GENERAL CONSIDERATIONS OF BLOOD BANKING AND TRANSFUSION IN DOG: A REVIEW
|
|
|
- Jasmin Nash
- 6 years ago
- Views:
Transcription
1 Scientific Works. Series C. Veterinary Medicine. Vol. LX (2) ISSN , ISSN Online , ISSN CD-ROM , ISSN-L GENERAL CONSIDERATIONS OF BLOOD BANKING AND TRANSFUSION IN DOG: A REVIEW C t lin IVA CU, Dorin OGOE, C t lin MIC A, Teodora Diana SUPEANU, Alexandru ONEA Faculty of Veterinary Medicine Bucharest, 105 Splaiul Independen ei, , District 5, Bucharest, Romania; ktathevet2018@gmail.com; sonea.alexandru@medu.edu.ro mcscatalin@yahoo.com; dtogoe@yahoo.com; supeanu.teodora@gmail.com Corresponding author: tel , ktathevet2018@gmail.com Abstract This review is a practical aspect of transfusion therapy for dogs and of the blood banking process and transfusion standards. Many of these aspects are based of the current veterinary and human standards. The first documented transfusion occurred in 1665, and was made by withdrawing blood from one dog and replaced it with blood from another dog. Since then veterinary transfusion medicine has made remarkable progress, following close to our human contra parts. Whole blood refers to blood that has not been separated. Blood products are composed from blood components and these are prepared either by centrifugation or by apheresis. The use of blood components allows several patients to benefit from one donation and reduces the risk of transfusion reactions to unnecessary components. Both whole blood and blood components may be used shortly after the collection or after storage, Blood banking allowing the user access to both blood and blood components immediately. This procedures may be feasible to obtain and process blood on demand. However for emergency clinics with a large requiring caseload of transfusion therapy, blood banking is essential. Key words: transfusion, blood, blood banking, donor, blood group. INTRODUCTION Transfusion is defined as an intravenous therapy with blood products or whole blood. Blood banking allows immediate access to whole blood and blood components in any cases (Abrams-Ogg, 2000; Gibson, 2007) The blood banking procedures that must be followed refer to the blood donors, the dog blood groups, the blood typing, crossmatching anticoagulant ( preservative solution ), the blood donation procedure, collection systems, the blood product preparation and storage, transfusion of dog whole blood and blood components. (Abrams-Ogg, 2000; Gibson, 2007). 38 Blood donors The source of blood donors may be obtained from clinic owned donors (depending on the anticipated needs), from a donor program (client owned pets that donate for benefits ), animal controlled facilities ( animal shelters or pounds that may allow stray dogs to donate ), terminal donors ( animal that are being euthanized for behavior problems or medical disorders that do not affect the quality of the donated blood ). (Gibson, 2007; Abrams-Ogg, 2000; Wardrop, et al., 2005; Slichter, et al., 1986) An ideal blood donor should be clinically normal, large breed with normal weight (25 28kg), friendly and has easily accessible veins
2 and a universal blood type, has to be current on the vaccination status, free of parasites and infectious disease (depending on the geographical location) and do not suffer of other disorders (immune mediated, cancer, systemic disorders, organ failure) and did not receive any drug therapy or had previous transfusions. (Gibson, 2007; Palmer, et al., 2014; Abrams-Ogg, 2000; Palmer, et al., 2014; Chervier, et al., 2012; Horgan, et al., 2009; Hackner, 2015) Blood groups The definition of blood groups are made by the inherited antigens of the RBC surface. They are of crucial importance in the transfusion medicine because of the risk of hemolytic reactions that occur when there is an antibody directed against a blood group antigen, depending on the complement activation by IgM and IgG. (Abrams-Ogg, 2000; Slichter, et al., 1986; Lynel, et al., 2009). The blood groups are classified in the DEA ( Dog Erythrocyte Antigen ) and there are sex antigens ( DEA 1.1, 1.2, 3, 4, 5, 7) defined by the current standardization antisera and a new antigen Dal, but 20 or more specificities have been described. DEA`s have not been extensively characterized for composition and structure. (Corato, et al., 1997; Abrams-Ogg, 2000; Gibson, 2007; Blais, et al., 2007). Blood typing The compatibility of donor recipient should be considered when selected a donor. To prevent DEA incompatibility is to blood type the donors using antisera. The antisera is produced by allow-immunizing a dog negative for a given DEA. Blood typing is not performed at a regular basis in veterinary medicine because of the prices and is performed on a small number of donors. The universal blood donor is negative to DEA 1.1, 1.2, 3, 5 and 7 and the minimal requirement to prevent a moderate or a severe DEA hemolytic reaction needs a minimum for the donor to be negative for DEA 1.1. (Lynel, et al., 2009; Abrams-Ogg, 2000; Gibson, 2007). Cross-matching The cross-matching procedure test for anti- RBC antibodies through hemolysis and agglutination. Cross-matching is an adjunct to blood typing and is not a substitute, but probably is the only incompatibility test available. (Lynel, et al., 2009; Abrams-Ogg, There are numerous cross-matching procedures, but two of these methods are adapted into the general practice, the rapid slide method and the tube method. Both of this methods function on the agglutination and hemolysis process. (Beth, 2013; Abrams- Ogg, 2000; Gibson, 2007). Blood donation system The estimated blood volume that can be donated safely it is between 15% and 20%, the maximum donation it is about ml/kg. Determine for a standard donation in the dog is 450ml referred as a canine unit of blood. Dogs can donate every month as long as they receive a good nutrition and iron supplementation in the diet. In case of the client own dogs the usual donation period it is every two months and does not require a nutritional supplementation. (Gibson, 2007) (McMichael, 2015) (Abrams- In the recent studies, guidelines have not been established of how frequently a dog could donate plasma or pellets if the RBC are returned to the donor. The standard suggestion is that we should use the whole blood donation guidelines. (McMichael, 2015) (Abrams- Collection system The collection system refers to the standard human blood collection packs and they are the most suitable collection packs, using a closed system that has no potential for environmental contact with the blood as it flows from the 39
