National Competent Authorities for the implementation of Directive 2010/63/EU on the protection of animals used for scientific purposes
|
|
|
- Lucinda Gordon
- 5 years ago
- Views:
Transcription
1 National Competent Authorities for the implementation of Directive 2010/63/EU on the protection of animals used for scientific purposes Working document on specific articles in Directive 2010/63/EU Brussels, 6-7 October 2011 The National Contact Points of the Member States responsible for the implementation of Directive 2010/63/EU on the protection of animals used for scientific purposes and the Commission agreed to discuss a number of articles contained in the Directive with a view to finding a common approach throughout the EU. The consensus on the understanding of the articles discussed at the meeting of 6-7 October 2011 is presented below to promote uniform implementation and application of the Directive. This document covers the following articles: Article 1(5) Article 3 Article 16 Article 40 Article 41 - Practices that are exempted from the scope of the Directive - Definitions for a procedure and project - Use, re-use and continued use - Multiple generic projects - Complex or multi-disciplinary projects Disclaimer: The following is intended as guidance to assist the Member States and others affected by this Directive to arrive at a common understanding of the provisions contained in the Directive. All comments should be considered within the context of Directive 2010/63/EU on the protection of animals used for scientific purposes. Only the Court of Justice of the European Union is entitled to interpret EU law with legally binding authority.
2 Article 1(5) - Practices that are exempted from the scope of the Directive Background Directive 2010/63/EU establishes measures for the protection of animals used for scientific or educational purposes. Article 1(5) lists a number of practices which are exempted from the requirements of the Directive. These are as follows: (a) (b) (c) (d) (e) (f) non-experimental agricultural practices; non-experimental clinical veterinary practices; veterinary clinical trials required for the marketing authorisation of a veterinary medicinal product; practices undertaken for the purposes of recognised animal husbandry; practices undertaken for the primary purpose of identification of an animal; practices not likely to cause pain, suffering, distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle according to good veterinary practice. The terms non-experimental, agricultural and clinical veterinary practices were used under the previous Directive 86/609/EEC. Article 1 describes the Subject matter and Scope of the Directive with paragraph 5 of this Article describing the circumstances under which this Directive would not apply. In determining whether or not an investigation falls within the scope of the Directive the key question to address is: Is the study to be undertaken for a scientific or educational purpose involving live vertebrates (including foetal forms of mammals in the last third of development and independently feeding larval forms) or live cephalopods? If the answer is yes, then the clauses in Paragraph 5 needs to be reviewed to determine whether or not there is an exemption from the scope of the Directive. If the practices are being undertaken as part of routine agricultural, animal husbandry or veterinary practice to manage health, welfare and care practices, are being applied for the primary purpose of identification, or are unlikely to cause pain, suffering, distress or lasting harm, these do not fall within the scope of Directive 2010/63/EU. (a) Non experimental agricultural practice Agriculture can be defined as the production of food, feed, fibre and other goods by the systematic raising of domesticated plants and animals. Agriculture covers all activities essential to food/feed/fibre production, including all techniques for raising and "processing" livestock. Agriculture includes agronomy, animal husbandry, and aquaculture. Agriculture practices are simply practices used in agriculture to facilitate farming. 2
3 A number of Council Directives set requirements for the protection of animals kept for farming practices [for example general standards are contained in 98/58/EC, and minimum standards set for calves in 2008/119/EC, pigs in 2001/88/EC and 2001/93/EC, laying hens in 1999/74/EC, and chickens kept for meat production in 2007/43/EC]. Examples of agricultural practices include disbudding/dehorning of cattle, castration of lambs, pigs and cattle, debeaking in poultry, nutritional manipulation of weight in broiler replacement breeders, rearing and weaning practices in dairy and veal calves, restraint around parturition e.g. pigs and advanced breeding techniques for agricultural purposes, such as embryo transfer and vasectomy to, for example, improve health or genetics of the flock or herd. Simple observational studies of commercial agricultural practices which do not include any additional practices/interventions which may cause pain, suffering, distress or lasting harm do not fall within the scope of Directive 2010/63/EU. For example, a study to compare the effects of intensive and extensive rearing systems on production and behavioural indices in growing pigs. Study consists of simple observations on commercial farms using different rearing systems (which comply with national/eu legislation), and subsequent comparison of behaviour and production records. (b) Non experimental clinical veterinary practice Clinical veterinary practice can be defined as procedures and techniques performed by veterinary surgeons in the course of their professional duties which ensure the health and welfare of animals committed to their care. Examples include - taking blood samples from an animal, or animals within a herd, to assist in clinical management e.g. disease diagnosis, metabolic/biochemical profile. - taking a series of biopsies from an animal for diagnosis and monitoring the efficacy of treatment - imaging to assist in diagnosis and monitoring of treatment - giving veterinary treatment, including to animals undergoing scientific procedures when treatment is for the animal's benefit and not part of a scientific procedure (c) Veterinary clinical trials required for the marketing authorisation of a veterinary medicinal product During the development of veterinary medicines, a great deal of work will have been performed on animals authorised under 2010/63/EU. There usually comes a point when it is necessary to test the efficacy and safety of new preparations in the target species under field conditions. Pharmaceutical companies need to generate these data in order to support an application for a marketing authorisation. 3
4 Directive 2001/82/EC details the requirements for veterinary clinical trials. Animals used in such trials are under veterinary care and appropriate clinical care, including alternative treatments, is provided should the test product prove ineffective or animal welfare is compromised. (d) Practices undertaken for the purposes of recognised animal husbandry Animal husbandry may be defined as the system of taking care of domestic animals, including those held and used in laboratories. This definition encompasses all husbandry and care practices including housing conditions and colony management, monitoring reproductive, growth and health indices. N.B. Further information will be made available on legal interpretation of "recognised" husbandry. For example: Single housing of males may be necessary to minimise aggression; Vaginal swabbing (mice/dogs) or blood sampling (dogs) to determine stage of oestrus and optimum time for mating; Single housing on grid floors to check for mating plugs (rats & mice); Weighing fish under general anaesthesia to monitor growth to facilitate dietary and stocking management; Management of diet (composition, quantity and availability) to meet requirements of animals e.g. managing obesity in older animals; infrequent feeding of snakes to mimic biological needs. Simple observational comparisons of different animal husbandry practices, which do not include any additional practices/interventions which may cause pain, suffering, distress or lasting harm, do not fall within the scope of directive 2010/63/EU. For example, a study to compare the effects of cage changing frequency on growth rates and behaviour in mice. (e) Practices undertaken for the primary purpose of identification of an animal Animals are identified for a number of reasons, for example - to facilitate identification of individual animals held in groups, to facilitate routine stock and breeding management and to facilitate tracing of animals for health and disease control. Practices undertaken for the primary purpose of identification are not within the scope of the Directive. This should not be confused with the requirements of Article 32. For animals used within the scope of the Directive, there is a requirement that each dog, cat and non- 4
