutches were used in this experiment, from which eggs were assigned to treatment or control groups in an effort to
118 105 spread each clutch among as many treatment groups as possible. Eggs were placed at the desired incubation temperature and treated prior to the developmental period during which sex determination takes place. One group of eggs was incubated at 30 C, a female producing temperature, to produce normal females for comparison. All other eggs were incubated at 33 C, a male producing temperature. Similarly, untreated eggs incubated at 33 C, were used to produce normal, control males. Estradiol-17β treated eggs were used as positive control for sex reversal (male to female), and thus for the effectiveness of all treatments and to produce females at a high temperature (33 C rather than 30 C) and thereby provide a temperature control within female alligators. Ecologically relevant compounds that have previously been shown to interact with sex steroid receptors from the alligator were chosen as treatment compounds. The three xenobiotic compounds used are commonly found in the aquatic ecosystems in Florida. Dicofol is a mitocide used extensively to control mites in citrus orchards. It was a major component of the material released into Lake Apopka during a chemical spill in 1980 (EPA, unpublished report). A persistent component of technical grade chlordane, trans-nonachlor, was chosen due to its prevalence in alligator tissue (Guillette et al., 1999a). An additional component of the 1980 chemical spill, p,p -DDD was selected as a persistent breakdown product of DDT that is found in alligator tissue and eggs. All compounds used to treat alligator eggs were shown to have an affinity for the alligator estrogen receptor (Vonier et al., 1997). The following experimental treatments were performed on eggs incubated at the male producing temperature of 33 C: 1) trans-nonachlor, 2) dicofol, 3) p,p -DDD, 4) a mixture of p,p -DDD and dicofol. The mixture was used to begin to examine potential interaction of xenobiotic compounds, because compounds are rarely present alone, if
119 106 ever. Additional treatment data including sample sizes and doses are summarized in Table 3.1 All treatment compounds were dissolved in 95% ethanol. Treatment consisted of placing 50 µl of the ethanol-treatment solution onto the surface of the egg and allowing it to be absorbed into the egg. Dosages were calculated by assuming an average egg weight of 90 g. Treatments were all accomplished at developmental stage 19.5 (from the developmental staging sequence for the American alligator; Ferguson, 1985) which is well before sex determination which occurs at stage This treatment method was developed for turtles by Wibbels, et el. (Wibbels et al., 1991a) and has been successfully used on alligators in our lab (Crain et al., 1997; Guillette et al., 1997b; Matter et al., 1998). Alligators were tagged with unique toe tags upon hatching and held in plastic cages (40 x 30cm) for 13 to 18 days. Animals were provided with several cm of water and no food during this period. Alligators normally live off of their internalized yolk mass for approximately 20 days after hatching (Ferguson, 1985). Therefore, food was not necessary and food was not provided to preserve the unique influence of treatment related effects in the animals from this experiment. The neonatal alligators then were killed with a lethal injection of sodium pentobarbital (Sigma P-3761) on days and the thymus and spleen were removed. Histology The body of the right thymus was removed and placed in Bouin s fixative in preparation for standard light microscopy. The alligator thymus is identical to that of other crocodilians; it is a paired, elongate organ with a enlarged, body ventral-lateral to the anterior end of the heart and a long, thin cranial extension reaching to the base of the
120 107 skull (Cooper et al., 1985). The entire spleen was also fixed and prepared for light microscopy. Standard histological protocol was used for tissue processing (see chapter 4 for a more explicit summary). In short, tissue was fixed for 48 hours and then rinsed and stored in 75% ethanol. Tissues were than dehydrated through a graded ethanol series, cleared in two changes of Hemo-De, and infiltrated with paraffin under vacuum. The resulting paraffin-tissue blocks were sectioned at 7µm and stained with a modified trichrome of Harris (Humason, 1997). Analysis Thymus The cortical and medullary areas were taken to obtain a histological measurement of the immunological status of the thymus (Figure 5.1). Measurements of the relative areas of the cortex and medulla were performed with the aid of the Optimas 6.0 imaging system and an Olympus microscope using the 4x objective lens. The procedure consisted of obtaining a video capture of a cross section of the thymus and tracing the area of the medulla and the entire thymus area. Lobules were measured individually. Area measurements were added for the total medullary and total thymic area of a given cross section. Cortical area was found by subtracting the area of the medulla from the total area of the thymus. Three individual cross sections were digitized to obtain three separate measures of the cortex and medulla of each animal. The first cross section digitized was selected by choosing the last cross section mounted on a slide a cross section determined to approximate maximum area during sectioning and mounting of the tissue. The second and third sections digitized were selected in relation to the first section; each section was 175 ± 20 µm from the previous section digitized. Additionally, the thymus
121 108 glands of six animals were compared along the length of the body of the organ to explore possible regional variation. It should be noted that the chain-like cranial extension of the thymus was not analyzed nor included in these discussions. All histological measurements were taken by a single researcher using single blind procedures to prevent researcher bias. Spleen The white pulp structure selected for measurement was the T-lymphocyte associated lymphocyte sheaths (Figure 5.2). Although B-lymphocyte associated Malpighian bodies were present, they were rare and difficult to locate; therefore, they were not used for analyses. Lymphocyte sheaths are the structures created by the ring of lymphocytes surrounding arteries in the spleen. Measurements of the lymphocyte sheaths were taken with a Nikon microscope using the 40x objective lens and an ocular micrometer calibrated to µm using a stage micrometer. Although some reptilian research makes a distinction between periarteriolar lymphocyte sheaths (PALS) and periellipsoidal lymphocyte sheaths (PELS) (Kroese, 1983), we have demonstrated that there is no morphometric distinction between PALS and PELS in alligators using width measurements for comparison (see chapter 4). Therefore, no distinction between PALS and PELS were made when taking measurements of lymphocyte sheaths. Cross sections of these lymphocyte sheaths were as likely to be longitudinal with respect to the artery as they were to be perpendicular and additional complications were posed by the complexity of the arterial branching patterns. It was hypothesized that area measurements would overestimate measurements of lymphocyte sheaths that were ellipsoidal; therefore, the thickness or width of the lymphocyte sheath was measured rather than the area. Efforts
122 109 were also made to measure the representative width of the lymphocyte ring by avoiding unusually thick or unusually thin areas of a given lymphocyte sheath. The thickness of lymphocyte sheaths was measured from the boundary of the consanguine artery and the lymphocyte sheath to the outer edge of the lymphocyte sheath. Nine lymphocyte sheaths were measured per spleen. Within each section, lymphocyte sheaths were chosen at random and measured. Measurements were taken from three sections separated by least 350 µm to avoid regional bias. In addition, regional variation was directly tested by the comparison of lymphocyte sheaths from six animals along the entire length of the organ. Data Analysis All statistical analyses were performed on Statview 5.0 (SAS Institute Inc., Cary, NC). Thymic analyses involve the comparison of relative areas of cortex and medulla; therefore, these ratio data were ArcSin transformed prior to analysis to achieve homoscedasticity (Sokal & Rohlf, 1995). If the data were homoscedastic, ANOVA were used for analyses of clutch, sex and dose effects. Initial analyses of length, weight, and clutch association with immune data were completed using regression analysis. Heteroscedastic data was compared with nonparametric analyses Mann Whitney U test for two factor analyses such as sex effects within a treatment and Kruskal-Wallis test for egg treatment and dose effect analyses. A probability of less than 0.05 was considered significant.
123 110 Results Dose, Allometric, Sex and Clutch Effects There was no dose effect on either thymic or splenic data as determined by ANOVA within any treatment. There was no correlation between lymphocyte sheath width and snout-vent length (r 2 = 0.002), total length (r 2 = 0.015) or weight (r 2 = 0.023). There was no correlation between thymic ratios and snout-vent length (r 2 = 0.005), total length (r 2 = 0.025) or weight (r 2 = 0.001). Interaction between clutch and treatment effect could not be tested with analysis of covariance because egg clutches were not of sufficient size to have at least one member of each clutch in every treatment. Clutch had no effect on lymphocyte sheath width (p = ) when alligators were compared irrespective of treatment; however, clutch had an effect on thymic ratio (p = ). The experimental design minimized this effect by spreading clutches throughout treatments. Several possible sex effects were built into the experiment. First, the expected sex of control eggs incubated at high and low temperatures was male and female respectively: eggs incubated at 33 C become male and eggs incubated at 30 C become female. Although 100% of control eggs incubated at 30 C were female, only 75% of the control eggs incubated at 33 C were male (see Table 5.1 and Figure 5.3). Second, estradiol-17β treatment of eggs incubated at a male temperature alters the sex of the developing embryo resulting in a female. Only 25% of eggs incubated at the male producing temperature were male when treated with estradiol-17β. Third, the other chemical treatments used (dicofol, p,p -DDD, trans-nonachlor) on eggs incubated at a male-producing temperature have all been shown to bind to the alligator estrogen receptor (Vonier et al., 1996).