3 vein to the container. The same collection packs are also available for preparation of blood components, these bags having satellite bags for the extraction of the erythroconcentrate. In an emergency, if the regular systems are not available they can be collected using a 60ml syringes collected to an infusion kit. (Gibson, 2007) (Abrams- The blood collection procedure is made with a venipuncture of the jugular vein, cephalic vein (large breed dogs) or the femoral artery also can be used, but technically the femoral artery puncture is the most difficult and it has an increased chance of hematoma formation and scarring of the vessels. (Abrams-Ogg, The blood collection procedure has a minimum requirement of four people: a phlebotomist, two restrainers and another person to handle the collecting bag. (Abrams- After care of the donor, refers to the observation of the dog for 15 to 30 minutes for weakness, pale mucous membranes, weak pulse or other signs of hypotension. In case of hypotension we can replace the volume with saline or a similar crystalloid solution after the donation. It is most necessary to apply moderate pressure over the vein puncture sight for about five minutes and to apply a neck bandage. (Gibson, 2007) (Abrams-Ogg, Blood product preparation and storage Whole blood packed RBC, fresh frozen plasma and frozen plasma are the most important blood products. Collected blood can be given for transfusion as fresh whole blood, stored or transferred into various components to be used fresh or refrigerated. (Abrams- The latest veterinary and human blood banking practices requires that only the blood collected in the closed system should be used for storage or blood product preparation and this should be standardized to minimize the risk of microbial growth. (Abrams-Ogg, 2000) (Gibson, 2007). The open collection system should be only used for immediate transfusions or emergency transfusion procedures. The requirements of blood product preparation after the collection of the blood refers that the pack should be held at room temperature while awaiting the separation into the components. (Mollison, 2000); (Abrams-Ogg, 2000); (Gibson, 2007). Every blood product should be labeled with the type of product, the donor, the blood type if it is known and the collection and expiration dates. (Gibson, 2007); (Abrams-Ogg, RBC products are represented by: whole blood and packed RBC. Whole blood is the most common used blood product and should be kept under refrigeration at 1 to 4 degrees Celsius and should be kept on a dedicated refrigerator. (Abrams- The principal indication of whole blood, fresh or stored is for acute hemorrhage and for replacement of RBC plasma in case if volume deficit appears. The whole blood volume that should be transferred it is estimated on ongoing future losses. The usual dosage that should be administrated is between 10 and 22 ml/kg and the volume should not be modified or exceed unless the outgoing losses are severe, in this case we should consider using massive transfusion. (Jutkowitz, 2015) (Gibson, 2007). Packed RBC. There are two principals to obtain packed RBC from fresh whole blood: centrifugation or sedimentation. Centrifugation procedure requires a 5000G`s for 5 minutes at 4 degrees Celsius. On slower centrifuges 2000G`s for 10 minutes and the plasma should be removed with a plasma extractor and kept on a designed refrigerator for a maximum of 30 days. The sedimentation process for whole blood it`s obtained by vertically suspension of the blood pack in a refrigerator for a minimum of 12 hours, procedure that lasts from 3 days to 2 weeks. To speed up the process we should 40
4 consider the addition of a synthetic colloid that would maximize the RBC and plasma separation. (Gibson, 2007). The RBC transfusion is indicated in cases of anemia without hypovolemia or deficits in other blood components, but has the benefits of not overloading the circulatory system. (Abrams- Plasma products are obtained the separation of RBC from the whole blood product. This could be processed into various products. (Abrams- Fresh plasma and fresh frozen plasma. The fresh frozen plasma is the plasma that has been separated and placed at -18 degrees Celsius in maximum 8 hours from the collection. Keep in mind the time restrictions, this product should be normally prepared by centrifugation. (Hackner, 2015); (Abrams- The transfusion of fresh frozen plasma has an interval between 10 to 30 ml/kg and it should be supplemented if a hemorrhage or a coagulation deficit persists. Frozen plasma. Liquid plasma should be stored under 1 to 6 degrees Celsius refrigeration and the maximum storage time is approximately 6 weeks. (Abrams-Ogg, 2000) (Gibson, 2007). Transfusion of whole blood and blood products The whole blood and blood components that had been refrigerated should be warmed before administration. The requirement is to warm the products gradually to room temperature during the administration, but to proceed with extreme caution because the excessive warming may decrease the RBC viability and increase the risk microbial growth. (Abrams- The whole blood should be mixed by a gentile inversion before the transfusion. The canine packed RBC`s that had been stored in CPDA1 has a PCV of approximately 70-80% and a very high viscosity that makes the product difficult to transfuse, even allows the formation of RBC clumps. To reduce this problem we should add 100ml of NaCl at 37 degrees Celsius, to facilitate the transfusion process. (Abrams-Ogg, 2000) (Gibson, 2007). Frozen plasma should be reheated in an incubator or a water bath at degrees Celsius for approximately 30 minutes per unit of canine plasma. Frozen plasma products may be more rapidly defrosted even in a microwave oven (Hurst et al. 1987); (Abrams- Transfusion rates The initial transfusion rate of whole blood and its components should be 0.25ml/kg/hour for the first 30 minutes and then should be transfused at a rate of 5 to 10 ml/kg/hour. For the cardiac patient it should not exceed 0.3 to 3 ml/kg/hour. (Gibson, 2007). The maximum rate of transfusion should not exceed 22 ml/kg/hour used in emergency situations except the situations where massive transfusion is required, at a maximum of 90 ml/kg/24hours. (Jutkowitz, 2015); (Gibson, 2007). It is recommended that during the higher transfusion rates that the patient should be connected to a monitor and it should be monitored during the transfusion. In the presence of the increased risk of volume overload the transfusion rates must be slowed down. To minimize the bacteria proliferation in the RBC and plasma products the veterinary and human transfusion standards recommend that every transfusion should be completed in a maximum of 4 hours. (Abrams-Ogg, 2000); (Jutkowitz, 2015); (Abrams- Delivery The delivery of whole blood and its components in most veterinary clinics is by gravity and it is recommended that we use infusion pumps for an accurate dosage. Alternatively the blood product may be gently 41
