Stability of Nafcillin Sodium Solutions in the Accufuser Elastomeric Infusion Device
|
|
|
- Isabel Page
- 5 years ago
- Views:
Transcription
1 Pharmacology & Pharmacy, 2013, 4, Published Online January 2013 ( 57 Stability of Nafcillin Sodium Solutions in the Accufuser Elastomeric Infusion Device Min-A Kang 1, Ju-Seop Kang 2 1 Department of Nursing, College of Nursing, Yonsei University, Seoul, South Korea; 2 Department of Pharmacology & Clinical Pharmacology Lab, College of Medicine and Division of Molecular Therapeutics Development, Hanyang Biomedical Research Institute, Hanyang University, Seoul, South Korea. jskang@hanyang.ac.kr Received October 25 th, 2012; revised November 30 th, 2012; accepted December 18 th, 2012 ABSTRACT The aim of this study was to investigate the stabilities of two kinds of solutions of nafcillin sodium (2.5 mg/ml) in 0.9% sodium chloride solution (NS, normal saline) and in injectable 5% dextrose water (D 5 W) in the intravenous elastomeric infusion device (Accufuser ) based on recommended solutions and storage periods. The injectable nafcillin solutions (NS- and D 5 W-nafcillin) in the Accufuser device were stored and evaluated at controlled temperatures (room temperature, RT, 5 C ± 2 C and cold temperature, CT, 4 C ± 2 C) during 6 weeks. Effects of the periods of storage (from 0 to 6 weeks) and the temperatures of storage (RT and CT) on the physicochemical appearances and concentrations of active compounds were determined. The visual clarity, ph, and concentrations of nafcillin sodium were determined by stability-indicating high-performance liquid chromatography (HPLC)-ultraviolet (UV) detection. The results showed that in NS and D 5 W solutions, the amount of nafcillin slightly changed and remained 92.66% and 97.30% of their initial amounts at CT during 6 weeks, respectively. On the other hand, in NS and D 5 W solutions at RT, the amount significantly decreased with time and reached 27.66% and 31.97% of their initial amounts during 4 weeks, respectively. Slight decrement of ph was observed in CT storage while significant change was observed in the RT storage. Moreover, in CT, no significant changes in physical appearances and colors of the solutions were observed during the study. However, the solutions changed into yellowish color and some particles were detected in both kinds of nafcillin solutions (NS and D 5 W) after 1.5 weeks in RT conditions. To sum up, under CT two kinds of nafcillin sodium solutions (NS and D 5 W) were stable with time in Accufuser without any significant physical changes and retained almost all of the initial concentrations up to 6 weeks. However, the solutions were not stable in RT storage. We suggest that nafcillin sodium solutions in an Accufuser should be preferentially diluted in NS and D 5 W while storing in CT condition. Keywords: Stability; Nafcillin Sodium; Intravenous Elastomeric Infusion Device (Accufuser ) 1. Introduction The disposable silicon balloon infusion device (Accufuser, Woo Young Medical Co. LTD., Seoul, South Korea) is a well-established simplified silicon-based elastomeric system for the administration of antibiotics and other drugs or nutrients that are suitable for patients as well as healthcare providers. An increasing number of patients are being treated as outpatients, and for them, drugs are often infused using portable pumps or infusion devices outside the hospital [1,2]. The disposable silicon balloon infusion device is advantageous in home health care setting because it is disposable, relatively inexpensive compared to electronic pumps, and it is easy for the patient to fill and manipulate [3]. The increasing popularity of the use of portable infusion devices outside hospital raises the issue of the stability of drugs likely to be administered with such devices. Conditions surrounding the infusion out- side hospitals are very different from the conditions of the infusion in hospital settings. Concentrations of drug in solution, temperatures of the environment immediately outside the drug container, bore of the tubing, and fluid flow rates may differ. In contrast to hospital practice, infusion-pump drug reservoirs in temporary clinic or home care setting are routinely loaded with sufficient doses of a drug, which may last for 24 hours or more, and it is not easy to check the solutions for the stability of the drug content [4]. To be suitable for self-administration by home-based patient, the drug should be stable in solution for a number of days under home storage conditions. Therefore, it is necessary to determine the physical and chemical stabilities of the admixtures in the infusion system before they are used. The study was done with nafcillin sodium solutions made with NS and D 5 W because these are the most available infusion solu-
2 58 Stability of Nafcillin Sodium Solutions in the Accufuser Elastomeric Infusion Device tions for nafcillin sodium administration. The purpose of this study was to evaluate the physicochemical stability of nafcillin sodium (Figure 1) solutions (2.5 mg/ml, normal saline, NS and 5% dextrose water, D 5 W) packed in sterile Accufuser device in which each samples were stored and evaluated at appropriate intervals up to 6 weeks under different storage conditions (room temperature, RT, 25 C ± 2 C and cold temperature, CT, 4 C ± 2 C). 2. Materials and Methods 2.1. Materials Nafcillin sodium was purchased from Sigma Co., USA. Normal saline (NS, 0.9% NaCl in water) and 5% dextrose (D 5 W, injectable 5% dextrose water) were purchased from Choongwae Pharmaceutical Co. Ltd., South Korea. Disposable silicon balloon infusion device (Accufuser ) was obtained from Woo Young Medical Co., Ltd, South Korea. Potassium phosphate monobasic was purchased from Sigma Co., USA, and all the chemicals for HPLC analysis were all HPLC-grade and were prepared immediately before use Preparation and Sampling of Solutions To prepare the test samples, the appropriate amounts of nafcillin sodium were added to a portion of the infusion solutions and were brought to a final volume of 100 ml with NS and D 5 W. The test solutions were packed in sterile Accufuser system for testing. All manipulations were aseptically performed under laminar airflow in a biological safety cabinet. The nominal nafcillin sodium concentration for the sample used in this testing was 2.5 mg/ml. The Accufuser systems were each filled with 100 ml of drug solution. Triplicate test solutions were prepared and stored in RT and CT conditions. After the Accufuser systems were filled, an extension-tubing set was attached and primed. About 3 ml of drug solutions was collected in a 4 ml glass vial with a Teflon-lined screw cap and stored in a freezer at 70 C until the analyzing process. One sample (approximately 3 ml) was obtained from the distal end of the tubing after 48 h, 1, 2, 4, and 6 weeks at RT and CT and stored in the manner described for the time-zero samples (Table 1) Methods The physical stability of the nafcillin sodium solutions were assessed by visual examination and HPLC analysis. Visual examinations were performed in normal diffuse fluorescent room light with unaided eye and high-intensity monodirectional light. The ph of the solutions was measured by a stainless electrode ph meter (Thermo Scientific Co., MA, USA). The drug concentrations were determined using a stability-indicating HPLC assay method based on several references [4-7]. The HPLC system [4, 7] consisted of an isocratic solvent delivery pump (Model 515, Waters Scientific Co. USA) which pumped a mixture (v/v, 65/35, ph 3.0) of acetonitrile (ACN, Sigma Co. USA) in 0.05M potassium phosphate (Sigma Co. USA) Figure 1. Chemical Structure of nafcillin sodium (C 21 H 22 N 2 NaO 5 S H 2 O), MW = Table 1. Study designs for the stability testing of nafcillin sodium solutions in Accufuser system. Conditions Time Solutions 0 h 48 h 1 st wk 2 nd wk 4 th wk 6 th wk O * O O O O O NS RT (25 C ± 2 C) D 5 W NS CT (4 C ± 2 C) D 5 W * O: processed sample, RT: room temperature, CT: cold temperature, NS: normal saline, D 5 W: 5% dextrose water.