124 111 Therefore, all treatment compounds were hypothesized to induce sex reversal (male to female). Contaminant treatment, irrespective of dose, altered sex of the treated eggs from 71% males (controls) to: 40% males (dicofol), 19% males (trans-nonachlor), 33% males (p,p -DDD) and 38% males (the mixture of trans-nonachlor and p,p -DDD). All treatment compounds reversed the sex of the alligators incubated at 33 C, resulting in a lower percent males for each treatment relative to control male alligators (p < for all comparisons). Therefore, in addition to possible sex effects between control males and females, sex differences within treatment groups are considered for each tissue type below. For example, nine of the 21 alligators treated with dicofol were female despite being incubated at 33 C, a male producing temperature. The lymphocyte sheath width did not differ between male and female hatchling alligators treated with dicofol (p = ). Thymus The control females (30 C and 31.5 C) have a higher thymic ratio of medulla to cortex than control males (30 C p < ; 31.5 C p = ) or 33 C incubated eggs treated with dicofol (30 C p < ; 31.5 C p = ), estradiol-17β (30 C p < ; 31.5 C p = ), trans-nonachlor (30 C p < ; 31.5 C p = ), p,p -DDD (30 C p < ; 31.5 C p = ) or a mixture of p,p -DDD and transnonachlor (30 C p < ; 31.5 C p = ; Figure 5.4). Eggs incubated at 33 C treated with trans-nonachlor had a significantly higher thymic ratio than control males (p = ). There was no other difference between any group incubated at 33 C. When a ratio was not used, and the medulla and cortex were examined separately, there
125 112 was no difference between treatments ( p for paired comparisons between treatments). Average thymic medullary and cortical cross sectional area is presented graphically for untreated control female alligators from the three incubation temperatures (Figure 5.5). There was no difference in the thymic ratio by dose within any treatment group. There was also no difference in thymic ratio by sex for any treatment groups (control males p = ; dicofol p = ; estradiol-17β p = ; transnonachlor p = ; p,p -DDD p = ; and the mixture of trans-nonachlor and p,p -DDD p = ). Spleen No difference was found in the lymphocyte sheath width between control males and control females (p = ; Figure 5.6). Dicofol (p = ) and trans-nonachlor (p = ) treated alligators had smaller lymphocyte sheath widths than the control males. No difference was found in any other comparison among control alligators or treatments. No effect of sex was found within any treatment for the lymphocyte sheath width of the spleen (dicofol p = ; estradiol-17β p = ; trans-nonachlor p = ; p,p -DDD p = ; and the mixture of trans-nonachlor and p,p -DDD p = ). Discussion The control female hatchling alligators having a larger ratio of medullary to cortical area of the thymus than the control males. The control female alligators were obtained by incubating the eggs at 30 C and 31.5 C taking advantage of the temperature dependent nature of sex determination in alligators. All other eggs (treatment and male
126 113 controls) were incubated at 33 C, a male producing temperature. The thymic ratio of control females was larger than all treatment groups incubated at 33 C, a male-producing temperature. When the cortex and medulla were examined separately, no effect of treatment was detected. Only when the thymic ratio was calculated was there a treatment effect. This indicates that the cross sectional area of the thymus was not different between treatments, but the relative proportion of the cortical and medullary regions of the thymus were different. Cross sectional areas were used because of the impossibility of obtaining thymus weight in small alligators. The thymus in alligators is an elongate organ extending from the chest to the back of the skull. The cranial extensions are much smaller than the body, which lies on the cranio-ventral side of the heart. Accurate weights of the thymus gland were not possible due to the difficulty in removing the long, thin cranial thymic extensions. The higher thymic ratio of females suggests a sex effect; however several experimental controls lead to the conclusion that the results display a temperature dependence in thymic growth, rather than a true sex effect. First, the untreated eggs incubated at 33 C resulted in two female and six male alligators. There was no difference in the thymic ratio between the sexes of these control alligators incubated at 33 C as would be expected if there was a sexual dimorphism in the thymic ratio. Second, both sexes of control alligators incubated at 33 C had a smaller thymic ratio than the control female alligators incubated at 30 C or 31.5 C. Third, the estradiol-17β treated eggs incubated at 33 C that were sex-reversed were female. These sex-reversed females had a smaller thymic ratio than the low incubation temperature females, and did not differ from the control males (also incubated at 33 C) in thymic ratio. The
127 114 effectiveness of the estradiol-induced females in representing control females raised at a high incubation temperature relies on the assumption that sex-reversed females are fully female. There is evidence from other reptiles to suggest that these sex-reversed estradiol-17β treated alligators are, indeed, normal females. In the turtle, Trachemys scripta, temperature and estradiol-17β appear to have a synergistic effect on sex determination suggesting a common pathway for estrogens and temperature control of sex determination (Wibbels et al., 1991b). In the turtle, Chelydra serpentina, eight month old estradiol induced female animals have sex steroid profiles consistent with the gonadal morphology suggesting that the sex reversed, estradiol-treated individuals have normal female gonads (Rhen et al., 1996). Therefore, we hypothesize that the thymic ratio in alligators is decreased by higher incubation temperature, in the range of temperatures used in the experiment. Incubation temperature is a critical factor during the development of reptiles, with permanent effects evident in some species. The observed temperature effects on the thymus in hatchling alligators is supported by many examples of temperature-dependent developmental effects in reptiles. Reptiles incubated at higher temperatures tend to grow faster (Deeming & Ferguson, 1988), and these effects can be maintained in the adults. The developmental effects on the alligator thymus were observed within 18 days of hatching; the length of time that the temperature effect on the thymus could last is unknown at this time. However, temperature has several long-term developmental effects in reptiles, including crocodilians. Alligators incubated at higher temperatures experience more rapid rates of morphogenesis and then more rapid growth rates as well (Deeming & Ferguson, 1989). Furthermore, mugger crocodiles raised at optimal temperatures not only
128 115 grew faster, but reproduced earlier, and produced more fertile eggs than animals incubated at sub-optimal temperatures (Lang, 1998). In the leopard gecko, adult behavior is developmentally determined by temperature (Flores et al., 1994). Female leopard geckos raised at a temperature that predominantly produces males are more likely to exhibit aggression as adults than females from other incubation temperatures (Flores et al., 1994). Juvenile growth rate in Chelydra serpentina is negatively correlated with incubation temperature (O'Steen, 1998). There is also a strong effect of incubation temperature on habitat choice in C. serpentina. Turtles from eggs incubated at low temperatures preferred higher water temperatures; whereas turtles from eggs incubated at high temperatures preferred lower water temperatures (O'Steen, 1998). Water temperature preference was maintained even after a 6 month hibernation period, suggesting that long-lasting differences in behavioral physiology can result from developmental temperature differences. In addition to the effect of incubation temperature on the thymus, several of the treatment compounds modified the development of the thymus and spleen. Embryonic exposure to trans-nonachlor in eggs incubated at 33 C, a male-producing temperature, resulted in an increased thymic ratio as well as decreased lymphocyte sheath width in the spleen. Embryonic exposure to dicofol also decreased the lymphocyte sheath width in the spleen, but had no effect on the ratio of the medulla to the cortex in the thymus. Both trans-nonachlor and dicofol have been shown to bind to the alligator estrogen receptor (Vonier et al., 1996). Therefore, the two EDCs may be indirectly modifying the splenic and thymic tissue in these hatchling alligators through modifications of the endocrine system. Previous research has shown that estrogenic effects on the immune system of
129 116 mice is often mediated through the thymus (Stimson & Hunter, 1980; Luster et al., 1984b). Thymus development is environmentally regulated (Bockman, 1997). Neonatal treatment of rats with estradiol benzoate caused a thymic involution (Leceta et al., 1988). The development of immune tissue is critical to the functional ability of the immune system throughout life. For example, neonatal estrogen treatment caused long-term impairment of the mitogenic response in mouse spleen (Kalland et al., 1979). An additional possibility for indirect immune regulation would be through a corticosteroid receptor rather than an estrogen receptor. Corticosterone has strong immunosuppressive effects on reptiles causing involution of the thymus and depletion of lymphocyte elements of the spleen (Saad & El Ridi, 1988). Prenatal exposure of mice to chlordane (0.16 mg/kg/day of gestation), an organochlorine pesticide that often contains transnonachlor, caused a persistent increase in plasma corticosterone (Cranmer et al., 1984). The rise in plasma corticosterone was present in male and female mice through 400 days of age, and therefore persisted into adulthood (Cranmer et al., 1984). Plasma corticosterone was not measured as part of the current study. Developmental exposure to chlordane also exhibits direct effects on immune function in mice including reduced macrophage function (Theus et al., 1992). Developmental exposure to chlordane also displays sex-specific immune effects. In utero exposure to chlordane significantly decreased the mixed lymphocyte reactivity of spleen cells in male mice, whereas females were unaffected (Barnett et al., 1985).