5 drawn of the bag in 60ml syringe and then slowly administrated. (Abrams- Record keeping of each transfusion should be kept for each transfusion and also it would have to contain general information from the blood product recipient, the date of the transfusion and transfusion reaction if they appear. A highly visible notation should be added to the medical record to show that the patient received the transfusion. (Abrams- Ogg, 2000); (Gibson, 2007). REFERENCES Abrams-Ogg, Anthony BSAVA Manual of Canine and Feline Haematology and Transfusion Medicine. Hampshire : British Small Animal Veterinary Association, Beth, Davidow Transfusion Medicine in Small Animals. Veterinary Clinics of North America: Small Animal Practice. July 2013, pp Blais, MC, et al Canine Dal blood type: A red cell antigen lacking in some Dalmatians. Journal of Veterinary Internal Medicine. 2007, pp Chervier, C, et al Causes of anaemia other than acute blood loss and their clinical significance in dogs. Journal of Small Animal Practice. 2012, pp Corato, A., et al Biochemical characterization of canine blood group antigens: immunoprecipitation of DEA 1.2, 4 and 7 and identification of a dog erythrocyte membrane antigen homologous to human Rhesus. Immunology and Immunopathology. s.l. : Elsevier, Gibson, Gillian BSAVA Manual of Canine and Feline Emergency and Critical Care. Hampshire : British Samll Animal Veterinary Association, Vol. II. Hackner, G. Susan Bleeding Disorders. Small Animal Critical Care Medicine. s.l. : Elsevier, 2015, pp Horgan, JE, Roberts, BK and Schermerhorn, T Splenectomy as an adjunctive treatment for dogs with immune-mediated hemolytic anemia. Journal of Veterinary Emergency and Critical Care. 2009, pp Jutkowitz, L. Ari Massive Transfusion. Small Animal Critical Care Medicine. s.l. : Elsevier, 2015, pp Lynel, J. Tocci and Patty, J. Ewing Increasing Patient Safety in veterinary Transfusion Medicine: an Overview of Pretransfusion Testing. Journal of Veterinary Emergency and Critical Care. 2009, pp McMichael, Maureen Prevention and Treatment of Transfusion reactions. Small Animal Critical Care Medicine. s.l. : Elsevier, 2015, pp Mollison, PL The introduction of citrate as an anticoagulant for transfusion and of glucose as red cell preservative. British Journal of Haematology. 2000, pp Palmer, Lee and Martin, Linda Traumatic Coagulopathy - Part 2: Resuscitative Stategies. Journal of Veterinary Emergency and Critical Care. 2014, pp Slichter, SJ., et al Canine platelet alloimmunization: The role of donor selection. British Journal of Haematology. 1986, pp Wardrop, K. Jane, et al Canine and Feline Blo od Donor Screening for Infectious Disease. Journal of Veterinary Internal Medicine. 2005, pp
Feline blood transfusions: preliminary considerations
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Feline blood transfusions: preliminary considerations Author : Andrea Harvey Categories : RVNs Date : September 1, 2011 ABSTRACT
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Feline blood transfusions: preliminary considerations Author : Andrea Harvey Categories : RVNs Date : September 1, 2011 ABSTRACT
Feline Blood Groups & Blood Transfusion
 Feline Blood Groups & Blood Transfusion Supervisor: Dr. Neshat Compiler : Sina Taefehshokr Veterinary Medicine Faculty Islamic Azad University Tabriz Branch, Iran Blood Types (Overview) Only 1 blood groups
Feline Blood Groups & Blood Transfusion Supervisor: Dr. Neshat Compiler : Sina Taefehshokr Veterinary Medicine Faculty Islamic Azad University Tabriz Branch, Iran Blood Types (Overview) Only 1 blood groups
Proceedings of the World Small Animal Veterinary Association Mexico City, Mexico 2005
 Close this window to return to IVIS Proceedings of the World Small Animal Veterinary Association Mexico City, Mexico 2005 Hosted by: Reprinted in the IVIS website with the permission of the WSAVA Laboratory
Close this window to return to IVIS Proceedings of the World Small Animal Veterinary Association Mexico City, Mexico 2005 Hosted by: Reprinted in the IVIS website with the permission of the WSAVA Laboratory
FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT
![FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT](/thumbs/93/113164093.jpg) FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 22 APR 2018 Biogal Galed Laboratories Acs Ltd. tel: 972-4-9898605. fax: 972-4-9898690 e-mail:info@biogal.co.il
FELINE CORONAVIRUS (FCoV) [FIP] ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 22 APR 2018 Biogal Galed Laboratories Acs Ltd. tel: 972-4-9898605. fax: 972-4-9898690 e-mail:info@biogal.co.il
Australian and New Zealand College of Veterinary Scientists. Membership Examination. Veterinary Anaesthesia and Critical Care Paper 1
 Australian and New Zealand College of Veterinary Scientists Membership Examination June 2015 Veterinary Anaesthesia and Critical Care Paper 1 Perusal time: Fifteen (15) minutes Time allowed: Two (2) hours
Australian and New Zealand College of Veterinary Scientists Membership Examination June 2015 Veterinary Anaesthesia and Critical Care Paper 1 Perusal time: Fifteen (15) minutes Time allowed: Two (2) hours
SOP: Blood Collection in Swine
 SOP: Blood Collection in Swine These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol
SOP: Blood Collection in Swine These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol
Feline Heartworm Antibody Test Kit. In vitro diagnostic test for the detection of antibodies to Dirofilaria immitis in feline serum or plasma.
 Feline Heartworm Antibody Test Kit In vitro diagnostic test for the detection of antibodies to Dirofilaria immitis in feline serum or plasma. Heartworm in Cats Feline heartworm infection (Dirofilaria immitis)
Feline Heartworm Antibody Test Kit In vitro diagnostic test for the detection of antibodies to Dirofilaria immitis in feline serum or plasma. Heartworm in Cats Feline heartworm infection (Dirofilaria immitis)
Xenotransfusion with canine blood in the feline species: review of the literature
 0530JFM15210.1177/1098612X12460530Journal of Feline Medicine and SurgeryBovens and Gruffydd-Jones Review Xeno with canine blood in the feline species: review of the literature Journal of Feline Medicine
0530JFM15210.1177/1098612X12460530Journal of Feline Medicine and SurgeryBovens and Gruffydd-Jones Review Xeno with canine blood in the feline species: review of the literature Journal of Feline Medicine
B. PACKAGE LEAFLET 1
 B. PACKAGE LEAFLET 1 PACKAGE LEAFLET FOR: Cadorex 300 mg/ml solution for injection for cattle, sheep and pigs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION
B. PACKAGE LEAFLET 1 PACKAGE LEAFLET FOR: Cadorex 300 mg/ml solution for injection for cattle, sheep and pigs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION
Fluid Therapy and Heat Injuries in Multi Purpose Canines (MPC) PFN: SOMVML0R. Terminal Learning Objective. References. Hours: Instructor:
 Fluid Therapy and Heat Injuries in Multi Purpose Canines (MPC) PFN: SOMVML0R Hours: Instructor: Slide 1 Terminal Learning Objective Action: Communicate knowledge of fluid therapy and heat injuries in Multi
Fluid Therapy and Heat Injuries in Multi Purpose Canines (MPC) PFN: SOMVML0R Hours: Instructor: Slide 1 Terminal Learning Objective Action: Communicate knowledge of fluid therapy and heat injuries in Multi
SOP: Blood Collection in the Horse
 SOP: Blood Collection in the Horse These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
SOP: Blood Collection in the Horse These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER
 Canine VacciCheck INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER IgG ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 13 JUL 2015 Biogal Galed Laboratories Acs. Ltd., tel: 972-4-9898605.
