TOLERABILITY OF IVERMECTIN IN GNATHOSTOMIASIS
|
|
|
- Shavonne Holmes
- 6 years ago
- Views:
Transcription
1 SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH TOLERABILITY OF IVERMECTIN IN GNATHOSTOMIASIS Valai Bussaratid 1, Srivicha Krudsood 2, Udomsak Silachamroon 1 and Sornchai Looareesuwan 1 1 Department of Clinical Tropical Medicine, 2 Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand Abstract. At present, no universally-accepted effective treatment for cutaneous gnathostomiasis is available. At the Hospital for Tropical Diseases, Mahidol University, albendazole 400 mg twice a day for 14 days is commonly prescribed for patients diagnosed with cutaneous gnathostomiasis. The efficacy of albendazole to induce outward migration of the parasite was less than or around 20% in 2 studies. Research for alternative, more efficacious treatment, is needed. In this prospective openlabeled study, we assessed the safety of ivermectin in 20 Thai patients diagnosed with cutaneous gnathostomiasis. Ivermectin, one time only, at dosages of 50, 100, 150, or 200 µg/kg bodyweight, was given orally to 4 groups of patients, 5 patients each group. Adverse events were recorded and laboratory tests were obtained before and after treatment. No serious adverse events occurred in this study. Forty adverse events were possibly related to ivermectin. The adverse events were malaise (35%), myalgia (30%), drowsiness (30%), pruritus (20%), nausea/vomiting (20%), dizziness (15%), diarrhea (15%), feeling of shortness of breath (10%), feeling of palpitations (10%), constipation (5%), anorexia (5%), and headache (5%). These adverse events were self-limited and not doserelated. Laboratory abnormalities were found in 3 patients (15%). Transient microscopic hematuria, pyuria, and mildly elevated liver enzymes were found in 1 patient each. Ivermectin single dose, of 50, 100, 150, and 200 µg/kg bodyweight, is considered safe in Thai patients. Future trials of ivermectin on human gnathostomiasis may be performed using dosages up to 200 µg/kg bodyweight. INTRODUCTION Human cutaneous gnathostomiasis, caused by advanced third-stage larva, and in some cases young adult Gnathostoma spinigerum, presents as an intermittent migratory subcutaneous swelling. It is not an uncommon tissue parasitic disease in Thailand. In 1889, Levinson first described the disease when he found gnathostoma larva from an infested Thai woman (Radomyos et al, 1996). During the period , Professor Svasti Daengsvang and Professor Chalerm Prommas described the complete life cycle of the parasite (Radomyos et al, 1996). Humans are accidental hosts when they eat raw freshwater fish, snake, frog, chicken, reptiles, and rodents contaminated with the larval parasite (Radomyos et al, 1996). In the 1980s, the estimated prevalence of human gnathostomiasis in Bangkok was 4 per 1,000 humans (Suntharasamai, 1987). Currently, at the Correspondence: Dr Valai Bussaratid, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, 420/6 Rajvithi Road, Bangkok 10400, Thailand. tmvbs@mahidol.ac.th Hospital for Tropical Diseases, Out-patient Department, about 3-5 patients have been followed up each week (0-5 new cases/week). Until now, there is no universally accepted antiparasitic agent for treatment of the disease. Currently, we use albendazole 400 mg once or twice daily for 2-3 weeks. The treatment had resulted in outward migration of the parasite in 3 of 41 patients treated with albendazole 400 mg twice a day for 14 days (Suntharasamai et al, 1992) and 21 of 100 patients treated with either albendazole 400 mg once or twice daily for 21 days (Kraivichian et al, 1992). The efficacy of albendazole is not proven to be high, and the drug at a dosage of 400 mg twice daily for 14 days can also cause transient elevation of liver enzymes (Inkatanuwat et al, 1998). Human gnathostomiasis can involve the eyes and rarely involves the central nervous system, causing eosinophilic myeloencephalitis or eosinophilic meningoencephalitis. Therefore, it is necessary to search for a more effective treatment. A study of ivermectin on advanced third stage larvae of Gnathostoma spinigerum was 644 Vol 36 No. 3 May 2005
2 IVERMECTIN TOLERABILITY IN GNATHOSTOMIASIS conducted in rabbits by Anantaphruti et al (1992). Infected rabbits were treated with either a single dose (0.2 mg/kg) or multiple doses of ivermectin. By the 28 th week after treatment, it was found that the reductions of worm load were 74.2% and 84.2% for the single-dose and multiple-dose groups, respectively. These data prompted trials of ivermectin in human gnathostomiasis. In this study, we assessed the safety and tolerability of ivermectin in human gnathostomiasis in 20 Thai patients with serologically confirmed diagnosis of cutaneous gnathostomiasis. MATERIALS AND METHODS This was a prospective, open-labeled study of ivermectin in gnathostomiasis, to assess the safety and tolerability of ivermectin. The study site was at the Hospital for Tropical Diseases, Mahidol University in Bangkok. The study protocol was approved by the Ethics Committee on Clinical Research of the Faculty of Tropical Medicine, Mahidol University. Subjects Twenty patients, aged years, who presented with active symptoms of recurrent migratory swelling, having the diagnosis of gnathostomiasis confirmed by Western blot analysis for serum antibodies against the 24 kda somatic antigen of the 3 rd stage larvae of G. spinigerum, were enrolled with their consent. Pregnant or nursing women, patients who had used any antiparasitic agents within the past 14 days, or patients with underlying diseases, such as pulmonary diseases (especially asthma), renal diseases (especially hematuria), history of seizure, liver diseases including elevated abnormal liver function tests (above maximum normal range), cardiac diseases (especially tachycardia with heart rate >100/minute.), or blood disorders [anemic (Hct <25%), or leukopenia (WBC <3,500/mm 3 )], or thrombocytopenia (platelet < 100,000/mm 3 ), or hypotension (blood pressure < 90/60 mmhg) were not eligible for this study. Drugs and doses The dosage of ivermectin used in this study was given at increments of 50 µg/kg bodyweight single dose. Dosages ranged from 50 µg/kg bodyweight to a maximum dose of 200 µg/kg, which is the maximum dose used in humans in the literature. Each dose was given to 5 consecutive patients. Monitoring and evaluations Safety and tolerability were assessed and recorded. Each patient was observed for immediate adverse reactions at the Out-patient Department for 30 minutes. Other symptoms were recorded for 7 days in a diary card given to the patient. The severity of a symptom was classified as mild, moderate, or severe. Mild symptom meant that it was self-limited without any treatment. Moderate symptom meant that the symptom was relieved with medication or an outpatient visit was required. Severe symptom meant that the patient needed hospitalization. Laboratory tests (CBC, AST, ALT, BUN, creatinine, and urine examination) were obtained at baseline and on the 7 th day to assess any immediate laboratory adverse events that may have occurred. If an abnormality occurred, the laboratory test was repeated periodically until it returned to normal. A complete physical examination was performed at baseline, on the 7 th day, and on the 28 th day. Since there was not enough information to definitely determine whether the causality of adverse events was related to ivermectin, we categorized adverse events into 2 groups: possibly-related to and not-related to ivermectin. Any adverse events that might be equally caused by ivermectin or other causes were called possibly related, while adverse events that were definitely related to other causes than ivermectin were called not related. After treatment with ivermectin, any patient who developed recurrent subcutaneous migratory swelling after 7 days of treatment initiation, and within 6 months, would be considered a treatment failure. These patients were evaluated for the need of further antiparasitic treatment (albendazole 400 mg twice daily for 14 days). RESULTS Twenty-one patients were enrolled. One patient withdrew consent prior to treatment initiation. There were 14 females and 6 males. Their Vol 36 No. 3 May