130 117 Figure 5.1: Cross section of the thymus from a hatchling alligator. Note septa (S), cortex (C) and medulla (M). Bar equals 500 µm. Figure 5.2: Cross section of the spleen from a hatchling alligator. Note the indicated lymphocyte sheaths (LS) and arteries. Bar equals 100 µm.
A flexible, reversible alternative to surgical castration
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk A flexible, reversible alternative to surgical castration Author : Virbac Categories : Canine, Companion animal, General,
Vet Times The website for the veterinary profession https://www.vettimes.co.uk A flexible, reversible alternative to surgical castration Author : Virbac Categories : Canine, Companion animal, General,
The Physiological Effects and Projected Outcomes of. Urbanization and Pollution on Reptiles. Dale Rodney Lockman. General Honors 401
 The Physiological Effects and Projected Outcomes of Urbanization and Pollution on Reptiles The Physiological Effects and Projected Outcomes of Urbanization and Pollution on Reptiles Dale Rodney Lockman
The Physiological Effects and Projected Outcomes of Urbanization and Pollution on Reptiles The Physiological Effects and Projected Outcomes of Urbanization and Pollution on Reptiles Dale Rodney Lockman
How Does Photostimulation Age Alter the Interaction Between Body Size and a Bonus Feeding Program During Sexual Maturation?
 16 How Does Photostimulation Age Alter the Interaction Between Body Size and a Bonus Feeding Program During Sexual Maturation? R A Renema*, F E Robinson*, and J A Proudman** *Alberta Poultry Research Centre,
16 How Does Photostimulation Age Alter the Interaction Between Body Size and a Bonus Feeding Program During Sexual Maturation? R A Renema*, F E Robinson*, and J A Proudman** *Alberta Poultry Research Centre,
Influence of Experimentally- induced clinical mastitis on Reproductive Performance of Dairy Cattle
 Influence of Experimentally- induced clinical mastitis on Reproductive Performance of Dairy Cattle Dr. Mitch Hockett Department of Animal Science North Carolina State University Characteristics of Mastitis
Influence of Experimentally- induced clinical mastitis on Reproductive Performance of Dairy Cattle Dr. Mitch Hockett Department of Animal Science North Carolina State University Characteristics of Mastitis
Endocrine Disrupting Chemical on R 1
 ... 40(2) 346 355 (2555) KKU Sci. J. 40(2) 346-355 (2012) Endocrine Disrupting Chemical on R Effect 1 1 E-mail: ksarun@kku.ac.th ABSTRACT Endocrine disrupting chemicals (EDCs) are the groups of synthetic
... 40(2) 346 355 (2555) KKU Sci. J. 40(2) 346-355 (2012) Endocrine Disrupting Chemical on R Effect 1 1 E-mail: ksarun@kku.ac.th ABSTRACT Endocrine disrupting chemicals (EDCs) are the groups of synthetic
University of Canberra. This thesis is available in print format from the University of Canberra Library.
 University of Canberra This thesis is available in print format from the University of Canberra Library. If you are the author of this thesis and wish to have the whole thesis loaded here, please contact
University of Canberra This thesis is available in print format from the University of Canberra Library. If you are the author of this thesis and wish to have the whole thesis loaded here, please contact
SINGLE ANNUAL IMPLANT
 Manage pet ferret adrenal cortical disease with a SINGLE ANNUAL IMPLANT NOT APPROVED BY FDA Legally marketed as an FDA Indexed Product under MIF 900-013. FOR USE IN FERRETS ONLY. Extra-label use is prohibited.
Manage pet ferret adrenal cortical disease with a SINGLE ANNUAL IMPLANT NOT APPROVED BY FDA Legally marketed as an FDA Indexed Product under MIF 900-013. FOR USE IN FERRETS ONLY. Extra-label use is prohibited.
Hormones and Reproduction in Fishes, Amphibians, and Reptiles
 Hormones and Reproduction in Fishes, Amphibians, and Reptiles Hormones and Reproduction in Fishes, Amphibians, and Reptiles Edited by David O. Norris and Richard E. Jones University of Colorado Boulder,
Hormones and Reproduction in Fishes, Amphibians, and Reptiles Hormones and Reproduction in Fishes, Amphibians, and Reptiles Edited by David O. Norris and Richard E. Jones University of Colorado Boulder,
Integration of Embryonic Zebrafish and Passive Sampling Device Extracts to Explore Mixture Toxicity
 Integration of Embryonic Zebrafish and Passive Sampling Device Extracts to Explore Mixture Toxicity Margaret M. Corvi 1 R.L. Tanguay 2 K. A. Anderson 2 1 BioResource Research 2 Environmental and Molecular
Integration of Embryonic Zebrafish and Passive Sampling Device Extracts to Explore Mixture Toxicity Margaret M. Corvi 1 R.L. Tanguay 2 K. A. Anderson 2 1 BioResource Research 2 Environmental and Molecular
Course Curriculum for Master Degree in Internal Medicine/ Faculty of Veterinary Medicine
 Course Curriculum for Master Degree in Internal Medicine/ Faculty of Veterinary Medicine The Master Degree in Internal Medicine/Faculty of Veterinary Medicine is awarded by the Faculty of Graduate Studies
Course Curriculum for Master Degree in Internal Medicine/ Faculty of Veterinary Medicine The Master Degree in Internal Medicine/Faculty of Veterinary Medicine is awarded by the Faculty of Graduate Studies
Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine
 Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine The Master Degree in Theriogenology & Artificial Insemination /Faculty of Veterinary Medicine
Course Curriculum for Master Degree Theriogenology & Artificial Insemination/Faculty of Veterinary Medicine The Master Degree in Theriogenology & Artificial Insemination /Faculty of Veterinary Medicine
Course Curriculum for Master Degree in Poultry Diseases/Veterinary Medicine
 Course Curriculum for Master Degree in Poultry Diseases/Veterinary Medicine The Master Degree in Poultry Diseases /Veterinary Medicine, is awarded by the Faculty of Graduate Studies at Jordan University
Course Curriculum for Master Degree in Poultry Diseases/Veterinary Medicine The Master Degree in Poultry Diseases /Veterinary Medicine, is awarded by the Faculty of Graduate Studies at Jordan University
Evolutionary and Functional Aspects of Pituitary Gonadotropins in the Green Turtle, Chelonia Mydas 1
 AMER. ZOOL., 20:565-574 (1980) Evolutionary and Functional Aspects of Pituitary Gonadotropins in the Green Turtle, Chelonia Mydas 1 PAUL LICHT Department of Zoology, University of California, Berkeley,
AMER. ZOOL., 20:565-574 (1980) Evolutionary and Functional Aspects of Pituitary Gonadotropins in the Green Turtle, Chelonia Mydas 1 PAUL LICHT Department of Zoology, University of California, Berkeley,
SOAR Research Proposal Summer How do sand boas capture prey they can t see?