Canine VacciCheck INFECTIOUS HEPATITIS, PARVOVIRUS & DISTEMPER IgG ANTIBODY TEST KIT INSTRUCTION MANUAL Sufficient for 12/120 assays 13 JUL 2015 Biogal Galed Laboratories Acs. Ltd., tel: 972-4-9898605.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Acecare 2mg/ml Solution for Injection for Dogs and Cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution contains
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Acecare 2mg/ml Solution for Injection for Dogs and Cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution contains
Administering wormers (anthelmintics) effectively
 COWS www.cattleparasites.org.uk Administering wormers (anthelmintics) effectively COWS is an industry initiative promoting sustainable control strategies for parasites in cattle Wormer administration Dec
COWS www.cattleparasites.org.uk Administering wormers (anthelmintics) effectively COWS is an industry initiative promoting sustainable control strategies for parasites in cattle Wormer administration Dec
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, BG, CY, CZ, DE, EE, EL, ES, FR, HR, HU, IE, IT, LT, LU, NL, PT, RO, SK, UK: Kelaprofen 100 mg/ml, solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, BG, CY, CZ, DE, EE, EL, ES, FR, HR, HU, IE, IT, LT, LU, NL, PT, RO, SK, UK: Kelaprofen 100 mg/ml, solution for injection
PACKAGE LEAFLET: INFORMATION FOR THE USER. Amikacin 250 mg/ml Injection
 PACKAGE LEAFLET: INFORMATION FOR THE USER Amikacin 250 mg/ml Injection Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again. If you
PACKAGE LEAFLET: INFORMATION FOR THE USER Amikacin 250 mg/ml Injection Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again. If you
Syringe and aggregate filter administration does not affect survival of transfused autologous fresh feline red blood cells
 Syringe and aggregate filter administration does not affect survival of transfused autologous fresh feline red blood cells Heikes, B. W., & Ruaux, C. G. (2014). Effect of syringe and aggregate filter administration
Syringe and aggregate filter administration does not affect survival of transfused autologous fresh feline red blood cells Heikes, B. W., & Ruaux, C. G. (2014). Effect of syringe and aggregate filter administration
Australian and New Zealand College of Veterinary Scientists. Membership Examination. Veterinary Emergency and Critical Care Paper 1
 Australian and New Zealand College of Veterinary Scientists Membership Examination June 2014 Veterinary Emergency and Critical Care Paper 1 Perusal time: Fifteen (15) minutes Time allowed: Two (2) hours
Australian and New Zealand College of Veterinary Scientists Membership Examination June 2014 Veterinary Emergency and Critical Care Paper 1 Perusal time: Fifteen (15) minutes Time allowed: Two (2) hours
SUMMARY OF PRODUCT CHARACTERISTICS. NUFLOR 300 mg/ml solution for injection for cattle and sheep
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NUFLOR 300 mg/ml solution for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NUFLOR 300 mg/ml solution for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Blood Collection Healthcare
 Blood Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms
Blood Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
EC-AH-011v1 January 2018 Page 1 of 5. Standard Operating Procedure Equine Center Clemson University
 EC-AH-011v1 January 2018 Page 1 of 5 Standard Operating Procedure Equine Center Clemson University SOP ID: EC-AH-011v1 January 2018 Title: Injection Techniques Author(s): Julia Tagher, CU Equine Center
EC-AH-011v1 January 2018 Page 1 of 5 Standard Operating Procedure Equine Center Clemson University SOP ID: EC-AH-011v1 January 2018 Title: Injection Techniques Author(s): Julia Tagher, CU Equine Center
Australian and New Zealand College of Veterinary Scientists. Fellowship Examination. Veterinary Anaesthesia and Critical Care Paper 1
 Australian and New Zealand College of Veterinary Scientists Fellowship Examination June 2016 Veterinary Anaesthesia and Critical Care Paper 1 Perusal time: Twenty (20) minutes Time allowed: Three (3) hours
Australian and New Zealand College of Veterinary Scientists Fellowship Examination June 2016 Veterinary Anaesthesia and Critical Care Paper 1 Perusal time: Twenty (20) minutes Time allowed: Three (3) hours
Withdrawal period: 93 days Milk: Not authorised for use in animals producing milk for human consumption.
 A. LABELLING PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE CARTON BOX AND LABELS OF 100 ml and 250 ml 1. NAME OF THE VETERINARY MEDICINAL PRODUCT TILKOMAY 300 mg/ml + 90 mg/ml solution
A. LABELLING PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE CARTON BOX AND LABELS OF 100 ml and 250 ml 1. NAME OF THE VETERINARY MEDICINAL PRODUCT TILKOMAY 300 mg/ml + 90 mg/ml solution
Welcome! 10/26/2015 1
 Welcome! Audio for this event is available via ReadyTalk Internet Streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Welcome! Audio for this event is available via ReadyTalk Internet Streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Effect of syringe and aggregate filter administration on survival of transfused autologous fresh feline red blood cells
 Original Study Journal of Veterinary Emergency and Critical Care 24(2) 2014, pp 162 167 doi: 10.1111/vec.12115 Effect of syringe and aggregate filter administration on survival of transfused autologous
Original Study Journal of Veterinary Emergency and Critical Care 24(2) 2014, pp 162 167 doi: 10.1111/vec.12115 Effect of syringe and aggregate filter administration on survival of transfused autologous
EXOTIC SMALL MAMMAL ANESTHETIC TECHNIQUES
 EXOTIC SMALL MAMMAL ANESTHETIC TECHNIQUES Jody Nugent-Deal, RVT, VTS (Anesthesia) and (Clinical Practice Exotic Companion Animal) Veterinary Medical Teaching Hospital University of California, Davis, CA
EXOTIC SMALL MAMMAL ANESTHETIC TECHNIQUES Jody Nugent-Deal, RVT, VTS (Anesthesia) and (Clinical Practice Exotic Companion Animal) Veterinary Medical Teaching Hospital University of California, Davis, CA
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
Heartworm Disease in Dogs
 Customer Name, Street Address, City, State, Zip code Phone number, Alt. phone number, Fax number, e-mail address, web site Heartworm Disease in Dogs Basics OVERVIEW Disease caused by infestation with heartworms
Customer Name, Street Address, City, State, Zip code Phone number, Alt. phone number, Fax number, e-mail address, web site Heartworm Disease in Dogs Basics OVERVIEW Disease caused by infestation with heartworms
Understanding your pet s LIVER CONDITION
 Understanding your pet s LIVER CONDITION Why is the liver so important? What causes liver disease in dogs and cats? The liver is one of the largest organs in your pet s body, and it s vital for their good
Understanding your pet s LIVER CONDITION Why is the liver so important? What causes liver disease in dogs and cats? The liver is one of the largest organs in your pet s body, and it s vital for their good
VIZSLA EPILEPSY RESEARCH PROJECT General Information
 General Information INTRODUCTION In March 1999, the AKC Canine Health Foundation awarded a grant to researchers at the University of Minnesota College of Veterinary Medicine to study the molecular genetics
General Information INTRODUCTION In March 1999, the AKC Canine Health Foundation awarded a grant to researchers at the University of Minnesota College of Veterinary Medicine to study the molecular genetics
Copper-Storage Liver Disease Basics
 Copper-Storage Liver Disease Basics OVERVIEW Abnormal accumulation of copper in the liver, causing sudden (acute) inflammation of the liver (hepatitis) or long-term (chronic) hepatitis and eventually progressive
Copper-Storage Liver Disease Basics OVERVIEW Abnormal accumulation of copper in the liver, causing sudden (acute) inflammation of the liver (hepatitis) or long-term (chronic) hepatitis and eventually progressive
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Revised: January 2012 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Blackleg Vaccine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance(s): per ml Five strains
SUMMARY OF PRODUCT CHARACTERISTICS Revised: January 2012 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Blackleg Vaccine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance(s): per ml Five strains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYTOPOINT 10 mg solution for injection for dogs CYTOPOINT 20 mg solution for injection for dogs CYTOPOINT 30 mg
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CYTOPOINT 10 mg solution for injection for dogs CYTOPOINT 20 mg solution for injection for dogs CYTOPOINT 30 mg
Irish Medicines Board
 IRISH MEDICINES BOARD ACT 1995, as amended European Communities (Animal Remedies) (No. 2) Regulations 2007 VPA: 10999/033/001A Case No: 7006569 The in exercise of the powers conferred on it by Animal Remedies
IRISH MEDICINES BOARD ACT 1995, as amended European Communities (Animal Remedies) (No. 2) Regulations 2007 VPA: 10999/033/001A Case No: 7006569 The in exercise of the powers conferred on it by Animal Remedies
Summary of product characteristics As per Annex C. SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1
 Summary of product characteristics As per Annex C SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1 1. NAME OF THE MEDICINAL PRODUCT. ANNEXURE C to MODULE I Tetanus vaccine (Adsorbed) I.P. 2.