3 SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH mean age was 32.3 ± 9.35 years. Their mean bodyweight in kilograms was ± Sixteen patients resided in Bangkok and it vicinities. Two patients were from the central region and one patient each was from the western and northeastern regions. Of the 20 patients, 2 were lost to follow-up before the 7 th day visit, four patients had no adverse events, and the other 14 patients reported adverse events. Three patients had only 1 adverse event. The other 11 patients had more than one adverse event. A total of 48 adverse events were reported. No severe adverse events occurred during the study. Five adverse events were of moderate severity, but none required an out-patient visit. Forty and eight adverse events were considered possibly related and not-related to ivermectin, respectively. Malaise (7/20), myalgia (6/20), drowsiness (6/20), pruritus (location not specified) (4/20), nausea/vomiting (4/ 20), dizziness (3/20), diarrhea (3/20), feeling of breathing discomfort (2/20), feeling of palpitations (2/20), constipation (1/20), anorexia (1/20), and headache (1/20) were possibly related to ivermectin (Table 1). Adverse events that were not related to ivermectin were localized rash (3/ 20), common cold (3/20), localized pruritus (1/ 20), and feeling of palpitations (1/20). All of these symptoms were transient. They lasted for hours (Table 2). Three patients reported palpitations. Two of them were in the 50 µg/kg group and 1 was taking ivermectin at a dosage of 200 µg/kg. One patient complained of palpitations during the 30 minute observation period. On physical examination, the heart rate and rhythm were regular. The other 2 patients had symptoms at home. These 3 patients also reported multiple other symptoms during the first 7 days. Two patients stated that they had a feeling of breathing discomfort, which occurred on the first and second days after administration of ivermectin. The symptoms were mild and selflimited and did not occur within the 30-minutes observation period. Three patients reported mild dizziness, which occurred during the first 3 days, and lasted for hours. Laboratory abnormalities were found in 3 patients. Transient microscopic hematuria and pyuria were found on the 7 th day follow-up visit in 1 patient each. The patient with transient microscopic hematuria (10-15 cells/hpf) and the one with transient pyuria (5-20 cells/hpf) were taking ivermectin 200 and 100 µg/kg, respectively. The abnormal findings became normal by the 28 th day follow-up visit. Transient elevation of liver enzymes (AST=44, ALT = 66) was found in 1 patient taking ivermectin 150 µg/kg on the 7 th day follow-up visit. There was no evidence of other concomitant medication or alcohol use in this patient. The liver enzymes returned to nor- Table 1 Adverse events possibly related to ivermectin. Adverse events 50 µg/kg 100 µg/kg 150 µg/kg 200 µg/kg Total patients Malaise Myalgia Drowsiness Pruritus Nausea/vomiting Dizziness Diarrhea Feel shortness of breath Feel palpitations Constipation Anorexia Headache Total Vol 36 No. 3 May 2005
4 IVERMECTIN TOLERABILITY IN GNATHOSTOMIASIS Table 2 Onset and duration of adverse events after administration of ivermectin. Symptoms Onset Onset Duration of Total patients less than 3 days after 3 days symptoms (No. of patients) (No. of patients) (hours) Malaise Myalgia Drowsiness Pruritus Nausea/vomiting Dizziness Diarrhea 1 2 Less than 24 3 Feel shortness of breath 2 0 Less than 24 2 Feel palpitations 3 0 Less than Constipation 1 0 Less than 24 1 Anorexia 1 0 Less than 24 1 Headache 1 0 Less than 24 1 Localized rash Common cold mal values on the 28 th day follow-up visit. This patient remained healthy throughout the 6-month follow-up. During 6 months of follow-up, we found that 3 patients most likely had idiopathic urticaria/ localized angioedema rather than gnathostomiasis. Of these 3 patients, 2 had facial swelling about once a month, while another developed generalized urticaria by the 7 th day follow-up visit. Seventeen patients with a clinically and serologically confirmed diagnosis of cutaneous gnathostomiasis, had been actively symptomatic for months to over 1 year prior to enrollment. Ten patients had been symptomatic for less than 3 months, while the other 7 patients had recurrent subcutaneous swelling for over 1 year. They had subcutaneous swelling located on the upper extremities (n=10), head and neck (n=4), and feet (n=1), respectively. The other 2 patients had no recorded swelling locations. After treatment, 6 patients experienced recurrent subcutaneous swelling at the previous location, occurring within 7 days. These swellings were mild and self-limited, lasting from 1-6 days (mean=3.8 days). Nine patients had no recurrent subcutaneous swelling during the first 7 days. Two patients did not have the 7 th day follow-up visit. DISCUSSION Ivermectin was discovered in 1975 by Merck Research Laboratory in New Jersey, USA. Ivermectin, a derivative of avermectins, is a potent macrocyclic lactone with antiparasitic activity. It causes paralysis in many nematodes and arthropods through an influx of chloride ions across the cell membrane. The chloride influx is mediated through the GABA receptors (Ottesen and Campbell, 1994). Due to broad antiparasitic activity for both ecto- and endoparasites in cattle, swine, horses, and companion animals, and acceptable toxicological features, ivermectin was introduced to the veterinary market in In 1995, ivermectin was the world s largest-selling animal health product, with more than 5 billion doses sold worldwide (Merck, 1995). Ivermectin use in humans started in 1978, after Campbell and colleagues at Merck & Co, Inc observed its macrofilaricidal effect on Onchocerca cervicalis in horses. The first clinical trial of ivermectin in humans began in 1981 in Senegal (Aziz et al, 1982). That study of 32 patients started with a very low ivermectin dose of Vol 36 No. 3 May
5 SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH 5 µg/kg bodyweight and increased in small increments until it reached 50 µg/kg bodyweight. The second study was done in Paris, France on 12 West African immigrants (Coulard et al, 1983). Both studies showed that a single oral administration of ivermectin with a dose range to a maximum of 200 µg/kg bodyweight was well tolerated. No serious adverse events were observed during these initial 2 studies. Two previous community-based open-labeled studies on ivermectin and onchocerciasis involved approximately 59,000 participants. Most were age 5-55 years old, in West Africa (De sole et al, 1989; Pacque et al, 1989). The study subjects were given ivermectin at a single dose of µg/kg bodyweight. Monitoring of self-reported adverse events was conducted under an active surveillance system for at least 72 hours, and certain groups of the study subjects were followed for 4 weeks. About 9% of 31,260 patients and 1.3% of 7,699 patients, in these 2 separate studies, respectively, reported adverse events. The most common adverse events were various pain conditions (6.3%), pruritus and rash (3.7%), fever (3.3%), subcutaneous swelling (3%), painful lymphadenopathy (1.5%), headache (<1%), dizziness (<1%), nausea (<1%), weakness (<1%), and diarrhea (<1%). Severe adverse events occurred in 0.24%. These reactions included severe symptomatic postural hypotension (inability of a patient to stand for at least 2 minutes owing to severe dizziness or weakness attributable to a marked drop in blood pressure) (0.147%), high fever (0.090%), and severe dyspnea (0.009%). Severe symptomatic postural hypotension mostly occurred during the first day. It was not dose related. Although the symptoms, which occurred in a total of 49 patients, were transient and self-limited, 9 patients required intravenous injections of hydrocortisone. For severe dyspnea, the relationship with ivermectin treatment was uncertain. Two patients had a history of asthma, but were in remission at the time of treatment. One had active preexisting respiratory symptoms prior to the severe episode of laryngeal edema. Since then, ivermectin has been considered a beneficial antiparasitic agent for onchocerciasis. It has been used as a drug of choice for onchocerciasis in millions of humans since It has also been used for loiasis, other lymphatic filariases, some