5 human primate shall be provided with a permanent individual identification mark in the least painful manner possible (Article 32). For reasons of good welfare, consideration should be given to the method chosen for identification, and should be the most refined technique appropriate to the need. For example, where it is only necessary to identify an animal for a short period of time, the use of a non-toxic dye or fur clipping would not cause any pain or discomfort. Alternatively, there may be a legal requirement in farm species for two separate methods to be employed, for example an ear tag and a transponder. (f) Practices not likely to cause pain, suffering, distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle according to good veterinary practice Practices undertaken for a scientific or educational purpose which do not reach a threshold of pain, suffering, distress or lasting harm equivalent to or higher than that caused by the introduction of a needle according to good veterinary practice do not fall within the scope of the Directive. Annex VIII provides some examples of equivalent thresholds for other classes of procedure, such as dietary manipulations and psychological stress: assessing body composition by non-invasive measures and minimal restraint; monitoring ECG with non-invasive techniques with minimal or no restraint of habituated animals; application of external telemetry devices that are expected to cause no impairment to socially adapted animals and do not interfere with normal activity and behaviour; breeding genetically altered animals which are expected to have no clinically detectable adverse phenotype; adding inert markers in the diet to follow passage of digesta; withdrawal of food for <24h in adult rats; open field testing. In addition it is important to note that a series or combination of below threshold techniques together may have the effect of causing an animal pain, suffering, distress or lasting harm. Using the food withdrawal as an example, if this was repeated frequently, it is likely that there would be adverse welfare consequences for the animal. Similarly, multiple or cumulative minor changes to an animal s environment may cause sufficient disturbance to the animal to be considered a mild procedure. 5
6 Article 3 - Definitions for a procedure and project Project According to the Directive, a project means a programme of work having a defined scientific objective and involving one or more procedures. Projects can vary in size and complexity, for example, from the work of a single scientist consisting of a single blood harvest procedure in a single species, to an entire department s drug discovery programme, which involves many scientists, multiple complex procedures and a wide range of species. Procedure According to the Directive, procedure means any use, invasive or non-invasive, of an animal for experimental or other scientific purposes, with known or unknown outcome, or educational purposes, which may cause the animal a level of pain, suffering or distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle according to good veterinary practice. Within a project, procedures will be performed to meet a defined scientific purpose. Procedures may be simple or complex depending on the purpose. The purpose may be achieved by using a single step procedure (for example withdrawal of blood), but much more commonly requires a number of steps used in combination to achieve a single outcome, and which requires the use of the same animal (for example antibody production would generally require a number of antigen injections to stimulate antibody production, and a number of blood samples to achieve the desired outcome). Examples of Procedures A single subcutaneous injection of a test substance may be given in a pharmaceutical project to attain the objective of understanding the drug distribution within the body tissues. The animal is then killed by a method listed in Annex IV. This project comprises of one procedure (the injection of the test substance) which may cause the animal pain, suffering, distress or lasting harm. In contrast, a procedure to assess the effect of the test substance on blood pressure using telemetry would require a number of separate technical steps to be carried out to meet this single scientific purpose (multi step procedure). The animal would need to be anaesthetised, blood pressure transducer implanted and, following a suitable recovery period, administered the test substance by subcutaneous injection. The animal is then killed by a method listed in Annex IV. In this example three steps namely anaesthesia, surgical implantation of blood pressure transducer and injection of the test substance) need to be used in combination 6
7 to meet the single scientific purpose of understanding the effects of the substance on blood pressure. 1 All the steps need to be made in the correct sequence and using the same animal to achieve the objective of the study neither omitting a step nor using a different animal at any stage would allow the objective to be met. 1 In this example, each step, if used in isolation to meet a single scientific purpose, would be considered a procedure as each may cause the animal pain, suffering, distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle in accordance with good veterinary practice. 7
8 Article 16 Use and re-use Use The use of an animal within a project extends from the time the procedure (or first procedure/technique in a series) is applied to it, to the time when the observations, or the collection of data (or other products) for a particular scientific purpose (usually a single experiment or test), are completed. Re-use Re-use is a term to indicate the subsequent use of an animal which has already completed a procedure (or series of procedures/techniques) for a particular scientific purpose. Article 16 on re-use defines it as a use when a different animal on which no procedure has previously been carried out could also be used. Article 16 also defines the circumstances under which an animal may be re-used. Continued Use This is a term not included in the Directive, but can be used to describe the situation when the single use of an animal extends over more than one project or across different procedures within the same project. This arrangement can simplify project applications and avoid undue repetition. Example 1 - Re-use Purpose 1: to obtain sheep blood to make diagnostic plates for bacteriology. Purpose 2: to determine the effect on blood parameters in sheep of a test dietary supplement, which may have adverse effects. First use A sheep is used on the first procedure to obtain a blood sample for use in preparation of diagnostic plates. Second use The same animal is then used on a second unrelated project to study the metabolism of a dietary supplement, blood samples are taken for analysis and other non-invasive measurements are made. The two studies are not related. 8
9 Any naive sheep could have been used for the second study. Use of the sheep for the dietary study would constitute re-use of that animal. Example 2 Re-use Purpose: The use of a sheep on more than one occasion to provide blood samples for use in preparation of diagnostic plates. The first blood sample obtained by jugular venepuncture constitutes the first use the purpose is attained. As a different animal could be used on each occasion, the second sample (procedure) and each subsequent sample (procedure) is classified as re-use. There is no scientific need to take multiple samples from the same animal. Example 3 - Use and continued use Purpose: To determine the effects of genetic defect X in mice by measuring changes in blood parameters with age and undertaking a histological analysis of adult brain structure. Use This could be undertaken within a single study. Step 1 - Production and genotyping of GM mouse Step 2 - Blood sampling or Continued use Split between a procedure (on the same or different project) under which the mouse with the genetic defect was produced and genotyped (Step 1), with the use continued under a second procedure (Step 2), possibly on a different project, on which the sampling and final preparation for histology are carried out. In this example Step 1 may be on a project at an establishment that specialises in breeding mice with genetic alterations (under a generic project authorisation) and Step 2 on the project of the scientist studying that particular genetic defect. 9
10 Example 4 More complicated situations Depending on the scientist s intention and the specific design of the study, it may not be immediately clear whether some studies should be regarded as reuse or continued use. Example: If the metabolism of a series of drugs is studied in an individual animal this will constitute continued use if serial-data from individual animals which had been used for each preceding study is needed to interpret each subsequent study (a within animal design) and data from a different animal would not have satisfied the scientific objective. However, if each study is to be interpreted independently of the others and without reference to earlier findings (and therefore any animal could have been used) this will constitute re-use. What actually happens to the animal is the same in each series of studies, but the way in which data are analysed determines whether the subsequent use is regarded as re-use or continued use. 10