Summary of product characteristics As per Annex C SUMMARY OF PRODUCT CHARACTERISTICS Doc. No. SPC/71108 Ver.1 1. NAME OF THE MEDICINAL PRODUCT. ANNEXURE C to MODULE I Tetanus vaccine (Adsorbed) I.P. 2.
Senior Pet Care and Early Disease Detection
 Senior Pet Care and Early Disease Detection Thanks to advances in veterinary medicine, pets are living longer than ever before. However, with this increased lifespan comes an increase in the types of ailments
Senior Pet Care and Early Disease Detection Thanks to advances in veterinary medicine, pets are living longer than ever before. However, with this increased lifespan comes an increase in the types of ailments
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Marbocare 20 mg/ml solution for injection for cattle and pigs (UK, IE, FR) Odimar 20 mg/ml solution for injection for cattle
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Marbocare 20 mg/ml solution for injection for cattle and pigs (UK, IE, FR) Odimar 20 mg/ml solution for injection for cattle
IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee. Blood Collection Guidelines
 IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee Blood Collection Guidelines Purpose To provide Iowa State University (ISU) Institutional Animal Care and Use Committee (IACUC) guidelines
IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee Blood Collection Guidelines Purpose To provide Iowa State University (ISU) Institutional Animal Care and Use Committee (IACUC) guidelines
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 10 Suspension for injection for sheep and cattle 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Potency
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 10 Suspension for injection for sheep and cattle 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances Potency
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Kelacyl 100 mg/ml, solution for injection for cattle and pigs (BG, CY, CZ, DE, EL, FR, HU, IE, IT, LT, PL, PT, RO, SK, UK)
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Kelacyl 100 mg/ml, solution for injection for cattle and pigs (BG, CY, CZ, DE, EL, FR, HU, IE, IT, LT, PL, PT, RO, SK, UK)
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT COASTAL ALABAMA COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: New application Amendment
Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220
 Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220 Introduction Enzootic Bovine Leukosis is a transmissible disease caused by the Enzootic Bovine Leukosis Virus (BLV)
Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220 Introduction Enzootic Bovine Leukosis is a transmissible disease caused by the Enzootic Bovine Leukosis Virus (BLV)
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Issued March 2017 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats Recicort vet 1.77 mg/ml + 17.7 mg/ml
SUMMARY OF PRODUCT CHARACTERISTICS Issued March 2017 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats Recicort vet 1.77 mg/ml + 17.7 mg/ml
Pathogenesis of E. canis
 Tick-born disease Rhipicephalus sanguineus brown dog tick Rickettsia Ehrlichia canis Ehrlichia platys Anaplasma platys Pathogenesis of E. canis Incubation period: 8 20 days Mononuclear cells Liver, spleen,
Tick-born disease Rhipicephalus sanguineus brown dog tick Rickettsia Ehrlichia canis Ehrlichia platys Anaplasma platys Pathogenesis of E. canis Incubation period: 8 20 days Mononuclear cells Liver, spleen,
Metacam 1.5 mg/ml oral suspension for dogs
 Metacam 1.5 mg/ml oral suspension for dogs Species:Dogs Therapeutic indication:pharmaceuticals: Neurological preparations: Analgesics, Other NSAIDs, Locomotor (including navicular and osteoarthritis) Active
Metacam 1.5 mg/ml oral suspension for dogs Species:Dogs Therapeutic indication:pharmaceuticals: Neurological preparations: Analgesics, Other NSAIDs, Locomotor (including navicular and osteoarthritis) Active
Blood Type Synchronization Using Dipstick Membrane in Domestic Cats (Felis domestica) on the Persian Cat (Felis silvestris) in Surabaya
 J. ppl. Environ. Biol. Sci., 8(4)-6, 018 018, TextRoad Publication ISSN: 090-474 Journal of pplied Environmental and Biological Sciences www.textroad.com Blood Type Synchronization Using Dipstick Membrane
J. ppl. Environ. Biol. Sci., 8(4)-6, 018 018, TextRoad Publication ISSN: 090-474 Journal of pplied Environmental and Biological Sciences www.textroad.com Blood Type Synchronization Using Dipstick Membrane
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, CZ, EE, ES, FR, IE, IS, IT, LT, LU, LV, NO, PL, PT, RO, SE, SI, SK, UK: Genestran 75 micrograms/ml solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT AT, BE, CZ, EE, ES, FR, IE, IS, IT, LT, LU, LV, NO, PL, PT, RO, SE, SI, SK, UK: Genestran 75 micrograms/ml solution for injection
Rapid Diagnostic Test for pet
 In vitro Diagnostic Rapid Diagnostic Test for pet Canine / Feline Rapid Test offers highly sensitive and specificity for the detection of antigen and antibody from various kinds of easily obtainable specimen.