3 Stability of Nafcillin Sodium Solutions in the Accufuser Elastomeric Infusion Device 59 as a mobile phase through a Capsell Pak C 18 UG120 ( mm, 5 μm, Shiseido Co., Japan) column at 1200 µl/min. The ratio of ACN to 0.05 M potassium phosphate (65/35) was held constant during the chromatographic run. The samples of 3.0 µl were injected into the HPLC system using an autosampler (Nanospace SI-2, Shiseido Co., Japan). The effluent from the column was monitored with a variable wavelength ultraviolet detector (Nanospace SI-1, Shiseido Co., Japan) at 220 nm. The integration of the chromatograms was performed by dschrom software (Do-Nam Instrumental Co., Seoul, Korea). The method was validated for linearity, precision (inter-day and intra-day), accuracy and selectivity [7]. The experiment was repeated three times on the same day and additionally on two consecutive days to determine inter- and intra-day precisions. Assays of control solutions from nafcillin sodium solutions (2.5 mg/ml) were undertaken to calculate the intra-day and inter-day variations using external standard method. Linearity was evaluated by serial dilutions of nafcillin sodium solutions with NS and D 5 W for analysis. The stability of nafcillin sodium infusion solutions was determined in disposable Accufuser infusion device during 6 weeks of storage under RT and CT conditions. Periodically, samples were evaluated for appearances, visible particles, ph and chromatographic analysis. We analyzed the concentration of nafcillin sodium in two solutions at 0, 48 h, 1, 2, 4, and 6 weeks after the preparation of solutions by HPLC- UV method. On each day, 1.0 ml of samples of nafcillin sodium solution with a nominal concentration were drawn from Accufuser infusion device for chromatographic analysis and 3.0 µl were directly injected into HPLC- UV system. Three aliquots of each solution were processed. Statistical analysis was performed using one-way ANOVA with the level of significance set at 0.05 (PCS, v. 4.0, Springer-Verlag, New York, USA). 3. Results 3.1. Method Validation The linearity of calibration curves of nafcillin sodium solution was established which showed good linearity over the range concentration of 0.5 ~ 2.8 mg/ml (r 2 = , Figure 2). Table 2 lists the relative standard deviation (R.S.D.) data obtained from the analysis of the samples on the same day (n = 5) and on consecutive days (n = 5). The R.S.D values were <1.26% and <2.35% for intra-day and inter-day results, respectively, meaning that the method was sufficiently precise Physical Appearance and ph Status There were no significant changes in physical appearances and clarities of the solutions in CT condition. The color of the samples was transparent with no color discoloration. Particles were not detected. However, the color of NS solutions became opaque after 10 days of study in RT condition. After 2 weeks, solutions changed into yellowish color and particles were detected. On the 4 th week, the filter of the silicone balloon infuser was partially clogged, and the injected samples did not flow out with the normal flow rate of 2.0 ml/h. The flow rate was less than the half of the normal flow rate. At the 6 th week, the filter of the silicone balloon infuser was completely clogged and the injected samples did not flow out at all. Similarly, the color of D 5 W solutions in RT condition became opaque after 2 weeks of study and changed into yellowish color at the 3 rd week. At the 4 th week, particles were detected and at the 6 th week, the filter of the silicone balloon infuser was completely clogged and the injected samples did not flow out at all. The ph of D 5 W and NS solutions slightly decreased from 6.84 to 6.06 Area ( 10 8 ) y = x R 2 = Nafcillin concentration (mg/ml) Figure 2. Calibration curve for the determination of nafcillin sodium concentrations in study solutions. Range (0.5 ~ 2.8 mg/ml), Area = Peak area. Table 2. Intra-day and Inter-day precision studies (n = 5). Parameter Group RT Nafcillin (25 C ± 2 C) CT Nafcillin (4 C ± 2 C) D 5 W NS D 5 W NS Intra-day (n = 5) Inter-day (n = 5) Accuracy (%) ± 1.47 * ± ± ± 0.81 CV (%) 1.22 ± ± ± ± 0.64 Accuracy (%) ± ± ± ± 0.23 CV (%) 1.26 ± ± ± ± 0.20
4 60 Stability of Nafcillin Sodium Solutions in the Accufuser Elastomeric Infusion Device and from 6.51 to 5.87 in the CT condition, but significantly (p < 0.05) decreased from 6.85 to 4.49 and from 6.57 to 4.11 in the RT condition during 6 weeks (Table 3) Concentration The typical HPLC chromatogram of nafcillin sodium (2.5 mg/ml) is shown in Figure 3. The retention time for nafcillin sodium was about 10.2 min. The initial concen- tration and the percentage of the remaining concentration which were observed at analytic time during 6 weeks for each nafcillin sodium solutions and storage conditions are listed in Table 4. The concentration of nafcillin sodium decreased significantly with time and reached 31.97% in D 5 W and 27.66% in NS after 4 weeks under RT condition. However, 97.30% of initial concentration remained in D 5 W and 92.66% remained in NS under CT condition. Table 3. The change of ph (Mean ± SD, %) of nafcillin sodium solutions according to the time of storage. RT 1) Nafcillin (25 C ± 2 C) CT 2) Nafcillin (4 C ± 2 C) Group D 5 W 3) NS 4) D 5 W NS Time Concentration (%) Concentration (%) Concentration (%) Concentration (%) 0 h 6.85 ± 0.03 (100.00) 6.57 ± 0.03 (100.00) 6.84 ± 0.02 (100.00) 6.51 ± 0.05 (100.00) 48 h 6.26 ± 0.04 (91.38) 5.97 ± 0.03 (90.87) 6.76 ± 0.03 (98.83) 6.30 ± 0.01 (96.77) 1 wk 5.81 ± 0.01 (84.82) 5.30 ± 0.02 (80.67) * 6.72 ± 0.04 (98.25) 6.29 ± 0.01 (96.62) 2 wks 5.40 ± 0.04 (78.83) * 4.71 ± 0.06 (71.69) * 6.58 ± 0.03 (96.20) 6.11 ± 0.02 (93.86) 4 wks 5.12 ± 0.01 (74.74) * 4.50 ± 0.01 (68.49) * 6.40 ± 0.01 (93.57) 5.92 ± 0.02 (90.94) 6 wks 4.49 ± 0.00 (65.17) * 4.11 ± 0.00 (62.56) * 6.06 ± 0.10 (88.60) 5.87 ± 0.04 (90.17) 1) RT: room temperature, 2) CT: cold temperature, 3) D 5 W: 5% dextrose, 4) NS: normal saline, * p < 0.05 vs 0 h. Table 4. The retention of the amount (%) of nafcillin sodium in solutions (NS and D 5 W) according to each storage temperature (RT and CT) and time (0 ~ 6 weeks). Group RT nafcillin sodium (25 C ± 2 C) CT nafcillin sodium (4 C ± 2 C) Time D 5 W NS D 5 W NS 0 hr ± ± ± ± hr ± ± ± ± week ± ± ± ± weeks ± ± 0.71 * ± ± weeks ± 0.07 * ± 0.12 * ± ± weeks ± ± 0.81 Figure 3. Typical chromatogram of standard solutions (NS and D 5 W) containing nafcillin sodium of 2.5 mg/ml, Y-axis (Absorbance Units), X-axis (Retention Time, min).
5 Stability of Nafcillin Sodium Solutions in the Accufuser Elastomeric Infusion Device Discussion and Conclusion Drugs that are altered from their original form (e.g. diluted, reconstituted or mixed with other drugs) or aliquoted into a new container do not usually keep their original expiration dates. Diluting, mixing, or transferring to a new container may cause the drugs to lose potency and degrade due to changes in ph, buffering, atmosphere (some drugs are packed under special gases such as argon or nitrogen), or precipitation. In addition, drugs that are kept in a sealed, sterile container may be contaminated and subjected to bacterial degradation [8]. Over the past 20 years, outpatient parenteral anti-microbial therapy has proved to be effective, safe, and economical for patients with a wide range of infectious diseases [9,10]. Outpatient parenteral anti-microbial therapy exemplifies the change of focus from inpatient care to outpatient, ambulatory care. A great number of new modified catheters and infusion devices are available. Therefore, further studies of drug stability under conditions of the usage of various infusion devices are necessary [11]. Stability of drug in solutions is highly influenced by many factors, including temperature, ph, medium and final concentration. Stability is defined as the retention of >90% of the initial drug concentration. If any drug or drug mixture is aliquoted into an unsealed or non-sterile container or if it is contaminated by non-aseptic technique, it would have a maximum of 24-hour expiration period unless it immediately becomes frozen. Frozen aliquots can usually be kept indefinitely (at 90 C), but once thawed, they have a maximum of 24-hour shelf life. IV fluids (e.g. NS, lactated Ringers) expire after 24 hours of opening if kept unrefrigerated, and may be kept up to 1 week if refrigerated. The stability of nafcillin sodium (20 mg/l) in normal saline solution maintained for 24 hours, 4 days and 12 weeks in the three storage temperature of RT, CT and 20 C, respectively [3]. Ascertaining the stability not only ensures that the active ingredient(s) are present, but also limits the concentrations of degradation products, which may have toxicological significance [12,13]. Therefore, stability-indicating assays for labeled active ingredients in dosage forms are essential for the safety of such formulations [14]. In this study, we tried to investigate the more various serial periods of storage conditions and to confirm stability of low concentration of nafcillin sodium (2.5 mg/ml) because of improvement of range of options of storage period and concentrations of nafcillin sodium in clinical situations than other papers [3,4,11]. Table 4 presents the better stability of nafcillin sodium in D 5 W under CT than NS under RT condition. Storage temperature may be the main effect on drug stability. It shows the maintenance of its stability during 2 weeks and 1 week in the NS and D 5 W solution, respectively under RT condition and 6 weeks in both solutions under CT condition. 