11 Article 40 - Multiple generic projects Some classes of projects involve a series of standard procedures being applied for a particular purpose. These are sometimes referred to as multiple generic projects. The procedures are generally well-established and the likely consequences on the animals are well-understood and can be minimised appropriately. There are unlikely to be particular novel or contentious issues raised during project evaluation. As in the case of simplified administrative procedure under Article 42, the procedures considered under multiple generic projects are required to satisfy regulatory requirements or needed for production or diagnostic purposes with established methods. Regulatory toxicology projects are required to deliver certain data for the evaluation of the safety of novel compounds on man, animals and the environment. The Directive 2010/63/EU includes articles which limits the use of animals, but at present, and for the foreseeable future there will remain a need for some such studies. There is generally a prescribed list of requirements for each class of compound which need to be followed to meet the needs of regulatory requirements such as for the production and marketing of pharmaceuticals, chemical substances or for testing the safety of food and feed additives. The animal studies remain reasonably consistent for each class of compound, and the scientific objective remains the same. Other examples which could be included in this category are projects for antibody production (using well established procedures applying minimum severity procedures) to provide a high quality product for use in specified areas e.g. disease diagnostic kits, and for projects with the objective of producing defined genetically modified lines for a particular purpose e.g. manipulation of genes involved in immune function. Project evaluation of multiple generic projects should look into the availability of alternative methods taking into consideration the types of compounds to be tested as well as the ways reduction and refinement can be applied in the context of standardised methods. As with any scientific study, before any animals are used within the framework of such an authorisation, the person responsible for the project must be able to demonstrate that the use of animals is justified for each individual study, that no alternatives could be used, that the minimum numbers of animals are used consistent with the scientific objective and that each study has been designed to reduce to the minimum any pain, suffering, distress or lasting harm. It is advisable in the framework of the project authorisation of multiple generic projects to draw the attention of the person responsible for the authorised project to the obligation to replace, reduce and refine methods throughout the course of the project to ensure that any changes in the availability of alternatives are duly considered, and utilised as soon as practicable. 11
12 Article 41 - Complex or multi-disciplinary projects Projects vary significantly in their scale and scope; may involve many procedures; may require a variety of species including non-human primates; may include severe procedures likely to have a significant impact on the animals; may raise novel scientific issues or introduce novel issues with regard to animal use. Projects with many complicated objectives and procedures, which raise unusual (out of the ordinary issues) with regard to the use and care of animals, in particular where high severity is expected, are likely to require more time for detailed consideration during the project evaluation, and in these circumstances there is the provision within Article 41 to extend the period for the authorisation decision to be made. 12
Use of animals for scientific or educational purposes principles in Finland
 Use of animals for scientific or educational purposes principles in Finland Eila Kaliste Project Authorisation Board (ELLA) Chief presenting officer Regional Administrative Agency for Southern Finland
Use of animals for scientific or educational purposes principles in Finland Eila Kaliste Project Authorisation Board (ELLA) Chief presenting officer Regional Administrative Agency for Southern Finland
ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION
 ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION 2015 CONTENTS 1. Introduction 2. Summary 3. Results 3.1 Species and numbers of naïve animals used in
ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION 2015 CONTENTS 1. Introduction 2. Summary 3. Results 3.1 Species and numbers of naïve animals used in
ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION
 ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION 2013 CONTENTS 1. Introduction 2. Summary 3. Results 3.1 Species and numbers of naive animals used in
ANNUAL STATISTICAL REPORT FOR ANIMALS USED IN IRELAND UNDER SCIENTIFIC ANIMAL PROTECTION LEGISLATION 2013 CONTENTS 1. Introduction 2. Summary 3. Results 3.1 Species and numbers of naive animals used in
Guide to Use of Animals for Educational Purposes under Scientific Animal Protection Legislation
 Guide to Use of Animals for Educational Purposes under Scientific Animal Protection Legislation AUT-G0117-3 14 JULY 2014 This guide does not purport to be an interpretation of law and/or regulations and
Guide to Use of Animals for Educational Purposes under Scientific Animal Protection Legislation AUT-G0117-3 14 JULY 2014 This guide does not purport to be an interpretation of law and/or regulations and
Regulating the scientific use of animals taken from the wild Implementation of Directive 2010/63/EU
 Regulating the scientific use of animals taken from the wild Implementation of Directive 2010/63/EU Dr Kim Willoughby, Mr Peter Gray, Dr Kate Garrod. Presented by: Dr Kim Willoughby Date: 26 October 2017
Regulating the scientific use of animals taken from the wild Implementation of Directive 2010/63/EU Dr Kim Willoughby, Mr Peter Gray, Dr Kate Garrod. Presented by: Dr Kim Willoughby Date: 26 October 2017
The Animal Welfare offi cer in the European Union
 The Animal Welfare offi cer in the European Union 2 1. INTRODUCTION The new animal welfare EU regulation applicable to slaughterhouses (Regulation 1099/2009) requires that slaughterhouse operators appoint
The Animal Welfare offi cer in the European Union 2 1. INTRODUCTION The new animal welfare EU regulation applicable to slaughterhouses (Regulation 1099/2009) requires that slaughterhouse operators appoint
ANIMAL CARE AND USE STANDARD
 ANIMAL ETHICS ANIMAL CARE AND USE STANDARD The Animal Care & Use Standards are designed to provide guidance regarding good practice to institutional animal users and carers, as well as Animal Ethics Committees
ANIMAL ETHICS ANIMAL CARE AND USE STANDARD The Animal Care & Use Standards are designed to provide guidance regarding good practice to institutional animal users and carers, as well as Animal Ethics Committees
Animal Research Ethics Procedure
 Animal Research Ethics Procedure Policy Hierarchy link Responsible Officer Contact Officer Superseded Documents UNSW Research Code of Conduct Director, Research Ethics & Compliance Support Coordinator,
Animal Research Ethics Procedure Policy Hierarchy link Responsible Officer Contact Officer Superseded Documents UNSW Research Code of Conduct Director, Research Ethics & Compliance Support Coordinator,
DIRECTIVE 2010/63/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 20.10.2010 Official Journal of the European Union L 276/33 DIRECTIVES DIRECTIVE 2010/63/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 22 September 2010 on the protection of animals used for scientific
20.10.2010 Official Journal of the European Union L 276/33 DIRECTIVES DIRECTIVE 2010/63/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 22 September 2010 on the protection of animals used for scientific
2012 No. 153 ANIMALS
 STATUTORY RULES OF NORTHERN IRELAND 2012 No. 153 ANIMALS ANIMAL WELFARE The Welfare of Animals (Permitted Procedures by Lay Persons) Regulations (Northern Ireland) 2012 Laid before the Assembly in draft
STATUTORY RULES OF NORTHERN IRELAND 2012 No. 153 ANIMALS ANIMAL WELFARE The Welfare of Animals (Permitted Procedures by Lay Persons) Regulations (Northern Ireland) 2012 Laid before the Assembly in draft
European Association of Establishments for Veterinary Document approved by the Executive Committee on January Education
 Education European Association of Establishments for Veterinary Education and Training requirements for veterinarians in Laboratory animal science and medicine (LASM): Minimum requirements to guarantee
Education European Association of Establishments for Veterinary Education and Training requirements for veterinarians in Laboratory animal science and medicine (LASM): Minimum requirements to guarantee
ruma Cattle Responsible use of antimicrobials in Cattle production GUIDELINES
 ruma RESPONSIBLE USE OF MEDICINES IN AGRICULTURE ALLIANCE GUIDELINES Cattle Responsible use of antimicrobials in Cattle production RUMA guidelines for the responsible use of antimicrobials by cattle farmers
ruma RESPONSIBLE USE OF MEDICINES IN AGRICULTURE ALLIANCE GUIDELINES Cattle Responsible use of antimicrobials in Cattle production RUMA guidelines for the responsible use of antimicrobials by cattle farmers
Regulating Animal Welfare in the EU.the EU.
 Regulating Animal Welfare in the EU.the EU. Andrea Gavinelli Unit G3 Animal Welfare Directorate General 1 Animal Welfare 1. An expanding policy area. 2. An issue of high public concern and political relevance.
Regulating Animal Welfare in the EU.the EU. Andrea Gavinelli Unit G3 Animal Welfare Directorate General 1 Animal Welfare 1. An expanding policy area. 2. An issue of high public concern and political relevance.
Challenges in Farm Animal Research: the Protectionist s View
 NORECOPA-Consensus Meeting: Harmonisation of the Care and Use of Agricultural Animals in Research 26-28 September 2012, Gardermoen, Norway Challenges in Farm Animal Research: the Protectionist s View Dipl.
NORECOPA-Consensus Meeting: Harmonisation of the Care and Use of Agricultural Animals in Research 26-28 September 2012, Gardermoen, Norway Challenges in Farm Animal Research: the Protectionist s View Dipl.
STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: REPRODUCTION TESTING
 VICH GL22 (SAFETY: REPRODUCTION) Revision 1 May 2004 For implementation at Step 7 STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: REPRODUCTION TESTING Recommended for Implementation
VICH GL22 (SAFETY: REPRODUCTION) Revision 1 May 2004 For implementation at Step 7 STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: REPRODUCTION TESTING Recommended for Implementation
Approved by Research Committee in November 2016.
