Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC)
|
|
|
- Darlene Bishop
- 5 years ago
- Views:
Transcription
1 Supplement for Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC) December, 2016 Family Planning Division Ministry of Health and Family Welfare Government of India
2
3 1. Overview Intramuscular injection of Medroxy Progesterone Acetate (MPA) is being widely used as a three monthly Progestin only Injectable contraceptive for over 50 years. A lower dose MPA is also available for subcutaneous use; called MPA-SC. Recently it has been registered in India for use after approval by the DCGI. This lower dose MPA (104 mg/0.65 ml) administered by subcutaneous (SC) route is therapeutically equivalent to the intramuscular formulation. Clinical studies indicate that the product effectively suppresses ovulation for at least 13 weeks regardless of race, ethnicity, BMI and return of fertility is no longer than MPA -IM. 2. Composition The composition of MPA-SC is same as MPA-IM, which is 17 alfa hydroxyprogesterone derivative progestine, medroxyprogesterone acetate. 3. Mechanism of Action Subcutaneous MPA acts in similar way to Intramuscular MPA by: Inhibiting Ovulation Thickening of cervical mucus Thinning of endometrial lining Refer to chapter 2 of 'Reference Manual for Injectable Contraceptive DMPA 2016' 4. Uniject System for Subcutaneous MPA Subcutaneous MPA is available as a single dose in prefilled auto disable injection device (Uniject system). Needle Shield Gap Port Injector Reservoir Fig 1: Subcutaneous Medroxy Progesterone Acetate in Uniject system 4.1 Key Features of Uniject Injection System This Uniject system has a thermoformed plastic laminate reservoir with ultra-thin needle attached by a polyethylene port. It is designed for single use and immediate disposal as it has a one-way valve and collapsible reservoir that cannot be re-filled. Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC) 1
4 4.2 Advantages of Uniject Injection System It is easy to use because it is prefilled and very easy to inject as squeezing the bulb pushes fluid into the needle. It is non-reusable, hence one to one transmission of blood borne pathogens through needle reuse is eliminated. It provides logistical benefits because Uniject is compact and smaller than a syringe and vial, it is easier to transport and store. A study commissioned by PATH found that MPA- SC in Uniject is 62 percent lighter and 25 percent less voluminous than MPA-IM packed with vial and syringe. The formulation for subcutaneous injection provides slower and more sustained absorption of the progestin than intramuscular MPA. This enables a 30 percent lower dose of progestin (104 versus 150 mg) and reduces peak blood levels by half, but with the same duration of effect as intramuscular MPA. A waste management assessment showed that SC- MPA in Uniject generates 70 percent less waste by volume than the standard auto-disable syringe with an empty MPA vial. 5. Safety and Efficacy Based on a systematic review of the evidence, the World Health Organization's (WHO) Medical Eligibility for Contraceptive Use confirms that both Subcutaneous and Intramuscular MPA have a similar safety and efficacy profile. Medical Eligibility Criteria (MEC) is identical to those for MPA- IM. Effectiveness is similar to MPA-IM and is 99.7%. (Refer to chapter 2 of 'Reference Manual for Injectable Contraceptive DMPA 2016') 6. Benefits and Limitations, Return of fertility, Medical Eligibility and Client Assessment, Counselling, Storage Reference Manual for Injectable Contraceptive DMPA 2016). is similar to MPA-IM. (Refer to 7. Time of Initiation Contraceptive DMPA 2016) same as for MPA-IM. Refer to (Reference Manual for Injectable 7.1 Switching from other methods is same as for MPA-IM. (Refer to Reference Manual for Injectable Contraceptive DMPA 2016). 7.2 Switching from MPA- SC to MPA- IM or Vice Versa As the active ingredient in the IM and SC is identical, it is safe to switch back and forth between IM and SC every three months with the same level of contraceptive protection. This switching is safe and it does not decrease effectiveness, though switching should not be a routine practice. If switching is necessary, the injection of the alternate mode should be administered on the due date and duly recorded. Clients need to be explained and told about 2 Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC)
5 changed mode of administration i.e. SC to IM or vice versa and the scheduled date for next injection. MPA-SC should not be used for IM administration and similarly MPA-IM should not be used for MPA-SC administration. 7.3 Comparing MPA Subcutaneous with MPA Intramuscular Features Packaging MPA-SC Available in single dose prefilled auto disabled injection device (Uniject) MPA-IM Available in single dose vials Dosage Single dose contains 104 mg / 0.65 ml of Medroxy Progesterone Acetate, to be administered every 3 months. Single dose contains 150 mg / ml of Medroxy Progesterone Acetate, to be administered every 3 months. Site of Administration Administered subcutaneously in outer anterior portion of thigh, abdomen or upper outer portion of arm. Administered intramuscularly in upper arm, hip or outer side of thigh. Mode of Administration 1. Pierces the epidermal and dermal layers of the skin and delivers drug in loose subcutaneous tissue. 2. Short needle 3. Ease of administration-more surface area available & fewer landmarks needed for SC injection. 1. Given deep intra muscular 2. Longer needle 3. Ease of administration-less surface area and particular landmarks needed to locate specific muscle. 8. Administration of MPA-SC 8.1 Pre-Injection Preparation Site Selection and Preparation of Site of Injection The injection site is subcutaneous fat of anterior outer part of thigh, abdomen (Below Umblicus) or upper and outer part of arm. The preferred, easily accessible and acceptable site should be taken into consideration before administration of injection. Avoid bony areas and the umbilicus. Change the injection site with each injection. The area of skin must be free from scars and skin conditions such as eczema or psoriasis. Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC) 3
6 A. Abdomen (below umbilicus) B. Anterior outer part of thigh C. Upper and Outer part of arm Fig 2: Sites of administration of MPA- SC: A. Abdomen; B. Anterior Outer part of thigh; C. Back of Upper Arm Use an antiseptic swab to wipe the skin in the injection area, chosen by the client. Allow the skin to dry Preparing the Injector When provider is ready to administer the injection, carefully tear open the foil pouch and remove the injector. Do not remove the needle shield at this stage. Check the injector as follows The needle shield should be in the position shown in the diagram below (Fig 3). There should be a gap between the needle shield and the port. If there is no gap discard the injector and use a new one. If the needle shield has come off the needle or is missing altogether, discard the injector and use a new one. Reservoir Port Gap Injector Needle Shield Fig 3: Diagrammatic representation of parts of Uniject Mixing MPA-SC in Reservoir Hold the injector firmly by the port. Shake the injector vigorously for 30 seconds to mix the medicine thoroughly. If there is any delay between mixing the medicine and proceeding through the next step, repeat the mixing procedure as above. Check the injector. The liquid contents should appear white to off white and uniform. There should be no leakage from any part. If any problem is observed discard the injector and use a new one. 4 Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC)