 SOAR Research Proposal Summer 2016 How do sand boas capture prey they can t see? Faculty Mentor: Dr. Frances Irish, Assistant Professor of Biological Sciences Project start date and duration: May 31, 2016
SOAR Research Proposal Summer 2016 How do sand boas capture prey they can t see? Faculty Mentor: Dr. Frances Irish, Assistant Professor of Biological Sciences Project start date and duration: May 31, 2016
Neutering Your Dog or Bitch
 Neutering Your Dog or Bitch We would like to advise you that the information contained in this document has been obtained from several different sources and is intended for information purposes only. No
Neutering Your Dog or Bitch We would like to advise you that the information contained in this document has been obtained from several different sources and is intended for information purposes only. No
Mastitis and the link to infertility
 CONTINUING EDUCATION I LARGE ANIMAL Mastitis and the link to infertility Mastitis and infertility are the two most common disease complexes in dairy cattle worldwide. Both are major reasons for culling
CONTINUING EDUCATION I LARGE ANIMAL Mastitis and the link to infertility Mastitis and infertility are the two most common disease complexes in dairy cattle worldwide. Both are major reasons for culling
FOLLICULAR GROWTH PATTERN IN BUFFALOES SYNCHRONIZED TO ESTRUS WITH PROGESTERONE IMPREGNATED INTRAVAGINAL SPONGES
 International Journal of Science, Environment and Technology, Vol. 3, No 3, 2014, 960 965 ISSN 2278-3687 (O) FOLLICULAR GROWTH PATTERN IN BUFFALOES SYNCHRONIZED TO ESTRUS WITH PROGESTERONE IMPREGNATED
International Journal of Science, Environment and Technology, Vol. 3, No 3, 2014, 960 965 ISSN 2278-3687 (O) FOLLICULAR GROWTH PATTERN IN BUFFALOES SYNCHRONIZED TO ESTRUS WITH PROGESTERONE IMPREGNATED
REQUEST FOR STATEMENTS OF INTEREST SOUTH FLORIDA-CARIBBEAN CESU NETWORK NUMBER W912HZ-16-SOI-0007 PROJECT TO BE INITIATED IN FY 2016
 REQUEST FOR STATEMENTS OF INTEREST SOUTH FLORIDA-CARIBBEAN CESU NETWORK NUMBER W912HZ-16-SOI-0007 PROJECT TO BE INITIATED IN FY 2016 Project Title: Evaluating Alligator Status as a System-wide Ecological
REQUEST FOR STATEMENTS OF INTEREST SOUTH FLORIDA-CARIBBEAN CESU NETWORK NUMBER W912HZ-16-SOI-0007 PROJECT TO BE INITIATED IN FY 2016 Project Title: Evaluating Alligator Status as a System-wide Ecological
Correlation of. Animal Science Biology & Technology, 3/E, by Dr. Robert Mikesell/ MeeCee Baker, 2011, ISBN 10: ; ISBN 13:
 Correlation of Animal Science Biology & Technology, 3/E, by Dr. Robert Mikesell/ MeeCee Baker, 2011, ISBN 10: 1435486374; ISBN 13: 9781435486379 to Indiana s Agricultural Education Curriculum Standards
Correlation of Animal Science Biology & Technology, 3/E, by Dr. Robert Mikesell/ MeeCee Baker, 2011, ISBN 10: 1435486374; ISBN 13: 9781435486379 to Indiana s Agricultural Education Curriculum Standards
PHYSIOLOGICAL PRINCIPLES UNDERLYING SYNCHRONIZATION OF ESTRUS
 PHYSIOLOGICAL PRINCIPLES UNDERLYING SYNCHRONIZATION OF ESTRUS M.F. Smith, G.A. Perry, J.A. Atkins, M.E. Risley, D.C. Busch, and D.J. Patterson Division of Animal Sciences, University of Missouri, Columbia
PHYSIOLOGICAL PRINCIPLES UNDERLYING SYNCHRONIZATION OF ESTRUS M.F. Smith, G.A. Perry, J.A. Atkins, M.E. Risley, D.C. Busch, and D.J. Patterson Division of Animal Sciences, University of Missouri, Columbia
Understanding Postpartum Anestrus and Puberty
 Understanding Postpartum Anestrus and Puberty Dr. Jack C. Whittier, Colorado State University Dr. Jim Berardinelli, Montana State University Dr. Les Anderson, University of Kentucky 2008 Robert E. Taylor
Understanding Postpartum Anestrus and Puberty Dr. Jack C. Whittier, Colorado State University Dr. Jim Berardinelli, Montana State University Dr. Les Anderson, University of Kentucky 2008 Robert E. Taylor
Jeff Baier MS DVM Birds of Prey Foundation Broomfield, CO
 Jeff Baier MS DVM Birds of Prey Foundation Broomfield, CO drjeffbaier@gmail.com Squamates Chelonians Snakes Lizards Varanids Monitor Lizards Crocodilians Reptilian adaptations Anaerobic glycolysis Low
Jeff Baier MS DVM Birds of Prey Foundation Broomfield, CO drjeffbaier@gmail.com Squamates Chelonians Snakes Lizards Varanids Monitor Lizards Crocodilians Reptilian adaptations Anaerobic glycolysis Low
ECTS II. semester Anatomy with Organogenesis of Domestic Animals II.
 1 st year I. semester Physics and Biophysics 16 0 38 0 5 Medical Chemistry 20 0 34 0 5 Zoology 15 20 40 0 5,5 Botany in Veterinary Medicine 10 0 10 0 1,5 Anatomy with Organogenesis of Domestic 18 0 64
1 st year I. semester Physics and Biophysics 16 0 38 0 5 Medical Chemistry 20 0 34 0 5 Zoology 15 20 40 0 5,5 Botany in Veterinary Medicine 10 0 10 0 1,5 Anatomy with Organogenesis of Domestic 18 0 64
A-l. Students shall examine the circulatory and respiratory systems of animals.
 Animal Science A-l. Students shall examine the circulatory and respiratory systems of animals. 1. Discuss the pathway of blood through the heart and circulatory system. 2. Describe and compare the functions
Animal Science A-l. Students shall examine the circulatory and respiratory systems of animals. 1. Discuss the pathway of blood through the heart and circulatory system. 2. Describe and compare the functions
Early lambing with: Improved fertility Improved fecundity Improved prolificacy Compact lambing period Normal return to season Normal sexual cycle
 Early lambing with: Improved fertility Improved fecundity Improved prolificacy Compact lambing period Normal return to season Normal sexual cycle Presentation: Regulin is a yellow cylindrical implant containing
Early lambing with: Improved fertility Improved fecundity Improved prolificacy Compact lambing period Normal return to season Normal sexual cycle Presentation: Regulin is a yellow cylindrical implant containing
Hudson, a 10-year-old MC Cocker spaniel, was referred for evaluation of severe polyuria and polydipsia (PU/PD) of 3 months in duration...
 VCAWLAspecialty.com David Bruyette, DVM, DACVIM Hudson, a 10-year-old MC Cocker spaniel, was referred for evaluation of severe polyuria and polydipsia (PU/PD) of 3 months in duration... 1. Physical Examination
VCAWLAspecialty.com David Bruyette, DVM, DACVIM Hudson, a 10-year-old MC Cocker spaniel, was referred for evaluation of severe polyuria and polydipsia (PU/PD) of 3 months in duration... 1. Physical Examination
STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: REPRODUCTION TESTING
 VICH GL22 (SAFETY: REPRODUCTION) Revision 1 May 2004 For implementation at Step 7 STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: REPRODUCTION TESTING Recommended for Implementation
VICH GL22 (SAFETY: REPRODUCTION) Revision 1 May 2004 For implementation at Step 7 STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: REPRODUCTION TESTING Recommended for Implementation
Reproductive physiology and eggs
 Reproductive physiology and eggs Class Business Reading for this lecture Required. Gill: Chapter 14 1. Reproductive physiology In lecture I will only have time to go over reproductive physiology briefly,
Reproductive physiology and eggs Class Business Reading for this lecture Required. Gill: Chapter 14 1. Reproductive physiology In lecture I will only have time to go over reproductive physiology briefly,
Fate and Transport of Hormones & Antimicrobials
 Fate and Transport of Hormones & Antimicrobials Linda S. Lee Purdue University Dept. of Agronomy April 25, 2008 1 Basic Properties & Source Concentrations Fate Processes Transport Processes 2 Hormones:
Fate and Transport of Hormones & Antimicrobials Linda S. Lee Purdue University Dept. of Agronomy April 25, 2008 1 Basic Properties & Source Concentrations Fate Processes Transport Processes 2 Hormones:
reproductive life History and the effects of sex and season on morphology in CRoTALus oreganus (northern PaCifiC RATTLESNAKES)
 reproductive life History and the effects of sex and season on morphology in CRoTALus oreganus (northern PaCifiC RATTLESNAKES) Benjamin Kwittken, Student Author dr. emily n. taylor, research advisor abstract