5. Acknowledgements This study was supported by Division of Molecular Therapeutics Development, Hanyang Biomedical Research Institute, Hanyang University and Woo Young Medical Co. LTD., Seoul, South Korea. REFERENCES [1] M. A. Kang and J. S. Kang, Stability Test of Ampicillin Sodium Solutions in the Accufuser Elastomeric Infusion Device Using HPLC-UV Method, Pharmacology & Pharmacy, Vol. 3, No. 4, 2012, pp [2] G. A. Lee, M. J. Kim, M. Kang, et al., Stability of Commonly Used Antibiotics Solutions in the Accufuser Elastometric Infusion Device under Recommended Storage and Used Conditions, The Open Nutraceuticals Journal, Vol. 4, 2011, pp doi: / [3] L. V. Allen Jr., M. L. Stiles, S. J. Prince and J. Smeeding, Stability of 14 Drugs in the Latex Reservoir of an Elastomeric Infusion Device, American Journal of Health- System Pharmacy, Vol. 53, No. 22, 1996, pp [4] M. L. Stiles and L. V. Allen Jr., Stability of Nafcillin Sodium, Oxacillin Sodium, Penicillin G Potassium, Pencillin G Sodium, and Tobramycin Sulfate in Polyvinyl Chloride Drug Reservoirs, American Journal of Health- System Pharmacy, Vol. 54, 1997, pp [5] Y. Zhang and L. A. Trissel, Physical Instability of Frozen Pemetrexed Solutions in PVC Bags, Annals of Pharmacotherapy, Vol. 40, No. 7-8, 2006, pp [6] L. A. Trissel and Y. Zhang, Stability of Methylprednisolone Sodium Succinate in Autodose Infusion System Bags, Journal of American Pharmacists Association, Vol. 42, No. 6, 2002, pp [7] V. Kumar, H. Bhutani and S. Singh, ICH Guidance in Practice: Validated Stability-Indicating HPLC Method for Simultaneous Determination of Ampicillin and Cloxacillin in Combination Drug Products, Journal of Pharmaceutical and Biomedical Analysis, Vol. 43, No. 2, 2007, pp [8] Research Committees of Boston University Research Copmliance, Conditional Use of Expired Medical Materials, elines/conditional-use-of-expired-medical-materials/ [9] A. Antoniskis, B. C. Anderson, E. J. van Volkinburg, J. M. Jackson and D. N. Gilbert, Feasibility of Outpatient Self-Administration of Parenteral Antibiotics, The Western Journal of Medicine, Vol. 128, 1978, pp [10] D. M. Poretz, D. Rich, J. O. Morales, L. V. Behren, et al., Outpatietns Use of Intravenous Antibiotics, American Journal of Medicine, Vol. 97, Suppl. 2A, 1994, pp doi: / (94) [11] A. J. J. Wood, Outpatient Parenteral Antimicrobial-Drug Therapy, New England Journal of Medicine, Vol. 337, No. 12, 1997, pp doi: /nejm
6 62 Stability of Nafcillin Sodium Solutions in the Accufuser Elastomeric Infusion Device [12] E. Boven, M. M. Nauta, F. P. Schulper, et al., Secondary Screening of Platinum Compounds in Human Ovarian Cancer Xenografts in Nude Mice, European Journal of Cancer and Clinical Oncology, Vol. 25, 1995, pp [13] J. P. Fee and G. H. Thompson, Comparative Tolerability of Profiles of the Inhaled Anesthetics, Drug Safety, Vol. 16, No. 3, 1997, pp [14] D. F. Driscoll, Stability and Compatibility Assessment Techniques for Total Parental Nutrition Admixtures: Setting the Bar According to Pharmacopeial Standards, Current Opinion in Clinical Nutrition & Metabolic Care, Vol. 8, 2005, pp doi: /01.mco
Isocratic Reverse Phase High Performance Liquid Chromatographic Estimation of Ramipril and Amlodipine in Pharmaceutical Dosage Form
 Isocratic Reverse Phase High Performance Liquid Chromatographic Estimation of Ramipril and Amlodipine in Pharmaceutical Dosage Form Manikanta Kumar. A, P. Vijay Kumar *, Mahesh Nasare, Venkateswar Rao,
Isocratic Reverse Phase High Performance Liquid Chromatographic Estimation of Ramipril and Amlodipine in Pharmaceutical Dosage Form Manikanta Kumar. A, P. Vijay Kumar *, Mahesh Nasare, Venkateswar Rao,
Development and validation of a HPLC analytical assay method for amlodipine besylate tablets: A Potent Ca +2 channel blocker
 Development and validation of a HPLC analytical assay method for amlodipine besylate tablets: A Potent Ca +2 channel blocker Richa Sah* and Saahil Arora 1. ISF College of Pharmacy, Moga, Punjab, India
Development and validation of a HPLC analytical assay method for amlodipine besylate tablets: A Potent Ca +2 channel blocker Richa Sah* and Saahil Arora 1. ISF College of Pharmacy, Moga, Punjab, India
AMOXICILLIN AND CLAVULANIC ACID TABLETS Draft proposal for The International Pharmacopoeia (February 2018)
 February 2018 Draft for comment 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 AMOXICILLIN AND CLAVULANIC ACID TABLETS Draft
February 2018 Draft for comment 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 AMOXICILLIN AND CLAVULANIC ACID TABLETS Draft
Journal of Global Trends in Pharmaceutical Sciences
 An Elsevier Indexed Journal ISSN-2230-7346 Journal of Global Trends in Pharmaceutical Sciences A NEW IMPROVED RP-HPLC METHOD FOR SIMULTANEOUS ESTIMATION OF HYDROCHLOROTHIAZIDE, AMLODIPINE BESYLATE AND
An Elsevier Indexed Journal ISSN-2230-7346 Journal of Global Trends in Pharmaceutical Sciences A NEW IMPROVED RP-HPLC METHOD FOR SIMULTANEOUS ESTIMATION OF HYDROCHLOROTHIAZIDE, AMLODIPINE BESYLATE AND
C 22 H 28 FNa 2 O 8 Pıı516.4
 SIMULTANEOUS DETERMINATION OF DEXAMETHASONE SODIUM PHOSPHATE AND CHLORAMPHENICOL IN OPHTHALMIC SOLUTIONS W.A. Shadoul, E.A. Gad Kariem, M.E. Adam, K.E.E. Ibrahim* Department of Pharmaceutical Chemistry,
SIMULTANEOUS DETERMINATION OF DEXAMETHASONE SODIUM PHOSPHATE AND CHLORAMPHENICOL IN OPHTHALMIC SOLUTIONS W.A. Shadoul, E.A. Gad Kariem, M.E. Adam, K.E.E. Ibrahim* Department of Pharmaceutical Chemistry,
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION OF ALISKIREN AND AMLODIPINE IN TABLET DOSAGE FORM
 Page288 Research Article Pharmaceutical Sciences DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION OF ALISKIREN AND AMLODIPINE IN TABLET DOSAGE FORM Divya P, Aleti P, Venisetty
Page288 Research Article Pharmaceutical Sciences DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR THE SIMULTANEOUS ESTIMATION OF ALISKIREN AND AMLODIPINE IN TABLET DOSAGE FORM Divya P, Aleti P, Venisetty
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR SIMULTANEOUS ESTIMATION OF AMLODIPINE BESYLATE AND IRBESARTAN
 Indexed in Cite Factor - Directory of International Research Journals in association with leading Universities DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR SIMULTANEOUS ESTIMATION OF AMLODIPINE BESYLATE
Indexed in Cite Factor - Directory of International Research Journals in association with leading Universities DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR SIMULTANEOUS ESTIMATION OF AMLODIPINE BESYLATE
Tamboli Ashpak Mubarak et al. IRJP 2 (8)
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN 2230 8407 Available online http://www.irjponline.com Research Article DEVELOPMENT AND VALIDATION OF STABILITY INDICATING HPLC METHOD FOR SIMULTANEOUS DETERMINATION
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN 2230 8407 Available online http://www.irjponline.com Research Article DEVELOPMENT AND VALIDATION OF STABILITY INDICATING HPLC METHOD FOR SIMULTANEOUS DETERMINATION
Sensitive and selective analysis of fipronil residues in eggs using Thermo Scientific GC-MS/MS triple quadrupole technology
 APPLICATION NOTE 10575 Sensitive and selective analysis of fipronil residues in eggs using Thermo Scientific GC-MS/MS triple quadrupole technology Authors Cristian Cojocariu, 1 Joachim Gummersbach, 2 and
APPLICATION NOTE 10575 Sensitive and selective analysis of fipronil residues in eggs using Thermo Scientific GC-MS/MS triple quadrupole technology Authors Cristian Cojocariu, 1 Joachim Gummersbach, 2 and
VALIDATED RP-HPLC METHOD FOR THE SIMULTANEOUS DETERMINATION OF AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM IN BULK AND PHARMACEUTICAL FORMULATION
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article VALIDATED RP-HPLC METHOD FOR THE SIMULTANEOUS DETERMINATION OF AMLODIPINE BESYLATE AND ATORVASTATIN
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article VALIDATED RP-HPLC METHOD FOR THE SIMULTANEOUS DETERMINATION OF AMLODIPINE BESYLATE AND ATORVASTATIN
Multi-residue Method II for Veterinary Drugs by HPLC (Animal and Fishery Products)
 Multi-residue Method II for Veterinary Drugs by HPLC (Animal and Fishery Products) 1. Analytes See Table 8. 2. Instruments High performance liquid chromatograph-photodiode array detector (HPLC-DAD) High
Multi-residue Method II for Veterinary Drugs by HPLC (Animal and Fishery Products) 1. Analytes See Table 8. 2. Instruments High performance liquid chromatograph-photodiode array detector (HPLC-DAD) High
Compliance. Should you have any questions, please contact Praveen Pabba, Ph.D., ( or
 Doxycycline Hyclate Delayed-Release Tablets Type of Posting Revision Bulletin Posting Date 28 Jul 2017 Official Date 01 Aug 2017 Expert Committee Chemical Medicines Monographs 1 Reason for Revision Compliance
Doxycycline Hyclate Delayed-Release Tablets Type of Posting Revision Bulletin Posting Date 28 Jul 2017 Official Date 01 Aug 2017 Expert Committee Chemical Medicines Monographs 1 Reason for Revision Compliance
Outpatient parenteral antimicrobial treatment. Which antibiotics can be used?