7 8.1.4 Activating the Injector Hold the injector firmly by the port with one hand.take care not to squeeze the reservoir. Hold the needle shield with other hand. Gap will be visible between the port and the end of the needle shield. Push the needle shield towards the port. Continue to push firmly until gap is closed between needle shield and the port.the injector is now activated. Continue to hold the injector firmly by the port. Pull the needle shield away to remove it from needle and discard. 8.2 Administering/Injecting the Dose Gently grasp and squeeze a large area of skin in the chosen area between thumb and fore finger, pulling it away from body. Hold the injector by port. Keep it as upright as possible with needle pointing downwards. Insert the needle into the skin so that the needle tip is in the subcutaneous tissue and the port just touches the skin. Hold the reservoir firmly between the thumb and the forefinger. Squeeze the reservoir slowly to inject the medicine. It should take 5-7 seconds. After the dose has been completely injected and the reservoir has collapsed, gently pull the needle out of the skin. It is important that the full dose of medicine is given, however small traces of white liquid may remain visibile inside the edge. This is normal. 8.3 Post Injection Care: Check whether any medicine has leaked out of the injector or has appeared on the skin. If complete dose has not been administered then additional dose should not be given. Advise client to use back up contraceptive method. Do not replace the needle shield after use. Use a clean cotton swab to press lightly on the injection area for a few seconds. Do not rub or massage the injection site. Follow safe practice for disposal of used injector and needles. The MPA-SC Uniject syringe, after use, should be placed in the container/box for disposal of sharps. The injector is for a single injecion only. It should be never reused. Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC) 5
8 8.4 Post Injection Instructions: same as MPA- IM Instruct client that she must come after 90 days for a repeat injection and give her the scheduled date. Hand over the MPA Client Card to her after explaining its content to her. Inform the client that the effect of injection is immediate if given between 'day one' to 'day seven' of her menstrual cycle or abortion. But if given after 'day seven' a backup contraceptive method (e.g. condom) should be used for 7 days. Assure the client that she is welcome to come back any time, if she has any problem, wants another method, has a major menstrual change, has a major change in health status or thinks might be pregnant. Ensure post injection counselling. 9. Follow Up Care, Management of Side effects, Special Issues on MPA is similar to MPA- IM. Refer to 'Reference Manual for Injectable Contraceptive DMPA 2016' 10. Infection Prevention Practices Follow standard guidelines of GoI as mentioned in chapter 9 of 'Reference Manual for Injectable Contraceptive DMPA 2016' 6 Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC)
9 Frequently Asked Questions on Subcutaneous Injectable Contraceptive Medroxy Progesterone Acetate (FAQ on MPA-SC) 1. How is MPA-SC different from MPA-IM? A. There is no difference between MPA-SC and MPA-IM in terms of composition, mechanism of action, safety, efficacy, benefits and side effects, except for the amount of drug and route of administration. However, Uniject injection system of subcutaneous injectable contraceptive Medroxy Progesterone Acetate provides ease of administration which minimizes chances of infection transmission and the potential to benefit system-level logistics in terms of storage, transport, distribution and waste management. 2. Is there any difference in duration of effectivity of MPA-SC and MPA-IM? A. No, there is no difference as they both are 3 monthly contraceptive injections. 3. Is MPA-SC as effective as MPA-IM for contraceptive protection? A. Yes, MPA-SC is as effective as MPA-IM. Studies have demonstrated that subcutaneous injectable contraceptive Medroxy Progesterone Acetate (MPA-SC) provides efficacy, safety and immediacy of contraceptive effect equivalent to the MPA-IM. In clinical trials, it effectively suppressed ovulation for at least three months in all subjects regardless of ethnicity, race and body mass index. 4. Can MPA-SC be administered to those women who cannot be given MPA-IM and vice-versa? A. No, SC-MPA cannot be administered to those women who are not eligible to take MPA-IM and vice versa. 5. Are the menstrual changes with MPA-SC different from those of MPA-IM? A. No, both MPA-IM and MPA-SC cause similar menstrual changes as the hormonal level in blood is same. 6. Can a woman switch between MPA-IM and MPA-SC? A. Yes, if necessary, because the active ingredient in the IM and SC formulations is identical, it is safe to switch back and forth between these two formulations on a regular dosing schedule (i.e., every three months) with the same level of contraceptive protection. Switching injectable is safe, and it does not decrease effectiveness. If switching is necessary, the first injection of the new injectable should be given when the next injection of the old formulation was due. Clients need to be informed and explained about the name of the new injectable, and its injection schedule. Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC) 7
10 7. What will happen if subcutaneous injectable contraceptive Medroxy Progesterone Acetate is administered intramuscularly? A. To ensure three months of contraceptive protection, Subcutaneous Injectable Contraceptive Medroxy Progesterone Acetate must be administered subcutaneously only. If MPA is given in a muscle, it may not provide protection for full three moths. However, the needle of MPA-SC is smaller (3/8 inches) than the MPA-IM needle, so it is not likely to reach the muscle hence giving MPA-SC by IM route is practically not possible. 8. Does MPA-SC have a different effect on Bone Mineral Density compared to MPA-IM? A. No, the effect of MPA-SC on bone density is similar to MPA-IM. Most studies have found that women lose BMD while using MPA but regain all or partial BMD after discontinuation. According to WHO, for women aged 18 to 45 years, there should be no restrictions on the use of MPA, including no restrictions on the duration of its use; and the advantages for adolescents younger than 18 years of using MPA generally outweigh the theoretical or proven risks, so they can also be given MPA. 9. Does Body Mass Index (BMI) affect the efficacy of MPA-SC? A. No, Clinical studies to date demonstrate that the contraceptive efficacy of the active ingredient in MPA-SC is not affected by body mass index (weight-to-height ratio). 8 Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC)
11 References 1. SAYANA PRESS 104 mg/0.65 ml suspension for injection. Aug Accessed on 28 Sep SAYANA PRESS 104 mg/0.65 ml suspension for injection, Package Leaflet: Information for the user. accessed on 28 Sep Jain J et al Contraception. Vol. 70(4): Sandwich, UK: Pfizer Ltd. SAYANA. Summary of Product Characteristics. July Government of India Reference Manual for Injectable Contraceptive DMPA. 6. Andrew M Kaunitz. Depot medroxyprogesterone acetate for contraception. Aug accessed on 5 Oct Bonnie Keith. Home-based administration of depo-subq provera 104 in the Uniject injection system. A literature review. July Medicines and Healthcare Products Regulatory Agency. Public Assessment report 2011 Sayanaject 104 mg suspension for injection (Medroxyprogestrone Acetate), Pfizer ltd UK/H/ 5497/001/ DC. Medroxy Progesterone Acetate-Subcutaneous Injectable Contraceptive (MPA-SC) 9