 1. Background Terms of Reference of the new DCU ANIMAL WELFARE BODY, 1.1 Legislation in the EU Approved by Research Committee in November 2016. Directive 2010/63/EU revising Directive 86/609/EEC on the
1. Background Terms of Reference of the new DCU ANIMAL WELFARE BODY, 1.1 Legislation in the EU Approved by Research Committee in November 2016. Directive 2010/63/EU revising Directive 86/609/EEC on the
PROTOCOL FOR THE HUMANE CARE AND USE OF LIVE VERTEBRATE ANIMALS
 PROTOCOL FOR THE HUMANE CARE AND USE OF LIVE VERTEBRATE ANIMALS Federal animal welfare regulations require that the Institutional Animal Care and Use Committee (IACUC) must review and approve all activities
PROTOCOL FOR THE HUMANE CARE AND USE OF LIVE VERTEBRATE ANIMALS Federal animal welfare regulations require that the Institutional Animal Care and Use Committee (IACUC) must review and approve all activities
Aide mémoire for environmental conditions and treatment of biological models
 I. Introduction This document was elaborated by experts and it is based on the current state of the art knowledge and OMCL in-house practices. The questions in the first column are addressed to the testing
I. Introduction This document was elaborated by experts and it is based on the current state of the art knowledge and OMCL in-house practices. The questions in the first column are addressed to the testing
Guideline on quality data requirements for veterinary medicinal products intended for minor use or minor species (MUMS)/limited market
 8 December 2016 EMA/CVMP/QWP/128710/2004-Rev.1 Committee for Medicinal Products for Veterinary Use (CVMP) Guideline on quality data requirements for veterinary medicinal products intended for minor use
8 December 2016 EMA/CVMP/QWP/128710/2004-Rev.1 Committee for Medicinal Products for Veterinary Use (CVMP) Guideline on quality data requirements for veterinary medicinal products intended for minor use
FARM ASSURANCE FOR SHEEP ONLY
 Farm Assurance FARM ASSURANCE FOR SHEEP ONLY 1) ANIMAL TREATMENTS The aim is to ensure that consumers of products produced at Blue Sky Meats have no risk as a result of animal health treatments on farms
Farm Assurance FARM ASSURANCE FOR SHEEP ONLY 1) ANIMAL TREATMENTS The aim is to ensure that consumers of products produced at Blue Sky Meats have no risk as a result of animal health treatments on farms
Guidance Document. Veterinary Operating Instructions. Guidance re: Requirements for Authorising Veterinarians Notice.
 Guidance Document Veterinary Operating Instructions Guidance re: Requirements for Authorising Veterinarians Notice 28 August 2015 A guidance document issued by the Ministry for Primary Industries Title
Guidance Document Veterinary Operating Instructions Guidance re: Requirements for Authorising Veterinarians Notice 28 August 2015 A guidance document issued by the Ministry for Primary Industries Title
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 1996L0022 EN 18.12.2008 002.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COUNCIL DIRECTIVE 96/22/EC of 29 April 1996 concerning
1996L0022 EN 18.12.2008 002.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COUNCIL DIRECTIVE 96/22/EC of 29 April 1996 concerning
LIFE.2.B EUROPEAN UNION. Brussels, 14 November 2018 (OR. en) 2014/0255 (COD) PE-CONS 43/18 AGRILEG 102 VETER 52 CODEC 1149
 EUROPEAN UNION THE EUROPEAN PARLIAMT THE COUNCIL Brussels, 14 November 2018 (OR. en) 2014/0255 (COD) PE-CONS 43/18 AGRILEG 102 VETER 52 CODEC 1149 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: REGULATION
EUROPEAN UNION THE EUROPEAN PARLIAMT THE COUNCIL Brussels, 14 November 2018 (OR. en) 2014/0255 (COD) PE-CONS 43/18 AGRILEG 102 VETER 52 CODEC 1149 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: REGULATION
COMMISSION OF THE EUROPEAN COMMUNITIES. Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 16.6.2009 COM(2009) 268 final 2009/0077 (COD) C7-0035/09 Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Regulation (EC)
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 16.6.2009 COM(2009) 268 final 2009/0077 (COD) C7-0035/09 Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Regulation (EC)
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 2003R2160 EN 27.10.2007 003.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 2160/2003 OF THE EUROPEAN
2003R2160 EN 27.10.2007 003.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 2160/2003 OF THE EUROPEAN
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 1996L0022 EN 18.12.2008 002.001 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COUNCIL DIRECTIVE 96/22/EC of 29 April 1996 concerning
1996L0022 EN 18.12.2008 002.001 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B COUNCIL DIRECTIVE 96/22/EC of 29 April 1996 concerning
Explanatory Memorandum to the Mutilations (Permitted Procedures) (Wales) (Amendment) Regulations 2008
 Explanatory Memorandum to the Mutilations (Permitted Procedures) (Wales) (Amendment) Regulations 2008 This Explanatory Memorandum has been prepared by the Office of the Chief Veterinary Officer and is
Explanatory Memorandum to the Mutilations (Permitted Procedures) (Wales) (Amendment) Regulations 2008 This Explanatory Memorandum has been prepared by the Office of the Chief Veterinary Officer and is
REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL
 EUROPEAN COMMISSION Brussels, 6.3.2018 COM(2018) 88 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL on the implementation of Article 5 of Regulation (EU) No 576/2013 on the
EUROPEAN COMMISSION Brussels, 6.3.2018 COM(2018) 88 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT AND THE COUNCIL on the implementation of Article 5 of Regulation (EU) No 576/2013 on the
2007 No. 256 ANIMALS
 SCOTTISH STATUTORY INSTRUMENTS 2007 No. 256 ANIMALS PREVENTION OF HARM The Prohibited Procedures on Protected Animals (Exemptions) (Scotland) Regulations 2007 Made - - - - 20th March 2007 Coming into force
SCOTTISH STATUTORY INSTRUMENTS 2007 No. 256 ANIMALS PREVENTION OF HARM The Prohibited Procedures on Protected Animals (Exemptions) (Scotland) Regulations 2007 Made - - - - 20th March 2007 Coming into force
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY
 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Brussels, 27 February 2018 NOTICE TO STAKEHOLDERS WITHDRAWAL OF THE UNITED KINGDOM AND EU RULES ON ANIMAL HEALTH AND WELFARE AND PUBLIC
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Brussels, 27 February 2018 NOTICE TO STAKEHOLDERS WITHDRAWAL OF THE UNITED KINGDOM AND EU RULES ON ANIMAL HEALTH AND WELFARE AND PUBLIC
CODE OF ETHICAL CONDUCT
 MASSEY UNIVERSITY CODE OF ETHICAL CONDUCT FOR THE USE OF LIVE ANIMALS FOR RESEARCH, TESTING AND TEACHING Revised Edition 2008 Page 1 CONTENTS 1. Revised Code of Ethical Conduct for the Use of Live Animals
MASSEY UNIVERSITY CODE OF ETHICAL CONDUCT FOR THE USE OF LIVE ANIMALS FOR RESEARCH, TESTING AND TEACHING Revised Edition 2008 Page 1 CONTENTS 1. Revised Code of Ethical Conduct for the Use of Live Animals
IACUC POLICIES, PROCEDURES, and GUIDELINES. HUMANE USE PAIN CLASSIFICATIONS (Pain Categories)
 Page 1 of 6 IACUC POLICIES, PROCEDURES, and GUIDELINES HUMANE USE PAIN CLASSIFICATIONS (Pain Categories) Purpose: This document provides guidelines for the classification of animal use into the Humane
Page 1 of 6 IACUC POLICIES, PROCEDURES, and GUIDELINES HUMANE USE PAIN CLASSIFICATIONS (Pain Categories) Purpose: This document provides guidelines for the classification of animal use into the Humane
Working for organic farming in Europe
 Working for organic farming in Europe International Federation of Organic Agriculture Movements EU Regional Group 9 st November 2012 President: Christopher Stopes Director: Marco Schlüter European Office
Working for organic farming in Europe International Federation of Organic Agriculture Movements EU Regional Group 9 st November 2012 President: Christopher Stopes Director: Marco Schlüter European Office
Official Journal of the European Union. (Acts whose publication is obligatory)
 12.12.2003 L 325/1 I (Acts whose publication is obligatory) REGULATION (EC) No 2160/2003 OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 17 November 2003 on the control of salmonella and other specified