intestinal nematodes, and scabies, for years without any report of serious adverse effects (Kumaraswami et al, 1988; Shikiya et al, 1992; Coutinho et al, 1994; Meinking et al, 1995; Bockarie et al, 1998; Huffam and Currie, 1998; Kombila et al, 1998;). Several community-based trials showed that ivermectin was largely well-accepted. It could be administered in mass treatment programs with minimum medical supervision (De Sole et al, 1989; Pacque et al, 1989). Previous studies demonstrated that a single dose of ivermectin (200 µg/kg body weight) was more effective, with fewer side effects, in treating strongyloidiasis than thiabendazole (Darty et al, 1994). A study by Pacque and colleagues (1990) showed no major effect on pregnancy outcomes after inadvertent ivermectin treatment of 203 unnoticeably pregnant women during community-based distribution. In our current study, 65% of patients reported adverse events within 7 days after a single dose of ivermectin. This high number of adverse events included events both possibly, and not, related to ivermectin. In addition, the diary-card containing specific questions whether the symptoms were present or absent might increase adverse event detection compared to the monitoring of self-reported adverse events under an active surveillance system in previous studies. In this study, adverse events possibly related to ivermectin were malaise (35%), myalgia (30%), drowsiness (30%), pruritus (20%) nausea/ vomiting (20%), dizziness (15%), diarrhea (15%), feeling of breathing discomfort (10%), feeling of palpitations (10%), constipation (5%), anorexia (5%), and headache (5%). These events were mild, self-limited, and not dose related. There were reports of tachycardia, orthostatic hypotension, worsening of bronchial asthma, hematuria, abnormal complete blood counts (CBC), elevated liver enzymes (AST and ALT) which were transient and occurred uncommonly (less than 3.5%)(Njoo et al, 1993). Various pain conditions and fever that were common adverse events in previous studies did not 648 Vol 36 No. 3 May 2005
6 IVERMECTIN TOLERABILITY IN GNATHOSTOMIASIS present in this study. We found one patient (5%) with transient microscopic hematuria, one patient (5%) with transient microscopic pyuria, and one patient (5%) with transient mildly-elevated liver enzymes. We were not able to confirm that these laboratory abnormalities were due to causes other than ivermectin. We observed recurrent subcutaneous swelling within 7 days of a single-dose ivermectin in 6 patients (30%). This reaction may have been a parasite-ivermectin interaction, but was not an indication of treatment success, as 2 of these 6 patients had recurrent migratory subcutaneous swelling during the 6-month follow-up. In summary, ivermectin is considered safe for further trials in human gnathostomiasis in the Thai population. Nevertheless, more clinical trials are needed to confirm safety and to assess efficacy. ACKNOWLEDGEMENTS We are grateful to Prof Pravan Suntharasamai and Paul Adams for reviewing this article. This study was supported by the Thailand- Tropical Diseases Research Program (T-2) and the Faculty of Tropical Medicine, Mahidol University. REFERENCES Anantaphruti M, Nuamtanong S, Waikagul J. Effect of ivermectin on experimental gnathostomiasis in rabbits. Trop Med Parasitol 1992; 43: Aziz MA, Diallo S, Diop IM, Lariviere M, Porta M. Efficacy and tolerance of ivermectin in human onchocerciasis. Lancet 1982; 2: Bockarie MJ, Alexander ND, Hyun P, et al. Randomised community-based trial of annual single-dose diethylcarbamazine with or without ivermectin against Wuchereria bancrofti infection in human beings and mosquitoes. Lancet 1998; 351: Coulard JP, Lariviere M, Gervais MC, et al. Treatment of human onchocerciasis with ivermectin. Bull Soc Pathol Exot Filiales 1983; 76: (In French). Coutinho A, Dreyer G, Mediros Z, et al. Ivermectin treatment of bancroftian filariasis in Recife, Brazil. Am J Trop Med Hyg 1994; 50: Darty A, Hilmarsdottir I, Mayorga-Sagastume R, et al. Treatment of Strongyloides stercoralis infection with ivermectin compared with albendazole: results of an open study of 60 cases. Trans R Soc Trop Med Hyg 1994; 88: De Sole G, Remme J, Awadzi K, et al. Adverse reactions after large-scale treatment of onchocerciasis with ivermectin: combined results from eight community trials. Bull WHO 1989; 67: Huffam SE, Currie BJ. Ivermectin for Sarcoptes scabei hyperinfestation. Int J Infect Dis 1998; 2: Inkatanuwat S, Suntharasamai P, Vutikes S, Riganti M. Changes of liver functions after albendazole treatment in human gnathostomiasis. J Med Assoc Thai 1998; 81: Kombila M, Duong T, Ferrer A, et al. Short-and longterm action of multiple doses of ivermectin on loiasis microfilaremia. Am J Trop Med Hyg 1998; 58: Kraivichian P, Kulkumthorn M, Yingyourd P, Akarabovorn P, Paireepai CC. Albendazole for the treatment of human gnathostomiasis. Trans R Soc Trop Med Hyg 1992; 86: Kumaraswami V, Ottesen EA, Vijayasekaran V, et al. Ivermectin for the treatment of Wuchereria bancrofti filariasis. Efficacy and adverse reactions. J Am Med Assoc 1988; 259: Meinking TL, Taplin D, Hermida JL, Pardo R, Kerdel FA. The treatment of scabies with ivermectin. N Engl J Med 1995; 333: Merck & Co, Inc. Merck Annual Report. 1995: Njoo FL, Belling GA, Oosting J, Vetter JC, Stilma JS, Kijlstra A. Concurrent parasitic infections in onchocerciasis and the occurrence of adverse reactions after ivermectin treatment. Am J Trop Med Hyg 1993; 48: Ottesen EA, Campbell WC. Ivermectin in human medicine. J Antimicrob Chemother 1994; 34: Pacque MC, Dukuly Z, Greene BM, et al. Communitybased treatment of onchocerciasis with ivermectin: acceptability and early adverse reactions. Bull WHO 1989; 67: Radomyos P, Supavej S, Looareesuwan S. Tissue parasites. In: Textbook of clinical parasitology. 1 st ed. Bangkok: Bangkok Medical Media Publishing; 1996: (In Thai). Shikiya K, Kinjo N, Uehara T, et al. Efficacy of ivermectin against Strongyloides stercoralis in humans. Intern Med 1992; 31: Suntharasamai P. Gnathostomiasis. In: Weatherall DJ, Ledingham JGG, Warrell DA, eds. Oxford textbook of medicine. 2 nd ed. London: Oxford University Press; 1987: Suntharasamai P, Riganti M, Chittamas S, Desakorn V. Albendazole stimulates outward migration of Gnathostoma spinigerum to the dermis in man. Southeast Asian J Trop Med Public Health 1992; 23: Vol 36 No. 3 May
Drug therapy of Filariasis. Dr. Shareef sm Asst. professor pharmacology
 Drug therapy of Filariasis Dr. Shareef sm Asst. professor pharmacology Signs and symptoms Lymphatic filariasis Fever Inguinal or axillary lymphadenopathy Testicular and/or inguinal pain Skin exfoliation
Drug therapy of Filariasis Dr. Shareef sm Asst. professor pharmacology Signs and symptoms Lymphatic filariasis Fever Inguinal or axillary lymphadenopathy Testicular and/or inguinal pain Skin exfoliation
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT IVOMEC Injection for Pigs 10 mg/ml 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active Substance: Ivermectin
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT IVOMEC Injection for Pigs 10 mg/ml 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active Substance: Ivermectin
TREATMENT OF CUTANEOUS GNATHOSTOMIASIS WITH IVERMECTIN
 Am. J. Trop. Med. Hyg., 71(5), 2004, pp. 623 628 Copyright 2004 by The American Society of Tropical Medicine and Hygiene TREATMENT OF CUTANEOUS GNATHOSTOMIASIS WITH IVERMECTIN KANYARAT KRAIVICHIAN, SURANG
Am. J. Trop. Med. Hyg., 71(5), 2004, pp. 623 628 Copyright 2004 by The American Society of Tropical Medicine and Hygiene TREATMENT OF CUTANEOUS GNATHOSTOMIASIS WITH IVERMECTIN KANYARAT KRAIVICHIAN, SURANG
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
. - many countries in Asia. Twenty species of Gnathostoma have been recorded in the literature although only
 SEATO ~edical ~esearch Studies on Gnathostomiasis in Thailand. Professor Svasti Daengsvang, M.D. Special Consultant to the Director. Principal investigator: Professor Svasti Daengsvang M.D. Associate Investigator:
SEATO ~edical ~esearch Studies on Gnathostomiasis in Thailand. Professor Svasti Daengsvang, M.D. Special Consultant to the Director. Principal investigator: Professor Svasti Daengsvang M.D. Associate Investigator:
SHE SINGS ALONG TO EVERY SONG...