12 December 2016 Developed with support from National Technical Support Unit (NTSU), Family Planning Division, Ministry of Health & Family Welfare, Government of India
EC-AH-011v1 January 2018 Page 1 of 5. Standard Operating Procedure Equine Center Clemson University
 EC-AH-011v1 January 2018 Page 1 of 5 Standard Operating Procedure Equine Center Clemson University SOP ID: EC-AH-011v1 January 2018 Title: Injection Techniques Author(s): Julia Tagher, CU Equine Center
EC-AH-011v1 January 2018 Page 1 of 5 Standard Operating Procedure Equine Center Clemson University SOP ID: EC-AH-011v1 January 2018 Title: Injection Techniques Author(s): Julia Tagher, CU Equine Center
Administering wormers (anthelmintics) effectively
 COWS www.cattleparasites.org.uk Administering wormers (anthelmintics) effectively COWS is an industry initiative promoting sustainable control strategies for parasites in cattle Wormer administration Dec
COWS www.cattleparasites.org.uk Administering wormers (anthelmintics) effectively COWS is an industry initiative promoting sustainable control strategies for parasites in cattle Wormer administration Dec
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Amfipen LA 100 mg/ml suspension for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Each ml contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Amfipen LA 100 mg/ml suspension for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Each ml contains:
How To Give Your Horse An Intramuscular Injection
 ANR-1018 A L A B A M A A & M A N D A U B U R N U N I V E R S I T I E S How To Give Your Horse An Intramuscular Injection Most horse owners occasionally must give their horse an injection. Fortunately,
ANR-1018 A L A B A M A A & M A N D A U B U R N U N I V E R S I T I E S How To Give Your Horse An Intramuscular Injection Most horse owners occasionally must give their horse an injection. Fortunately,
EXCEDE Sterile Suspension
 VIAL LABEL MAIN PANEL PRESCRIPTION ANIMAL REMEDY KEEP OUT OF REACH OF CHILDREN READ SAFETY DIRECTIONS FOR ANIMAL TREATMENT ONLY EXCEDE Sterile Suspension 200 mg/ml CEFTIOFUR as Ceftiofur Crystalline Free
VIAL LABEL MAIN PANEL PRESCRIPTION ANIMAL REMEDY KEEP OUT OF REACH OF CHILDREN READ SAFETY DIRECTIONS FOR ANIMAL TREATMENT ONLY EXCEDE Sterile Suspension 200 mg/ml CEFTIOFUR as Ceftiofur Crystalline Free
For the treatment of infections caused by a wide range of Gram-positive and Gramnegative pathogenic bacteria including:
 SUMMARY OF PRODUCT CHARCTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Amoxycare Suspension for Injection 15% w/v 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Active Substance(s)
SUMMARY OF PRODUCT CHARCTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Amoxycare Suspension for Injection 15% w/v 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Active Substance(s)
Ear drops suspension. A smooth, uniform, white to off-white viscous suspension.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT OTOMAX EAR DROPS SUSPENSION 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of the veterinary medicinal product contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT OTOMAX EAR DROPS SUSPENSION 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of the veterinary medicinal product contains:
Primates: Cercopithecidae
 Primates: Cercopithecidae Fact Sheet Compiled by: Yedra Feltrer MSc MRCVS ZSL veterinary officer Last Updated: March 2014 Fact Sheet Reviewed by: Sally Boutelle MS Contraceptive methods: GnRH agonist (implant)
Primates: Cercopithecidae Fact Sheet Compiled by: Yedra Feltrer MSc MRCVS ZSL veterinary officer Last Updated: March 2014 Fact Sheet Reviewed by: Sally Boutelle MS Contraceptive methods: GnRH agonist (implant)
DEPOSEL Slow Release Selenium Injection for Cattle and Sheep
 Date of change: 21 October 2004 Page: 1 of 9 Carton (front panel). POISON KEEP OUT OF REACH OF CHILDREN FOR ANIMAL TREATMENT ONLY DEPOSEL Slow Release Selenium Injection for Cattle and Sheep Active ingredient:
Date of change: 21 October 2004 Page: 1 of 9 Carton (front panel). POISON KEEP OUT OF REACH OF CHILDREN FOR ANIMAL TREATMENT ONLY DEPOSEL Slow Release Selenium Injection for Cattle and Sheep Active ingredient:
PACKAGE LEAFLET: INFORMATION FOR THE USER. GENTAMICIN VISION 3 mg/g eye ointment Gentamicin
 PACKAGE LEAFLET: INFORMATION FOR THE USER GENTAMICIN VISION 3 mg/g eye ointment Gentamicin Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You may need to
PACKAGE LEAFLET: INFORMATION FOR THE USER GENTAMICIN VISION 3 mg/g eye ointment Gentamicin Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You may need to
SUMMARY OF PRODUCT CHARACTERISTICS. NUFLOR 300 mg/ml solution for injection for cattle and sheep
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NUFLOR 300 mg/ml solution for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NUFLOR 300 mg/ml solution for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Prescription Label. Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long):
 Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
SUMMARY OF PRODUCT CHARACTERISTICS. Lincomycin (as Lincomycin hydrochloride) Neomycin (as Neomycin sulphate) Excipients Disodium edetate
 SUMMARY OF PRODUCT CHARACTERISTICS AN: 00221/2013 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Lincocin Forte S Intramammary Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Lincomycin
SUMMARY OF PRODUCT CHARACTERISTICS AN: 00221/2013 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Lincocin Forte S Intramammary Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Lincomycin
SINGLE ANNUAL IMPLANT
 Manage pet ferret adrenal cortical disease with a SINGLE ANNUAL IMPLANT NOT APPROVED BY FDA Legally marketed as an FDA Indexed Product under MIF 900-013. FOR USE IN FERRETS ONLY. Extra-label use is prohibited.
Manage pet ferret adrenal cortical disease with a SINGLE ANNUAL IMPLANT NOT APPROVED BY FDA Legally marketed as an FDA Indexed Product under MIF 900-013. FOR USE IN FERRETS ONLY. Extra-label use is prohibited.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BLUEVAC BTV8 suspension for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BLUEVAC BTV8 suspension for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of
B. PACKAGE LEAFLET 1
 B. PACKAGE LEAFLET 1 PACKAGE LEAFLET FOR: Cadorex 300 mg/ml solution for injection for cattle, sheep and pigs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION
B. PACKAGE LEAFLET 1 PACKAGE LEAFLET FOR: Cadorex 300 mg/ml solution for injection for cattle, sheep and pigs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Medicinal product no longer authorised
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BTVPUR AlSap 1 suspension for injection for sheep and cattle. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BTVPUR AlSap 1 suspension for injection for sheep and cattle. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CLYNAV solution for injection for Atlantic salmon 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 0.05 ml dose
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CLYNAV solution for injection for Atlantic salmon 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 0.05 ml dose
Intramuscular injections are administered according to established procedure in the adult patient.