reproductive life History and the effects of sex and season on morphology in CRoTALus oreganus (northern PaCifiC RATTLESNAKES) Benjamin Kwittken, Student Author dr. emily n. taylor, research advisor abstract
Christie Ward - The Question of Cushings
 Many horse people are familiar with the classical symptom of advanced Cushing's disease in horses: a shaggy coat that refuses to shed out in the spring. But did you know that this hormonal disease can
Many horse people are familiar with the classical symptom of advanced Cushing's disease in horses: a shaggy coat that refuses to shed out in the spring. But did you know that this hormonal disease can
Course Offerings: Associate of Applied Science Veterinary Technology. Course Number Name Credits
 Course Offerings: Associate of Applied Science Veterinary Technology Course Number Name Credits Required Courses in Major: Fall Semester, First Year *VETT-101 Animal Health Careers 1-0-1 *VETT-102 Veterinary
Course Offerings: Associate of Applied Science Veterinary Technology Course Number Name Credits Required Courses in Major: Fall Semester, First Year *VETT-101 Animal Health Careers 1-0-1 *VETT-102 Veterinary
CONSERVATIONAL IMPLICATIONS OF TEMPERATURE-DEPENDENT SEX DETERMINATION CORIE L. THERRIEN THANE WIBBLES, COMMITTEE CHAIR KEN MARION LARRY BOOTS
 CONSERVATIONAL IMPLICATIONS OF TEMPERATURE-DEPENDENT SEX DETERMINATION by CORIE L. THERRIEN THANE WIBBLES, COMMITTEE CHAIR KEN MARION LARRY BOOTS A THESIS Submitted to the graduate faculty of The University
CONSERVATIONAL IMPLICATIONS OF TEMPERATURE-DEPENDENT SEX DETERMINATION by CORIE L. THERRIEN THANE WIBBLES, COMMITTEE CHAIR KEN MARION LARRY BOOTS A THESIS Submitted to the graduate faculty of The University
5 State of the Turtles
 CHALLENGE 5 State of the Turtles In the previous Challenges, you altered several turtle properties (e.g., heading, color, etc.). These properties, called turtle variables or states, allow the turtles to
CHALLENGE 5 State of the Turtles In the previous Challenges, you altered several turtle properties (e.g., heading, color, etc.). These properties, called turtle variables or states, allow the turtles to
Maternal Effects in the Green Turtle (Chelonia mydas)
 Maternal Effects in the Green Turtle (Chelonia mydas) SUBMITTED BY SAM B. WEBER TO THE UNIVERSITY OF EXETER AS A THESIS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY; 8 TH JUNE 2010 This thesis is
Maternal Effects in the Green Turtle (Chelonia mydas) SUBMITTED BY SAM B. WEBER TO THE UNIVERSITY OF EXETER AS A THESIS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY; 8 TH JUNE 2010 This thesis is
Overview. Mike Smith presentation Oct. 8, 2014 ARSBC PHYSIOLOGICAL PRINCIPLES UNDERLYING SYNCHRONIZATION OF ESTRUS
 Mike Smith presentation ct., 1 PHYSILGICAL PRINCIPLES UNDERLYING SYNCHRNIZATIN F ESTRUS M.F. Smith, G.A. Perry, J.A. Atkins, K.G. Pohler, R.M. Wallace, S.E. Dickinson, A.. Gatea and D.J. Patterson Division
Mike Smith presentation ct., 1 PHYSILGICAL PRINCIPLES UNDERLYING SYNCHRNIZATIN F ESTRUS M.F. Smith, G.A. Perry, J.A. Atkins, K.G. Pohler, R.M. Wallace, S.E. Dickinson, A.. Gatea and D.J. Patterson Division
The Friends of Nachusa Grasslands 2016 Scientific Research Project Grant Report Due June 30, 2017
 The Friends of Nachusa Grasslands 2016 Scientific Research Project Grant Report Due June 30, 2017 Name: Laura Adamovicz Address: 2001 S Lincoln Ave, Urbana, IL 61802 Phone: 217-333-8056 2016 grant amount:
The Friends of Nachusa Grasslands 2016 Scientific Research Project Grant Report Due June 30, 2017 Name: Laura Adamovicz Address: 2001 S Lincoln Ave, Urbana, IL 61802 Phone: 217-333-8056 2016 grant amount:
RESEARCH AND PROFESSIONAL EXPERIENCE
 Yu Ping Tang Department of Psychology 293 Farm Lane 108 Giltner Hall Michigan State University East Lansing, Michigan 48824 Tel: 517-4325113 Fax: 517-4322744 E-mail: tangyupi@msu.edu RESIDENCE 3931 Trailwood
Yu Ping Tang Department of Psychology 293 Farm Lane 108 Giltner Hall Michigan State University East Lansing, Michigan 48824 Tel: 517-4325113 Fax: 517-4322744 E-mail: tangyupi@msu.edu RESIDENCE 3931 Trailwood
AN OVERVIEW OF THE LATEST RESEARCH EXAMINING THE IMPACT OF STRESS ON THE HEALTH AND WELFARE OF BEEF CATTLE
 1 AN OVERVIEW OF THE LATEST RESEARCH EXAMINING THE IMPACT OF STRESS ON THE HEALTH AND WELFARE OF BEEF CATTLE Dr. Bernadette Earley, Animal and Bioscience Research Department, Animal & Grassland Research
1 AN OVERVIEW OF THE LATEST RESEARCH EXAMINING THE IMPACT OF STRESS ON THE HEALTH AND WELFARE OF BEEF CATTLE Dr. Bernadette Earley, Animal and Bioscience Research Department, Animal & Grassland Research
Acutely Restricting Nutrition Causes Anovulation and Alters Endocrine Function in Beef Heifers
 Acutely Restricting Nutrition Causes Anovulation and Alters Endocrine Function in Beef Heifers F.J. White, L.N. Floyd, C.A. Lents, N.H. Ciccioli, L.J. Spicer, and R.P. Wettemann Story in Brief The effects
Acutely Restricting Nutrition Causes Anovulation and Alters Endocrine Function in Beef Heifers F.J. White, L.N. Floyd, C.A. Lents, N.H. Ciccioli, L.J. Spicer, and R.P. Wettemann Story in Brief The effects
The following part explains the actual status of scientific investigations/knowledge.
 Sebaceaous Adenitis a mysterious skin disease Overview Sebaceous adenitis (SA) is an uncommon inflammatory disease centred on the destruction of the sebaceous glands. The disease has been reported in many
Sebaceaous Adenitis a mysterious skin disease Overview Sebaceous adenitis (SA) is an uncommon inflammatory disease centred on the destruction of the sebaceous glands. The disease has been reported in many
Overview PHYSIOLOGICAL PRINCIPLES UNDERLYING SYNCHRONIZATION OF ESTRUS
 PHYSILGICAL PRINCIPLES UNDERLYING SYNCHRNIZATIN F ESTRUS M.F. Smith, G.A. Perry, J.A. Atkins, E.M. Jinks, K.G. Pohler, and D.J. Patterson Division of Animal Sciences, University of Missouri, Columbia Department
PHYSILGICAL PRINCIPLES UNDERLYING SYNCHRNIZATIN F ESTRUS M.F. Smith, G.A. Perry, J.A. Atkins, E.M. Jinks, K.G. Pohler, and D.J. Patterson Division of Animal Sciences, University of Missouri, Columbia Department
Dominance/Suppression Competitive Relationships in Loblolly Pine (Pinus taeda L.) Plantations
 Dominance/Suppression Competitive Relationships in Loblolly Pine (Pinus taeda L.) Plantations by Michael E. Dyer Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and Stand University
Dominance/Suppression Competitive Relationships in Loblolly Pine (Pinus taeda L.) Plantations by Michael E. Dyer Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and Stand University
Update of Ferret Adrenal Disease: Etiology, Diagnosis, and Treatment
 Update of Ferret Adrenal Disease: Etiology, Diagnosis, and Treatment Cathy A. Johnson-Delaney, DVM, Dipl ABVP (Avian) Session #135 Affiliation: From Eastside Avian & Exotic Animal Medical Center, PLLC,
Update of Ferret Adrenal Disease: Etiology, Diagnosis, and Treatment Cathy A. Johnson-Delaney, DVM, Dipl ABVP (Avian) Session #135 Affiliation: From Eastside Avian & Exotic Animal Medical Center, PLLC,
HOW XTC IMPROVED MINOXIDIL PENETRATION - 5 WAYS!
 HOW XTC IMPROVED MINOXIDIL PENETRATION - 5 WAYS! What Hinders Minoxidil from Working Well 1. Sebum from sebaceous gland blocks the hair follicle. 2. Minoxidil therefore, cannot penetrate through the sebum
HOW XTC IMPROVED MINOXIDIL PENETRATION - 5 WAYS! What Hinders Minoxidil from Working Well 1. Sebum from sebaceous gland blocks the hair follicle. 2. Minoxidil therefore, cannot penetrate through the sebum
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
THE RELATIONSHIP BETWEEN SEASONAL STEROID HORMONE CONCENTRATIONS AND THE REPRODUCTIVE CYCLE IN THE NORTHERN PACIFIC RATTLESNAKE, CROTALUS OREGANUS
 THE RELATIONSHIP BETWEEN SEASONAL STEROID HORMONE CONCENTRATIONS AND THE REPRODUCTIVE CYCLE IN THE NORTHERN PACIFIC RATTLESNAKE, CROTALUS OREGANUS A Thesis Presented to The Faculty of California Polytechnic
THE RELATIONSHIP BETWEEN SEASONAL STEROID HORMONE CONCENTRATIONS AND THE REPRODUCTIVE CYCLE IN THE NORTHERN PACIFIC RATTLESNAKE, CROTALUS OREGANUS A Thesis Presented to The Faculty of California Polytechnic
INFO SHEET. Cull Eggs: What To Expect And How To Reduce The Incidence.
