Origin of West Indian Populations of the Geographically Widespread Boa Corallus enydris Inferred from Mitochondrial DNA Sequences
|
|
|
- Ferdinand Merritt
- 5 years ago
- Views:
Transcription
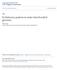
1 MOLECULAR PHYLOCENETICS AND EVOLUTION Vol. 4. No.1. March. pp Origin of West Indian Populations of the Geographically Widespread Boa Corallus enydris Inferred from Mitochondrial DNA Sequences ROBERT w. HENDERSON AND S. BLAIR HEDGES* Section of Vertebrate Zoology, Milwaukee Public Museum, 800 West Wells Street, Milwaukee, Wisconsin ; and Department of Biology, 208 Mueller Lab, Penn State Unil/ersity. University Park, Pennsylvania Received June ; revised Sept~'mber Corallus enydris (Serpentes: Boidae: Boinae) is an arboreal snake with an extremely wide mainland distribution from southern Costa Rica to southeastern Brazil arid is one of two boine species that has invaded the Lesser Antilles (Grenada Bank and St. Vincent). Mitochondrial DNA sequences of samples from seven geographically disparate localities provided evidence of phylogenetic relationships. The monophyly of C. enydris is corroborated and a major dichotomy between northern samples (Panama and Trinidad) and southern samples (Guyana, Peru, southeastern Brazil) was found and corresponds to the two currently recognized subspecies. Unexpectedly, the two samples from the West Indies (southern Lesser Antilles) cluster with the southern rather than the geographically closer northern samples (e.g., Trinidad). The results imply a fairly recent Guianan-Amazonian origin of West Indian populations. tl 1986 Academic Pre... lac. INTRODUCTION The neotropical mainland boine snake (Serpentes: Boidae: Boinae) fauna, currently comprised of 10 species, includes five species with widely sympatric ranges in Amazonia and the Guianas: Boa constrictor, Corall us caninus, Corollus enydris, Epicrotes cenchria, and Eunectes murinus. The ranges of B. constrictor, C. enydris, and E. cenchria are even broader, extending from Mexico and/or southern Central America to southeastern Brazil and/or northern Argentina; only B. constrictor and C. enydris have invaded the West Indies. Only C. caninus is regarded as monotypic, whereas B. constrictor and E. cenchria have 9 or 10 currently recognized subspecies. They are poorly defined geographically, taxonomically, and systematically, although recent advances have been made (e.g., Tolson, 1987; Kluge, 1991) or are in progress (R.W.H., in preparation). C. enydris, an arboreal species ranging from southern Costa Rica to southern Brazil, has two currently recognized subspecies (Fig. 1): C. e. enydris which occurs throughout most of the Amazonian lowlands, the Guianas, and the Atlantic coastal forest of Brazil and C. e. cooki which occurs in southern Central America, northern Colombia, northern Venezuela (including Isla Margarita), Trinidad and Tobago, and the Lesser Antilles as far north as St. Vincent. C. enydris is more variable in color and pattern than any other boine (e.g., Figs. 1-7 in Henderson, 1993). In this paper we present data regarding phylogenetic relationships in C. enydris based on mitochondrial DNA sequences of sample~ from seven geographically disparate localities and evidence for the origin of the West Indian populations. MATERIALS AND METHODS DNA was extracted from small amounts «50 mg) of liver or red blood cells from the following specimens of C. enydris: MPM (Milwaukee Public Museum) 26290, Panama, Canal Zone; MPM 23596, St. Vincent, St. Patrick, 2.0 miles ENE of Layou; MPM 25445, Grenada, St. Andrew, Pearls; MPM 23595, Trinidad, Hollis Reservoir; LSUMZ (Louisiana State University Museum of Zoology) 43139, Guyana, Jonestown; KU <University of Kansas) , Peru, Cuzco, Cuzco Amazonica; and IB (lnstituto Butantan) 55042, Brazil, Siio Paulo, 19uape (Fig. 1). Considering the broad