 Outpatient parenteral antimicrobial treatment Which antibiotics can be used? Franky Buyle SBIMC-BVIKM March 30th 2017 Brussels Pharmacy Multidisciplinary Infection Team Ghent University Hospital, Belgium
Outpatient parenteral antimicrobial treatment Which antibiotics can be used? Franky Buyle SBIMC-BVIKM March 30th 2017 Brussels Pharmacy Multidisciplinary Infection Team Ghent University Hospital, Belgium
Should you have any questions, please contact Edith Chang, Ph.D., Senior Scientific Liaison ( or
 Amlodipine and Tablets Type of Posting Posting Date Targeted Official Date Notice of Intent to Revise 26 Oct 2018 To Be Determined, Revision Bulletin Expert Committee Chemical Medicines Monographs 2 In
Amlodipine and Tablets Type of Posting Posting Date Targeted Official Date Notice of Intent to Revise 26 Oct 2018 To Be Determined, Revision Bulletin Expert Committee Chemical Medicines Monographs 2 In
Determination of ofloxacin in bulk drug and pharmaceutical dosage form by high performance liquid chromatography method
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (10):188-192 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (10):188-192 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
MOXIFLOXACIN HYDROCHLORIDE (MOXIFLOXACINI HYDROCHLORIDUM) Draft proposal for The International Pharmacopoeia. (January 2018)
 January 2018 DRAFT FOR COMMENT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 MOXIFLOXACIN HYDROCHLORIDE (MOXIFLOXACINI HYDROCHLORIDUM) Draft proposal
January 2018 DRAFT FOR COMMENT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 MOXIFLOXACIN HYDROCHLORIDE (MOXIFLOXACINI HYDROCHLORIDUM) Draft proposal
ABSTRACT. Usharani N, Divya K and Ashrtiha VVS. Original Article
 Original Article Development and Validation of UV-Derivative Spectroscopic and RP-HPLC Methods for the Determination of Amlodipine Besylate and Valsartan in Tablet Dosage form and Comparison of the Developed
Original Article Development and Validation of UV-Derivative Spectroscopic and RP-HPLC Methods for the Determination of Amlodipine Besylate and Valsartan in Tablet Dosage form and Comparison of the Developed
Streptomycin Sulfate According to USP
 Application Note Antibiotics The most reliable LC-EC applications for Antibiotics analysis Aminoglycosides Amikacin Framycetin Sulphate Gentamicin Sulphate Kanamycin Sulphate Lincomycin Neomycin Spectinomycin
Application Note Antibiotics The most reliable LC-EC applications for Antibiotics analysis Aminoglycosides Amikacin Framycetin Sulphate Gentamicin Sulphate Kanamycin Sulphate Lincomycin Neomycin Spectinomycin
Amlodipine, Valsartan, and Hydrochlorothiazide Tablets
 . Table Interim Revision Announcement Official November 1, 2017 Amlodipine 1 Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 2 (Continued) Tablet Strength Nominal Amlodipine/ Nominal Concentra-
. Table Interim Revision Announcement Official November 1, 2017 Amlodipine 1 Amlodipine, Valsartan, and Hydrochlorothiazide Tablets 2 (Continued) Tablet Strength Nominal Amlodipine/ Nominal Concentra-
Outpatient parenteral antimicrobial treatment. Which antibiotics can be used?
 Outpatient parenteral antimicrobial treatment Which antibiotics can be used? Franky Buyle SBIMC-BVIKM March 30th 2017 Brussels Pharmacy Multidisciplinary Infection Team Ghent University Hospital, Belgium
Outpatient parenteral antimicrobial treatment Which antibiotics can be used? Franky Buyle SBIMC-BVIKM March 30th 2017 Brussels Pharmacy Multidisciplinary Infection Team Ghent University Hospital, Belgium
HPLC method for simultaneous determination of Albendazole metabolites in plasma
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(11): 860-865 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 HPLC method for simultaneous determination of
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(11): 860-865 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 HPLC method for simultaneous determination of
A Unique Approach to Managing the Problem of Antibiotic Resistance
 A Unique Approach to Managing the Problem of Antibiotic Resistance By: Heather Storteboom and Sung-Chul Kim Department of Civil and Environmental Engineering Colorado State University A Quick Review The
A Unique Approach to Managing the Problem of Antibiotic Resistance By: Heather Storteboom and Sung-Chul Kim Department of Civil and Environmental Engineering Colorado State University A Quick Review The
International Journal of Advances in Pharmacy and Biotechnology Vol.3, Issue-2, 2017, 1-7 Research Article Open Access.
 I J A P B International Journal of Advances in Pharmacy and Biotechnology Vol.3, Issue-2, 2017, 1-7 Research Article Open Access. ISSN: 2454-8375 COMPARISON OF ANTIMICROBIAL ACTIVITY AND MIC OF BRANDED
I J A P B International Journal of Advances in Pharmacy and Biotechnology Vol.3, Issue-2, 2017, 1-7 Research Article Open Access. ISSN: 2454-8375 COMPARISON OF ANTIMICROBIAL ACTIVITY AND MIC OF BRANDED
Development and Validation of Amlodipine Impurities in Amlodipine Tablets Using Design Space Computer Modeling
 American Journal of Analytical Chemistry, 2016, 7, 918-926 http://www.scirp.org/journal/ajac ISSN Online: 2156-8278 ISSN Print: 2156-8251 Development and Validation of Amlodipine Impurities in Amlodipine
American Journal of Analytical Chemistry, 2016, 7, 918-926 http://www.scirp.org/journal/ajac ISSN Online: 2156-8278 ISSN Print: 2156-8251 Development and Validation of Amlodipine Impurities in Amlodipine
Pharma Research Library. 2013, Vol. 1(1):19-29
 Available online at www.pharmaresearchlibrary.com Pharma Research Library International Journal of Current Trends in Pharmaceutical Research 2013, Vol. 1(1):19-29 Pharma Research Library Method development
Available online at www.pharmaresearchlibrary.com Pharma Research Library International Journal of Current Trends in Pharmaceutical Research 2013, Vol. 1(1):19-29 Pharma Research Library Method development
ANTIBIOTICS IN PLASMA
 by LC/MS Code LC79010 (Daptomycin, Vancomycin, Streptomycin, Linezolid, Levofloxacin, Ciprofloxacin, Gentamicin, Amikacin, Teicoplanin) INTRODUCTION Technically it defines "antibiotic" a substance of natural
by LC/MS Code LC79010 (Daptomycin, Vancomycin, Streptomycin, Linezolid, Levofloxacin, Ciprofloxacin, Gentamicin, Amikacin, Teicoplanin) INTRODUCTION Technically it defines "antibiotic" a substance of natural
SPECTROPHOTOMETRIC ESTIMATION OF MELOXICAM IN BULK AND ITS PHARMACEUTICAL FORMULATIONS
 SPECTROPHOTOMETRIC ESTIMATION OF MELOXICAM IN BULK AND ITS PHARMACEUTICAL FORMULATIONS B.DHANDAPANI, S.ESWARA MURALI, N. SUSRUTHA, RAMA SWETHA, S K. SONIA RANI, T. SARATH BABU, G.V. SEETHARAMANJANEYULU,
SPECTROPHOTOMETRIC ESTIMATION OF MELOXICAM IN BULK AND ITS PHARMACEUTICAL FORMULATIONS B.DHANDAPANI, S.ESWARA MURALI, N. SUSRUTHA, RAMA SWETHA, S K. SONIA RANI, T. SARATH BABU, G.V. SEETHARAMANJANEYULU,
DETERMINATION OF ACTIVE SUBSTANCES IN MULTICOMPONENT VETERINARY PREPARATIONS OF ANTIPARASITIC ACTION BY HPLC METHOD
 Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 67 No. 5 pp. 463ñ468, 2010 ISSN 0001-6837 Polish Pharmaceutical Society DETERMINATION OF ACTIVE SUBSTANCES IN MULTICOMPONENT VETERINARY PREPARATIONS OF
Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 67 No. 5 pp. 463ñ468, 2010 ISSN 0001-6837 Polish Pharmaceutical Society DETERMINATION OF ACTIVE SUBSTANCES IN MULTICOMPONENT VETERINARY PREPARATIONS OF
Determination, Confirmation and Quantitation of Multi-Class Antibiotic Residues in Milk by UHPLC MS/MS
 APPLICATION NOTE Liquid Chromatography/ Mass Spectrometry Authors: Avinash Dalmia PerkinElmer, Inc. Shelton, CT Determination, Confirmation and Quantitation of Multi-Class Antibiotic Residues in Milk by
APPLICATION NOTE Liquid Chromatography/ Mass Spectrometry Authors: Avinash Dalmia PerkinElmer, Inc. Shelton, CT Determination, Confirmation and Quantitation of Multi-Class Antibiotic Residues in Milk by
A Simple Sample Preparation with HPLC UV Method for Estimation of Amlodipine from Plasma: Application to Bioequivalence Study
 22 The Open Chemical and Biomedical Methods Journal, 2008, 1, 22-27 Open Access A Simple Sample Preparation with HPLC UV Method for Estimation of Amlodipine from Plasma: Application to Bioequivalence Study
22 The Open Chemical and Biomedical Methods Journal, 2008, 1, 22-27 Open Access A Simple Sample Preparation with HPLC UV Method for Estimation of Amlodipine from Plasma: Application to Bioequivalence Study
Ultra-Fast Analysis of Contaminant Residue from Propolis by LC/MS/MS Using SPE
 Ultra-Fast Analysis of Contaminant Residue from Propolis by LC/MS/MS Using SPE Matthew Trass, Philip J. Koerner and Jeff Layne Phenomenex, Inc., 411 Madrid Ave.,Torrance, CA 90501 USA PO88780811_L_2 Introduction
Ultra-Fast Analysis of Contaminant Residue from Propolis by LC/MS/MS Using SPE Matthew Trass, Philip J. Koerner and Jeff Layne Phenomenex, Inc., 411 Madrid Ave.,Torrance, CA 90501 USA PO88780811_L_2 Introduction
Mouse Formulary. The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.