 Prevention. Protection. SHE SINGS ALONG TO EVERY SONG... Protect your best friend with the 5-IN-1 HEARTWORM MEDICINE THAT USES LUFENURON TO STOP FLEAS BEFORE THEY START. Prevention. Protection. POWERED
Prevention. Protection. SHE SINGS ALONG TO EVERY SONG... Protect your best friend with the 5-IN-1 HEARTWORM MEDICINE THAT USES LUFENURON TO STOP FLEAS BEFORE THEY START. Prevention. Protection. POWERED
Period of study: 12 Nov 2002 to 08 Apr 2004 (first subject s first visit to last subject s last visit)
 Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency of Bayer's
Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website and is provided for patients and healthcare professionals to increase the transparency of Bayer's
A review of Filariasis
 International Journal of Current Research in Medical Sciences ISSN: 2454-5716 P-ISJN: A4372-3064, E -ISJN: A4372-3061 www.ijcrims.com Review Article Volume 5, Issue 2-2019 DOI: http://dx.doi.org/10.22192/ijcrms.2019.05.02.005
International Journal of Current Research in Medical Sciences ISSN: 2454-5716 P-ISJN: A4372-3064, E -ISJN: A4372-3061 www.ijcrims.com Review Article Volume 5, Issue 2-2019 DOI: http://dx.doi.org/10.22192/ijcrms.2019.05.02.005
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
 BOEHRINGER INGELHEIM VETMEDICA, INC. USA Product Label http://www.vetdepot.com 2621 NORTH BELT HIGHWAY, ST. JOSEPH, MO, 64506 2002 Telephone: 800 325 9167 Fax: 816 236 2717 Email: www.bi vetmedica.com
BOEHRINGER INGELHEIM VETMEDICA, INC. USA Product Label http://www.vetdepot.com 2621 NORTH BELT HIGHWAY, ST. JOSEPH, MO, 64506 2002 Telephone: 800 325 9167 Fax: 816 236 2717 Email: www.bi vetmedica.com
Heartworm Disease in Dogs
 Customer Name, Street Address, City, State, Zip code Phone number, Alt. phone number, Fax number, e-mail address, web site Heartworm Disease in Dogs Basics OVERVIEW Disease caused by infestation with heartworms
Customer Name, Street Address, City, State, Zip code Phone number, Alt. phone number, Fax number, e-mail address, web site Heartworm Disease in Dogs Basics OVERVIEW Disease caused by infestation with heartworms
MOXIDECTIN SPOT-ON SOLUTION FOR KITTENS AND SMALL CATS. 280 mg/ml FLURALANER 14 mg/ml MOXIDECTIN Also contains: 339 mg/ml DIMETHYLACETAMIDE (solvent)
 Product Name: BRAVECTO PLUS FLEA, TICK AND WORM 112.5 MG FLURALANER AND 5.6 MG MOXIDECTIN SPOT-ON SOLUTION FOR KITTENS AND SMALL CATS APVMA Approval No: 85418/113229 Label Name: BRAVECTO PLUS FLEA, TICK
Product Name: BRAVECTO PLUS FLEA, TICK AND WORM 112.5 MG FLURALANER AND 5.6 MG MOXIDECTIN SPOT-ON SOLUTION FOR KITTENS AND SMALL CATS APVMA Approval No: 85418/113229 Label Name: BRAVECTO PLUS FLEA, TICK
BENDEX Plus Tablets (Ivermectin + Albendazole)
 Published on: 22 Sep 2014 BENDEX Plus Tablets ( + ) Composition BENDEX Plus Tablets Each uncoated tablet contains:, BP...6 mg, IP...400 mg Dosage Form Tablet Pharmacology Pharmacodynamics The principal
Published on: 22 Sep 2014 BENDEX Plus Tablets ( + ) Composition BENDEX Plus Tablets Each uncoated tablet contains:, BP...6 mg, IP...400 mg Dosage Form Tablet Pharmacology Pharmacodynamics The principal
WHO/FIU Distr.: Limited English only
 WHO/FIU98.194 Distr.: Limited English only WHO/FIL/98.194 English only This document is not issued to the general public, and all rights are reserved by the World Health Organization (WHO). The document
WHO/FIU98.194 Distr.: Limited English only WHO/FIL/98.194 English only This document is not issued to the general public, and all rights are reserved by the World Health Organization (WHO). The document
Questions and answers on serious non-fatal adverse events and reporting rules
 12 April 2017 EMA/CVMP/PhVWP/303762/2012-Rev.1 Committee for Medicinal Products for Veterinary Use Questions and answers on serious non-fatal adverse events and reporting rules This questions and answers
12 April 2017 EMA/CVMP/PhVWP/303762/2012-Rev.1 Committee for Medicinal Products for Veterinary Use Questions and answers on serious non-fatal adverse events and reporting rules This questions and answers
A Field Study on Efficacy of Albendazole (Albezol ) Against Gastro-intestinal Nematodes in Ruminants
 Kasetsart J. (Nat. Sci.) 39 : 647-651 (25) A Field Study on Efficacy of Albendazole (Albezol ) Against Gastro-intestinal Nematodes in Ruminants Theera Rukkwamsuk 1, Anawat Sangmalee 1, Korawich Anukoolwuttipong
Kasetsart J. (Nat. Sci.) 39 : 647-651 (25) A Field Study on Efficacy of Albendazole (Albezol ) Against Gastro-intestinal Nematodes in Ruminants Theera Rukkwamsuk 1, Anawat Sangmalee 1, Korawich Anukoolwuttipong
HOOKWORM FAQ SHEET (rev ) Adapted from the CDC Fact Sheet
 HOOKWORM FAQ SHEET (rev 3-1-10) Adapted from the CDC Fact Sheet Hookworm Infection FAQ Sheet Contents What is hookworm? Where are hookworms commonly found? How do I get a hookworm infection? Who is at
HOOKWORM FAQ SHEET (rev 3-1-10) Adapted from the CDC Fact Sheet Hookworm Infection FAQ Sheet Contents What is hookworm? Where are hookworms commonly found? How do I get a hookworm infection? Who is at
Dear Doctor: Our sincerest thanks, Stephen A. Connell, DVM Director, Technical, Academic and Consumer Services Elanco Companion Animal Health
 Dear Doctor: As a trained professional, you understand the loss of a pet is incredibly difficult. Every pet owner responds differently as they grieve. We believe the recent negative media coverage of Trifexis
Dear Doctor: As a trained professional, you understand the loss of a pet is incredibly difficult. Every pet owner responds differently as they grieve. We believe the recent negative media coverage of Trifexis
New Insights into the Treatment of Leishmaniasis
 New Insights into the Treatment of Leishmaniasis Eric Zini Snow meeting, 14 March 2009 Few drugs available for dogs Initially developed to treat human leishmaniasis, later adopted in dogs None eradicates
New Insights into the Treatment of Leishmaniasis Eric Zini Snow meeting, 14 March 2009 Few drugs available for dogs Initially developed to treat human leishmaniasis, later adopted in dogs None eradicates
Tolerance and safety of enalapril
 Br. J. clin. Pharmac. (1984), 18, 249S-253S Tolerance and safety of enalapril W. McFATE SMITH, R. 0. DAVIES, M. A. GABRIEL, D. M. KRAMSCH, F. MONCLOA, JANET E. RUSH & J. F. WALKER Merck Sharp & Dohme Research
Br. J. clin. Pharmac. (1984), 18, 249S-253S Tolerance and safety of enalapril W. McFATE SMITH, R. 0. DAVIES, M. A. GABRIEL, D. M. KRAMSCH, F. MONCLOA, JANET E. RUSH & J. F. WALKER Merck Sharp & Dohme Research
PACKAGE LEAFLET Page 1 of 6
 PACKAGE LEAFLET Page 1 of 6 Package leaflet: Information for the patient Desloratadine Mylan 5 mg film-coated tablets desloratadine Read all of this leaflet carefully before you start taking this medicine
PACKAGE LEAFLET Page 1 of 6 Package leaflet: Information for the patient Desloratadine Mylan 5 mg film-coated tablets desloratadine Read all of this leaflet carefully before you start taking this medicine
Amoxicillin Introduction: Mechanism of action: Pharmacology: Indications: Dosage: 12 Weeks ( 3 Months):
 Amoxicillin Introduction: A semisynthetic antibiotic, an analog of ampicillin, with a broad spectrum of bactericidal activity against many gram-positive and gram-negative microganisms. Mechanism of action:
Amoxicillin Introduction: A semisynthetic antibiotic, an analog of ampicillin, with a broad spectrum of bactericidal activity against many gram-positive and gram-negative microganisms. Mechanism of action:
COMMON MANGE IN DOGS AND CATS days spent on the dog Females burrow tunnels in the stratum corneum to lay eggs
 COMMON MANGE IN DOGS AND CATS Sarcoptic Mange LIFE CYCLE OF Sarcoptes scabiei 17 21 days spent on the dog Females burrow tunnels in the stratum corneum to lay eggs CLINICAL SIGNS Intense pruritus Papular
COMMON MANGE IN DOGS AND CATS Sarcoptic Mange LIFE CYCLE OF Sarcoptes scabiei 17 21 days spent on the dog Females burrow tunnels in the stratum corneum to lay eggs CLINICAL SIGNS Intense pruritus Papular
HEARTWORM DISEASE AND THE DAMAGE DONE
 HEARTWORM DISEASE AND THE DAMAGE DONE Stephen Jones, DVM There are now more months of the year where environmental conditions favor mosquito survival and reproduction. Warmer temperatures Indoor environments
HEARTWORM DISEASE AND THE DAMAGE DONE Stephen Jones, DVM There are now more months of the year where environmental conditions favor mosquito survival and reproduction. Warmer temperatures Indoor environments
General introduction
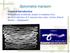 Spirometra mansoni General introduction Distributed worldwide, mainly in southeast Asia. Larval infection of S. mansoni may cause serious clinical disease ---Sparganosis Morphology Adult worm measures
Spirometra mansoni General introduction Distributed worldwide, mainly in southeast Asia. Larval infection of S. mansoni may cause serious clinical disease ---Sparganosis Morphology Adult worm measures
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Revised: December 2011 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Dectomax 10 mg/ml Solution for Injection for Pigs (UK) Zearl 10 mg/ml Solution for Injection for Pigs
SUMMARY OF PRODUCT CHARACTERISTICS Revised: December 2011 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Dectomax 10 mg/ml Solution for Injection for Pigs (UK) Zearl 10 mg/ml Solution for Injection for Pigs
US Federal law restricts this drug to use by or on the order of a licensed veterinarian.