 INFO SHEET Cull Eggs: What To Expect And How To Reduce The Incidence info.hybrid@hendrix-genetics.com www.hybridturkeys.com Introduction Over the years, several Hybrid customers have inquired about the
INFO SHEET Cull Eggs: What To Expect And How To Reduce The Incidence info.hybrid@hendrix-genetics.com www.hybridturkeys.com Introduction Over the years, several Hybrid customers have inquired about the
Overview. Clinical signs. Will you treat? Owner willing to treat? Surgical vs. Medical. Medical options
 Part II (cushing s disease is hard to diagnose) Cushing s Disease Is Easy To Treat Why test? When to test? How to test? Will you treat? How to treat? Overview Thomas Schermerhorn, VMD, DACVIM(SAIM) Kansas
Part II (cushing s disease is hard to diagnose) Cushing s Disease Is Easy To Treat Why test? When to test? How to test? Will you treat? How to treat? Overview Thomas Schermerhorn, VMD, DACVIM(SAIM) Kansas
Elanco Osurnia US. New Case - Pet Owner
 Elanco Osurnia US New Case - Pet Owner Elanco Osurnia US Recheck - Pet Owner Elanco Osurnia US Recurring - Pet Owner 617786 USA 921849 Otic gel Antibacterial, antifungal, anti-inflammatory For Otic Use
Elanco Osurnia US New Case - Pet Owner Elanco Osurnia US Recheck - Pet Owner Elanco Osurnia US Recurring - Pet Owner 617786 USA 921849 Otic gel Antibacterial, antifungal, anti-inflammatory For Otic Use
EFFECTS OF SEASON AND RESTRICTED FEEDING DURING REARING AND LAYING ON PRODUCTIVE AND REPRODUCTIVE PERFORMANCE OF KOEKOEK CHICKENS IN LESOTHO
 EFFECTS OF SEASON AND RESTRICTED FEEDING DURING REARING AND LAYING ON PRODUCTIVE AND REPRODUCTIVE PERFORMANCE OF KOEKOEK CHICKENS IN LESOTHO By SETSUMI MOTŠOENE MOLAPO MSc (Animal Science) NUL Thesis submitted
EFFECTS OF SEASON AND RESTRICTED FEEDING DURING REARING AND LAYING ON PRODUCTIVE AND REPRODUCTIVE PERFORMANCE OF KOEKOEK CHICKENS IN LESOTHO By SETSUMI MOTŠOENE MOLAPO MSc (Animal Science) NUL Thesis submitted
Faculty Mentor, Department of Integrative Biology, Oklahoma State University
 Sex Recognition in Anole Lizards Authors: Shelby Stavins and Dr. Matthew Lovern * Abstract: Sexual selection is the process that furthers a species, and either improves the genetic variability or weakens
Sex Recognition in Anole Lizards Authors: Shelby Stavins and Dr. Matthew Lovern * Abstract: Sexual selection is the process that furthers a species, and either improves the genetic variability or weakens
Endocrine and reproductive responses to implants of deslorein acetate in horses
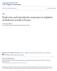 Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2002 Endocrine and reproductive responses to implants of deslorein acetate in horses Carrie Ann Johnson Louisiana
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2002 Endocrine and reproductive responses to implants of deslorein acetate in horses Carrie Ann Johnson Louisiana
REPORT OF ACTIVITIES TURTLE ECOLOGY RESEARCH REPORT Crescent Lake National Wildlife Refuge 31 May to 4 July 2017
 REPORT OF ACTIVITIES 2017 TURTLE ECOLOGY RESEARCH REPORT Crescent Lake National Wildlife Refuge 31 May to 4 July 2017 A report submitted to Refuge Biologist Marlin French 15 July 2017 John B Iverson Dept.
REPORT OF ACTIVITIES 2017 TURTLE ECOLOGY RESEARCH REPORT Crescent Lake National Wildlife Refuge 31 May to 4 July 2017 A report submitted to Refuge Biologist Marlin French 15 July 2017 John B Iverson Dept.
Primates: Cercopithecidae
 Primates: Cercopithecidae Fact Sheet Compiled by: Yedra Feltrer MSc MRCVS ZSL veterinary officer Last Updated: March 2014 Fact Sheet Reviewed by: Sally Boutelle MS Contraceptive methods: GnRH agonist (implant)
Primates: Cercopithecidae Fact Sheet Compiled by: Yedra Feltrer MSc MRCVS ZSL veterinary officer Last Updated: March 2014 Fact Sheet Reviewed by: Sally Boutelle MS Contraceptive methods: GnRH agonist (implant)
MEDICAL CENTER POLICY NO.
 Vice President and Chief Executive Officer of the Medical Center MEDICAL CENTER POLICY NO. 0246 A. SUBJECT: Animals in the Medical Center B. EFFECTIVE DATE: January 1, 2014 (R) C. POLICY: The University
Vice President and Chief Executive Officer of the Medical Center MEDICAL CENTER POLICY NO. 0246 A. SUBJECT: Animals in the Medical Center B. EFFECTIVE DATE: January 1, 2014 (R) C. POLICY: The University
Avian Reproductive System Female
 extension Avian Reproductive System Female articles.extension.org/pages/65372/avian-reproductive-systemfemale Written by: Dr. Jacquie Jacob, University of Kentucky For anyone interested in raising chickens
extension Avian Reproductive System Female articles.extension.org/pages/65372/avian-reproductive-systemfemale Written by: Dr. Jacquie Jacob, University of Kentucky For anyone interested in raising chickens
Australian and New Zealand College of Veterinary Scientists. Fellowship Examination. Veterinary Anaesthesia and Critical Care Paper 1
 Australian and New Zealand College of Veterinary Scientists Fellowship Examination June 2016 Veterinary Anaesthesia and Critical Care Paper 1 Perusal time: Twenty (20) minutes Time allowed: Three (3) hours
Australian and New Zealand College of Veterinary Scientists Fellowship Examination June 2016 Veterinary Anaesthesia and Critical Care Paper 1 Perusal time: Twenty (20) minutes Time allowed: Three (3) hours
Anestrus and Estrous Detection Aids
 Anestrus and Estrous Detection Aids IRM-7 Dairy Integrated Reproductive Management Dr. M.A. Varner University of Maryland The accurate and efficient detection of estrus (heat) in dairy cattle is an important
Anestrus and Estrous Detection Aids IRM-7 Dairy Integrated Reproductive Management Dr. M.A. Varner University of Maryland The accurate and efficient detection of estrus (heat) in dairy cattle is an important
Sheikh Muhammad Abdur Rashid Population ecology and management of Water Monitors, Varanus salvator (Laurenti 1768) at Sungei Buloh Wetland Reserve,
 Author Title Institute Sheikh Muhammad Abdur Rashid Population ecology and management of Water Monitors, Varanus salvator (Laurenti 1768) at Sungei Buloh Wetland Reserve, Singapore Thesis (Ph.D.) National
Author Title Institute Sheikh Muhammad Abdur Rashid Population ecology and management of Water Monitors, Varanus salvator (Laurenti 1768) at Sungei Buloh Wetland Reserve, Singapore Thesis (Ph.D.) National
JoJoKeKe s Herpetology Exam
 ~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~~*~*~*~*~*~*~*~*~*~*~*~*~*~*~ JoJoKeKe s Herpetology Exam (SSSS) 2:30 to be given at each station- B/C Station 1: 1.) What is the family & genus of the shown
~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~~*~*~*~*~*~*~*~*~*~*~*~*~*~*~ JoJoKeKe s Herpetology Exam (SSSS) 2:30 to be given at each station- B/C Station 1: 1.) What is the family & genus of the shown
Dairy Science 305 Lactation Physiology Fall 2014 Syllabus
 Dairy Science 305 Lactation Physiology Fall 2014 Syllabus Instructor: Laura Hernandez, Ph.D. Office: 864 Animal Science Phone: 263-9867 Email: llhernandez@wisc.edu Office Hours: By appointment Lecture:
Dairy Science 305 Lactation Physiology Fall 2014 Syllabus Instructor: Laura Hernandez, Ph.D. Office: 864 Animal Science Phone: 263-9867 Email: llhernandez@wisc.edu Office Hours: By appointment Lecture:
INTRODUCTION TO ANIMAL AND VETERINARY SCIENCE CURRICULUM. Unit 1: Animals in Society/Global Perspective
 Chariho Regional School District - Science Curriculum September, 2016 INTRODUCTION TO ANIMAL AND VETERINARY SCIENCE CURRICULUM Unit 1: Animals in Society/Global Perspective Students will gain an understanding
Chariho Regional School District - Science Curriculum September, 2016 INTRODUCTION TO ANIMAL AND VETERINARY SCIENCE CURRICULUM Unit 1: Animals in Society/Global Perspective Students will gain an understanding
Livestock and Poultry Environmental Learning Center Webcast Series March 28, 2008
 Antibiotic and Hormone Use in Livestock Production Paul Ebner Assistant Professor Department of Animal Sciences Purdue University Presentation Outline Antibiotics and Hormones a. How they are used b. Quantities