12.12.2003 L 325/1 I (Acts whose publication is obligatory) REGULATION (EC) No 2160/2003 OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 17 November 2003 on the control of salmonella and other specified
Official Journal of the European Union L 280/5
 24.10.2007 Official Journal of the European Union L 280/5 COMMISSION REGULATION (EC) No 1237/2007 of 23 October 2007 amending Regulation (EC) No 2160/2003 of the European Parliament and of the Council
24.10.2007 Official Journal of the European Union L 280/5 COMMISSION REGULATION (EC) No 1237/2007 of 23 October 2007 amending Regulation (EC) No 2160/2003 of the European Parliament and of the Council
V E T E R I N A R Y C O U N C I L O F I R E L A N D ETHICAL VETERINARY PRACTICE
 V E T E R I N A R Y C O U N C I L O F I R E L A N D ETHICAL VETERINARY PRACTICE ETHICAL VETERINARY PRACTICE The term Ethical Veterinary Practice is a wide ranging one, implying as it does, compliance with
V E T E R I N A R Y C O U N C I L O F I R E L A N D ETHICAL VETERINARY PRACTICE ETHICAL VETERINARY PRACTICE The term Ethical Veterinary Practice is a wide ranging one, implying as it does, compliance with
Animal Care & Ethics Committee
 \ Animal Care & Ethics Committee Animal Usage and Annual Compliance Form Guidelines Animal Usage and Compliance Forms DUE 1 FEBRUARY ANNUALLY As the Chief Investigator of a UNSW Animal Care and Ethics
\ Animal Care & Ethics Committee Animal Usage and Annual Compliance Form Guidelines Animal Usage and Compliance Forms DUE 1 FEBRUARY ANNUALLY As the Chief Investigator of a UNSW Animal Care and Ethics
Delegating to Auxiliaries in Food Animal & Equine Practice
 Delegating to Auxiliaries in Food Animal & Equine Practice Approved by Council: June 2004; September 2006; June 2011 Indirect definition modified June 9, 2010 Publication Date: Update September 2004, Website
Delegating to Auxiliaries in Food Animal & Equine Practice Approved by Council: June 2004; September 2006; June 2011 Indirect definition modified June 9, 2010 Publication Date: Update September 2004, Website
ANNEXES. to the Proposal. for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 EUROPEAN COMMISSION Brussels, XXX SANCO/12328/2013 Rev. 4 ANNEX (POOL/G1/2013/12328/12328R4-EN ANNEX.doc) [ ](2014) XXX draft ANNEXES 1 to 6 ANNEXES to the Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT
EUROPEAN COMMISSION Brussels, XXX SANCO/12328/2013 Rev. 4 ANNEX (POOL/G1/2013/12328/12328R4-EN ANNEX.doc) [ ](2014) XXX draft ANNEXES 1 to 6 ANNEXES to the Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY
 Ref. Ares(2018)2119965-20/04/2018 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health and food audits and analysis DG(SANTE) 2017-6296 FINAL REPORT OF AN AUDIT CARRIED OUT IN DENMARK
Ref. Ares(2018)2119965-20/04/2018 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health and food audits and analysis DG(SANTE) 2017-6296 FINAL REPORT OF AN AUDIT CARRIED OUT IN DENMARK
Office of Research Services
 Responsible Officer Approved by Deputy Vice Chancellor (Research) Vice-Chancellor Approved and commenced September, 2015 Review by September, 2018 Relevant Legislation, Ordinance, Rule and/or Governance
Responsible Officer Approved by Deputy Vice Chancellor (Research) Vice-Chancellor Approved and commenced September, 2015 Review by September, 2018 Relevant Legislation, Ordinance, Rule and/or Governance
EN SANCO/745/2008r6 EN EN
 SANCO/745/2008r6 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, C(2008) Commission staff working document GUIDANCE DOCUMT On the minimum requirements for Salmonella control programmes to be recognised
SANCO/745/2008r6 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, C(2008) Commission staff working document GUIDANCE DOCUMT On the minimum requirements for Salmonella control programmes to be recognised
TEXTS ADOPTED Provisional edition. P8_TA-PROV(2018)0429 Animal welfare, antimicrobial use and the environmental impact of industrial broiler farming
 European Parliament 204-209 TEXTS ADOPTED Provisional edition P8_TA-PROV(208)0429 Animal welfare, antimicrobial use and the environmental impact of industrial broiler farming European Parliament resolution
European Parliament 204-209 TEXTS ADOPTED Provisional edition P8_TA-PROV(208)0429 Animal welfare, antimicrobial use and the environmental impact of industrial broiler farming European Parliament resolution
Policy on the use of animals in research and education at SLU
 Only the Swedish version is the oficial version. GOVERNING DOCUMENT SLU ID: SLU.ua 2015.1.1.1-4840 Subject area: Research and doctoral education and Undergraduate and Master's education Document type:
Only the Swedish version is the oficial version. GOVERNING DOCUMENT SLU ID: SLU.ua 2015.1.1.1-4840 Subject area: Research and doctoral education and Undergraduate and Master's education Document type:
(Text with EEA relevance)
 L 225/76 19.8.2016 COMMISSION REGULATION (EU) 2016/1396 of 18 August 2016 amending certain Annexes to Regulation (No 999/2001 of the European Parliament and of the Council laying down rules for the prevention,
L 225/76 19.8.2016 COMMISSION REGULATION (EU) 2016/1396 of 18 August 2016 amending certain Annexes to Regulation (No 999/2001 of the European Parliament and of the Council laying down rules for the prevention,
MODEL STANDARDS FOR PET SHOP LICENCE CONDITIONS
 ANIMAL WELFARE ACT 2006 PET ANIMALS ACT 1951 MODEL STANDARDS FOR PET SHOP LICENCE CONDITIONS Reptiles, Amphibians, Fish and other Aquatic Invertebrates h&e314v2 The Standard Licence Conditions N.B. Reptiles,
ANIMAL WELFARE ACT 2006 PET ANIMALS ACT 1951 MODEL STANDARDS FOR PET SHOP LICENCE CONDITIONS Reptiles, Amphibians, Fish and other Aquatic Invertebrates h&e314v2 The Standard Licence Conditions N.B. Reptiles,
RESPONSIBILITIES OF THE PRESCRIBING VETERINARIAN
 APPENDIX 15 AUSTRALIAN VETERINARY ASSOCIATION (AVA) CODE OF PRACTICE FOR PRESCRIPTION AND USE OF PRODUCTS WHICH CONTAIN ANTIMICROBIAL AGENTS [Adopted 7 May 2008] INTRODUCTION The purpose of this Code of
APPENDIX 15 AUSTRALIAN VETERINARY ASSOCIATION (AVA) CODE OF PRACTICE FOR PRESCRIPTION AND USE OF PRODUCTS WHICH CONTAIN ANTIMICROBIAL AGENTS [Adopted 7 May 2008] INTRODUCTION The purpose of this Code of
COMMISSION. (Text with EEA relevance) (2009/712/EC)
 19.9.2009 Official Journal of the European Union L 247/13 COMMISSION COMMISSION DECISION of 18 September 2009 implementing Council Directive 2008/73/EC as regards Internet-based information pages containing
19.9.2009 Official Journal of the European Union L 247/13 COMMISSION COMMISSION DECISION of 18 September 2009 implementing Council Directive 2008/73/EC as regards Internet-based information pages containing
Research with Animals
 Research with Animals Matthew Olugbenga Oyeyemi momattyemi@gmail.com +2348038059952 Research with Animals 1 Objectives Describe situations when animals may be research subjects Identify laws and regulations
Research with Animals Matthew Olugbenga Oyeyemi momattyemi@gmail.com +2348038059952 Research with Animals 1 Objectives Describe situations when animals may be research subjects Identify laws and regulations
A Guide for Lay Members of Animal Ethics Committees
 A Guide for Lay Members of Animal Ethics Committees Disclaimer While every effort has been made to ensure the information in this Guide is accurate, the Ministry of Agriculture and Forestry (MAF) and National
A Guide for Lay Members of Animal Ethics Committees Disclaimer While every effort has been made to ensure the information in this Guide is accurate, the Ministry of Agriculture and Forestry (MAF) and National
Further memorandum submitted by the Department for Environment, Food and Rural Affairs
 Further memorandum submitted by the Department for Environment, Food and Rural Affairs Follow-up to the evidence session on 5 November 2008: [Bee research] I am writing in response to your letter of 10
Further memorandum submitted by the Department for Environment, Food and Rural Affairs Follow-up to the evidence session on 5 November 2008: [Bee research] I am writing in response to your letter of 10
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/477/03/Final COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS POSITION PAPER REGARDING AVAILABILITY OF PRODUCTS FOR MINOR USES AND MINOR