 Mouse Formulary The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.): Intraperitoneal (IP) doses should not exceed 80 ml/kg
Mouse Formulary The maximum recommended volume of a drug given depends on the route of administration (Formulary for Laboratory Animals, 3 rd ed.): Intraperitoneal (IP) doses should not exceed 80 ml/kg
OPAT discharge navigator and laboratory monitoring Select OPAT button for ALL patients that discharge on intravenous antimicrobials
 Clinical Monitoring of Outpatient Parenteral Antimicrobial Therapy (OPAT) and Selected Oral Antimicrobial Agents Adult Inpatient/Ambulatory Clinical Practice Guideline Appendix A. Coordinating an OPAT
Clinical Monitoring of Outpatient Parenteral Antimicrobial Therapy (OPAT) and Selected Oral Antimicrobial Agents Adult Inpatient/Ambulatory Clinical Practice Guideline Appendix A. Coordinating an OPAT
Consider the patient, the drug and the device how do you choose?
 Consider the patient, the drug and the device how do you choose? Tim Hills Lead Pharmacist Antimicrobials and Infection Control Nottingham University Hospitals NHS Trust OPAT Recommendations Drug Therapy
Consider the patient, the drug and the device how do you choose? Tim Hills Lead Pharmacist Antimicrobials and Infection Control Nottingham University Hospitals NHS Trust OPAT Recommendations Drug Therapy
European Journal of Biomedical and Pharmaceutical ISSN Sciences
 ejbps, 2016, Volume 3, Issue 11, 272-281. Research Article SJIF Impact Factor 3.881 Sharma et al. European Journal of Biomedical AND Pharmaceutical sciences European Journal of Biomedical and Pharmaceutical
ejbps, 2016, Volume 3, Issue 11, 272-281. Research Article SJIF Impact Factor 3.881 Sharma et al. European Journal of Biomedical AND Pharmaceutical sciences European Journal of Biomedical and Pharmaceutical
SUMMARY OF PRODUCT CHARACTERISTICS. Bottle of powder: Active substance: ceftiofur sodium mg equivalent to ceftiofur...
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT WONDERCEF powder and solvent for solution for injection for horses not intended for the production of foods for human consumption.
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT WONDERCEF powder and solvent for solution for injection for horses not intended for the production of foods for human consumption.
Development and validation of HPLC method for simultaneous estimation of Amlodipine besylate and Enalapril maleate in solid dosage form
 World Journal of Pharmaceutical Sciences ISS (Print): 2321-3310; ISS (nline): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ riginal
World Journal of Pharmaceutical Sciences ISS (Print): 2321-3310; ISS (nline): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ riginal
International Journal of Pharmaceutical Research & Analysis
 13 International Journal of Pharmaceutical Research & Analysis e-issn: 2249 7781 Print ISSN: 2249 779X www.ijpra.com RP-HPLC METHOD DEVELOPMENT AND VALIDATION FOR SIMULTANEOUS ESTIMATION OF AMLODIPINE
13 International Journal of Pharmaceutical Research & Analysis e-issn: 2249 7781 Print ISSN: 2249 779X www.ijpra.com RP-HPLC METHOD DEVELOPMENT AND VALIDATION FOR SIMULTANEOUS ESTIMATION OF AMLODIPINE
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE RESPONSE TO OFFICE ACTION
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE Applicant(s) Roychowdhury et al. Customer No. 62965 Serial No. 13/541,524 Confirmation No. 8238 Filed July 3, 2012 Group Art Unit 1629 Examiner For Polansky,
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE Applicant(s) Roychowdhury et al. Customer No. 62965 Serial No. 13/541,524 Confirmation No. 8238 Filed July 3, 2012 Group Art Unit 1629 Examiner For Polansky,
METHOD DEVELOPMENT AND VALIDATION FOR THE SIMULTANEOUS ESTIMATION OF OFLOXACIN AND ORNIDAZOLE IN TABLET DOSAGE FORM BY RP-HPLC
 METHOD DEVELOPMENT AND VALIDATION FOR THE SIMULTANEOUS ESTIMATION OF OFLOXACIN AND ORNIDAZOLE IN TABLET DOSAGE FORM BY RP-HPLC B.Dhandapani *1, N.Thirumoorthy 2, Shaik Harun Rasheed 3, M.Rama kotaiah 3
METHOD DEVELOPMENT AND VALIDATION FOR THE SIMULTANEOUS ESTIMATION OF OFLOXACIN AND ORNIDAZOLE IN TABLET DOSAGE FORM BY RP-HPLC B.Dhandapani *1, N.Thirumoorthy 2, Shaik Harun Rasheed 3, M.Rama kotaiah 3
PO. Vasan, Gandhinagar District, Gujarat, India, 3 Dean at Faculty of Pharmacy, Dharmsinh Desai University, Nadiad, Gujarat, India.