range of Corollus enydris, we acknowledge the relatively small number of samples in our analysis. Those we have, however, are geographically strategic (Fig. 1), being near (1) the northern edge of the mainland range (Panama), (2) the southern end of the mainland range (Atlantic coastal forest in southeastern Brazil), and (3) the western edge in the Upper Amazon of Peru and from (4) two West Indian islands, including one from the northernmost edge of the range (St. Vincent) and (5) Trinidad. The Trinidad sample (C. e. cooki) is critical because of its geographic proximity to the Lesser Antilles and because of its morphological similarity to Venezuelan populations occurring between /95 $6.00 Copyright It 1995 by Academic Press. Inc. All rights of reproduction in any fonn reserved. 88
2 ORIGIN OF ANTILLEAN POPULATIONS OF THE BOA Corallus enydris 89 o KM 1'1 1,1 I I I I FIG. 1. Geographic distribution of the boid snake Corallus enydris, showing the locations of samples used in this study. The more northern, darker stippling indicates the range of C. e. cooki, and the more southern, lighter stippling indicates the range of C. e. enydris. The numbered localities indicate sites represented in our sample: (1) Panama, (2) St. Vincent, (31 Grenada, (4) Trinidad, (5) Guyana, 161 southern Peru, and (7) southeastern Brazil. See Materials and Methods for more precise locality data. the Caribbean coast and the Rio Orinoco (Henderson and Boos, 1993). Despite long distances between Guianan-Amazonian sample localities on the South American mainland (Guyana-Brazil, ca km; Guyana Peru, ca km) and the potential for wide rivers (e.g., Rio Amazonas) to act as barriers to dispersal, it is likely that the Guianan-Amazonian range (including Atlantic coastal forest in Brasil) of C. enydris is contiguous (Fig. 1; Henderson, 1993). An individual of C. caninus (Audubon Zoo 5902, locality unknown) was included for comparison, and the boid Epicrates striatus (S.B.H. Laboratory No , Dominican Republic, Samana, 7 km S. of Las Galeras) was included for the purpose of rooting the tree. Methods of DNA extraction, amplification, and dideoxy sequencing are described elsewhere (Hedges et al., 1991; Hedges and Bezy, 1993). Two oligonucleotide primers were used to amplify and sequence both complementary strands ofa 307-bp region of the mitochondrial cytochrome b gene (Hedges et az., 1992). In some cases, two other primers designed to amplify the same region (Kocher et al., 1989) also were used. Aerosolresistant tips were used in preparation of reagents in order to reduce the probability of contamination. There were no insertions or deletions and therefore alignment was straightforward. Phylogenetic analysis (distance and parsimony) was performed with MEGA (Kumar et az., 1993). The Jukes-Cantor (1969) distance was used with the neighbor-joining method (Saitou and Nei, 1987), and statistical confidence of the nodes on the trees was inferred by a t test for branch-length significance from zero, expressed as the complement of the probability, or confidence probability (CP; Rzhetsky and Nei, 1992; Kumar et az., 1993), and by the bootstrap method (Felsenstein, 1985) using 2000 replications (Hedges, 1992). Statistical significance was assessed at the 95% level. Maximum parsimony analysis was done with the modified branch and bound algorithm in MEGA. Sites containing ambiguities were not included in the analyses. RESULTS A total of 271 aligned sites (nine taxa) could be scored from autoradiograms and were used in the phylogenetic analyses (Fig. 2). Of these, 74 were variable and 28 were parsimony sites (those sites informative under the conditions of parsimony). Pairwise corrected distances among the specimens of C. enydris were low «0.08), supporting the use of a Jukes-Cantor distance rather than a more complicated correction (Nei, 1991).