 POLICY & PROCEDURE TITLE: IM Injection in Adult Patient Scope/Purpose: To document the standard of care in administering adult intramuscular injections in HealthPoint clinics. Division/Department: All
POLICY & PROCEDURE TITLE: IM Injection in Adult Patient Scope/Purpose: To document the standard of care in administering adult intramuscular injections in HealthPoint clinics. Division/Department: All
EPA Est. No IL-001. Assurity and Elanco. EPA Reg. No
 Monthly application is recommended to prevent and treat flea infestations. Assurity starts working in 30 minutes, kills adult fleas before they can lay eggs and continues killing fleas for a full month
Monthly application is recommended to prevent and treat flea infestations. Assurity starts working in 30 minutes, kills adult fleas before they can lay eggs and continues killing fleas for a full month
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT COXEVAC suspension for injection for cattle and goats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT COXEVAC suspension for injection for cattle and goats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Giving IV Medication by Balloon System (EIS)
 Giving IV Medication by Balloon System (EIS) Medication: Click here to enter text. What do I do when I get the medication from HomeMed? Always check the prescription and label for final instructions. o
Giving IV Medication by Balloon System (EIS) Medication: Click here to enter text. What do I do when I get the medication from HomeMed? Always check the prescription and label for final instructions. o
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Revised: January 2012 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Blackleg Vaccine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance(s): per ml Five strains
SUMMARY OF PRODUCT CHARACTERISTICS Revised: January 2012 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Blackleg Vaccine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance(s): per ml Five strains
Error! Reference source not found. I. SUMMARY OF PRODUCT CHARACTERISTICS
 PRODUCTNAME NOBIVAC RABIES 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Nobivac Rabies 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active components: Rabies strain Pasteur RIV; at least 2 I.U. per dose
PRODUCTNAME NOBIVAC RABIES 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Nobivac Rabies 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active components: Rabies strain Pasteur RIV; at least 2 I.U. per dose
adult fleas flea eggs flea larvae adult ticks tick nymphs tick larvae KILLS & REPELS: mosquitoes KEEP OUT OF REACH OF CHILDREN CAUTION
 For Use Only on Medium 15-30 lbs. Dogs/Puppies & 12 weeks of age or older 3 MONTH SUPPLY Flea & Tick Spot On For DOGS Includes Applicator KILLS: adult fleas flea eggs flea larvae adult ticks Applicator
For Use Only on Medium 15-30 lbs. Dogs/Puppies & 12 weeks of age or older 3 MONTH SUPPLY Flea & Tick Spot On For DOGS Includes Applicator KILLS: adult fleas flea eggs flea larvae adult ticks Applicator
Guidelines for the administration of SureSeal
 Guidelines for the administration of SureSeal WHAT IS SURESEAL AND WHAT ARE THE INDICATIONS SureSeal contains the inert substance bismuth subnitrate 2.6g suspension and PVP iodine as a preservative in
Guidelines for the administration of SureSeal WHAT IS SURESEAL AND WHAT ARE THE INDICATIONS SureSeal contains the inert substance bismuth subnitrate 2.6g suspension and PVP iodine as a preservative in
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Acecare 2mg/ml Solution for Injection for Dogs and Cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution contains
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Acecare 2mg/ml Solution for Injection for Dogs and Cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution contains
Scientific Discussion post-authorisation update for Rheumocam extension X/007
 5 May 2011 EMA/170257/2011 Veterinary Medicines and Product Data Management Scientific Discussion post-authorisation update for Rheumocam extension X/007 Scope of extension: addition of 20 mg/ml solution
5 May 2011 EMA/170257/2011 Veterinary Medicines and Product Data Management Scientific Discussion post-authorisation update for Rheumocam extension X/007 Scope of extension: addition of 20 mg/ml solution
for For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs. for CATS
 Effectively breaks the flea life cycle For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs. For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs ACTIVE INGREDIENTS: Imidacloprid 9.10%
Effectively breaks the flea life cycle For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs. For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs ACTIVE INGREDIENTS: Imidacloprid 9.10%
Package leaflet: Information for the user. GENTAMICIN VISION 3 mg/ml eye drops, solution Gentamicin
 Package leaflet: Information for the user GENTAMICIN VISION 3 mg/ml eye drops, solution Gentamicin Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the user GENTAMICIN VISION 3 mg/ml eye drops, solution Gentamicin Read all of this leaflet carefully before you start taking this medicine because it contains important
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION Pr PENTAMYCETIN Chloramphenicol Ophthalmic Solution USP 0.25%, 0.5% Chloramphenicol Ophthalmic Ointment USP 1% Antibiotic Pr PENTAMYCETIN/HC Chloramphenicol and Hydrocortisone Eye
PRESCRIBING INFORMATION Pr PENTAMYCETIN Chloramphenicol Ophthalmic Solution USP 0.25%, 0.5% Chloramphenicol Ophthalmic Ointment USP 1% Antibiotic Pr PENTAMYCETIN/HC Chloramphenicol and Hydrocortisone Eye
Mass Delivery of Nonsurgical Sterilants
 Neutersol - What Works? What Targets? What Next? Nonsurgical Sterilization Presentation By: Sean Hawkins, President November 11, 2006 ACC&D Annual Conference Mass Delivery of Nonsurgical Sterilants Fun
Neutersol - What Works? What Targets? What Next? Nonsurgical Sterilization Presentation By: Sean Hawkins, President November 11, 2006 ACC&D Annual Conference Mass Delivery of Nonsurgical Sterilants Fun
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Pro Penstrep Suspension for Injection for Cattle, Sheep and Pigs. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Pro Penstrep Suspension for Injection for Cattle, Sheep and Pigs. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYTOPOINT 10 mg solution for injection for dogs CYTOPOINT 20 mg solution for injection for dogs CYTOPOINT 30 mg
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYTOPOINT 10 mg solution for injection for dogs CYTOPOINT 20 mg solution for injection for dogs CYTOPOINT 30 mg
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Carofertin 10 mg/ml Emulsion for injection for cattle and pigs 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: Active
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Carofertin 10 mg/ml Emulsion for injection for cattle and pigs 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: Active
Starts working through contact
 DO NOT USE ON CATS 81356777 108 x 34 x 120 Once-A-Month Topical Treatment for Fleas and Lice For Use Only on Dogs and Puppies 7 Weeks and Older and Weighing 11 20 lbs. READ THE ENTIRE LABEL BEFORE EACH
DO NOT USE ON CATS 81356777 108 x 34 x 120 Once-A-Month Topical Treatment for Fleas and Lice For Use Only on Dogs and Puppies 7 Weeks and Older and Weighing 11 20 lbs. READ THE ENTIRE LABEL BEFORE EACH
Early lambing with: Improved fertility Improved fecundity Improved prolificacy Compact lambing period Normal return to season Normal sexual cycle
 Early lambing with: Improved fertility Improved fecundity Improved prolificacy Compact lambing period Normal return to season Normal sexual cycle Presentation: Regulin is a yellow cylindrical implant containing
Early lambing with: Improved fertility Improved fecundity Improved prolificacy Compact lambing period Normal return to season Normal sexual cycle Presentation: Regulin is a yellow cylindrical implant containing
USA Product Label
 BAYER HEALTHCARE LLC Animal Health Division USA Product Label http://www.vetdepot.com P.O. BOX 390, SHAWNEE MISSION, KS, 66201 0390 Customer Service Tel.: 800 633 3796 Customer Service Fax: 800 344 4219
BAYER HEALTHCARE LLC Animal Health Division USA Product Label http://www.vetdepot.com P.O. BOX 390, SHAWNEE MISSION, KS, 66201 0390 Customer Service Tel.: 800 633 3796 Customer Service Fax: 800 344 4219
Package leaflet: Information for the user. HYDROCORTISON CUM CHLORAMPHENICOL 5 mg/g + 2 mg/g eye ointment hydrocortisone acetate, chloramphenicol
 Package leaflet: Information for the user HYDROCORTISON CUM CHLORAMPHENICOL 5 mg/g + 2 mg/g eye ointment hydrocortisone acetate, chloramphenicol Read all of this leaflet carefully before you start using
Package leaflet: Information for the user HYDROCORTISON CUM CHLORAMPHENICOL 5 mg/g + 2 mg/g eye ointment hydrocortisone acetate, chloramphenicol Read all of this leaflet carefully before you start using
Summary of product characteristics As per Annex C. SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1
 Summary of product characteristics As per Annex C SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1 1. NAME OF THE MEDICINAL PRODUCT. ANNEXURE C to MODULE I Tetanus vaccine (Adsorbed) I.P. 2.