 International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN : 0974-4290 Vol.6, No.5, pp 2615-2619, Aug-Sept 2014 Development and Validation of Simultaneous Estimation of Cefpodoxime proxetil and
International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN : 0974-4290 Vol.6, No.5, pp 2615-2619, Aug-Sept 2014 Development and Validation of Simultaneous Estimation of Cefpodoxime proxetil and
Development and Validation of RP-HPLC Method for Determination of Related Substances of Medetomidine in Bulk Drug
 Human Journals Research Article July 2016 Vol.:6, Issue:4 All rights are reserved by Nuzhath Fathima et al. Development and Validation of RP-HPLC Method for Determination of Related Substances of Medetomidine
Human Journals Research Article July 2016 Vol.:6, Issue:4 All rights are reserved by Nuzhath Fathima et al. Development and Validation of RP-HPLC Method for Determination of Related Substances of Medetomidine
Journal of Applied Pharmaceutical Research ISSN No
 SIMULTANEOUS ESTIMATION OF PYRANTEL PAMOATE, PRAZIQUANTEL & FEBANTEL BY HIGH PERFORMANCE LIQUID CHROMATOGRAPHY USING DUAL WAVELENGTH Rupali Sajjanwar (Rupali Jitendra Paranjape)*, Shyamala Bhaskaran, Kulesh
SIMULTANEOUS ESTIMATION OF PYRANTEL PAMOATE, PRAZIQUANTEL & FEBANTEL BY HIGH PERFORMANCE LIQUID CHROMATOGRAPHY USING DUAL WAVELENGTH Rupali Sajjanwar (Rupali Jitendra Paranjape)*, Shyamala Bhaskaran, Kulesh
Development and Validation of UV Spectrophotometric Area Under Curve (AUC) method for estimation of Pyrantel Pamoate in Bulk and Tablet Dosage Form
 International Journal of Interdisciplinary and Multidisciplinary Studies (IJIMS), 2014, Vol 1, No.7, 70-76. 70 Available online at http://www.ijims.com ISSN: 2348 0343 Development and Validation of UV
International Journal of Interdisciplinary and Multidisciplinary Studies (IJIMS), 2014, Vol 1, No.7, 70-76. 70 Available online at http://www.ijims.com ISSN: 2348 0343 Development and Validation of UV
Deptt of Pharma Science SGRR ITS Patel Nagar, Dehradun (UK)
 METHOD DEVELOPMENT AND ITS VALIDATION FOR SIMULTANEOUS ESTIMATION OF ATORVASTATIN AND AMLODIPINE IN COMBINATION IN TABLET DOSAGE FORM BY UV SPECTROSCOPY, USING MULTI-COMPONENT MODE OF ANALYSIS V. Juyal
METHOD DEVELOPMENT AND ITS VALIDATION FOR SIMULTANEOUS ESTIMATION OF ATORVASTATIN AND AMLODIPINE IN COMBINATION IN TABLET DOSAGE FORM BY UV SPECTROSCOPY, USING MULTI-COMPONENT MODE OF ANALYSIS V. Juyal
Stability of Tylosin in Honey Impact on Residue Analysis Don Noot, Tom Thompson
 Stability of Tylosin in Honey Impact on Residue Analysis Don Noot, Tom Thompson Background Information collaboration with Agriculture and Agri-Food Canada project leader: Dr. Steve Pernal (Beaverlodge,
Stability of Tylosin in Honey Impact on Residue Analysis Don Noot, Tom Thompson Background Information collaboration with Agriculture and Agri-Food Canada project leader: Dr. Steve Pernal (Beaverlodge,
A VALIDATED HPLC-ASSAY FOR THE DETERMINATION OF MELOXICAM IN PRESENCE OF ITS DEGRADATION PRODUCTS
 Sc!entia Pharmaceutica (Sci. Pharm.) 72, 213-220 (2004) 21 3 O Osterreichische Apotheker-Verlagsgesellschaft m. b. H., Wien, Printed in Austria A VALIDATED HPLC-ASSAY FOR THE DETERMINATION OF MELOXICAM
Sc!entia Pharmaceutica (Sci. Pharm.) 72, 213-220 (2004) 21 3 O Osterreichische Apotheker-Verlagsgesellschaft m. b. H., Wien, Printed in Austria A VALIDATED HPLC-ASSAY FOR THE DETERMINATION OF MELOXICAM
Rapid LC-MS/MS Method for the Analysis of Fipronil and Amitraz Insecticides and Associated Metabolites in Egg and Other Poultry Products
 Rapid LC-MS/MS Method for the Analysis of Fipronil and Amitraz Insecticides and Associated Metabolites in Egg and Other Poultry Products Ashley Sage 1, Jianru Stahl-Zeng 2, Jason Causon 1, Mike Whitmore
Rapid LC-MS/MS Method for the Analysis of Fipronil and Amitraz Insecticides and Associated Metabolites in Egg and Other Poultry Products Ashley Sage 1, Jianru Stahl-Zeng 2, Jason Causon 1, Mike Whitmore
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Amfipen LA 100 mg/ml suspension for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Each ml contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Amfipen LA 100 mg/ml suspension for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Each ml contains:
Triline Pumps. Vacuum & Pressure Gas moving Engineers. Diaphragm Pumps EVM Series
 Vacuum & Pressure Gas moving Engineers Diaphragm Pumps EVM Series EVM Diaphragm Pumps & Accessories has evolved over the years by working in partnership with many leading manufactures, to develop Triline
Vacuum & Pressure Gas moving Engineers Diaphragm Pumps EVM Series EVM Diaphragm Pumps & Accessories has evolved over the years by working in partnership with many leading manufactures, to develop Triline
Determination of Acaricides in Korean Honey Bull. Korean Chem. Soc. 2008, Vol. 29, No
 Determination of Acaricides in Korean Honey Bull. Korean Chem. Soc. 2008, Vol. 29, No. 5 1043 Simultaneous Determination of Amitraz, Bromopropylate, Coumaphos, Cymiazole and 2,4-Dimethylaniline in Korean
Determination of Acaricides in Korean Honey Bull. Korean Chem. Soc. 2008, Vol. 29, No. 5 1043 Simultaneous Determination of Amitraz, Bromopropylate, Coumaphos, Cymiazole and 2,4-Dimethylaniline in Korean
Available online International Journal of Pharmaceutical Research & Allied Sciences, 2016, 5(4):37-44.
 Available online www.ijpras.com International Journal of Pharmaceutical Research & Allied Sciences, 2016, 5(4):37-44 Research Article ISSN : 2277-3657 CODEN(USA) : IJPRPM DEVELOPMENT AND VALIDATION OF
Available online www.ijpras.com International Journal of Pharmaceutical Research & Allied Sciences, 2016, 5(4):37-44 Research Article ISSN : 2277-3657 CODEN(USA) : IJPRPM DEVELOPMENT AND VALIDATION OF
SZENT ISTVÁN UNIVERSITY. Doctoral School of Veterinary Science
 SZENT ISTVÁN UNIVERSITY Doctoral School of Veterinary Science Comparative pharmacokinetics of the amoxicillinclavulanic acid combination in broiler chickens and turkeys, susceptibility and stability tests
SZENT ISTVÁN UNIVERSITY Doctoral School of Veterinary Science Comparative pharmacokinetics of the amoxicillinclavulanic acid combination in broiler chickens and turkeys, susceptibility and stability tests
SIMPLE U.V. SPECTROPHOTOMETRIC METHODS FOR THE ESTIMATION OF OFLOXACIN IN PHARMACEUTICAL FORMULATIONS
 Int. J. Chem. Sci.: 8(2), 2010, 983-990 SIMPLE U.V. SPECTROPHOTOMETRIC METHODS FOR THE ESTIMATION OF OFLOXACIN IN PHARMACEUTICAL FORMULATIONS C. SOWMYA *, Y. PADMANABHA REDDY, J. RAVINDRA REDDY, M. SIVA
Int. J. Chem. Sci.: 8(2), 2010, 983-990 SIMPLE U.V. SPECTROPHOTOMETRIC METHODS FOR THE ESTIMATION OF OFLOXACIN IN PHARMACEUTICAL FORMULATIONS C. SOWMYA *, Y. PADMANABHA REDDY, J. RAVINDRA REDDY, M. SIVA
Development and Validation of a RP-HPLC Method for Simultaneous Determination of Levofloxacin and Moxifloxacin in Pharmaceutical Dosage Forms
 Journal of Basic & Applied Sciences, 2013, 9, 633-638 633 Development and Validation of a RP-HPLC Method for Simultaneous Determination of Levofloxacin and Moxifloxacin in Pharmaceutical Dosage Forms Farjahan
Journal of Basic & Applied Sciences, 2013, 9, 633-638 633 Development and Validation of a RP-HPLC Method for Simultaneous Determination of Levofloxacin and Moxifloxacin in Pharmaceutical Dosage Forms Farjahan
Gliding Motility Assay for P. berghei Sporozoites
 Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Pharmacokinetics of Amoxicillin/Clavulanic Acid Combination after Oral Administration of New Suspension Formulations in Human Volunteers
 R Iranian Journal of Pharmaceutical Sciences Summer 2006: 2(3): 129-136 www.ijps.ir Original Article Pharmacokinetics of Amoxicillin/Clavulanic Acid Combination after Oral Administration of New Suspension
R Iranian Journal of Pharmaceutical Sciences Summer 2006: 2(3): 129-136 www.ijps.ir Original Article Pharmacokinetics of Amoxicillin/Clavulanic Acid Combination after Oral Administration of New Suspension
Chandra Mohan Rao Kota et al INTERNATIONAL JOURNAL OF RESEARCH AND REVIEWS IN PHARMACY AND APPLIED SCIENCES
 Research Article Chandra Mohan Rao Kota et al I S S N 2249-1236 VOL 1, ISSUE (2) INTERNATIONAL JOURNAL OF RESEARCH AND REVIEWS IN PHARMACY AND APPLIED SCIENCES A SIMPLE GRADIENT RP-HPLC METHOD FOR THE
Research Article Chandra Mohan Rao Kota et al I S S N 2249-1236 VOL 1, ISSUE (2) INTERNATIONAL JOURNAL OF RESEARCH AND REVIEWS IN PHARMACY AND APPLIED SCIENCES A SIMPLE GRADIENT RP-HPLC METHOD FOR THE
GASTRO-INTESTINAL (ENTERAL)
 GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 95 A D Gastrostomy Feeding Tubes & Accessories Tri-Funnel Replacement Gastrostomy Tube Green inflator cuff, sterile. ard 12 French, 5 cc balloon.
GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 95 A D Gastrostomy Feeding Tubes & Accessories Tri-Funnel Replacement Gastrostomy Tube Green inflator cuff, sterile. ard 12 French, 5 cc balloon.