3 90 HENDERSON AND HEDGES 1 E.strfetua 2 cenf,.. 3 p.".. 4 Trinided 5 Guyene 6 Bruil 8 Grenldli 1 E.strfetua 2 cenl,.. 3 Penema 4 Trinided 5 Guyena 6 Irull 8 Grenada 1 E.strletus 2 cenlnus 3P_ 6 Brezil 8 Grenade 9 St.Vincent 1 E.str!etus 2 caninus 3 P IINIIIIIl 6 Brazil a Grenade 46 C TTC GGA TCC ATA CTA CTT GCT TGe TTA TCT CTA CAA CTA CTT ACA T r T C A.C T.C. G.C G A G G.. A..... A... A.. A... A.,...C A.C C. G.C T.G G G A.e C. G.e T... G A C A.C CG G.C G G C A.C CG G.C G G C A.C CG G.C G....G C A.C CG G.C G.G G... C A.C CG G.C G... G 91 GGA TTC TTC TTA GeT GTA CAC TAC ACA GCA MT ATT MC CTA GCA C T C C T..C C G T C.. r C....C.. C G.. T... G C C....C.. C... T... G C '" C....C.. C G.. T.... C C....C.. C G.. r.. C C....C.. C G.. T.... C C....C.. C G.. r.... C C....C C G.. T TTC TCA TCT ATC ATC CAC ATT ACC CGA GAT GTC CCA TAT GGA TGA.. C G.. G... T.T...C.. C G.T G...TA.. C.. C... G.T G...TA... C.. C G.T G...TA... C.. C G.T G...TA... C.. C... G.T G...TA... C.. C G.T G...TA... C.. C '" G.T G...TA... C.. C '" 181 ATA ATA CAA MC CTT CAC GeT ArT GGG GCC TCA GTA rta TTT ATT A C A A C C C.. T.. C.. C.. A... A... C.. C.. C.. T.. C.. C.. G... A... C.. C.. C T....T C T C A A A C C C.. T.. A.. T.. C.. A... A... C.. C.. C 1 E.strietus 2 caninus 3 PaNIIIIII 6 Brazil 8 Grenada 9 St.Vincent 1 E.strietus 2 canlnus 3 p.".,.. 4 Trinic:\act 6 Brez! l 8 Grenade 226 TGT ATT TAT ATC CAT ATC GCA CGA GGC TTG TAT TAT GGG TCG TAC.. C.. C.. C... C.A.. C.. A T.. C.. C T... C... C.. C.. C.. C..... C.. C C....C.. C.. C.. C..... C.. T C T C.A.. C.. C.. T.. T.. C.. T C C.A.. C.. C.. T.. r.. C.. C.. T.. C C... C.. C.. T T.. C.. C.. T.. C T C.A.. C.. C.. T.. T.. C.. C.. T.. C... C.A.. C.. C.. T.. T 271 TTA MC AM GAA ACC TGA CTC TCA GGT ACC ACA TTA CTA ATC ATA C....A... C... T. T... C....A... G.. A... T... T... C....A A A C....A A A T r c....a..a A T.. T C....G.. A... A.. A... C.. T... T C....G.. A... A.. A... T... T C....G.. A... A.. A... T... T FIG. 2. DNA sequences of a portion of the mitochondrial cytochrome b gene in nine boid snakes: (1) Epicrates striatu8, (2) Corallus caninus, and (3-9) geographic representatives of the species C. enydris.
4 ORIGIN OF ANTILLEAN POPULATIONS OF THE BOA Corallus enydris 91 r GreNlda L St. Vincent 98/97 - Peru Panama Trinidad ' _- Corallu5 caninus \ Epicrates striatus d o.bs FIG. 3. Phylogenetic relationships of Corallus enydris from seven widely distributed localities, inferred from a neighbor'joining analysis of DNA sequences (Jukes-Cantor distance) of a portion of the mitochondrial cytochrome b gene. The numbers on the tree are statistical estimates of confidence of each node: the "confidence probability" (Rzhetsky and Nei. 1992; Kumar et oz. 1993) derived from the standard error estimate of the branch length (left of slash) and the bootstrap P value (Felsenstein, 1985) based on 2000 replications (right of slash). The West Indian boine species Epicrates striatus was used to root the tree. d, distance. A phylogenetic tree (Fig. 3) corroborates the monophyly of C. enydris (CP = 98%) and shows a major dichotomy between samples from Panama and Trinidad (79%) and those from other areas (98%). With two exceptions, this split corresponds to a morphological subdivision within the species recognized at the subspecific level. The Panama and Trinidad samples are assigned to the subspecies C. e. cooki, whereas the other samples are placed in C. e. enydris <Henderson, 1993). The exceptions are the two samples from the Lesser Antilles (St. Vincent and Grenada). Although these West Indian populations have been assigned to the subspecies C. e. cooki. they cluster strongly (98%) with the samples of C. e. enydris. The two Lesser Antillean samples cluster with a mainland population (Guyana) farther to the south rather than the geographically proximal Trinidad. The maximum parsimony analysis resulted in six equally most-parsimonius trees of length 41 (Fig. 4). All showed the same major dichotomy within C. enydris as found in the neighbor-joining analysis. DISCUSSION The most unexpected result of the DNA sequence analysis was the implication of a fairly