Summary of product characteristics As per Annex C SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1 1. NAME OF THE MEDICINAL PRODUCT. ANNEXURE C to MODULE I Tetanus vaccine (Adsorbed) I.P. 2.
[Version 7.2, 12/2008] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
![[Version 7.2, 12/2008] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS [Version 7.2, 12/2008] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS](/thumbs/92/108778510.jpg) [Version 7.2, 12/2008] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT RABORAL V-RG 2. QUALITATIVE AND QUANTITATIVE COMPOSITION For 1 dose: Active substance: -
[Version 7.2, 12/2008] ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT RABORAL V-RG 2. QUALITATIVE AND QUANTITATIVE COMPOSITION For 1 dose: Active substance: -
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Porcilis ColiClos suspension for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose of 2 ml
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Porcilis ColiClos suspension for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each dose of 2 ml
= 0.5 mg. In vitro toxin neutralisation test based on haemolysis of sheep erythrocytes. For a full list of excipients, see section 6.1.
 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 8 Suspension for injection for sheep and cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances: Potency value/quantity/ml C. perfringens
1 NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 8 Suspension for injection for sheep and cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances: Potency value/quantity/ml C. perfringens
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Oncept IL-2 lyophilisate and solvent for suspension for injection for cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Oncept IL-2 lyophilisate and solvent for suspension for injection for cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
7/25/2014. Proper Injection Technique. Review Pork Quality Assurance Plus. Contact Information. Why are injections given?
 Breeding Herd Education Series 2011-12 Timely, relevant & convenient learning Thank you for participating in SowBridge 2011-12. To start this presentation, advance one slide by pressing enter or the down
Breeding Herd Education Series 2011-12 Timely, relevant & convenient learning Thank you for participating in SowBridge 2011-12. To start this presentation, advance one slide by pressing enter or the down
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Eurican Herpes 205 powder and solvent for emulsion for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Eurican Herpes 205 powder and solvent for emulsion for injection. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active
Gentamicin Bladder Irrigation
 Patient and Family Education Gentamicin Bladder Irrigation What is Gentamicin bladder irrigation? Gentamicin is an antibiotic medicine that is used to treat or prevent urinary tract infections (UTIs).
Patient and Family Education Gentamicin Bladder Irrigation What is Gentamicin bladder irrigation? Gentamicin is an antibiotic medicine that is used to treat or prevent urinary tract infections (UTIs).
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Dipen 100ml Suspension for Injection for cattle, sheep and pigs 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Substance
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Dipen 100ml Suspension for Injection for cattle, sheep and pigs 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Substance
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, CZ, EE, ES, FR, IE, IS, IT, LT, LU, LV, NO, PL, PT, RO, SE, SI, SK, UK: Genestran 75 micrograms/ml solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, CZ, EE, ES, FR, IE, IS, IT, LT, LU, LV, NO, PL, PT, RO, SE, SI, SK, UK: Genestran 75 micrograms/ml solution for injection
Blood Collection Healthcare
 Blood Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms
Blood Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Medicinal product no longer authorised
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Zubrin 50 mg oral lyophilisates for dogs Zubrin 100 mg oral lyophilisates for dogs Zubrin 200 mg oral lyophilisates
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Zubrin 50 mg oral lyophilisates for dogs Zubrin 100 mg oral lyophilisates for dogs Zubrin 200 mg oral lyophilisates
This drug SHOULD NOT be used in: XXPregnant or nursing animals. XXDogs that are weak, old, or frail.