Stability indicating HPLC Method Validation for the Assay of Dexmedetomidine in Dexmedetomidine Hydrochloride Injection
 International Journal of TechnoChem Research ISSN:2395-4248 www.technochemsai.com Vol.02, No.01, pp 54-61, 2016 Stability indicating HPLC Method Validation for the Assay of Dexmedetomidine in Dexmedetomidine
International Journal of TechnoChem Research ISSN:2395-4248 www.technochemsai.com Vol.02, No.01, pp 54-61, 2016 Stability indicating HPLC Method Validation for the Assay of Dexmedetomidine in Dexmedetomidine
Dosing Your Cat with Azithromycin Pediatric Suspension. By Lorraine Shelton
 Dosing Your Cat with Azithromycin Pediatric Suspension By Lorraine Shelton To join a community of cat fanciers and health professionals interested in cattery related health issues, visit http://groups.yahoo.com/group/fanciershealth
Dosing Your Cat with Azithromycin Pediatric Suspension By Lorraine Shelton To join a community of cat fanciers and health professionals interested in cattery related health issues, visit http://groups.yahoo.com/group/fanciershealth
COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE
 European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/211249/2005-FINAL July 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE DIHYDROSTREPTOMYCIN (Extrapolation to all ruminants)
European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/211249/2005-FINAL July 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR VETERINARY USE DIHYDROSTREPTOMYCIN (Extrapolation to all ruminants)
MEDICATION ADMINSITRATION: ANTIBIOTIC LOCK THERAPY GUIDELINE
 MEDICATION ADMINSITRATION: ANTIBIOTIC LOCK THERAPY GUIDELINE I. PURPOSE Central venous catheters are an integral part in medical management for patients requiring long-term total parenteral nutrition,
MEDICATION ADMINSITRATION: ANTIBIOTIC LOCK THERAPY GUIDELINE I. PURPOSE Central venous catheters are an integral part in medical management for patients requiring long-term total parenteral nutrition,
Detection of residues of quinolones in milk
 Food Safety and Monitoring of Safety Aspects 77 Detection of residues of quinolones in milk Gertraud Suhren and P. Hammer Federal Dairy Research Centre, Institute for Hygiene, Hermann-Weigmann-Str. 1,
Food Safety and Monitoring of Safety Aspects 77 Detection of residues of quinolones in milk Gertraud Suhren and P. Hammer Federal Dairy Research Centre, Institute for Hygiene, Hermann-Weigmann-Str. 1,
Fluoroquinolones ELISA KIT
 Fluoroquinolones ELISA KIT Cat. No.:DEIA6883 Pkg.Size:96T Intended use The Fluoroquinolones ELISA KIT is an immunoassay for the detection of Fluoroquinolones in contaminated samples including water, fish
Fluoroquinolones ELISA KIT Cat. No.:DEIA6883 Pkg.Size:96T Intended use The Fluoroquinolones ELISA KIT is an immunoassay for the detection of Fluoroquinolones in contaminated samples including water, fish
single intravenous and oral doses and after 14 repeated oral
 Br. J. clin. Pharmac. (1986), 22, 21-25 The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily J. K. FAULKNER
Br. J. clin. Pharmac. (1986), 22, 21-25 The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily J. K. FAULKNER
Pradhan Prasanna Kumar et al. Int. Res. J. Pharm. 2014, 5 (9) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article ANALYTICAL METHOD DEVELOPMENT AND VALIDATION FOR SIMULTANEOUS ESTIMATION OF FENAC SODIUM AND FLOXACIN IN THEIR
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article ANALYTICAL METHOD DEVELOPMENT AND VALIDATION FOR SIMULTANEOUS ESTIMATION OF FENAC SODIUM AND FLOXACIN IN THEIR
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Cefenil 50 mg/ml Powder and Solvent for Solution for Injection for and. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Powder vial
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Cefenil 50 mg/ml Powder and Solvent for Solution for Injection for and. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Powder vial
Determination of Benzimidazole Residues in Animal Tissue by Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry
 PO-CON1472E Determination of Benzimidazole Residues in Animal Tissue by Ultra High Performance Liquid Chromatography Tandem ASMS 14 TP 21 Yin Huo, Jinting Yao, Changkun Li, Taohong Huang, Shin-ichi Kawano,
PO-CON1472E Determination of Benzimidazole Residues in Animal Tissue by Ultra High Performance Liquid Chromatography Tandem ASMS 14 TP 21 Yin Huo, Jinting Yao, Changkun Li, Taohong Huang, Shin-ichi Kawano,
Concentration of Enrofloxacin Residue from Tilapia (Oreochromis niloticus) Muscular That Infected by Aeromonas salmonicida
 Journal of Agricultural Science and Technology A 4 (2014) 750-754 Earlier title: Journal of Agricultural Science and Technology, ISSN 1939-1250 doi: 10.17265/2161-6256/2014.09.005 D DAVID PUBLISHING Concentration
Journal of Agricultural Science and Technology A 4 (2014) 750-754 Earlier title: Journal of Agricultural Science and Technology, ISSN 1939-1250 doi: 10.17265/2161-6256/2014.09.005 D DAVID PUBLISHING Concentration
Quantification of Chloramphenicol in Chicken Using Xevo TQD with RADAR Technology
 Quantification of Chloramphenicol in Chicken Using Xevo TQD with RADAR Technology Dimple Shah, Marian Twohig, and Jennifer A. Burgess Waters Corporation, Milford, MA, U.S.A. A P P L I C AT ION B E N E
Quantification of Chloramphenicol in Chicken Using Xevo TQD with RADAR Technology Dimple Shah, Marian Twohig, and Jennifer A. Burgess Waters Corporation, Milford, MA, U.S.A. A P P L I C AT ION B E N E
Method development and validation for simultaneous estimation of telmisartan and amlodipine by RP-HPLC
 World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
International Journal of Pharmacy and Pharmaceutical Sciences. Research Article
 Academic Sciences International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 4, Suppl 3, 2012 Research Article A NOVEL AND HIGH-THROUGHPUT METHOD FOR THE SIMULTANEOUS DETERMINATION
Academic Sciences International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 4, Suppl 3, 2012 Research Article A NOVEL AND HIGH-THROUGHPUT METHOD FOR THE SIMULTANEOUS DETERMINATION
Application of Peristaltic Filling for Flexibility and Accuracy
 E03 - Aseptic Processing Technology 2008 Case Study: Application of Peristaltic Filling for Flexibility and Accuracy by Ted Kemnitz Automated Machine Technologies, Inc. AMT (919) 361 0121 Ted.Kemnitz@AMTLiquidFilling.com
E03 - Aseptic Processing Technology 2008 Case Study: Application of Peristaltic Filling for Flexibility and Accuracy by Ted Kemnitz Automated Machine Technologies, Inc. AMT (919) 361 0121 Ted.Kemnitz@AMTLiquidFilling.com
GASTRO-INTESTINAL (ENTERAL)
 GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 97 D Gastrostomy Feeding Tubes & Accessories Feeding Tube 18 French, 7-10 ml balloon. 058436 Each Tri-Funnel Replacement Gastrostomy Tube Green inflator
GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 97 D Gastrostomy Feeding Tubes & Accessories Feeding Tube 18 French, 7-10 ml balloon. 058436 Each Tri-Funnel Replacement Gastrostomy Tube Green inflator
The Guide for the Care and Use of Laboratory Animals, 8th Edition, November Euthanasia. pp
 Euthanasia Policy IACUP Policy Effective Date: October 2015 I. Purpose This policy establishes the standards for euthanasia of laboratory animals at UCSF. This policy has been created to ensure that euthanasia
Euthanasia Policy IACUP Policy Effective Date: October 2015 I. Purpose This policy establishes the standards for euthanasia of laboratory animals at UCSF. This policy has been created to ensure that euthanasia
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
Specific and Simple HPLC Assay of Ecofriendly Meloxicam in Pharmaceutical Formulations K.T. Mahmood 1, B.Khan 2, M. Ashraf 3 and I. U.