Antibiotic and Hormone Use in Livestock Production Paul Ebner Assistant Professor Department of Animal Sciences Purdue University Presentation Outline Antibiotics and Hormones a. How they are used b. Quantities
Everglades Invasive Reptile and Amphibian Monitoring Program 1
 WEC386 Everglades Invasive Reptile and Amphibian Monitoring Program 1 Rebecca G. Harvey, Mike Rochford, Jennifer Ketterlin, Edward Metzger III, Jennifer Nestler, and Frank J. Mazzotti 2 Introduction South
WEC386 Everglades Invasive Reptile and Amphibian Monitoring Program 1 Rebecca G. Harvey, Mike Rochford, Jennifer Ketterlin, Edward Metzger III, Jennifer Nestler, and Frank J. Mazzotti 2 Introduction South
VETERINARY BIOMEDICAL SCIENCES (VBSC)
 Veterinary Biomedical Sciences (VBSC) 1 VETERINARY BIOMEDICAL SCIENCES (VBSC) VBSC 5000 Master s Research and Thesis Prerequisites: Graduate standing. Description: Research problem for meeting requirements
Veterinary Biomedical Sciences (VBSC) 1 VETERINARY BIOMEDICAL SCIENCES (VBSC) VBSC 5000 Master s Research and Thesis Prerequisites: Graduate standing. Description: Research problem for meeting requirements
Spot the Difference: Using the domestic cat as a model for the nutritional management of captive cheetahs. Katherine M. Bell
 Spot the Difference: Using the domestic cat as a model for the nutritional management of captive cheetahs Katherine M. Bell Edited by Lucy A. Tucker and David G. Thomas Illustrated by Justine Woosnam and
Spot the Difference: Using the domestic cat as a model for the nutritional management of captive cheetahs Katherine M. Bell Edited by Lucy A. Tucker and David G. Thomas Illustrated by Justine Woosnam and
CCAC guidelines on: the care and use of fish in research, teaching and testing
 CCAC guidelines on: the care and use of fish in research, teaching and testing Gilly Griffin, PhD Guidelines Program Director Harmonisation of the Care and Use of Fish in Research Gardermoen, Norway May
CCAC guidelines on: the care and use of fish in research, teaching and testing Gilly Griffin, PhD Guidelines Program Director Harmonisation of the Care and Use of Fish in Research Gardermoen, Norway May
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Maprelin 75 µg/ml solution for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Maprelin 75 µg/ml solution for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Suprelorin 4.7 mg implant for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Deslorelin (as
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Suprelorin 4.7 mg implant for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Deslorelin (as
Evolution of Dog. Celeste, Dan, Jason, Tyler
 Evolution of Dog Celeste, Dan, Jason, Tyler Early Canid Domestication: Domestication Natural Selection & Artificial Selection (Human intervention) Domestication: Morphological, Physiological and Behavioral
Evolution of Dog Celeste, Dan, Jason, Tyler Early Canid Domestication: Domestication Natural Selection & Artificial Selection (Human intervention) Domestication: Morphological, Physiological and Behavioral
2 nd Term Final. Revision Sheet. Students Name: Grade: 11 A/B. Subject: Biology. Teacher Signature. Page 1 of 11
 2 nd Term Final Revision Sheet Students Name: Grade: 11 A/B Subject: Biology Teacher Signature Page 1 of 11 Nour Al Maref International School Riyadh, Saudi Arabia Biology Worksheet (2 nd Term) Chapter-26
2 nd Term Final Revision Sheet Students Name: Grade: 11 A/B Subject: Biology Teacher Signature Page 1 of 11 Nour Al Maref International School Riyadh, Saudi Arabia Biology Worksheet (2 nd Term) Chapter-26
Biology Slide 1 of 50
 Biology 1 of 50 2 of 50 What Is a Reptile? What are the characteristics of reptiles? 3 of 50 What Is a Reptile? What Is a Reptile? A reptile is a vertebrate that has dry, scaly skin, lungs, and terrestrial
Biology 1 of 50 2 of 50 What Is a Reptile? What are the characteristics of reptiles? 3 of 50 What Is a Reptile? What Is a Reptile? A reptile is a vertebrate that has dry, scaly skin, lungs, and terrestrial
Chasing Chickens: 40 Years of Pecking and Scratching. Nelson A. Cox ARS-PMSRU Russell Research Center, Athens GA 30607
 Chasing Chickens: 40 Years of Pecking and Scratching Nelson A. Cox USDA-ARS ARS-PMSRU Russell Research Center, Athens GA 30607 Education (LSU) B. S. (1966) Bacteriology M. S. (1968) Food Science (Microbiology
Chasing Chickens: 40 Years of Pecking and Scratching Nelson A. Cox USDA-ARS ARS-PMSRU Russell Research Center, Athens GA 30607 Education (LSU) B. S. (1966) Bacteriology M. S. (1968) Food Science (Microbiology
Call of the Wild. Investigating Predator/Prey Relationships
 Biology Call of the Wild Investigating Predator/Prey Relationships MATERIALS AND RESOURCES EACH GROUP calculator computer spoon, plastic 100 beans, individual pinto plate, paper ABOUT THIS LESSON This
Biology Call of the Wild Investigating Predator/Prey Relationships MATERIALS AND RESOURCES EACH GROUP calculator computer spoon, plastic 100 beans, individual pinto plate, paper ABOUT THIS LESSON This
Current Status of Amphibian Populations. Amphibian biology - characteristics making
 Global Amphibian Declines: What Have We Done? Mike Tyler Steve Holmer Nikki Maxwell University of Tennessee Knoxville Department of Forestry, Wildlife and Fisheries Graduate Student Seminar 15 October
Global Amphibian Declines: What Have We Done? Mike Tyler Steve Holmer Nikki Maxwell University of Tennessee Knoxville Department of Forestry, Wildlife and Fisheries Graduate Student Seminar 15 October
Weaver Dunes, Minnesota
 Hatchling Orientation During Dispersal from Nests Experimental analyses of an early life stage comparing orientation and dispersal patterns of hatchlings that emerge from nests close to and far from wetlands
Hatchling Orientation During Dispersal from Nests Experimental analyses of an early life stage comparing orientation and dispersal patterns of hatchlings that emerge from nests close to and far from wetlands
2009 MN Cattle Feeder Days Jolene Kelzer University of Minnesota Beef Team
 2009 MN Cattle Feeder Days Jolene Kelzer University of Minnesota Beef Team 101.8 M total US cattle and calves (July 1) Down 1% from 2008 (103.3 M) 11.6 M total US cattle on feed (July 1) Down 5% from 2008
2009 MN Cattle Feeder Days Jolene Kelzer University of Minnesota Beef Team 101.8 M total US cattle and calves (July 1) Down 1% from 2008 (103.3 M) 11.6 M total US cattle on feed (July 1) Down 5% from 2008
COMPARING BODY CONDITION ESTIMATES OF ZOO BROTHER S ISLAND TUATARA (SPHENODON GUNTHERI) TO THAT OF THE WILD, A CLINICAL CASE
 COMPARING BODY CONDITION ESTIMATES OF ZOO BROTHER S ISLAND TUATARA (SPHENODON GUNTHERI) TO THAT OF THE WILD, A CLINICAL CASE Kyle S. Thompson, BS,¹, ²* Michael L. Schlegel, PhD, PAS² ¹Oklahoma State University,
COMPARING BODY CONDITION ESTIMATES OF ZOO BROTHER S ISLAND TUATARA (SPHENODON GUNTHERI) TO THAT OF THE WILD, A CLINICAL CASE Kyle S. Thompson, BS,¹, ²* Michael L. Schlegel, PhD, PAS² ¹Oklahoma State University,
Homework Case Study Update #3
 Homework 7.1 - Name: The graph below summarizes the changes in the size of the two populations you have been studying on Isle Royale. 1996 was the year that there was intense competition for declining
Homework 7.1 - Name: The graph below summarizes the changes in the size of the two populations you have been studying on Isle Royale. 1996 was the year that there was intense competition for declining
Failure of Gonadotropin Therapy to Induce Estrus in Gilts Treated with a GnRH Analog to Suppress Ovarian Activity
 Failure of Gonadotropin Therapy to Induce Estrus in Gilts Treated with a GnRH Analog to Suppress Ovarian Activity Antonio Garcia, DVM, PhD (deceased) a Mark J. Estienne, PhD b Allen F. Harper, PhD b James
Failure of Gonadotropin Therapy to Induce Estrus in Gilts Treated with a GnRH Analog to Suppress Ovarian Activity Antonio Garcia, DVM, PhD (deceased) a Mark J. Estienne, PhD b Allen F. Harper, PhD b James
Therapeutic apheresis in veterinary
 Therapeutic apheresis in veterinary 1 I.P.Pavlov First St.-Petersburg State Medical University, Saint-Petersburg, Russia. Voinov V.A. A. By types of animals on the basis of anatomical and physiological