In vitro Diagnostic Rapid Diagnostic Test for pet Canine / Feline Rapid Test offers highly sensitive and specificity for the detection of antigen and antibody from various kinds of easily obtainable specimen.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Medicinal product no longer authorised
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Zubrin 50 mg oral lyophilisates for dogs Zubrin 100 mg oral lyophilisates for dogs Zubrin 200 mg oral lyophilisates
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Zubrin 50 mg oral lyophilisates for dogs Zubrin 100 mg oral lyophilisates for dogs Zubrin 200 mg oral lyophilisates
Acute Hemorrhagic Diarrhea Syndrome (AHDS) A Cause of Bloody Feces in Dogs
 Acute Hemorrhagic Diarrhea Syndrome (AHDS) A Cause of Bloody Feces in Dogs No dog parent wants to clean up diarrhea. Cleaning up bloody diarrhea is even more unpleasant. Unfortunately, the development
Acute Hemorrhagic Diarrhea Syndrome (AHDS) A Cause of Bloody Feces in Dogs No dog parent wants to clean up diarrhea. Cleaning up bloody diarrhea is even more unpleasant. Unfortunately, the development
AVIAN & EXOTIC NURSING Darlene H. Geekie, RVT
 AVIAN & EXOTIC NURSING Darlene H. Geekie, RVT EXOTICS Objectives Client communication Review of restraint technique and challenges Review of phlebotomy techniques and basic nursing care Client Communication
AVIAN & EXOTIC NURSING Darlene H. Geekie, RVT EXOTICS Objectives Client communication Review of restraint technique and challenges Review of phlebotomy techniques and basic nursing care Client Communication
Illustrated Articles Northwestern Veterinary Hospital
 Page 1 of 5 First Aid in Cats Medical emergencies occur suddenly and without warning. It is important for all cat owners to have a basic understanding of common veterinary medical emergencies and basic
Page 1 of 5 First Aid in Cats Medical emergencies occur suddenly and without warning. It is important for all cat owners to have a basic understanding of common veterinary medical emergencies and basic
RVC OPEN ACCESS REPOSITORY COPYRIGHT NOTICE
 RVC OPEN ACCESS REPOSITORY COPYRIGHT NOTICE This author s accepted manuscript may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. The full details
RVC OPEN ACCESS REPOSITORY COPYRIGHT NOTICE This author s accepted manuscript may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. The full details
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BLUEVAC BTV8 suspension for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT BLUEVAC BTV8 suspension for injection for cattle and sheep 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of
= 0.5 mg. In vitro toxin neutralisation test based on haemolysis of sheep erythrocytes. For a full list of excipients, see section 6.1.
 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 8 Suspension for injection for sheep and cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances: Potency value/quantity/ml C. perfringens
1 NAME OF THE VETERINARY MEDICINAL PRODUCT Covexin 8 Suspension for injection for sheep and cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active substances: Potency value/quantity/ml C. perfringens
EXCEDE Sterile Suspension
 VIAL LABEL MAIN PANEL PRESCRIPTION ANIMAL REMEDY KEEP OUT OF REACH OF CHILDREN READ SAFETY DIRECTIONS FOR ANIMAL TREATMENT ONLY EXCEDE Sterile Suspension 200 mg/ml CEFTIOFUR as Ceftiofur Crystalline Free
VIAL LABEL MAIN PANEL PRESCRIPTION ANIMAL REMEDY KEEP OUT OF REACH OF CHILDREN READ SAFETY DIRECTIONS FOR ANIMAL TREATMENT ONLY EXCEDE Sterile Suspension 200 mg/ml CEFTIOFUR as Ceftiofur Crystalline Free
Proceedings of the Southern European Veterinary Conference - SEVC -
 Close this window to return to IVIS www.ivis.org Proceedings of the Southern European Veterinary Conference - SEVC - Sep. 30-Oct. 3, 2010, Barcelona, Spain Next SEVC Conference: Sep. 30-Oct. 2, 2011 -
Close this window to return to IVIS www.ivis.org Proceedings of the Southern European Veterinary Conference - SEVC - Sep. 30-Oct. 3, 2010, Barcelona, Spain Next SEVC Conference: Sep. 30-Oct. 2, 2011 -
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Pro Penstrep Suspension for Injection for Cattle, Sheep and Pigs. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Pro Penstrep Suspension for Injection for Cattle, Sheep and Pigs. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
MARBOCYL 10% SUMMARY OF PRODUCT CHARACTERISTICS
 MARBOCYL 10% SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL 10%, solution for injection for cattle and swine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Marbofloxacin...100.0
MARBOCYL 10% SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL 10%, solution for injection for cattle and swine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Marbofloxacin...100.0
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Oncept IL-2 lyophilisate and solvent for suspension for injection for cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Oncept IL-2 lyophilisate and solvent for suspension for injection for cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CLYNAV solution for injection for Atlantic salmon 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 0.05 ml dose
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CLYNAV solution for injection for Atlantic salmon 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 0.05 ml dose
SUMMARY OF PRODUCT CHARACTERISTICS. Animeloxan 1.5 mg/ml oral suspension for dogs. Active substance: Meloxicam 1.5 mg (equivalent to 0.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Animeloxan 1.5 mg/ml oral suspension for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of suspension contains:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Animeloxan 1.5 mg/ml oral suspension for dogs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of suspension contains:
BreenLab - Molecular Cytogenetic Investigation of Soft Tissue Sarcoma General information and sample submission requirements
 PARTICIPANTS NEEDED FOR RESEARCH ON CANINE CANCER THE STUDY The research project Cellular Genomics- A molecular cytogenetics investigation of canine soft tissue sarcoma is part of Dr. Matthew Breen s laboratory
PARTICIPANTS NEEDED FOR RESEARCH ON CANINE CANCER THE STUDY The research project Cellular Genomics- A molecular cytogenetics investigation of canine soft tissue sarcoma is part of Dr. Matthew Breen s laboratory
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Rifen 100 mg/ml solution for injection for horses, cattle and swine. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Rifen 100 mg/ml solution for injection for horses, cattle and swine. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml contains:
Dosing Your Cat with Azithromycin Pediatric Suspension. By Lorraine Shelton
 Dosing Your Cat with Azithromycin Pediatric Suspension By Lorraine Shelton To join a community of cat fanciers and health professionals interested in cattery related health issues, visit http://groups.yahoo.com/group/fanciershealth
Dosing Your Cat with Azithromycin Pediatric Suspension By Lorraine Shelton To join a community of cat fanciers and health professionals interested in cattery related health issues, visit http://groups.yahoo.com/group/fanciershealth
CAUTION KEEP OUT OF REACH OF CHILDREN READ SAFETY DIRECTIONS FOR ANIMAL TREATMENT ONLY FRONTLINE
 037 002768 - A 0008 DIRECTIONS FOR USE: READ THE DETAILED DIRECTIONS FOR USE BEFORE USING FRONTLINE. Dose rate: 100 ml Pack (0.5 ml/pump - 200 pumps per pack) Fleas: 3-6 ml/kg = 6-12 spray pumps/kg Flea
037 002768 - A 0008 DIRECTIONS FOR USE: READ THE DETAILED DIRECTIONS FOR USE BEFORE USING FRONTLINE. Dose rate: 100 ml Pack (0.5 ml/pump - 200 pumps per pack) Fleas: 3-6 ml/kg = 6-12 spray pumps/kg Flea
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS Revised: December 2013 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Flunixin 50 mg/ml Solution for Injection for Cattle, Horses and Pigs (United Kingdom, Germany, Iceland)
SUMMARY OF PRODUCT CHARACTERISTICS Revised: December 2013 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Flunixin 50 mg/ml Solution for Injection for Cattle, Horses and Pigs (United Kingdom, Germany, Iceland)
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Melosolute 20 mg/ml solution for injection for cattle, pigs and horses. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Melosolute 20 mg/ml solution for injection for cattle, pigs and horses. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One ml contains:
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site:
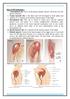 Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
loopfull is removed from each dilution and transferred to capable of killing the test organism in 10 minutes but not GERMICIDAL SUBSTANCES
 A NEW METHOD FOR THE EVALUATION OF GERMICIDAL SUBSTANCES A. J. SALLE, W. A. McOMIE AND I. L. SHECHMEISTER Department of Bacteriology, University of California, Berkeley, California Received for publication
A NEW METHOD FOR THE EVALUATION OF GERMICIDAL SUBSTANCES A. J. SALLE, W. A. McOMIE AND I. L. SHECHMEISTER Department of Bacteriology, University of California, Berkeley, California Received for publication
DEPOSEL Slow Release Selenium Injection for Cattle and Sheep
 Date of change: 21 October 2004 Page: 1 of 9 Carton (front panel). POISON KEEP OUT OF REACH OF CHILDREN FOR ANIMAL TREATMENT ONLY DEPOSEL Slow Release Selenium Injection for Cattle and Sheep Active ingredient:
Date of change: 21 October 2004 Page: 1 of 9 Carton (front panel). POISON KEEP OUT OF REACH OF CHILDREN FOR ANIMAL TREATMENT ONLY DEPOSEL Slow Release Selenium Injection for Cattle and Sheep Active ingredient:
SUMMARY OF PRODUCT CHARACTERISTICS. Bottle of powder: Active substance: ceftiofur sodium mg equivalent to ceftiofur...