 PFIZER INC. PFIZER ANIMAL HEALTH USA Product Label http://www.vetdepot.com 235 E. 42ND ST., NEW YORK, NY, 10017 Telephone: 269 833 4000 Customer Service: 800 733 5500 and 800 793 0596 Veterinary Medical
PFIZER INC. PFIZER ANIMAL HEALTH USA Product Label http://www.vetdepot.com 235 E. 42ND ST., NEW YORK, NY, 10017 Telephone: 269 833 4000 Customer Service: 800 733 5500 and 800 793 0596 Veterinary Medical
Intestinal parasitic infections are a serious
 Paediatrica Indonesiana VOLUME 54 March NUMBER 2 Original Article Albendazole alone vs. albendazole and diethylcarbamazine combination therapy for trichuriasis Windya Sari Nasution, Muhammad Ali, Ayodhia
Paediatrica Indonesiana VOLUME 54 March NUMBER 2 Original Article Albendazole alone vs. albendazole and diethylcarbamazine combination therapy for trichuriasis Windya Sari Nasution, Muhammad Ali, Ayodhia
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYTOPOINT 10 mg solution for injection for dogs CYTOPOINT 20 mg solution for injection for dogs CYTOPOINT 30 mg
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYTOPOINT 10 mg solution for injection for dogs CYTOPOINT 20 mg solution for injection for dogs CYTOPOINT 30 mg
CURRICULUM VITAE. Piyanan Taweethavonsawat. University, Bangkok, Thailand M.Sc. (Pathobiology) Faculty of Veterinary Medicine,
 CURRICULUM VITAE Personal Data Name Piyanan Taweethavonsawat Date of Birth July 11, 1974 Place of Birth Civil status Nationality Bangkok, Thailand Single Thai Academic qualifications 1991-1996 D.V.M. Faculty
CURRICULUM VITAE Personal Data Name Piyanan Taweethavonsawat Date of Birth July 11, 1974 Place of Birth Civil status Nationality Bangkok, Thailand Single Thai Academic qualifications 1991-1996 D.V.M. Faculty
LABELLING AND PACKAGE LEAFLET
 LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE CARTON BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MILTEFORAN 20 mg/ml oral solution for dogs miltefosine 2.
LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE CARTON BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MILTEFORAN 20 mg/ml oral solution for dogs miltefosine 2.
Helminth Infections. Pinworms
 Helminth Infections Pinworms Helminths Worm classified as a parasite Contaminate food, water, air, feces, pets, wild animals, toilet seats and door handles Prevention: Frequent hand washing Frequent cleaning
Helminth Infections Pinworms Helminths Worm classified as a parasite Contaminate food, water, air, feces, pets, wild animals, toilet seats and door handles Prevention: Frequent hand washing Frequent cleaning
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT COXEVAC suspension for injection for cattle and goats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT COXEVAC suspension for injection for cattle and goats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
JMSCR Vol 05 Issue 03 Page March 2017
 www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i3.219 Comparative Study of Adverse Effect of
www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i3.219 Comparative Study of Adverse Effect of
ESSENTIAL HEARTWORM PREVENTION GUIDE PROTECT YOUR DOG FROM HEARTWORM WITHOUT HARMFUL MEDS INFORMATION PROVIDED BY PETER DOBIAS DVM
 ESSENTIAL HEARTWORM PREVENTION GUIDE PROTECT YOUR DOG FROM HEARTWORM WITHOUT HARMFUL MEDS INFORMATION PROVIDED BY PETER DOBIAS DVM REASONS WHY YOU WANT TO AVOID HEARTWORM MEDS Here are the adverse events
ESSENTIAL HEARTWORM PREVENTION GUIDE PROTECT YOUR DOG FROM HEARTWORM WITHOUT HARMFUL MEDS INFORMATION PROVIDED BY PETER DOBIAS DVM REASONS WHY YOU WANT TO AVOID HEARTWORM MEDS Here are the adverse events
SUMMARY OF PRODUCT CHARACTERISTICS. 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Emdocam 20 mg/ml solution for injection for cattle, pigs and horses
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Emdocam 20 mg/ml solution for injection for cattle, pigs and horses 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Emdocam 20 mg/ml solution for injection for cattle, pigs and horses 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
TB Grand Rounds. MDR-TB: Management of Adverse Drug Reactions. Reynard J. McDonald, M.D. September 18, Patient History
 TB Grand Rounds MDR-TB: Management of Adverse Drug Reactions Reynard J. McDonald, M.D. September 18, 2007 Patient History This 30 y/o H/M was born in Ecuador and immigrated to the US in 2001 On 11-22-05
TB Grand Rounds MDR-TB: Management of Adverse Drug Reactions Reynard J. McDonald, M.D. September 18, 2007 Patient History This 30 y/o H/M was born in Ecuador and immigrated to the US in 2001 On 11-22-05
Clinical Manifestations and Treatment of Plague Dr. Jacky Chan. Associate Consultant Infectious Disease Centre, PMH
 Clinical Manifestations and Treatment of Plague Dr. Jacky Chan Associate Consultant Infectious Disease Centre, PMH Update of plague outbreak situation in Madagascar A large outbreak since 1 Aug 2017 As
Clinical Manifestations and Treatment of Plague Dr. Jacky Chan Associate Consultant Infectious Disease Centre, PMH Update of plague outbreak situation in Madagascar A large outbreak since 1 Aug 2017 As
For the treatment and prevention of infections caused by:
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYDECTIN 0.1 % W/V ORAL SOLUTION for sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Active substance Moxidectin
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYDECTIN 0.1 % W/V ORAL SOLUTION for sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Active substance Moxidectin
Drug combinations against soiltransmitted
 Jennifer Keiser Helminth Drug Development Unit Department of Medical Parasitology and Infection Biology Swiss TPH Winter Symposium 2017 Helminth Infection from Transmission to Control Drug combinations
Jennifer Keiser Helminth Drug Development Unit Department of Medical Parasitology and Infection Biology Swiss TPH Winter Symposium 2017 Helminth Infection from Transmission to Control Drug combinations
Indicated for the treatment of pruritus associated with allergic dermatitis and the clinical manifestations of atopic dermatitis in dogs.
 Zoetis UK Limited Telephone: 0845 300 8034 Website: www.zoetis.co.uk Email: customersupportuk@zoetis.com Apoquel film-coated for dogs Species: Therapeutic indication: Active ingredient: Product: Product
Zoetis UK Limited Telephone: 0845 300 8034 Website: www.zoetis.co.uk Email: customersupportuk@zoetis.com Apoquel film-coated for dogs Species: Therapeutic indication: Active ingredient: Product: Product
Job Title Name Signature Date
 Supply of Trimethoprim 200mg tablets By Community Pharmacists for the Management of Uncomplicated Urinary Tract Infections in Female Patients Protocol number 473 Version 1 Date protocol prepared: November
Supply of Trimethoprim 200mg tablets By Community Pharmacists for the Management of Uncomplicated Urinary Tract Infections in Female Patients Protocol number 473 Version 1 Date protocol prepared: November
What causes heartworm disease?