European Medicines Agency Veterinary Medicines and Inspections EMEA/CVMP/477/03/Final COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS POSITION PAPER REGARDING AVAILABILITY OF PRODUCTS FOR MINOR USES AND MINOR
Requirements for the Protection of Animals Kept for Farming Purposes which are Intended for Slaughter
 Republic of Latvia Cabinet Regulation No. 21 Adopted 8 January 2013 Requirements for the Protection of Animals Kept for Farming Purposes which are Intended for Slaughter Issued pursuant to Section 10,
Republic of Latvia Cabinet Regulation No. 21 Adopted 8 January 2013 Requirements for the Protection of Animals Kept for Farming Purposes which are Intended for Slaughter Issued pursuant to Section 10,
Ministry for Primary Industries Manato Ahu Matua
 Ministry for Primary Industries Manato Ahu Matua SCR17-0004 lan McKelvie Chairperson Primary Production Committee Dear lan McKelvie Government Response to Petition of Tara Jackson on behalf of the New
Ministry for Primary Industries Manato Ahu Matua SCR17-0004 lan McKelvie Chairperson Primary Production Committee Dear lan McKelvie Government Response to Petition of Tara Jackson on behalf of the New
UPEI / AVC Guidelines for Categories of Invasiveness and Rest Periods for Teaching Animals
 UPEI / AVC Guidelines for Categories of Invasiveness and Rest Periods for Teaching Animals Created: 1996 Revised: April 2011 Background The UPEI Animal Care Committee (ACC) recognizes that animals can
UPEI / AVC Guidelines for Categories of Invasiveness and Rest Periods for Teaching Animals Created: 1996 Revised: April 2011 Background The UPEI Animal Care Committee (ACC) recognizes that animals can
No July 2000 REGULATION. respecting veterinarians authorisations to prescribe drugs SECTION II
 REGULATION respecting veterinarians authorisations to prescribe drugs SECTION I Scope and definitions Article 1 This Regulation contains special provisions applying to veterinarians authorisations to prescribe
REGULATION respecting veterinarians authorisations to prescribe drugs SECTION I Scope and definitions Article 1 This Regulation contains special provisions applying to veterinarians authorisations to prescribe
Animal Health and Welfare policies in the EU Status quo and tendencies
 Animal Health and Welfare policies in the EU Status quo and tendencies The views expressed here are purely those of the writer and may not in any circumstances be regarded as stating an official position
Animal Health and Welfare policies in the EU Status quo and tendencies The views expressed here are purely those of the writer and may not in any circumstances be regarded as stating an official position
Statistics of Scientific Procedures on Living Animals Northern Ireland 2012
 Statistics of Scientific Procedures on Living Animals Northern Ireland 2012 Statistics of Scientific Procedures on Living Animals Northern Ireland 2012 Prepared pursuant to section 21(7) of the Animals
Statistics of Scientific Procedures on Living Animals Northern Ireland 2012 Statistics of Scientific Procedures on Living Animals Northern Ireland 2012 Prepared pursuant to section 21(7) of the Animals
Agvet Chemicals Task Group Veterinary Prescribing and Compounding Rights Working Group
 Agvet Chemicals Task Group Veterinary Prescribing and Compounding Rights Working Group Submission from the Australian Veterinary Association Ltd www.ava.com.au The Australian Veterinary Association Limited
Agvet Chemicals Task Group Veterinary Prescribing and Compounding Rights Working Group Submission from the Australian Veterinary Association Ltd www.ava.com.au The Australian Veterinary Association Limited
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY
 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health and food audits and analysis DG(SANTE) 2017-6110 FINAL REPORT OF A FACT-FINDING MISSION CARRIED OUT IN TURKEY FROM 05 SEPTEMBER
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health and food audits and analysis DG(SANTE) 2017-6110 FINAL REPORT OF A FACT-FINDING MISSION CARRIED OUT IN TURKEY FROM 05 SEPTEMBER
CODE OF ETHICAL CONDUCT
 MASSEY UNIVERSITY CODE OF ETHICAL CONDUCT FOR THE USE OF LIVE ANIMALS FOR RESEARCH, TESTING AND TEACHING Revised Edition 2013 Page 1 CONTENTS 1. Revised Code of Ethical Conduct for the Use of Live Animals
MASSEY UNIVERSITY CODE OF ETHICAL CONDUCT FOR THE USE OF LIVE ANIMALS FOR RESEARCH, TESTING AND TEACHING Revised Edition 2013 Page 1 CONTENTS 1. Revised Code of Ethical Conduct for the Use of Live Animals
Key considerations in the breeding of macaques and marmosets for scientific purposes
 Key considerations in the breeding of macaques and marmosets for scientific purposes Key considerations in the breeding of macaques and marmosets for scientific purposes Laboratory Animal Science Association
Key considerations in the breeding of macaques and marmosets for scientific purposes Key considerations in the breeding of macaques and marmosets for scientific purposes Laboratory Animal Science Association
Animal Liberation Queensland Submission on Australian Animal Welfare Standards and Guidelines Section A: Cattle 04/05/13
 Animal Liberation Queensland Submission on Australian Animal Welfare Standards and Guidelines Section A: Cattle 04/05/13 Chapter 1: Responsibilities S1.1 A person must take reasonable actions to ensure
Animal Liberation Queensland Submission on Australian Animal Welfare Standards and Guidelines Section A: Cattle 04/05/13 Chapter 1: Responsibilities S1.1 A person must take reasonable actions to ensure
Guideline on the conduct of efficacy studies for intramammary products for use in cattle
 1 2 3 18 October 2013 EMEA/CVMP/EWP/141272/2011 Committee for Medicinal products for Veterinary Use (CVMP) 4 5 6 Guideline on the conduct of efficacy studies for intramammary products for use in cattle
1 2 3 18 October 2013 EMEA/CVMP/EWP/141272/2011 Committee for Medicinal products for Veterinary Use (CVMP) 4 5 6 Guideline on the conduct of efficacy studies for intramammary products for use in cattle
Public consultation on Proposed Revision of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes 2004
 RESEARCH INTEGRITY Animal Ethics Committee Web: http://sydney.edu.au/research_support/ethics Project Officer Australian code of practice for the care and use of animals for scientific purposes Health and
RESEARCH INTEGRITY Animal Ethics Committee Web: http://sydney.edu.au/research_support/ethics Project Officer Australian code of practice for the care and use of animals for scientific purposes Health and
Animal Welfare Assessment Transfers Checklist
 Animal Welfare Assessment Transfers Checklist Our Animal Welfare Commitment The believes that consideration of an animal s welfare must include its physical, physiological and mental state and that good
Animal Welfare Assessment Transfers Checklist Our Animal Welfare Commitment The believes that consideration of an animal s welfare must include its physical, physiological and mental state and that good
L 39/12 Official Journal of the European Union
 L 39/12 Official Journal of the European Union 10.2.2009 COMMISSION REGULATION (EC) No 119/2009 of 9 February 2009 laying down a list of third countries or parts thereof, for imports into, or transit through,
L 39/12 Official Journal of the European Union 10.2.2009 COMMISSION REGULATION (EC) No 119/2009 of 9 February 2009 laying down a list of third countries or parts thereof, for imports into, or transit through,
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY
 Ref. Ares(2016)105284-08/01/2016 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Directorate F - Food and Veterinary Office DG(SANTE) 2015-7426 - MR FINAL REPORT OF AN AUDIT CARRIED
Ref. Ares(2016)105284-08/01/2016 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Directorate F - Food and Veterinary Office DG(SANTE) 2015-7426 - MR FINAL REPORT OF AN AUDIT CARRIED
COUNCIL REGULATION (EEC) No 2377/90
 -W- -- 18. 8. 90 Official Journal of the ~uroiean Communities No L 224/P - - (Acts whose publication is obligatory) COUNCIL REGULATION (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure
-W- -- 18. 8. 90 Official Journal of the ~uroiean Communities No L 224/P - - (Acts whose publication is obligatory) COUNCIL REGULATION (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure
Council of the European Union Brussels, 15 September 2014 (OR. en) Mr Uwe CORSEPIUS, Secretary-General of the Council of the European Union
 Council of the European Union Brussels, 15 September 2014 (OR. en) Interinstitutional File: 2014/0255 (COD) 13196/14 AGRILEG 179 VETER 84 CODEC 1813 PROPOSAL From: date of receipt: 15 September 2014 To:
Council of the European Union Brussels, 15 September 2014 (OR. en) Interinstitutional File: 2014/0255 (COD) 13196/14 AGRILEG 179 VETER 84 CODEC 1813 PROPOSAL From: date of receipt: 15 September 2014 To:
UACC Policy and Procedures on Animal Use Frequency for Teaching Animals and Resident Herds/Colonies
 UACC Policy and Procedures on Animal Use Frequency for Teaching Animals and Resident Herds/Colonies BACKGROUND The CCAC guidelines suggest institutional Animal Care Committees (ACCs) develop clear procedures
UACC Policy and Procedures on Animal Use Frequency for Teaching Animals and Resident Herds/Colonies BACKGROUND The CCAC guidelines suggest institutional Animal Care Committees (ACCs) develop clear procedures
MONITORING SHEETS STEP-BY-STEP INSTRUCTIONS
 MONITORING SHEETS STEP-BY-STEP INSTRUCTIONS This is a 3 step guide to designing a practical and relevant welfare monitoring package for an AEC application. The AEC endorsed monitoring package includes:
MONITORING SHEETS STEP-BY-STEP INSTRUCTIONS This is a 3 step guide to designing a practical and relevant welfare monitoring package for an AEC application. The AEC endorsed monitoring package includes:
POSITION DESCRIPTION. Organisational Context: Important Functional Relationships: Page 1. Job Title: Reports To: Direct Reports: Position Purpose:
 Page 1 POSITION DESCRIPTION Job Title: Reports To: Direct Reports: Position Purpose: Keeper Level One, Two and /Animal Care Manager Nil A Zoo Keeper is responsible for providing quality animal husbandry
Page 1 POSITION DESCRIPTION Job Title: Reports To: Direct Reports: Position Purpose: Keeper Level One, Two and /Animal Care Manager Nil A Zoo Keeper is responsible for providing quality animal husbandry
Recommendation for the basic surveillance of Eudravigilance Veterinary data
 1 2 3 25 May 2010 EMA/CVMP/PhVWP/471721/2006 Veterinary Medicines and Product Data Management 4 5 6 Recommendation for the basic surveillance of Eudravigilance Veterinary data Draft 7 Draft agreed by Pharmacovigilance
1 2 3 25 May 2010 EMA/CVMP/PhVWP/471721/2006 Veterinary Medicines and Product Data Management 4 5 6 Recommendation for the basic surveillance of Eudravigilance Veterinary data Draft 7 Draft agreed by Pharmacovigilance
Animal Care Resource Guide Veterinary Care Issue Date: August 18, 2006
 Veterinary Care Issue Date: August 18, 2006 Subject: Veterinary Care Policy #3 Expired Medical Materials Pharmaceutical-Grade Compounds in Research Surgery Pre- and Post- Procedural Care Program of Veterinary
Veterinary Care Issue Date: August 18, 2006 Subject: Veterinary Care Policy #3 Expired Medical Materials Pharmaceutical-Grade Compounds in Research Surgery Pre- and Post- Procedural Care Program of Veterinary
Official Journal of the European Union
 11.6.2003 L 143/23 COUNCIL DIRECTIVE 2003/43/EC of 26 May 2003 amending Directive 88/407/EEC laying down the animal health requirements applicable to intra- Community trade in and imports of semen of domestic
11.6.2003 L 143/23 COUNCIL DIRECTIVE 2003/43/EC of 26 May 2003 amending Directive 88/407/EEC laying down the animal health requirements applicable to intra- Community trade in and imports of semen of domestic
Reflection paper on promotion of pharmacovigilance reporting
 13 July 2017 EMA/CVMP/PhVWP/390033/2014-Rev.1 Committee for Medicinal Products for Veterinary Use (CVMP) Reflection paper on promotion of pharmacovigilance reporting Draft agreed by CVMP Pharmacovigilance
13 July 2017 EMA/CVMP/PhVWP/390033/2014-Rev.1 Committee for Medicinal Products for Veterinary Use (CVMP) Reflection paper on promotion of pharmacovigilance reporting Draft agreed by CVMP Pharmacovigilance
Recognition of Export Controls and Certification Systems for Animals and Animal Products. Guidance for Competent Authorities of Exporting Countries
 Recognition of Export Controls and Certification Systems for Animals and Animal Products Guidance for Competent Authorities of Exporting Countries Disclaimer This guidance does not constitute, and should
Recognition of Export Controls and Certification Systems for Animals and Animal Products Guidance for Competent Authorities of Exporting Countries Disclaimer This guidance does not constitute, and should
Franck Berthe Head of Animal Health and Welfare Unit (AHAW)
 EFSA s information meeting: identification of welfare indicators for monitoring procedures at slaughterhouses Parma, 30/01/2013 The role of EFSA in Animal Welfare Activities of the AHAW Unit Franck Berthe
EFSA s information meeting: identification of welfare indicators for monitoring procedures at slaughterhouses Parma, 30/01/2013 The role of EFSA in Animal Welfare Activities of the AHAW Unit Franck Berthe
Having regard to the Treaty establishing the European Community, and in particular Article 152(4)(b) thereof,
 14.10.2003 L 262/17 DIRECTIVE 2003/74/EC OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 22 September 2003 amending Council Directive 96/22/EC concerning the prohibition on the use in stockfarming of certain
14.10.2003 L 262/17 DIRECTIVE 2003/74/EC OF THE EUROPEAN PARLIAMT AND OF THE COUNCIL of 22 September 2003 amending Council Directive 96/22/EC concerning the prohibition on the use in stockfarming of certain
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
CODE OF RECOMMENDATIONS FOR THE WELFARE OF PET GERBILS DUTY OF CARE TO A PET GERBIL UNDER THE ANIMAL WELFARE (GUERNSEY) ORDINANCE, 2012
 CODE OF RECOMMENDATIONS FOR THE WELFARE OF PET GERBILS DUTY OF CARE TO A PET GERBIL UNDER THE ANIMAL WELFARE (GUERNSEY) ORDINANCE, 2012 Section 8 of the Animal Welfare (Guernsey) Ordinance, 2012 provides
CODE OF RECOMMENDATIONS FOR THE WELFARE OF PET GERBILS DUTY OF CARE TO A PET GERBIL UNDER THE ANIMAL WELFARE (GUERNSEY) ORDINANCE, 2012 Section 8 of the Animal Welfare (Guernsey) Ordinance, 2012 provides
Code of Practice for the Welfare of Gamebirds Reared for Sporting Purposes
 Code of Practice for the Welfare of Gamebirds Reared for Sporting Purposes Contents Page Preface 2 Introduction 4 Recommendations 5 1. Origin of Stock 5 2. Incubation and hatching 5 3. Inspection and Husbandry
Code of Practice for the Welfare of Gamebirds Reared for Sporting Purposes Contents Page Preface 2 Introduction 4 Recommendations 5 1. Origin of Stock 5 2. Incubation and hatching 5 3. Inspection and Husbandry
Use of the Animal Welfare Assessment Grid to assess the life time experience of animals and cumulative severity of procedures
 Use of the Animal Welfare Assessment Grid to assess the life time experience of animals and cumulative severity of procedures Sarah Wolfensohn OBE BSc MA VetMB CertLAS FSB DipECLAM DipECAWBM-WSEL MRCVS
Use of the Animal Welfare Assessment Grid to assess the life time experience of animals and cumulative severity of procedures Sarah Wolfensohn OBE BSc MA VetMB CertLAS FSB DipECLAM DipECAWBM-WSEL MRCVS
Animal Care Resource Guide Veterinary Care Issue Date: July 17, 2007
 Policies Animal Care Resource Guide Veterinary Care Issue Date: July 17, 2007 Subject: Veterinary Care: Expired Medical Materials Pharmaceutical-Grade Compounds in Research Surgery Pre- and Post- Procedural
Policies Animal Care Resource Guide Veterinary Care Issue Date: July 17, 2007 Subject: Veterinary Care: Expired Medical Materials Pharmaceutical-Grade Compounds in Research Surgery Pre- and Post- Procedural
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 2001R0999 EN 17.11.2012 036.001 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 999/2001 OF THE EUROPEAN PARLIAMENT
2001R0999 EN 17.11.2012 036.001 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 999/2001 OF THE EUROPEAN PARLIAMENT
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE ANIMAL PROTOCOL REVIEW QUESTIONNAIRE. Name Role on Protocol Department P. O.