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT WONDERCEF powder and solvent for solution for injection for horses not intended for the production of foods for human consumption.
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT WONDERCEF powder and solvent for solution for injection for horses not intended for the production of foods for human consumption.
BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT. Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin
 BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin 2. STATEMENT OF ACTIVE AND OTHER SUBSTANCES Each ml contains: Tilmicosin 300 mg;
BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin 2. STATEMENT OF ACTIVE AND OTHER SUBSTANCES Each ml contains: Tilmicosin 300 mg;
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Selectan 300 mg/ml solution for injection for cattle and swine. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Selectan 300 mg/ml solution for injection for cattle and swine. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
BEYOND MENDEL. Incomplete Dominance: Blue (BB) Red (RR) F 1 hybrids have appearance in between 2 parents Purple (BR)
 AP BIOLOGY EVOLUTION/HEREDITY UNIT Unit 1 Part 4 Chapter 14 Activity #5 NAME DATE PERIOD BEYOND MENDEL INCOMPLETE DOMINANCE Incomplete Dominance: Blue (BB) Red (RR) F 1 hybrids have appearance in between
AP BIOLOGY EVOLUTION/HEREDITY UNIT Unit 1 Part 4 Chapter 14 Activity #5 NAME DATE PERIOD BEYOND MENDEL INCOMPLETE DOMINANCE Incomplete Dominance: Blue (BB) Red (RR) F 1 hybrids have appearance in between
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
 BOEHRINGER INGELHEIM VETMEDICA, INC. USA Product Label http://www.vetdepot.com 2621 NORTH BELT HIGHWAY, ST. JOSEPH, MO, 64506 2002 Telephone: 800 325 9167 Fax: 816 236 2717 Email: www.bi vetmedica.com
BOEHRINGER INGELHEIM VETMEDICA, INC. USA Product Label http://www.vetdepot.com 2621 NORTH BELT HIGHWAY, ST. JOSEPH, MO, 64506 2002 Telephone: 800 325 9167 Fax: 816 236 2717 Email: www.bi vetmedica.com
What is Codominance?
 What is Codominance? Codominance Occurs when both alleles for a gene are expressed in a heterozygous individual. Roan Cattle Codominance There are two alleles for coat color in cattle. Yet, there are three
What is Codominance? Codominance Occurs when both alleles for a gene are expressed in a heterozygous individual. Roan Cattle Codominance There are two alleles for coat color in cattle. Yet, there are three
St George/Sutherland Hospitals And Health Services (SGSHHS)
 VASCATH INSTILLATION OF ANTICOAGULATION / ANTIBIOTIC LOCK Cross references (including NSW Health/ SESIAHS policy directives) NSW Health Policy for Medication Handling in NSW Public Hospitals PD207_007
VASCATH INSTILLATION OF ANTICOAGULATION / ANTIBIOTIC LOCK Cross references (including NSW Health/ SESIAHS policy directives) NSW Health Policy for Medication Handling in NSW Public Hospitals PD207_007
VETERINARY SCIENCE CURRICULUM. Unit 1: Safety and Sanitation
 Chariho Regional School District - Science Curriculum September, 2016 VETERINARY SCIENCE CURRICULUM Unit 1: Safety and Sanitation Students will gain an understanding of the types of hazards common in veterinary
Chariho Regional School District - Science Curriculum September, 2016 VETERINARY SCIENCE CURRICULUM Unit 1: Safety and Sanitation Students will gain an understanding of the types of hazards common in veterinary
ASVCP quality assurance guidelines: veterinary immunocytochemistry (ICC)
 ASVCP quality assurance guidelines: veterinary immunocytochemistry (ICC) Version 1.0 (Approved 11/2017) Developed by the American Society for Veterinary Clinical Pathology (ASVCP) Quality Assurance and
ASVCP quality assurance guidelines: veterinary immunocytochemistry (ICC) Version 1.0 (Approved 11/2017) Developed by the American Society for Veterinary Clinical Pathology (ASVCP) Quality Assurance and
RESULT OF STUDYING SOME ACUTE PHASE PROTEINS AND CORTISOL IN PREGNANT EWES
 Ulaankhuu.A and et al. (16) Mongolian Journal of Agricultural Sciences ¹19 (3): 27-31 27 RESULT OF STUDYING SOME ACUTE PHASE PROTEINS AND CORTISOL IN PREGNANT EWES A.Ulaankhuu 1*, G.Lkhamjav 2, Yoshio
Ulaankhuu.A and et al. (16) Mongolian Journal of Agricultural Sciences ¹19 (3): 27-31 27 RESULT OF STUDYING SOME ACUTE PHASE PROTEINS AND CORTISOL IN PREGNANT EWES A.Ulaankhuu 1*, G.Lkhamjav 2, Yoshio
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pentofel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Per dose of 1ml: Active components Inactivated Feline Panleukopenia
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pentofel 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Per dose of 1ml: Active components Inactivated Feline Panleukopenia
Top Gun Phlebotomy. Pseudohyperkalemia 9/10/2018. HOT TOPIC / Phlebotomy Top Gun, Pseudohyperkalemia HOT TOPIC / 2018
 Top Gun Phlebotomy Pseudohyperkalemia HOT TOPIC / 2018 Presenter: Brad Karon, M.D., Ph.D. Professor Laboratory Medicine and Pathology Department of Laboratory Medicine and Pathology, Mayo Clinic 1 Disclosure
Top Gun Phlebotomy Pseudohyperkalemia HOT TOPIC / 2018 Presenter: Brad Karon, M.D., Ph.D. Professor Laboratory Medicine and Pathology Department of Laboratory Medicine and Pathology, Mayo Clinic 1 Disclosure
SUMMARY OF PRODUCT CHARACTERISTICS. Pentoject, Pentobarbitone Sodium 200 mg/ml Solution for Injection
 SUMMARY OF PRODUCT CHARACTERISTICS Revised: June 2018 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pentoject, Pentobarbitone Sodium 200 mg/ml Solution for Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS Revised: June 2018 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Pentoject, Pentobarbitone Sodium 200 mg/ml Solution for Injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