 Heartworm Disease: What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs and cats. It is caused by a blood-borne parasite called Dirofilaria
Heartworm Disease: What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs and cats. It is caused by a blood-borne parasite called Dirofilaria
CANINE HEARTWORM DISEASE
 ! CANINE HEARTWORM DISEASE What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs. It is caused by a blood-borne parasite called Dirofilaria
! CANINE HEARTWORM DISEASE What causes heartworm disease? Heartworm disease (dirofilariasis) is a serious and potentially fatal disease in dogs. It is caused by a blood-borne parasite called Dirofilaria
SUMMARY OF PRODUCT CHARACTERISTICS. Cephacare flavour 50 mg tablets for cats and dogs. Excipients: For a full list of excipients, see section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Cephacare flavour 50 mg tablets for cats and dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Cephacare flavour 50 mg tablets for cats and dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active
Acute Hemorrhagic Diarrhea Syndrome (AHDS) A Cause of Bloody Feces in Dogs
 Acute Hemorrhagic Diarrhea Syndrome (AHDS) A Cause of Bloody Feces in Dogs No dog parent wants to clean up diarrhea. Cleaning up bloody diarrhea is even more unpleasant. Unfortunately, the development
Acute Hemorrhagic Diarrhea Syndrome (AHDS) A Cause of Bloody Feces in Dogs No dog parent wants to clean up diarrhea. Cleaning up bloody diarrhea is even more unpleasant. Unfortunately, the development
Outlines. Introduction Prevalence Resistance Clinical presentation Diagnosis Management Prevention Case presentation Achievements
 Amal Meas Al-Anizi, PharmD Candidate KSU, Infectious Disease Rotation 2014 Outlines Introduction Prevalence Resistance Clinical presentation Diagnosis Management Prevention Case presentation Achievements
Amal Meas Al-Anizi, PharmD Candidate KSU, Infectious Disease Rotation 2014 Outlines Introduction Prevalence Resistance Clinical presentation Diagnosis Management Prevention Case presentation Achievements
B. PACKAGE LEAFLET 1
 B. PACKAGE LEAFLET 1 PACKAGE LEAFLET FOR: Cadorex 300 mg/ml solution for injection for cattle, sheep and pigs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION
B. PACKAGE LEAFLET 1 PACKAGE LEAFLET FOR: Cadorex 300 mg/ml solution for injection for cattle, sheep and pigs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION
Federal law (U.S.A.) restricts this drug to use by or on the order of a licensed veterinarian.
 BAYER HEALTHCARE LLC Animal Health Division USA Product Label http://www.vetdepot.com P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390 Customer Service Tel.: 800-633-3796 Customer Service Fax: 800-344-4219
BAYER HEALTHCARE LLC Animal Health Division USA Product Label http://www.vetdepot.com P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390 Customer Service Tel.: 800-633-3796 Customer Service Fax: 800-344-4219
POST-OPERATIVE ANALGESIA AND FORMULARIES
 POST-OPERATIVE ANALGESIA AND FORMULARIES An integral component of any animal protocol is the prevention or alleviation of pain or distress, such as that associated with surgical and other procedures. Pain
POST-OPERATIVE ANALGESIA AND FORMULARIES An integral component of any animal protocol is the prevention or alleviation of pain or distress, such as that associated with surgical and other procedures. Pain
ENVIRACOR J-5 aids in the control of clinical signs associated with Escherichia coli (E. coli) mastitis
 GDR11136 ENVIRACOR J-5 aids in the control of clinical signs associated with Escherichia coli (E. coli) mastitis February 2012 Summary The challenge data presented in this technical bulletin was completed
GDR11136 ENVIRACOR J-5 aids in the control of clinical signs associated with Escherichia coli (E. coli) mastitis February 2012 Summary The challenge data presented in this technical bulletin was completed
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Efestad 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 5 mg desloratadine. Excipient: 31.5
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Efestad 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 5 mg desloratadine. Excipient: 31.5
Day 90 Labelling, PL LABELLING AND PACKAGE LEAFLET
 LABELLING AND PACKAGE LEAFLET A. LABELLING PARTICULARS TO APPEAR ON THE OUTER PACKAGE : Carton 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Alvegesic vet. 10 mg/ml Solution for injection for Horses, Dogs
LABELLING AND PACKAGE LEAFLET A. LABELLING PARTICULARS TO APPEAR ON THE OUTER PACKAGE : Carton 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Alvegesic vet. 10 mg/ml Solution for injection for Horses, Dogs
For the treatment of mixed parasitic infections in cats caused by roundworms and tapeworms of the following species:
 Printed from (http://www.noahcompendium.co.uk). (c) Copyright 2018. All Rights Reserved. Date: Wednesday, October 24, 2018 11:47 Bayer plc Telephone:0118 206 3000 Website:www.bayer.co.uk Email:animal.health@bayer.com
Printed from (http://www.noahcompendium.co.uk). (c) Copyright 2018. All Rights Reserved. Date: Wednesday, October 24, 2018 11:47 Bayer plc Telephone:0118 206 3000 Website:www.bayer.co.uk Email:animal.health@bayer.com
ZENTEL (Albendazole) PRODUCT INFORMATION
 ZENTEL (Albendazole) PRODUCT INFORMATION DESCRIPTION ZENTEL contains albendazole, which is methyl [5-(propylthio)-1H-benzimidazol-2-yl] carbamate. It is a member of the benzimidazole group of anthelmintic
ZENTEL (Albendazole) PRODUCT INFORMATION DESCRIPTION ZENTEL contains albendazole, which is methyl [5-(propylthio)-1H-benzimidazol-2-yl] carbamate. It is a member of the benzimidazole group of anthelmintic
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Marbocare 20 mg/ml solution for injection for cattle and pigs (UK, IE, FR) Odimar 20 mg/ml solution for injection for cattle
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Marbocare 20 mg/ml solution for injection for cattle and pigs (UK, IE, FR) Odimar 20 mg/ml solution for injection for cattle
TO ENSURE ADEQUATE ABSORPTION, ALWAYS ADMINISTER PROGRAM FLAVOR TABS IN CONJUNCTION WITH A NORMAL MEAL.
 NOVARTIS ANIMAL HEALTH US, INC. USA Product Label http://www.vetdepot.com 3200 NORTHLINE AVE. SUITE 300, GREENSBORO, NC, 27408 Customer Service: 800 332 2761 Professional Services: 800 637 0281 Fax: 336
NOVARTIS ANIMAL HEALTH US, INC. USA Product Label http://www.vetdepot.com 3200 NORTHLINE AVE. SUITE 300, GREENSBORO, NC, 27408 Customer Service: 800 332 2761 Professional Services: 800 637 0281 Fax: 336
Prescribing Guidelines for Outpatient Antimicrobials in Otherwise Healthy Children
 Prescribing Guidelines for Outpatient Antimicrobials in Otherwise Healthy Children Prescribing Antimicrobials for Common Illnesses When treating common illnesses such as ear infections and strep throat,
Prescribing Guidelines for Outpatient Antimicrobials in Otherwise Healthy Children Prescribing Antimicrobials for Common Illnesses When treating common illnesses such as ear infections and strep throat,
ISMP Canada HYDROmorphone Knowledge Assessment Survey
 ISMP Canada HYDROmorphone Knowledge Assessment Survey Knowledge Assessment Questions 1. In an equipotent dose, HYDROmorphone is more potent than morphine. True False Unsure 2. HYDROmorphone can be given
ISMP Canada HYDROmorphone Knowledge Assessment Survey Knowledge Assessment Questions 1. In an equipotent dose, HYDROmorphone is more potent than morphine. True False Unsure 2. HYDROmorphone can be given
Diagnosing intestinal parasites. Clinical reference guide for Fecal Dx antigen testing
 Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
ANNEX III LABELLING AND PACKAGE LEAFLET
 ANNEX III LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE Card box and package leaflet for brown glass bottle (Type 1) 1. NAME OF THE
ANNEX III LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE Card box and package leaflet for brown glass bottle (Type 1) 1. NAME OF THE
Intravenous Antibiotic Therapy Information Leaflet
 Scottish Adult Cystic Fibrosis Service Ninewells Hospital Dundee Intravenous Antibiotic Therapy Information Leaflet February 2008 Intravenous antibiotic therapy in cystic fibrosis Patients with cystic
Scottish Adult Cystic Fibrosis Service Ninewells Hospital Dundee Intravenous Antibiotic Therapy Information Leaflet February 2008 Intravenous antibiotic therapy in cystic fibrosis Patients with cystic
Health Products Regulatory Authority
 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Ketamidor 100 mg/ml solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: Active substance: Ketamine (as hydrochloride) Excipient:
1 NAME OF THE VETERINARY MEDICINAL PRODUCT Ketamidor 100 mg/ml solution for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains: Active substance: Ketamine (as hydrochloride) Excipient:
Diagnosing intestinal parasites. Clinical reference guide for Fecal Dx antigen testing
 Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
Diagnosing intestinal parasites Clinical reference guide for Fecal Dx antigen testing Screen every dog at least twice a year The Companion Animal Parasite Council (CAPC) guidelines recommend including
November 2017 Review Nov Signatures of those developing the Patient Group Direction Job Title Name Signature Date Doctor Stephanie Dundas