 VIRGINIA STATE UNIVERSITY Petersburg, Virginia 23806 FOR IACUC USE Review Month: Protocol Number: INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE ANIMAL PROTOCOL REVIEW QUESTIONNAIRE Submission Procedures:
VIRGINIA STATE UNIVERSITY Petersburg, Virginia 23806 FOR IACUC USE Review Month: Protocol Number: INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE ANIMAL PROTOCOL REVIEW QUESTIONNAIRE Submission Procedures:
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
COMMISSION DELEGATED REGULATION (EU) /... of XXX
 Ref. Ares(2017)4396495-08/09/2017 EUROPEAN COMMISSION Brussels, XXX SANTE/7009/2016 CIS Rev. 1 (POOL/G2/2016/7009/7009R1-EN CIS.doc) [ ](2016) XXX draft COMMISSION DELEGATED REGULATION (EU) /... of XXX
Ref. Ares(2017)4396495-08/09/2017 EUROPEAN COMMISSION Brussels, XXX SANTE/7009/2016 CIS Rev. 1 (POOL/G2/2016/7009/7009R1-EN CIS.doc) [ ](2016) XXX draft COMMISSION DELEGATED REGULATION (EU) /... of XXX
RSPCA (Victoria) Farm animal welfare The next 5 years
 RSPCA (Victoria) Farm animal welfare The next 5 years RSPCA Charter RSPCA Australia believes that animals must treated humanely. Where humans make use of animals or interferes with their habitat, they
RSPCA (Victoria) Farm animal welfare The next 5 years RSPCA Charter RSPCA Australia believes that animals must treated humanely. Where humans make use of animals or interferes with their habitat, they
The Guide for the Care and Use of Laboratory Animals Eighth Edition
 The Guide for the Care and Use of Laboratory Animals Eighth Edition Janet Garber, Committee Chair Lida Anestidou, Study Director Institute for Laboratory Animal Research The National Academies National
The Guide for the Care and Use of Laboratory Animals Eighth Edition Janet Garber, Committee Chair Lida Anestidou, Study Director Institute for Laboratory Animal Research The National Academies National
EUROPEAN LIVESTOCK AND MEAT TRADES UNION UECBV
 EUROPEAN LIVESTOCK AND MEAT TRADES UNION UECBV Slaughter of animals The role of industry organisations in the implementation of the Animal Welfare Standards Claudia Vinci Veterinary Advisor Table of content
EUROPEAN LIVESTOCK AND MEAT TRADES UNION UECBV Slaughter of animals The role of industry organisations in the implementation of the Animal Welfare Standards Claudia Vinci Veterinary Advisor Table of content
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
EU Programmes for Animal Welfare in the European region
 EU Programmes for Animal Welfare in the European region Andrea Gavinelli Unit G3 Animal Welfare Directorate General Health and Consumers 1 FUNDAMENTALS Animal Welfare Definition as agreed by OIE members
EU Programmes for Animal Welfare in the European region Andrea Gavinelli Unit G3 Animal Welfare Directorate General Health and Consumers 1 FUNDAMENTALS Animal Welfare Definition as agreed by OIE members
UiTM CARE APPLICATION FORM
 UiTM CARE APPLICATION FORM (Committee on Animal Research and Ethics) FOR UiTM CARE OFFICE USE ONLY Proposal No.:... Date of hard copy receipt:... INFORMATION FOR PRINICIPAL INVESTIGATOR Submit the duly
UiTM CARE APPLICATION FORM (Committee on Animal Research and Ethics) FOR UiTM CARE OFFICE USE ONLY Proposal No.:... Date of hard copy receipt:... INFORMATION FOR PRINICIPAL INVESTIGATOR Submit the duly
CODE OF RECOMMENDATIONS FOR THE WELFARE OF PET HAMSTERS DUTY OF CARE TO A PET HAMSTER UNDER THE ANIMAL WELFARE (GUERNSEY) ORDINANCE, 2012
 CODE OF RECOMMENDATIONS FOR THE WELFARE OF PET HAMSTERS DUTY OF CARE TO A PET HAMSTER UNDER THE ANIMAL WELFARE (GUERNSEY) ORDINANCE, 2012 Section 8 of the Animal Welfare (Guernsey) Ordinance, 2012 provides
CODE OF RECOMMENDATIONS FOR THE WELFARE OF PET HAMSTERS DUTY OF CARE TO A PET HAMSTER UNDER THE ANIMAL WELFARE (GUERNSEY) ORDINANCE, 2012 Section 8 of the Animal Welfare (Guernsey) Ordinance, 2012 provides
March 16, Guide's space recommendations as a minimum while always recognizing that performance standards also must be met.
 Comments of The American Association of Immunologists (AAI) to the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) Regarding the 8 th Edition of the Guide
Comments of The American Association of Immunologists (AAI) to the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) Regarding the 8 th Edition of the Guide
DP.1. Control tables
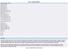 Data inclusion criteria Report year: 2015 Country: Croatia EU Submission: ALL Genetic status: ALL Animal Species: ALL Species grouping Level 1: ALL Species grouping Level 2: ALL Mammals: ALL Non-human
Data inclusion criteria Report year: 2015 Country: Croatia EU Submission: ALL Genetic status: ALL Animal Species: ALL Species grouping Level 1: ALL Species grouping Level 2: ALL Mammals: ALL Non-human
DP.1. Control tables
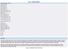 Data inclusion criteria Report year: 2014 Country: Croatia EU Submission: ALL Genetic status: ALL Animal Species: ALL Species grouping Level 1: ALL Species grouping Level 2: ALL Mammals: ALL Non-human
Data inclusion criteria Report year: 2014 Country: Croatia EU Submission: ALL Genetic status: ALL Animal Species: ALL Species grouping Level 1: ALL Species grouping Level 2: ALL Mammals: ALL Non-human