 Specific and Simple HPLC Assay of Ecofriendly Meloxicam in Pharmaceutical Formulations K.T. Mahmood 1, B.Khan 2, M. Ashraf 3 and I. U.Haq 4 1 DTL,Health Department Punjab, Lahore, 2 Lahore College for
Specific and Simple HPLC Assay of Ecofriendly Meloxicam in Pharmaceutical Formulations K.T. Mahmood 1, B.Khan 2, M. Ashraf 3 and I. U.Haq 4 1 DTL,Health Department Punjab, Lahore, 2 Lahore College for
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site:
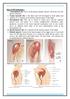 Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
Introduction Zosyn background information. History of reformulation of Zosyn
 REVIEW Zosyn (/) reformulation: Expanded compatibility and coadministration with lactated solutions and selected aminoglycosides Narendra R Desai Syed M Shah Jonathan Cohen Matthew McLaughlin Hema R Dalal
REVIEW Zosyn (/) reformulation: Expanded compatibility and coadministration with lactated solutions and selected aminoglycosides Narendra R Desai Syed M Shah Jonathan Cohen Matthew McLaughlin Hema R Dalal
European Public MRL assessment report (EPMAR)
 18 March 2016 EMA/CVMP/619817/2015 Committee for Medicinal Products for Veterinary Use European Public MRL assessment report (EPMAR) Gentamicin (all mammalian food producing species and fin fish) On 3
18 March 2016 EMA/CVMP/619817/2015 Committee for Medicinal Products for Veterinary Use European Public MRL assessment report (EPMAR) Gentamicin (all mammalian food producing species and fin fish) On 3
Determination of gentamicin and related impurities in gentamicin sulfate
 APPLICATION NOTE 767 Determination of gentamicin and related impurities in gentamicin sulfate Authors Jingli Hu and Jeffrey Rohrer Thermo Fisher Scientific Sunnyvale, CA Keywords Dionex IonPac AmG-µm C8
APPLICATION NOTE 767 Determination of gentamicin and related impurities in gentamicin sulfate Authors Jingli Hu and Jeffrey Rohrer Thermo Fisher Scientific Sunnyvale, CA Keywords Dionex IonPac AmG-µm C8
Quantification of Several Acidic Drugs in Equine Serum Using LC MS-MS
 Journal of Analytical Toxicology Advance Access published August 27, 2013 Journal of Analytical Toxicology 2013;1 5 doi:10.1093/jat/bkt069 Special Issue Quantification of Several Acidic Drugs in Equine
Journal of Analytical Toxicology Advance Access published August 27, 2013 Journal of Analytical Toxicology 2013;1 5 doi:10.1093/jat/bkt069 Special Issue Quantification of Several Acidic Drugs in Equine
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BLUEVAC BTV8 suspension for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BLUEVAC BTV8 suspension for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of
Analysis of Multiclass Veterinary Drugs in Baby Food by Ultra Fast Chromatography with High Performance Triple Quadrupole Mass Spectrometry
 Analysis of Multiclass Veterinary Drugs in Baby Food by Ultra Fast Chromatography with High Performance Triple Quadrupole Mass Spectrometry Charles Yang, 1 Dipankar Ghosh, 1 Mary Blackburn, 1 Jamie Humphries
Analysis of Multiclass Veterinary Drugs in Baby Food by Ultra Fast Chromatography with High Performance Triple Quadrupole Mass Spectrometry Charles Yang, 1 Dipankar Ghosh, 1 Mary Blackburn, 1 Jamie Humphries
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/389/98-FINAL July 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS ENROFLOXACIN (extension to
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/389/98-FINAL July 1998 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS ENROFLOXACIN (extension to
Introduction to Pharmacokinetics and Pharmacodynamics
 Introduction to Pharmacokinetics and Pharmacodynamics Diane M. Cappelletty, Pharm.D. Assistant Professor of Pharmacy Practice Wayne State University August, 2001 Vocabulary Clearance Renal elimination:
Introduction to Pharmacokinetics and Pharmacodynamics Diane M. Cappelletty, Pharm.D. Assistant Professor of Pharmacy Practice Wayne State University August, 2001 Vocabulary Clearance Renal elimination:
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Acecare 2mg/ml Solution for Injection for Dogs and Cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution contains
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Acecare 2mg/ml Solution for Injection for Dogs and Cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution contains
Novel RP-HPLC Method Development and Validation of Meloxicam Suppository
 Original Article Novel RP-HPLC Method Development and Validation of Meloxicam Suppository Sufiyan Ahmad 1 *, Sharma Deepika 1, Patil Amol 1, Warude Kapil 1, Md. Rageeb Md.Usman 2 1 Department of Quality
Original Article Novel RP-HPLC Method Development and Validation of Meloxicam Suppository Sufiyan Ahmad 1 *, Sharma Deepika 1, Patil Amol 1, Warude Kapil 1, Md. Rageeb Md.Usman 2 1 Department of Quality
PACKAGE LEAFLET: INFORMATION FOR THE USER. Amikacin 250 mg/ml Injection
 PACKAGE LEAFLET: INFORMATION FOR THE USER Amikacin 250 mg/ml Injection Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again. If you
PACKAGE LEAFLET: INFORMATION FOR THE USER Amikacin 250 mg/ml Injection Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again. If you
Clinical Practice Standard
 Clinical Practice Standard 1-20-6-1-010 TITLE: INTRAVENOUS TO ORAL CONVERSION FOR ANTIMICROBIALS A printed copy of this document may not reflect the current, electronic version on OurNH. APPLICABILITY:
Clinical Practice Standard 1-20-6-1-010 TITLE: INTRAVENOUS TO ORAL CONVERSION FOR ANTIMICROBIALS A printed copy of this document may not reflect the current, electronic version on OurNH. APPLICABILITY:
Development And Validation Of Methods For Estimation Of Pimobendan In Pharmaceutical Dosage Form
 International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.5, No.5, pp 2154-2164, July-Sept 2013 Development And Validation Of Methods For Estimation Of Pimobendan In Pharmaceutical
International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.5, No.5, pp 2154-2164, July-Sept 2013 Development And Validation Of Methods For Estimation Of Pimobendan In Pharmaceutical
Scientific Discussion post-authorisation update for Rheumocam extension X/007
 5 May 2011 EMA/170257/2011 Veterinary Medicines and Product Data Management Scientific Discussion post-authorisation update for Rheumocam extension X/007 Scope of extension: addition of 20 mg/ml solution
5 May 2011 EMA/170257/2011 Veterinary Medicines and Product Data Management Scientific Discussion post-authorisation update for Rheumocam extension X/007 Scope of extension: addition of 20 mg/ml solution
Determination of Amlodipine in Rat Plasma by UV Spectroscopy
 Determination of Amlodipine in Rat Plasma by UV Spectroscopy P. Srinivasulu 1*, B.K. Gowthami 2, T.N.V. Ganesh Kumar 1, D. Surya Narayana Raju 1, S. Vidyadhara 1 1 Chebrolu Hanumaiah Institute of Pharmaceutical
Determination of Amlodipine in Rat Plasma by UV Spectroscopy P. Srinivasulu 1*, B.K. Gowthami 2, T.N.V. Ganesh Kumar 1, D. Surya Narayana Raju 1, S. Vidyadhara 1 1 Chebrolu Hanumaiah Institute of Pharmaceutical
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, CZ, EE, ES, FR, IE, IS, IT, LT, LU, LV, NO, PL, PT, RO, SE, SI, SK, UK: Genestran 75 micrograms/ml solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, CZ, EE, ES, FR, IE, IS, IT, LT, LU, LV, NO, PL, PT, RO, SE, SI, SK, UK: Genestran 75 micrograms/ml solution for injection
Treatment of peritonitis in patients receiving peritoneal dialysis Antibiotic Guidelines. Contents
 Treatment of peritonitis in patients receiving Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Jude Allen (Pharmacist) Additional author(s): Dr David Lewis, Dr Dimitrios Poulikakos,
Treatment of peritonitis in patients receiving Antibiotic Guidelines Classification: Clinical Guideline Lead Author: Jude Allen (Pharmacist) Additional author(s): Dr David Lewis, Dr Dimitrios Poulikakos,
PROPYLENE GLYCOL FREE MINOXIDIL TOPICAL FORMULATION FOR HAIR LOSS BASED ON PATENTED TECHNOLOGY
 Page 1 of 7 LICENSING OPPORTUNITY PROPYLENE GLYCOL FREE MINOXIDIL TOPICAL FORMULATION FOR HAIR LOSS BASED ON PATENTED TECHNOLOGY NO PROPYLENE GLYCOL NO SCALP IRRITATION, NO GREASY HAIR BIOEQUIVALENT ABSORPTION
Page 1 of 7 LICENSING OPPORTUNITY PROPYLENE GLYCOL FREE MINOXIDIL TOPICAL FORMULATION FOR HAIR LOSS BASED ON PATENTED TECHNOLOGY NO PROPYLENE GLYCOL NO SCALP IRRITATION, NO GREASY HAIR BIOEQUIVALENT ABSORPTION
STANDARD OPERATING PROCEDURE #110 MOUSE ANESTHESIA
 STANDARD OPERATING PROCEDURE #110 MOUSE ANESTHESIA 1. PURPOSE This Standard Operating Procedure (SOP) describes methods for anesthetizing mice. 2. RESPONSIBILITY Principal Investigators (PIs) and their
STANDARD OPERATING PROCEDURE #110 MOUSE ANESTHESIA 1. PURPOSE This Standard Operating Procedure (SOP) describes methods for anesthetizing mice. 2. RESPONSIBILITY Principal Investigators (PIs) and their
Sci Pharm
 Sci Pharm www.scipharm.at Research article Open Access A Validated Stability-Indicating RP-UPLC Method for Simultaneous Determination of Desloratadine and Sodium Benzoate in Oral Liquid Pharmaceutical
Sci Pharm www.scipharm.at Research article Open Access A Validated Stability-Indicating RP-UPLC Method for Simultaneous Determination of Desloratadine and Sodium Benzoate in Oral Liquid Pharmaceutical
Quality of Veterinary Medicinal Products. How to ensure the quality of Veterinary Medicinal Products
 Quality of Veterinary Medicinal Products How to ensure the quality of Veterinary Medicinal Products Jean-Pierre Orand Anses/ANMV OIE Collaborating Centre on Veterinary medicinal products Jean-pierre.orand@anses.fr
Quality of Veterinary Medicinal Products How to ensure the quality of Veterinary Medicinal Products Jean-Pierre Orand Anses/ANMV OIE Collaborating Centre on Veterinary medicinal products Jean-pierre.orand@anses.fr