 Supply of Trimethoprim 200mg tablets by Community Pharmacists for the Management of Uncomplicated Urinary Tract Infections in Female Patients from 16 and 65 years of age. November 2017 Review Nov 2019
Supply of Trimethoprim 200mg tablets by Community Pharmacists for the Management of Uncomplicated Urinary Tract Infections in Female Patients from 16 and 65 years of age. November 2017 Review Nov 2019
Presentation of Quiz #85
 Presentation of Quiz #85 ***Reminder: Slides are copyrighted and cannot be copied for publication. A 36 year old male from Columbia was admitted to the hospital with seizures. This patient had previously
Presentation of Quiz #85 ***Reminder: Slides are copyrighted and cannot be copied for publication. A 36 year old male from Columbia was admitted to the hospital with seizures. This patient had previously
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Xylacare 2% w/v Solution for Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Qualitative composition
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Xylacare 2% w/v Solution for Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Qualitative composition
SUMMARY OF PRODUCT CHARACTERISTICS. Equimax Tabs Vet, 150 mg / 20 mg, Chewable tablet for Horses
 SUMMARY OF PRODUCT CHARACTERISTICS Revised: June 2013 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Equimax Tabs 150 mg / 20 mg Chewable tablet for Horses For DK, SE, FI, IS, NO : Equimax Tabs Vet, 150 mg
SUMMARY OF PRODUCT CHARACTERISTICS Revised: June 2013 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Equimax Tabs 150 mg / 20 mg Chewable tablet for Horses For DK, SE, FI, IS, NO : Equimax Tabs Vet, 150 mg
Please refer to Table 1 Dosage and Treatment Schedule TABLE 1 Species Product Number of Tubes Cats. Rabbits or Advantage 40 for Cats
 Advantage Introduction Company name: Bayer plc Address: Animal Health Division Bayer House, Strawberry Hill, Newbury Berkshire RG14 1JA Telephone: 01635 563000 Fax: 01635 563622 Email: animal.health@bayerhealthcare.com
Advantage Introduction Company name: Bayer plc Address: Animal Health Division Bayer House, Strawberry Hill, Newbury Berkshire RG14 1JA Telephone: 01635 563000 Fax: 01635 563622 Email: animal.health@bayerhealthcare.com
Anesthesia Check-off Form
 Anesthesia Check-off Form 5231 SW 91st Drive Gainesville, FL 32608 (352) 377-6003 The doctors and staff at Haile Plantation Animal Clinic would like to offer the most advanced medical care and services
Anesthesia Check-off Form 5231 SW 91st Drive Gainesville, FL 32608 (352) 377-6003 The doctors and staff at Haile Plantation Animal Clinic would like to offer the most advanced medical care and services
Strongyloidiasis: who should be screened, when to suspect, how to treat?
 Strongyloidiasis: who should be screened, when to suspect, how to treat? Zeno Bisoffi, Centro Malattie Tropicali, Ospedale S.Cuore - Negrar http://www.tropicalmed.eu/ NEGLECTED DISEASES AND GLOBAL HEALTH,
Strongyloidiasis: who should be screened, when to suspect, how to treat? Zeno Bisoffi, Centro Malattie Tropicali, Ospedale S.Cuore - Negrar http://www.tropicalmed.eu/ NEGLECTED DISEASES AND GLOBAL HEALTH,
Meningeal worm (deer, brain worm) Parelaphostrongylus tenuis by Dr. Mary Smith DVM & Dr. tatiana Stanton
 Meningeal worm (deer, brain worm) Parelaphostrongylus tenuis by Dr. Mary Smith DVM & Dr. tatiana Stanton Parasite of White-tailed Deer - Nonpathogenic Small ruminants are an abnormal host (sheep, goat,
Meningeal worm (deer, brain worm) Parelaphostrongylus tenuis by Dr. Mary Smith DVM & Dr. tatiana Stanton Parasite of White-tailed Deer - Nonpathogenic Small ruminants are an abnormal host (sheep, goat,
Invasive Group A Streptococcus (GAS)
 Invasive Group A Streptococcus (GAS) Cause caused by a bacterium commonly found on the skin and in the throat transmitted by direct, indirect or droplet contact with secretions from the nose, and throat
Invasive Group A Streptococcus (GAS) Cause caused by a bacterium commonly found on the skin and in the throat transmitted by direct, indirect or droplet contact with secretions from the nose, and throat
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS VIRBAGEN OMEGA - EN 1
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS VIRBAGEN OMEGA - EN 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Virbagen Omega 5 MU for dogs Virbagen Omega 10 MU for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS VIRBAGEN OMEGA - EN 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Virbagen Omega 5 MU for dogs Virbagen Omega 10 MU for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Patient Group Direction for METRONIDAZOLE (Version 03) Valid From 1 June May 2020
 Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
SPONTANEOUS EMERGENCE OF A GNATHOSTOMA SPINIGERUM ADULT WORM FROM THE ABDOMINAL SKIN OF A LAOTIAN WOMAN: A CASE REPORT
 SPONTANEOUS EMERGENCE OF A GNATHOSTOMA SPINIGERUM ADULT WORM FROM THE ABDOMINAL SKIN OF A LAOTIAN WOMAN: A CASE REPORT Rattanaphone Phetsouvanh 1, Shigehisa Habe 2, Poul Newton 1, Manivanh Vongsouvaht
SPONTANEOUS EMERGENCE OF A GNATHOSTOMA SPINIGERUM ADULT WORM FROM THE ABDOMINAL SKIN OF A LAOTIAN WOMAN: A CASE REPORT Rattanaphone Phetsouvanh 1, Shigehisa Habe 2, Poul Newton 1, Manivanh Vongsouvaht
The first human case of Trichinella spiralis infection in Korea
 111 The Korean Journal of Parasitology Vol. 38, No. 2, 111-115, June 2000 Brief Communication The first human case of Trichinella spiralis infection in Korea Woon-Mok SOHN 1) *, Han-Mo KIM 2), Dong-Il
111 The Korean Journal of Parasitology Vol. 38, No. 2, 111-115, June 2000 Brief Communication The first human case of Trichinella spiralis infection in Korea Woon-Mok SOHN 1) *, Han-Mo KIM 2), Dong-Il
Package leaflet: Information for the patient Genticin 80mg/2ml Solution for Injection Gentamicin
 Package leaflet: Information for the patient Genticin 80mg/2ml Solution for Injection Gentamicin Read all of this leaflet carefully before you are given this medicine because it contains important information
Package leaflet: Information for the patient Genticin 80mg/2ml Solution for Injection Gentamicin Read all of this leaflet carefully before you are given this medicine because it contains important information
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT ERAQUELL 18.7 mg/g Oral Paste (AT, BE, DE, EL, FI, FR, IT, IR, LU, NL, UK) ERAQUELL vet. 18.7 mg/g Oral Paste (NO, SE) EQUIMEL
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT ERAQUELL 18.7 mg/g Oral Paste (AT, BE, DE, EL, FI, FR, IT, IR, LU, NL, UK) ERAQUELL vet. 18.7 mg/g Oral Paste (NO, SE) EQUIMEL
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Cydectin 1% w/v Injectable Solution for Sheep 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Moxidectin Excipients
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Cydectin 1% w/v Injectable Solution for Sheep 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Moxidectin Excipients
Prescription Label. Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long):
 Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
PATIENT INFORMATION LEAFLET GENTAMICIN 10MG/ML SOLUTION FOR INJECTION OR INFUSION. and GENTAMICIN 40MG/ML SOLUTION FOR INJECTION OR INFUSION
 PATIENT INFORMATION LEAFLET GENTAMICIN 10MG/ML SOLUTION FOR INJECTION OR INFUSION and GENTAMICIN 40MG/ML SOLUTION FOR INJECTION OR INFUSION Read all of this leaflet carefully before you start taking this
PATIENT INFORMATION LEAFLET GENTAMICIN 10MG/ML SOLUTION FOR INJECTION OR INFUSION and GENTAMICIN 40MG/ML SOLUTION FOR INJECTION OR INFUSION Read all of this leaflet carefully before you start taking this
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Melosolute 20 mg/ml solution for injection for cattle, pigs and horses. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Melosolute 20 mg/ml solution for injection for cattle, pigs and horses. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
MARBOCYL 10% SUMMARY OF PRODUCT CHARACTERISTICS
 MARBOCYL 10% SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL 10%, solution for injection for cattle and swine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Marbofloxacin...100.0
MARBOCYL 10% SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL 10%, solution for injection for cattle and swine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Marbofloxacin...100.0
your dog Protect UP TO $50 AND SAVE COME SEE US TO [City, ST ZIP] [Street Address] [First Name Last Name] [Phone Number] [City, ST ZIP]
![your dog Protect UP TO $50 AND SAVE COME SEE US TO [City, ST ZIP] [Street Address] [First Name Last Name] [Phone Number] [City, ST ZIP] your dog Protect UP TO $50 AND SAVE COME SEE US TO [City, ST ZIP] [Street Address] [First Name Last Name] [Phone Number] [City, ST ZIP]](/thumbs/89/100184303.jpg) [City, ST ZIP] COME SEE US TO Protect your dog [First Name Last Name] [City, ST ZIP] AND SAVE UP TO $50 [Customer Name], [Dog Name deserves/your dogs deserve] powerful parasite protection. We recommend
[City, ST ZIP] COME SEE US TO Protect your dog [First Name Last Name] [City, ST ZIP] AND SAVE UP TO $50 [Customer Name], [Dog Name deserves/your dogs deserve] powerful parasite protection. We recommend
Metacam is an anti-inflammatory medicine used in cattle, pigs, horses, dogs, cats and guinea pigs.