 Fipronil with (S)-Methoprene & Pyripoxyfen, Topical (Dogs) (fip-roe-nil with meth-oh-preen and pye-ri-proks-i-fen) Category: Topical Agent to Treat & Control Fleas, Ticks, & Lice; Insect Growth Regulator
Fipronil with (S)-Methoprene & Pyripoxyfen, Topical (Dogs) (fip-roe-nil with meth-oh-preen and pye-ri-proks-i-fen) Category: Topical Agent to Treat & Control Fleas, Ticks, & Lice; Insect Growth Regulator
Dosing Your Cat with Azithromycin Pediatric Suspension. By Lorraine Shelton
 Dosing Your Cat with Azithromycin Pediatric Suspension By Lorraine Shelton To join a community of cat fanciers and health professionals interested in cattery related health issues, visit http://groups.yahoo.com/group/fanciershealth
Dosing Your Cat with Azithromycin Pediatric Suspension By Lorraine Shelton To join a community of cat fanciers and health professionals interested in cattery related health issues, visit http://groups.yahoo.com/group/fanciershealth
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site:
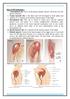 Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
SAMPLE NOT FOR SALE 8 Weeks and Older and 9 lbs. and Under 1 Pack x 20 x 145
 8 Weeks and Older 79836945 78 x 20 x 45 9 lbs. and Under recycled paper Imidacloprid... 9. % Other Ingredients... 90.9 % Total... 00.0 % One 0.04 fl oz (0.4 ml) Tube KEEP OUT OF REACH OF CHILDREN See back
8 Weeks and Older 79836945 78 x 20 x 45 9 lbs. and Under recycled paper Imidacloprid... 9. % Other Ingredients... 90.9 % Total... 00.0 % One 0.04 fl oz (0.4 ml) Tube KEEP OUT OF REACH OF CHILDREN See back
large dog 5-way protection against: fleas/ticks/biting flies/mosquitoes/lice WARNING pack flea & tick protection KEEP OUT OF REACH OF CHILDREN
 from the makers of 5-way protection against: fleas/ticks/biting flies/mosquitoes/lice Topical prevention and treatment of fleas, ticks, mosquitoes, biting flies, and lice for monthly use only on dogs and
from the makers of 5-way protection against: fleas/ticks/biting flies/mosquitoes/lice Topical prevention and treatment of fleas, ticks, mosquitoes, biting flies, and lice for monthly use only on dogs and
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 10 Suspension for injection for sheep and cattle 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Potency
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 10 Suspension for injection for sheep and cattle 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Potency
Ubroseal Dry Cow 2.6 g intramammary suspension for cattle
 Health Products Regulatory Authority 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Ubroseal Dry Cow 2.6 g intramammary suspension for cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 4g intramammary
Health Products Regulatory Authority 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Ubroseal Dry Cow 2.6 g intramammary suspension for cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 4g intramammary
Irish Medicines Board
 IRISH MEDICINES BOARD ACT 1995, as amended European Communities (Animal Remedies) (No. 2) Regulations 2007 VPA: 10988/081/002 Case No: 7007872 The Irish Medicines Board in exercise of the powers conferred
IRISH MEDICINES BOARD ACT 1995, as amended European Communities (Animal Remedies) (No. 2) Regulations 2007 VPA: 10988/081/002 Case No: 7007872 The Irish Medicines Board in exercise of the powers conferred
 Patient and Family Education How to Add Antibiotics and other Medicines to the Dialysate What do I do before I start? Gather these supplies and place them on a clean surface: Antibiotic vials and sterile
Patient and Family Education How to Add Antibiotics and other Medicines to the Dialysate What do I do before I start? Gather these supplies and place them on a clean surface: Antibiotic vials and sterile
Please refer to Table 1 Dosage and Treatment Schedule TABLE 1 Species Product Number of Tubes Cats. Rabbits or Advantage 40 for Cats
 Advantage Introduction Company name: Bayer plc Address: Animal Health Division Bayer House, Strawberry Hill, Newbury Berkshire RG14 1JA Telephone: 01635 563000 Fax: 01635 563622 Email: animal.health@bayerhealthcare.com
Advantage Introduction Company name: Bayer plc Address: Animal Health Division Bayer House, Strawberry Hill, Newbury Berkshire RG14 1JA Telephone: 01635 563000 Fax: 01635 563622 Email: animal.health@bayerhealthcare.com
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Marbocare 20 mg/ml solution for injection for cattle and pigs (UK, IE, FR) Odimar 20 mg/ml solution for injection for cattle
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Marbocare 20 mg/ml solution for injection for cattle and pigs (UK, IE, FR) Odimar 20 mg/ml solution for injection for cattle
Irish Medicines Board
 IRISH MEDICINES BOARD ACT 1995 EUROPEAN COMMUNITIES (ANIMAL REMEDIES) (No. 2) REGULATIONS 2007 (S.I. No. 786 of 2007) VPA: 10999/056/001 Case No: 7004318 The Irish Medicines Board in exercise of the powers
IRISH MEDICINES BOARD ACT 1995 EUROPEAN COMMUNITIES (ANIMAL REMEDIES) (No. 2) REGULATIONS 2007 (S.I. No. 786 of 2007) VPA: 10999/056/001 Case No: 7004318 The Irish Medicines Board in exercise of the powers
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 [Version 7.3.1, 11/2010] FINAL SPC, LABELLING AND PACKAGE LEAFLET ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CEVAC Clostridium Ovino suspension for injection
[Version 7.3.1, 11/2010] FINAL SPC, LABELLING AND PACKAGE LEAFLET ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CEVAC Clostridium Ovino suspension for injection
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pentofel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Per dose of 1ml: Active components Inactivated Feline Panleukopenia
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pentofel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Per dose of 1ml: Active components Inactivated Feline Panleukopenia
Arkansas Beef Quality Assurance Program Producer Certification Exam
 University of Arkansas, United States Department of Agriculture and County Governments Cooperating Arkansas Beef Quality Assurance Program Producer Certification Exam Please mark one answer per question
University of Arkansas, United States Department of Agriculture and County Governments Cooperating Arkansas Beef Quality Assurance Program Producer Certification Exam Please mark one answer per question
UNIVERSITY OF TENNESSEE AT MARTIN
 UNIVERSITY OF TENNESSEE AT MARTIN Sheep and Goat Teaching and Research Farm STANDARD OPERATING PROCEDURES EFFECTIVE: 5-20-05 REVISED: 5-17-10 TABLE OF CONTENTS The intent of this document is to describe
UNIVERSITY OF TENNESSEE AT MARTIN Sheep and Goat Teaching and Research Farm STANDARD OPERATING PROCEDURES EFFECTIVE: 5-20-05 REVISED: 5-17-10 TABLE OF CONTENTS The intent of this document is to describe
Package leaflet: Information for the user
 Text draft from 12.07.2018 Minoxidil BIO-H-TIN-Pharma 20 mg/ml Page 1 Package leaflet: Information for the user Minoxidil BIO-H-TIN-Pharma 20 mg/ml cutaneous spray, solution Minoxidil For women aged over
Text draft from 12.07.2018 Minoxidil BIO-H-TIN-Pharma 20 mg/ml Page 1 Package leaflet: Information for the user Minoxidil BIO-H-TIN-Pharma 20 mg/ml cutaneous spray, solution Minoxidil For women aged over
and Weighing 8 weeks and Older ONLY on Cats For use FOR CATS CONTAINS IMIDACLOPRID AND PYRIPROXYFEN
 113068 MONTH 4 113068 4 MONTH KILLS FLEAS AND FLEA EGGS PREVENTS REINFESTATION FRAGRANCE-FREE ACTIVE INGREDIENTS: Imidacloprid 9.10% Pyriproxyfen 0.46% OTHER INGREDIENTS 90.44% TOTAL 100.00% Convenient,
113068 MONTH 4 113068 4 MONTH KILLS FLEAS AND FLEA EGGS PREVENTS REINFESTATION FRAGRANCE-FREE ACTIVE INGREDIENTS: Imidacloprid 9.10% Pyriproxyfen 0.46% OTHER INGREDIENTS 90.44% TOTAL 100.00% Convenient,
Kirkland Signature Minoxidil Topical Solution USP, 5% Drug Facts