recent Guianan-Amazonian origin of West Indian populations of C. enydris as indicated by the very close genetic relationship between those samples and Guyana. Although it is possible that this result is due to allelic relationships rather than population relationships, the latter explanation is better supported when the biogeography of the region is considered. The strong South American influence in the Grenada Bank-St. Vincent fauna is Guyana Brlzi I Peru Pa... r Trinidad Coral Ius cani"". Epicrates striatus FIG. 4. Phylogenetic relationships of Corallus enydris inferred from a maximum parsimony analysis (branch-and-bound). The tree is a strict consensus of six most-parsimonious trees, each of length 41. well-documented, but Trinidad or northern Venezuela (e.g., the Orinoco Delta), possibly via Trinidad, has been assumed to be the origin (e.g., Lescure, 1987; Lescure et al., 1991). Trinidad had a direct land connection to South America during the late Pleistocene low sea level stand (Eshelman and Morgan, 1985) and is now separated from Venezuela by about 10.5 km of water. There is no geological evidence to indicate that the Grenada Bank ever had a continental connection (Maury et al. 1990) and C. enydris almost surely reached the West Indies by overwater dispersal from South America on the South Equatorial Current. Although the Grenada Bank is situated closer to Venezuela and Trinidad (ca. 145 km) than to the Guyana coast (ca. 650 km), there is evidence for the potential overwater dispersal to the southern Lesser Antilles (the Grenada Bank and St. Vincent) from northeastern Brazil and the Guianas (e.g., the mouth of the Essequibo River in Guyana) (Guppy, 1917). The reptile fauna of the Grenada Bank and St. Vincent has a number of geographically wide-ranging species in common with Trinidad, northern Venezula, and the Guianas, but little is known regarding their relationships. In light of these preliminary results, the relationships of the West Indian and mainland populations and the current taxonomy and systematics of C. enydris must be reevaluated. ACKNOWLEDGMENTS We thank H. E. A. BOO8 (Trinidad), W. E. Duellman and J. E. Simmons [University or Kansas (KU)J. G. Puorto [lnstituto Butantan (IB)]. A. S. Rand and L. Weight (Smithsonian Tropical Research Institute), and D. Reynolds and D. Rossman [Louisiana State University Museum of Zoology ILSUMZ>] for tissue samples of Corollus and F. de Barros Molina and A. M. Young for assisting in the delivery of the samples. The West Indian samples were collected by R.W.H. with the kind permission ofthe forestry departments of8t. Vincent and Grenada. The laboratory portion of this research was supported by a grant from the National Science Foundation <DEB ) to S.B.H. Analysis of morphological variables by R.W.H. entailed many loans from many institutions; all will be acknowledged accordingly in a future publication.
5 92 HENDERSON AND HEDGES REFERENCES Eshelman, R. E., and Morgan, G. S. (1985). Tobagan recent mammals, fossil vertebrates, and their zoogeographical implications. Natl. Geog. Soc. Res. Rep. 21: Felsenstein, J. (1985). Confidence limits on phylogeneis: An approach using the bootstrap. Euolution 39: Guppy, H. B. (1917). "Plants, Seeds, and Currents in the West Indies and Az.ores," Williams and Norgate, London. Hedges, S. B. (1992). The number of replications needed for accurate estimation of the bootstrap p-value in phylogenetic studies. Mol. Biol. Euol. 9(2): Hedges, S. B., and Bezy, R. L. (1993). Phylogeny ofxantusiid lizards: Concern for data and analysis. Mol. Phylogenet. Evol. 2: Hedges, S. B., Bezy, R. L., and Maxson, L. R. (1991). Phylogenetic relationships and biogeography of xantusiid lizards inferred from mitochondrial DNA sequences. Mol. Biol. Evol. 8: Hedges, S. B., Bogart, J. P., and Maxson, L. R. (1992). Ancestry of unisexual salamanders (genus Ambystoma). Nature 356: Henderson, R. W. (1993). Corallus enydris. Cat. Am. Amphibians Reptiles 576: 1-6. Henderson, R. W., and Boos, H. E. A. (1993). "The Tree Boa (Corallus enydris) on Trinidad and Tobago," Living World, J. Trinidad & Tobago Field Naturalists Club, pp Jukes, T. H., and Cantor, C. R. (1969). Evolution of protein molecules. In "Mammalian Protein Metabolism" (H. N. Munroe, Ed.) pp , Academic Press, New York. Kluge, A. G. (1991). "Boine Snake Phylogeny and Research Cycles," Misc. Publ. Mus. Zoo!. Univ. Michigan (178): iv + 58 pp. Kocher, T. D., Thomas, W. K., Meyer, A., Edwards, S. V., Paabo, S., Villablanca, F. X., and Wilson, A. C. U989). Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 86: Kumar, S., Tamura, K., and Nei, M. (1993). MEGA, molecular evolutionary genetic analysis software for microcomputers. CABIOS 10: Lescure, J. (1987). Le peuplement en Reptiles et Amphibiens des Petites Antilles. Bull. Soc. Zool. France 112: Lescure, J., Jeremie, J., Lourenc;o, W., Mauries, J.-P., Pierre, J., Bastre, C., and Thibaud, J. M. (1991). Biogeographie et insularite l'exerr..ple des Petites Antilles. C. R. Soc. Biogeogr. 87: Maury, R. C., Westbrook, G. K., Baker, P. E., Bouysse, Ph., and Westercamp, D. (1990). Geology of the Lesser Antilles. In "The Caribbean Region" (G. Dengo and J. E. Case, Eels.), Vol. H., pp , Geo!. Soc. America, Geol. of North America, Boulder, CO. Nei, M. (1991). Relative efficiencies of different tree-making methods for molecular data. In "Phylogenetic Analysis orona Sequences" (M. M. Miyamoto and J. Cracraft, Eds.), pp , Oxford Univ. Press, Oxford. Rzhetsky, A., and Nei, M. (1992). A simple method for estimating and testing minimum-evolution trees. Mol. Biol. Euol. 4: Saitou, N., and Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Bi()/. Evol. 4: Tolson, P. J. (1987). "Phylogenetics of the Boid Snake Genus Epicrates and Caribbean Vicariance Theory." Occ. Pap. Mus. Zoo!. Univ. Michigan (715): 1-68.
Supplemental Information. Discovery of Reactive Microbiota-Derived. Metabolites that Inhibit Host Proteases
 Cell, Volume 168 Supplemental Information Discovery of Reactive Microbiota-Derived Metabolites that Inhibit Host Proteases Chun-Jun Guo, Fang-Yuan Chang, Thomas P. Wyche, Keriann M. Backus, Timothy M.
Cell, Volume 168 Supplemental Information Discovery of Reactive Microbiota-Derived Metabolites that Inhibit Host Proteases Chun-Jun Guo, Fang-Yuan Chang, Thomas P. Wyche, Keriann M. Backus, Timothy M.
Development and characterization of 79 nuclear markers amplifying in viviparous and oviparous clades of the European common lizard
 https://doi.org/10.1007/s10709-017-0002-y SHORT COMMUNICATION Development and characterization of 79 nuclear markers amplifying in viviparous and oviparous clades of the European common lizard J. L. Horreo
https://doi.org/10.1007/s10709-017-0002-y SHORT COMMUNICATION Development and characterization of 79 nuclear markers amplifying in viviparous and oviparous clades of the European common lizard J. L. Horreo
A Taxonomic Review of the Corallus hortulanus Complex of Neotropical Tree Boas
 Caribbean Journal of Science, Vol. 33, No. 3 4, 198 221, 1997 Copyright 1997 College of Arts and Sciences University of Puerto Rico, Mayagüez A Taxonomic Review of the Corallus hortulanus Complex of Neotropical
Caribbean Journal of Science, Vol. 33, No. 3 4, 198 221, 1997 Copyright 1997 College of Arts and Sciences University of Puerto Rico, Mayagüez A Taxonomic Review of the Corallus hortulanus Complex of Neotropical
Molecular Phylogeny and Biogeography of West Indian Teiid Lizards of the Genus Ameiva
 Caribbean Journal of Science, Vol. 39, No. 3, 298-306, 2003 Copyright 2003 College of Arts and Sciences University of Puerto Rico, Mayagüez Molecular Phylogeny and Biogeography of West Indian Teiid Lizards
Caribbean Journal of Science, Vol. 39, No. 3, 298-306, 2003 Copyright 2003 College of Arts and Sciences University of Puerto Rico, Mayagüez Molecular Phylogeny and Biogeography of West Indian Teiid Lizards
Lecture 11 Wednesday, September 19, 2012
 Lecture 11 Wednesday, September 19, 2012 Phylogenetic tree (phylogeny) Darwin and classification: In the Origin, Darwin said that descent from a common ancestral species could explain why the Linnaean