 EMA/CVMP/259397/2006 EMEA/V/C/000033 An overview of Metacam and why it is authorised in the EU What is Metacam and what is it used for? Metacam is an anti-inflammatory medicine used in cattle, pigs, horses,
EMA/CVMP/259397/2006 EMEA/V/C/000033 An overview of Metacam and why it is authorised in the EU What is Metacam and what is it used for? Metacam is an anti-inflammatory medicine used in cattle, pigs, horses,
(sulfadiazine and pyrimethamine) Antiprotozoal Oral Suspension. The Frustrating Challenge of EPM
 (sulfadiazine and pyrimethamine) The Frustrating Challenge of EPM T H E C H A L L E N G E S Knows no geographical limitations» EPM is widespread throughout North and South America» ~50% of horses are seropositive
(sulfadiazine and pyrimethamine) The Frustrating Challenge of EPM T H E C H A L L E N G E S Knows no geographical limitations» EPM is widespread throughout North and South America» ~50% of horses are seropositive
Incredible. xng237353_techdetailer4thtick9x12_rsg.indd 1
 Incredible. xng237353_techdetailer4thtick9x12_rsg.indd 1 xng237353_techdetailer4thtick9x12_rsg.indd 2 For dog owners who prefer to help protect their pets from fleas and ticks with an oral product that
Incredible. xng237353_techdetailer4thtick9x12_rsg.indd 1 xng237353_techdetailer4thtick9x12_rsg.indd 2 For dog owners who prefer to help protect their pets from fleas and ticks with an oral product that
HOOKWORM INFECTIONS OF SCHOOLCHILDREN IN SOUTHERN THAILAND
 SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH HOOKWORM INFECTIONS OF SCHOOLCHILDREN IN SOUTHERN THAILAND Malinee T Anantaphruti, Wanna Maipanich, Chatree Muennoo, Somchit Pubampen and Surapol Sanguankiat Department
SOUTHEAST ASIAN J TROP MED PUBLIC HEALTH HOOKWORM INFECTIONS OF SCHOOLCHILDREN IN SOUTHERN THAILAND Malinee T Anantaphruti, Wanna Maipanich, Chatree Muennoo, Somchit Pubampen and Surapol Sanguankiat Department
Oral and intestinal candidiasis. As adjuvant treatment with other local nystatin preparations to prevent reinfection.
 1. NAME OF THE MEDICINAL PRODUCT Nystatin Orifarm, 100 000 IU/ml oral suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains 100 000 IU nystatin. Excipients with known effect: - Methyl parahydroxybenzoate
1. NAME OF THE MEDICINAL PRODUCT Nystatin Orifarm, 100 000 IU/ml oral suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains 100 000 IU nystatin. Excipients with known effect: - Methyl parahydroxybenzoate
Parasites in Sheep Flocks
 Parasites in Sheep Flocks 1 WHAT IS NEW IN PARASITE CONTROL FOR SHEEP FLOCKS? Drew E. Hunnisett, DVM Honeywood and Warder Veterinary Services 132 Commerce Park Drive, Unit N Barrie, Ontario L4N 8W8 705
Parasites in Sheep Flocks 1 WHAT IS NEW IN PARASITE CONTROL FOR SHEEP FLOCKS? Drew E. Hunnisett, DVM Honeywood and Warder Veterinary Services 132 Commerce Park Drive, Unit N Barrie, Ontario L4N 8W8 705
Prescription Label. Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long):
 Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
Prescription Label Patient Name: Species: Drug Name & Strength: Directions (amount to give how often & for how long): Prescribing Veterinarian's Name & Contact Information: Refills: [Content to be provided
Albendazole for the control and elimination of lymphatic filariasis: systematic review
 Tropical Medicine and International Health volume 10 no 9 pp 818 825 september 2005 Albendazole for the control and elimination of lymphatic filariasis: systematic review Julia Critchley 1, David Addiss
Tropical Medicine and International Health volume 10 no 9 pp 818 825 september 2005 Albendazole for the control and elimination of lymphatic filariasis: systematic review Julia Critchley 1, David Addiss
SUMMARY OF PRODUCT CHARACTERISTICS. NUFLOR 300 mg/ml solution for injection for cattle and sheep
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NUFLOR 300 mg/ml solution for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NUFLOR 300 mg/ml solution for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Cydectin. Fort Dodge PRODUCT DESCRIPTION
 Cydectin Fort Dodge moxidectin Injectable Solution for Beef and Nonlactating Dairy Cattle Antiparasitic Contains 10 mg moxidectin/ml Not for use in female dairy cattle of breeding age, veal calves, and
Cydectin Fort Dodge moxidectin Injectable Solution for Beef and Nonlactating Dairy Cattle Antiparasitic Contains 10 mg moxidectin/ml Not for use in female dairy cattle of breeding age, veal calves, and
Follow this and additional works at:
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2004 A randomized clinical trial comparing single- and multi-dose combination therapy with diethylcarbamazine and
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2004 A randomized clinical trial comparing single- and multi-dose combination therapy with diethylcarbamazine and
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Rycarfa 100 mg tablets for dogs (BE, DE, ES, FR, IE, IT, NL, PT, UK) Rycarfa vet 100 mg tablets for dogs (DK, FI) Carprox
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Rycarfa 100 mg tablets for dogs (BE, DE, ES, FR, IE, IT, NL, PT, UK) Rycarfa vet 100 mg tablets for dogs (DK, FI) Carprox
Oral and intestinal candidiasis. As adjuvant treatment with other local nystatin preparations to prevent reinfection.
 1. NAME OF THE MEDICINAL PRODUCT Nystimex, 100 000 IU/ml oral suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains 100 000 IU nystatin. Excipients: Methyl parahydroxybenzoate 1 mg Sodium
1. NAME OF THE MEDICINAL PRODUCT Nystimex, 100 000 IU/ml oral suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains 100 000 IU nystatin. Excipients: Methyl parahydroxybenzoate 1 mg Sodium
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Desloratadine Actavis 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 5 mg desloratadine.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Desloratadine Actavis 5 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 5 mg desloratadine.
SATISFACTION GUARANTEED.
 Happiness is powerful flea and tick control. The vet s #1 choice for their dogs and yours. 1 SATISFACTION GUARANTEED. Along with our FRONTLINE Plus and HEARTGARD Plus (ivermectin/pyrantel) pet health products,
Happiness is powerful flea and tick control. The vet s #1 choice for their dogs and yours. 1 SATISFACTION GUARANTEED. Along with our FRONTLINE Plus and HEARTGARD Plus (ivermectin/pyrantel) pet health products,
Double-Blind, Placebo-Controlled, Randomized Study of Dipyrone as a Treatment for Pyrexia in Horses
 Double-Blind, Placebo-Controlled, Randomized Study of Dipyrone as a Treatment for Pyrexia in Horses Emily Sundman, DVM Ming Yin, PhD Tianhua Hu, PhD Melinda Poole, DVM Disclosures Sundman, Yin, Hu, and
Double-Blind, Placebo-Controlled, Randomized Study of Dipyrone as a Treatment for Pyrexia in Horses Emily Sundman, DVM Ming Yin, PhD Tianhua Hu, PhD Melinda Poole, DVM Disclosures Sundman, Yin, Hu, and