 Kirkland Signature Minoxidil Topical Solution USP, 5% Drug Facts Active ingredient Minoxidil 5% w/v Purpose Hair regrowth treatment for men Use to regrow hair on the top of the scalp (vertex only, see
Kirkland Signature Minoxidil Topical Solution USP, 5% Drug Facts Active ingredient Minoxidil 5% w/v Purpose Hair regrowth treatment for men Use to regrow hair on the top of the scalp (vertex only, see
BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT. Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin
 BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin 2. STATEMENT OF ACTIVE AND OTHER SUBSTANCES Each ml contains: Tilmicosin 300 mg;
BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin 2. STATEMENT OF ACTIVE AND OTHER SUBSTANCES Each ml contains: Tilmicosin 300 mg;
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12 1. NAME OF THE VETERINARY MEDICINAL PRODUCT HALOCUR 0.5 mg/ml oral solution for calves 2. Qualitative and quantitative composition Active substance Halofuginone
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12 1. NAME OF THE VETERINARY MEDICINAL PRODUCT HALOCUR 0.5 mg/ml oral solution for calves 2. Qualitative and quantitative composition Active substance Halofuginone
and Weighing 8 weeks and Older ONLY on Cats For use FOR CATS CONTAINS IMIDACLOPRID AND PYRIPROXYFEN
 113068 MONTH 4 113068 4 MONTH KILLS FLEAS AND FLEA EGGS PREVENTS REINFESTATION FRAGRANCE-FREE ACTIVE INGREDIENTS: Imidacloprid 9.10% Pyriproxyfen 0.46% OTHER INGREDIENTS 90.44% TOTAL 100.00% Convenient,
113068 MONTH 4 113068 4 MONTH KILLS FLEAS AND FLEA EGGS PREVENTS REINFESTATION FRAGRANCE-FREE ACTIVE INGREDIENTS: Imidacloprid 9.10% Pyriproxyfen 0.46% OTHER INGREDIENTS 90.44% TOTAL 100.00% Convenient,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Maprelin 75 µg/ml solution for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Maprelin 75 µg/ml solution for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection
and older weighing Puppies 7 weeks on Dogs and For use ONLY FOR DOGS For use ONLY on Dogs and Puppies 7 weeks and older weighing lbs
 113072 MONTH 4 113072 4 MONTH WATERPROOF KILLS FLEAS, FLEA EGGS, LARVAE, PUPAE PREVENTS REINFESTATIONS Convenient, easy-to-apply and fragrance free monthly topical solution. NET CONTENTS: 0.336 fl. oz
113072 MONTH 4 113072 4 MONTH WATERPROOF KILLS FLEAS, FLEA EGGS, LARVAE, PUPAE PREVENTS REINFESTATIONS Convenient, easy-to-apply and fragrance free monthly topical solution. NET CONTENTS: 0.336 fl. oz
Swab Sampling Policy & Procedures
 Swab Sampling Policy & Procedures G. Fish Chief Steward G. Fish - Chief Steward Effective 3 rd April 2014 Table of Contents Policy Objectives... 3 Overview of the Procedures... 3 Security Measures Pre
Swab Sampling Policy & Procedures G. Fish Chief Steward G. Fish - Chief Steward Effective 3 rd April 2014 Table of Contents Policy Objectives... 3 Overview of the Procedures... 3 Security Measures Pre
IN THE DAILY LIFE of a veterinarian or
 Administering Medication and Care IN THE DAILY LIFE of a veterinarian or veterinary technician, the majority of animal care involves administering medication to sick animals, giving vaccines for viruses,
Administering Medication and Care IN THE DAILY LIFE of a veterinarian or veterinary technician, the majority of animal care involves administering medication to sick animals, giving vaccines for viruses,
Assuring Quality: A guide for youth livestock producers Activity for 2008
 Assuring Quality: A guide for youth livestock producers Activity for 2008 Daily Care and Management---Dairy Cow Activity 1: Proper Milking Procedures Resources Needed: Mud Bucket for water (ice cream pails
Assuring Quality: A guide for youth livestock producers Activity for 2008 Daily Care and Management---Dairy Cow Activity 1: Proper Milking Procedures Resources Needed: Mud Bucket for water (ice cream pails
PREFURRED ONE For Dogs
 FRONT PANEL PREFURRED ONE For Dogs [For Dogs & Puppies 8 weeks or older and up to 22 lbs.] [For Dogs 23-44 lbs.] [For Dogs 45-88 lbs.] [For Dogs 89-132 lbs.] Kills Fleas & Ticks for up to 4 weeks! Convenient
FRONT PANEL PREFURRED ONE For Dogs [For Dogs & Puppies 8 weeks or older and up to 22 lbs.] [For Dogs 23-44 lbs.] [For Dogs 45-88 lbs.] [For Dogs 89-132 lbs.] Kills Fleas & Ticks for up to 4 weeks! Convenient
Example 1: Quality Assurance Individual
 Example 1: Quality Assurance Individual Use the available medicine labels to answer the following questions: 1 What is the name of the chemical compound in the product? 2 Is refrigeration required for
Example 1: Quality Assurance Individual Use the available medicine labels to answer the following questions: 1 What is the name of the chemical compound in the product? 2 Is refrigeration required for
large dog lbs REPELS AND kills ticks, fleas and mosquitoes
 DO NOT USE ON CATS 81356823 108 x 34 x 120 Topical Prevention and Treatment of Ticks, Fleas, Mosquitoes, Biting Flies and Lice for Monthly Use Only on Dogs and Puppies 7 Weeks of Age and Older and Weighing
DO NOT USE ON CATS 81356823 108 x 34 x 120 Topical Prevention and Treatment of Ticks, Fleas, Mosquitoes, Biting Flies and Lice for Monthly Use Only on Dogs and Puppies 7 Weeks of Age and Older and Weighing
extra large dog 5-way protection 3 pack extra large dog WARNING extra large dog flea & tick protection over 55 lbs KEEP OUT OF REACH OF CHILDREN pack
 Seite 1: Layout aussen Seite 2: Layout innen Seite 3: Lack und Prägung against: fleas/ticks/biting flies/mosquitoes/lice flea & tick protection DO NOT USE ON CATS pack 3 81946760 108 x 34 x 120 11556-134_DefenseCare
Seite 1: Layout aussen Seite 2: Layout innen Seite 3: Lack und Prägung against: fleas/ticks/biting flies/mosquitoes/lice flea & tick protection DO NOT USE ON CATS pack 3 81946760 108 x 34 x 120 11556-134_DefenseCare
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Purevax RCPCh lyophilisate and solvent for suspension for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Purevax RCPCh lyophilisate and solvent for suspension for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS page 1 of 7 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Panacur PetPaste 187.5 mg/g oral paste for dogs and cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g oral
SUMMARY OF PRODUCT CHARACTERISTICS page 1 of 7 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Panacur PetPaste 187.5 mg/g oral paste for dogs and cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g oral
SUMMARY OF PRODUCT CHARACTERISTICS FOR CATS
 SUMMARY OF PRODUCT CHARACTERISTICS FOR CATS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT EFFIPRO 50 mg spot-on solution for cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One 0.5 ml pipette contains :
SUMMARY OF PRODUCT CHARACTERISTICS FOR CATS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT EFFIPRO 50 mg spot-on solution for cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One 0.5 ml pipette contains :
DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS
 DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS 1. TITLE SLIDE: DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS. Hands are one of the most common sources of the spread of pathogenic microorganisms. Hand
DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS 1. TITLE SLIDE: DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS. Hands are one of the most common sources of the spread of pathogenic microorganisms. Hand
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Issued March 2017 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats Recicort vet 1.77 mg/ml + 17.7 mg/ml
SUMMARY OF PRODUCT CHARACTERISTICS Issued March 2017 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats Recicort vet 1.77 mg/ml + 17.7 mg/ml
LABELLING AND PACKAGE LEAFLET
 LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE CARTON BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MILTEFORAN 20 mg/ml oral solution for dogs miltefosine 2.
LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE CARTON BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MILTEFORAN 20 mg/ml oral solution for dogs miltefosine 2.
LUTEOSYL(d)-Cloprostenol mg/ml Solution for injection for cattle and pigs
 SUMMARY OF PRODUCT CHARACTERISTICS SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT In France, Germany, Hungary, Italy, Poland, Spain and The Netherlands; LUTEOSYL 0.075 mg/ml
SUMMARY OF PRODUCT CHARACTERISTICS SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT In France, Germany, Hungary, Italy, Poland, Spain and The Netherlands; LUTEOSYL 0.075 mg/ml
ANNEX III LABELLING AND PACKAGE LEAFLET
 ANNEX III LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE Card box and package leaflet for brown glass bottle (Type 1) 1. NAME OF THE
ANNEX III LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE Card box and package leaflet for brown glass bottle (Type 1) 1. NAME OF THE
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Suprelorin 4.7 mg implant for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Deslorelin (as
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Suprelorin 4.7 mg implant for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Deslorelin (as
Beef Cattle Handbook
 Beef Cattle Handbook BCH-2320 Product of Extension Beef Cattle Resource Committee Estrous Synchronization for Beef Cattle Gene H. Deutscher, Extension Beef Specialist, University of Nebraska This Fact
Beef Cattle Handbook BCH-2320 Product of Extension Beef Cattle Resource Committee Estrous Synchronization for Beef Cattle Gene H. Deutscher, Extension Beef Specialist, University of Nebraska This Fact
SUMMARY OF PRODUCT CHARACTERISTICS. Pentoject, Pentobarbitone Sodium 200 mg/ml Solution for Injection
 SUMMARY OF PRODUCT CHARACTERISTICS Revised: June 2018 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pentoject, Pentobarbitone Sodium 200 mg/ml Solution for Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS Revised: June 2018 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pentoject, Pentobarbitone Sodium 200 mg/ml Solution for Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
x 3 CrossBlock II CrossBlock II 3-10 lbs 3-10 lbs For Dogs and Puppies For Dogs and Puppies 7 weeks or older 7 weeks or older
 x 3 Waterproof Kills Fleas Kills Flea Larvae Kills Flea Eggs CrossBlock II ACTIVE INGREDIENTS : Imidacloprid... 9.10% Pyriproxyfen... 0.46% OTHER INGREDIENTS:... 90.44% TOTAL... 100.00% EPA Est. No. 74720-DEU-01
x 3 Waterproof Kills Fleas Kills Flea Larvae Kills Flea Eggs CrossBlock II ACTIVE INGREDIENTS : Imidacloprid... 9.10% Pyriproxyfen... 0.46% OTHER INGREDIENTS:... 90.44% TOTAL... 100.00% EPA Est. No. 74720-DEU-01
medium dog 5-way protection 3 pack medium dog WARNING medium dog flea & tick protection KEEP OUT OF REACH OF CHILDREN pack lbs DO NOT USE ON CATS
 Seite 1: Layout aussen Seite 2: Layout innen Seite 3: Lack und Prägung against: fleas/ticks/biting flies/mosquitoes/lice flea & tick protection DO NOT USE ON CATS pack 3 81946450 108 x 34 x 120 11556-133_DefenseCare
Seite 1: Layout aussen Seite 2: Layout innen Seite 3: Lack und Prägung against: fleas/ticks/biting flies/mosquitoes/lice flea & tick protection DO NOT USE ON CATS pack 3 81946450 108 x 34 x 120 11556-133_DefenseCare
Minims Chloramphenicol
 Minims Chloramphenicol Eye Drops Chloramphenicol Eye Drops Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Minims Chloramphenicol, including how to
Minims Chloramphenicol Eye Drops Chloramphenicol Eye Drops Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Minims Chloramphenicol, including how to
Topical prevention and treatment of ticks, fleas, mosquitoes, biting flies and lice for monthly use on dogs and puppies 7 weeks of age and older
 BAYER HEALTHCARE LLC Animal Health Division P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390 Customer Service Tel.: 800-633-3796 Customer Service Fax: 800-344-4219 Website: www.bayer-ah.com Every effort has
BAYER HEALTHCARE LLC Animal Health Division P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390 Customer Service Tel.: 800-633-3796 Customer Service Fax: 800-344-4219 Website: www.bayer-ah.com Every effort has
Unshakeable confidence
 NEW PRODUCT OF THE YEAR as voted by vets for the 2nd year running** Unshakeable confidence Osurnia is the only otitis externa* treatment that applies like a liquid and stays like a gel. Right where you
NEW PRODUCT OF THE YEAR as voted by vets for the 2nd year running** Unshakeable confidence Osurnia is the only otitis externa* treatment that applies like a liquid and stays like a gel. Right where you
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Selectan 300 mg/ml solution for injection for cattle and swine. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Selectan 300 mg/ml solution for injection for cattle and swine. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
RABIES CONTROL PROGRAM DUTY TO REPORT
 RABIES CONTROL PROGRAM DUTY TO REPORT To: Physicians Registered Nurses in the Extended Class Date: April 7, 2017 Re: Duty to Report Animal Bite/Animal Contact This letter is being sent in accordance with
RABIES CONTROL PROGRAM DUTY TO REPORT To: Physicians Registered Nurses in the Extended Class Date: April 7, 2017 Re: Duty to Report Animal Bite/Animal Contact This letter is being sent in accordance with