Therapeutic apheresis in veterinary 1 I.P.Pavlov First St.-Petersburg State Medical University, Saint-Petersburg, Russia. Voinov V.A. A. By types of animals on the basis of anatomical and physiological
The estrous cycle. lecture 3. Dr. Wafer M. Salih Dr. Sadeq J. Zalzala Dr. Haydar A. AL-mutar Dr. Ahmed M. Zakri
 The estrous cycle lecture 3 By Dr. Wafer M. Salih Dr. Sadeq J. Zalzala Dr. Haydar A. AL-mutar Dr. Ahmed M. Zakri The estrous cycle Definition Sexual Puberty in the females is defined as the age at the
The estrous cycle lecture 3 By Dr. Wafer M. Salih Dr. Sadeq J. Zalzala Dr. Haydar A. AL-mutar Dr. Ahmed M. Zakri The estrous cycle Definition Sexual Puberty in the females is defined as the age at the
Evaluation of the hair growth and retention activity of two solutions on human hair explants
 activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
The color and patterning of pigmentation in cats, dogs, mice horses and other mammals results from the interaction of several different genes
 The color and patterning of pigmentation in cats, dogs, mice horses and other mammals results from the interaction of several different genes 1 Gene Interactions: Specific alleles of one gene mask or modify
The color and patterning of pigmentation in cats, dogs, mice horses and other mammals results from the interaction of several different genes 1 Gene Interactions: Specific alleles of one gene mask or modify
Naturally occurring hyperadrenocorticism is a wellrecognized
 Evaluation of twice-daily lower-dose trilostane treatment administered orally in dogs with naturally occurring hyperadrenocorticism Edward C. Feldman, dvm, dacvim Objective To evaluate effectiveness and
Evaluation of twice-daily lower-dose trilostane treatment administered orally in dogs with naturally occurring hyperadrenocorticism Edward C. Feldman, dvm, dacvim Objective To evaluate effectiveness and
Luteolysis and Pregnancy Outcomes in Dairy Cows after Treatment with Estrumate or Lutalyse
 Luteolysis and Pregnancy Outcomes in Dairy Cows after Treatment with Estrumate or Lutalyse J. S. Stevenson and A. P. Phatak Summary In Experiment, lactating dairy cows (n =,230) in 6 herds were treated
Luteolysis and Pregnancy Outcomes in Dairy Cows after Treatment with Estrumate or Lutalyse J. S. Stevenson and A. P. Phatak Summary In Experiment, lactating dairy cows (n =,230) in 6 herds were treated
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii. Yates, Lauren A.
 A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
Temperature-Dependent Sex Determination in Crocodilians
 THE JOURNAL OF EXPERIMENTAL ZOOLOGY 270:28-44 (1994) Temperature-Dependent Sex Determination in Crocodilians JEFFREY W. LANG AND HARRY V. ANDREWS Department of BioZogy, University of North Dakota, Grand
THE JOURNAL OF EXPERIMENTAL ZOOLOGY 270:28-44 (1994) Temperature-Dependent Sex Determination in Crocodilians JEFFREY W. LANG AND HARRY V. ANDREWS Department of BioZogy, University of North Dakota, Grand
A description of an Indo-Chinese rat snake (Ptyas korros [Schlegel, 1837]) clutch, with notes on an instance of twinning
![A description of an Indo-Chinese rat snake (Ptyas korros [Schlegel, 1837]) clutch, with notes on an instance of twinning A description of an Indo-Chinese rat snake (Ptyas korros [Schlegel, 1837]) clutch, with notes on an instance of twinning](/thumbs/86/93949508.jpg) 1 2 A description of an Indo-Chinese rat snake (Ptyas korros [Schlegel, 1837]) clutch, with notes on an instance of twinning 3 4 Simon Dieckmann 1, Gerrut Norval 2 * and Jean-Jay Mao 3 5 6 7 8 9 10 11
1 2 A description of an Indo-Chinese rat snake (Ptyas korros [Schlegel, 1837]) clutch, with notes on an instance of twinning 3 4 Simon Dieckmann 1, Gerrut Norval 2 * and Jean-Jay Mao 3 5 6 7 8 9 10 11
Lactation. Macroscopic Anatomy of the Mammary Gland. Anatomy AS 1124
 Lactation AS 1124 Macroscopic Anatomy of the Mammary Gland Species differences in numbers and locations of glands inguinal - caudal to the abdomen, between the hind legs (cow, mare, ewe) abdominal - along
Lactation AS 1124 Macroscopic Anatomy of the Mammary Gland Species differences in numbers and locations of glands inguinal - caudal to the abdomen, between the hind legs (cow, mare, ewe) abdominal - along
Station 1 1. (3 points) Identification: Station 2 6. (3 points) Identification:
 SOnerd s 2018-2019 Herpetology SSSS Test 1 SOnerd s SSSS 2018-2019 Herpetology Test Station 20 sounds found here: https://drive.google.com/drive/folders/1oqrmspti13qv_ytllk_yy_vrie42isqe?usp=sharing Station
SOnerd s 2018-2019 Herpetology SSSS Test 1 SOnerd s SSSS 2018-2019 Herpetology Test Station 20 sounds found here: https://drive.google.com/drive/folders/1oqrmspti13qv_ytllk_yy_vrie42isqe?usp=sharing Station
Metacam 1.5 mg/ml oral suspension for dogs
 Metacam 1.5 mg/ml oral suspension for dogs Species:Dogs Therapeutic indication:pharmaceuticals: Neurological preparations: Analgesics, Other NSAIDs, Locomotor (including navicular and osteoarthritis) Active
Metacam 1.5 mg/ml oral suspension for dogs Species:Dogs Therapeutic indication:pharmaceuticals: Neurological preparations: Analgesics, Other NSAIDs, Locomotor (including navicular and osteoarthritis) Active
Oral fertility control for grey squirrels
 Oral fertility control for grey squirrels Summary The National Wildlife Management Centre (NWMC), under the terms of a contract with the UK Squirrel Accord, is researching the development and delivery
Oral fertility control for grey squirrels Summary The National Wildlife Management Centre (NWMC), under the terms of a contract with the UK Squirrel Accord, is researching the development and delivery
March 16, Guide's space recommendations as a minimum while always recognizing that performance standards also must be met.
 Comments of The American Association of Immunologists (AAI) to the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) Regarding the 8 th Edition of the Guide
Comments of The American Association of Immunologists (AAI) to the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) Regarding the 8 th Edition of the Guide
COMMISSION OF THE EUROPEAN COMMUNITIES REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 20.1.2005 COM(2005) 7 final. REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT FOURTH REPORT ON THE STATISTICS ON THE NUMBER OF ANIMALS
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 20.1.2005 COM(2005) 7 final. REPORT FROM THE COMMISSION TO THE COUNCIL AND THE EUROPEAN PARLIAMENT FOURTH REPORT ON THE STATISTICS ON THE NUMBER OF ANIMALS
Veterinary Medical Terminology
 Curriculum Outline: Course # Required courses prior to admission Credit hours BIO 0 Principles of Biology I with Lab 4 CHM 0 General Chemistry I with Lab 4 ENG 110 or 111 or 1 Freshman Composition or Composition
Curriculum Outline: Course # Required courses prior to admission Credit hours BIO 0 Principles of Biology I with Lab 4 CHM 0 General Chemistry I with Lab 4 ENG 110 or 111 or 1 Freshman Composition or Composition
Title of Project: Distribution of the Collared Lizard, Crotophytus collaris, in the Arkansas River Valley and Ouachita Mountains
 Title of Project: Distribution of the Collared Lizard, Crotophytus collaris, in the Arkansas River Valley and Ouachita Mountains Project Summary: This project will seek to monitor the status of Collared
Title of Project: Distribution of the Collared Lizard, Crotophytus collaris, in the Arkansas River Valley and Ouachita Mountains Project Summary: This project will seek to monitor the status of Collared
Aquarium Department Celebrate, Connect, Care
 Aquarium Department Celebrate, Connect, Care Introduction Gary Violetta Curator of Fishes at SeaWorld Orlando Graduated from Bowling Green State University Major : Marine Science Minor: Chemistry SeaWorld
Aquarium Department Celebrate, Connect, Care Introduction Gary Violetta Curator of Fishes at SeaWorld Orlando Graduated from Bowling Green State University Major : Marine Science Minor: Chemistry SeaWorld