PCR detection of Leptospira in. stray cat and
 PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
PCR detection of Leptospira in 1 Department of Pathology, School of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, Shahrekord, Iran 2 Department of Microbiology, School of Veterinary
FELINE BEHAVIOR CONSULTATION QUESTIONNAIRE
 Name: Address: FELINE BEHAVIOR CONSULTATION QUESTIONNAIRE GENERAL INFORMATION Date of consultation: Postal (zip) code: Email: Phone: Home: ( ) Business: ( ) Fax: ( ) Veterinarian/clinic: Clinic address:
Name: Address: FELINE BEHAVIOR CONSULTATION QUESTIONNAIRE GENERAL INFORMATION Date of consultation: Postal (zip) code: Email: Phone: Home: ( ) Business: ( ) Fax: ( ) Veterinarian/clinic: Clinic address:
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NEFOTEK 100 mg/ml solution for injection for cattle, horses and pigs [AT, CZ, IE, PL, SK, UK, DE, FR, ES, HU, IT, SI] COXOFEN
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NEFOTEK 100 mg/ml solution for injection for cattle, horses and pigs [AT, CZ, IE, PL, SK, UK, DE, FR, ES, HU, IT, SI] COXOFEN
Some important information about the fetus and the newborn puppy
 Some important information about the fetus and the newborn puppy Dr. Harmon Rogers Veterinary Teaching Hospital Washington State University Here are a few interesting medical details about fetuses and
Some important information about the fetus and the newborn puppy Dr. Harmon Rogers Veterinary Teaching Hospital Washington State University Here are a few interesting medical details about fetuses and
Blood Cells of Reptiles. Blood Cells of Reptiles. Blood Cells of Reptiles. Blood Cells of Reptiles. Blood Cells of Reptiles
 INTRODUCTION TO REPTILE HEMATOLOGY & CYTOLOGY DVM. PhD Dec 14 2014 Leukocytes Thrombocytes Similar diagnostic principles as Mammals. Similar in function as Avian. Much more unknowns and variables in Reptiles.
INTRODUCTION TO REPTILE HEMATOLOGY & CYTOLOGY DVM. PhD Dec 14 2014 Leukocytes Thrombocytes Similar diagnostic principles as Mammals. Similar in function as Avian. Much more unknowns and variables in Reptiles.
Error! Reference source not found. I. SUMMARY OF PRODUCT CHARACTERISTICS
 PRODUCTNAME NOBIVAC RABIES 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Nobivac Rabies 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active components: Rabies strain Pasteur RIV; at least 2 I.U. per dose
PRODUCTNAME NOBIVAC RABIES 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Nobivac Rabies 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active components: Rabies strain Pasteur RIV; at least 2 I.U. per dose
A Simply Smart Choice for Point-of-Care Testing
 A Simply Smart Choice for Point-of-Care Testing The entire WITNESS line of canine and feline diagnostics tests are accurate, affordable, and easy to use WITNESS HEARTWORM WITNESS LH WITNESS RELAXIN Canine
A Simply Smart Choice for Point-of-Care Testing The entire WITNESS line of canine and feline diagnostics tests are accurate, affordable, and easy to use WITNESS HEARTWORM WITNESS LH WITNESS RELAXIN Canine
A long-acting, broad spectrum, injectable antibiotic for the treatment and control of
 APPROVED PACKAGE INSERT FOR BIVATOP 200 LA FOR ANIMAL USE ONLY BIVATOP 200 LA Reg. no.: G4115 (Act 36/1947) Namibia: V13/17.1.2/1224 (Act 13/2003) A long-acting, broad spectrum, injectable antibiotic for
APPROVED PACKAGE INSERT FOR BIVATOP 200 LA FOR ANIMAL USE ONLY BIVATOP 200 LA Reg. no.: G4115 (Act 36/1947) Namibia: V13/17.1.2/1224 (Act 13/2003) A long-acting, broad spectrum, injectable antibiotic for
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Maprelin 75 µg/ml solution for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Maprelin 75 µg/ml solution for injection for pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution for injection
Ubroseal Dry Cow 2.6 g intramammary suspension for cattle
 Health Products Regulatory Authority 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Ubroseal Dry Cow 2.6 g intramammary suspension for cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 4g intramammary
Health Products Regulatory Authority 1 NAME OF THE VETERINARY MEDICINAL PRODUCT Ubroseal Dry Cow 2.6 g intramammary suspension for cattle 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each 4g intramammary
SUMMARY OF PRODUCT CHARACTERISTICS. Florgane 300 mg/ml Suspension for Injection for Cattle and Pigs
 SUMMARY OF PRODUCT CHARACTERISTICS Revised November 2015 1. NAME OF THE VETERINARY MEDICINAL PRODUCT: Florgane 300 mg/ml Suspension for Injection for Cattle and Pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION:
SUMMARY OF PRODUCT CHARACTERISTICS Revised November 2015 1. NAME OF THE VETERINARY MEDICINAL PRODUCT: Florgane 300 mg/ml Suspension for Injection for Cattle and Pigs 2. QUALITATIVE AND QUANTITATIVE COMPOSITION:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT COXEVAC suspension for injection for cattle and goats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT COXEVAC suspension for injection for cattle and goats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains:
Hemorrhage Control in the Prehospital Arena
 Hemorrhage Control in the Prehospital Arena Eric M. Rudnick, MD, FACEP, FAAEM, FAEMS Medical Director Nor-Cal EMS Agency 1 Thank You Committee on Tactical Combat Casualty Care Loyola University Mark Belden
Hemorrhage Control in the Prehospital Arena Eric M. Rudnick, MD, FACEP, FAAEM, FAEMS Medical Director Nor-Cal EMS Agency 1 Thank You Committee on Tactical Combat Casualty Care Loyola University Mark Belden