Lecture 11 Wednesday, September 19, 2012 Phylogenetic tree (phylogeny) Darwin and classification: In the Origin, Darwin said that descent from a common ancestral species could explain why the Linnaean
Molecular study on Salmonella serovars isolated from poultry
 Molecular study on Salmonella serovars isolated from poultry presented by Enas Fathy mohamed Abdallah Under The Supervision of Prof. Dr. Mohamed Refai Professor of Microbiology Faculty of Veterinary Medicine,
Molecular study on Salmonella serovars isolated from poultry presented by Enas Fathy mohamed Abdallah Under The Supervision of Prof. Dr. Mohamed Refai Professor of Microbiology Faculty of Veterinary Medicine,
CLADISTICS Student Packet SUMMARY Phylogeny Phylogenetic trees/cladograms
 CLADISTICS Student Packet SUMMARY PHYLOGENETIC TREES AND CLADOGRAMS ARE MODELS OF EVOLUTIONARY HISTORY THAT CAN BE TESTED Phylogeny is the history of descent of organisms from their common ancestor. Phylogenetic
CLADISTICS Student Packet SUMMARY PHYLOGENETIC TREES AND CLADOGRAMS ARE MODELS OF EVOLUTIONARY HISTORY THAT CAN BE TESTED Phylogeny is the history of descent of organisms from their common ancestor. Phylogenetic
Modern Evolutionary Classification. Lesson Overview. Lesson Overview Modern Evolutionary Classification
 Lesson Overview 18.2 Modern Evolutionary Classification THINK ABOUT IT Darwin s ideas about a tree of life suggested a new way to classify organisms not just based on similarities and differences, but
Lesson Overview 18.2 Modern Evolutionary Classification THINK ABOUT IT Darwin s ideas about a tree of life suggested a new way to classify organisms not just based on similarities and differences, but
Phylogeographic assessment of Acanthodactylus boskianus (Reptilia: Lacertidae) based on phylogenetic analysis of mitochondrial DNA.
 Zoology Department Phylogeographic assessment of Acanthodactylus boskianus (Reptilia: Lacertidae) based on phylogenetic analysis of mitochondrial DNA By HAGAR IBRAHIM HOSNI BAYOUMI A thesis submitted in
Zoology Department Phylogeographic assessment of Acanthodactylus boskianus (Reptilia: Lacertidae) based on phylogenetic analysis of mitochondrial DNA By HAGAR IBRAHIM HOSNI BAYOUMI A thesis submitted in
Phylogeny of Xantusiid Lizards: Concern for Data and Analysis
 MOLECULAR PHYLOGENETICS AND EVOLUTION Vol. 2, No.1, March, pp. 76-87, 1993 Phylogeny of Xantusiid Lizards: Concern for Data and Analysis S. BLAIR HEDGES* AND ROBERT L. BEzvt * Department of Biology and
MOLECULAR PHYLOGENETICS AND EVOLUTION Vol. 2, No.1, March, pp. 76-87, 1993 Phylogeny of Xantusiid Lizards: Concern for Data and Analysis S. BLAIR HEDGES* AND ROBERT L. BEzvt * Department of Biology and
Introduction to phylogenetic trees and tree-thinking Copyright 2005, D. A. Baum (Free use for non-commercial educational pruposes)
 Introduction to phylogenetic trees and tree-thinking Copyright 2005, D. A. Baum (Free use for non-commercial educational pruposes) Phylogenetics is the study of the relationships of organisms to each other.
Introduction to phylogenetic trees and tree-thinking Copyright 2005, D. A. Baum (Free use for non-commercial educational pruposes) Phylogenetics is the study of the relationships of organisms to each other.
POPULATION GENETICS OF THE BIG BEND SLIDER (TRACHEMYS GAIGEAE GAIGEAE) AND THE RED EARED SLIDER (TRACHEMYS SCRIPTA ELEGANS) IN
 POPULATION GENETICS OF THE BIG BEND SLIDER (TRACHEMYS GAIGEAE GAIGEAE) AND THE RED EARED SLIDER (TRACHEMYS SCRIPTA ELEGANS) IN THE CONTACT ZONE IN THE LOWER RIO GRANDE DRAINAGE OF TEXAS by Lauren M. Schumacher,
POPULATION GENETICS OF THE BIG BEND SLIDER (TRACHEMYS GAIGEAE GAIGEAE) AND THE RED EARED SLIDER (TRACHEMYS SCRIPTA ELEGANS) IN THE CONTACT ZONE IN THE LOWER RIO GRANDE DRAINAGE OF TEXAS by Lauren M. Schumacher,
Title: Phylogenetic Methods and Vertebrate Phylogeny
 Title: Phylogenetic Methods and Vertebrate Phylogeny Central Question: How can evolutionary relationships be determined objectively? Sub-questions: 1. What affect does the selection of the outgroup have
Title: Phylogenetic Methods and Vertebrate Phylogeny Central Question: How can evolutionary relationships be determined objectively? Sub-questions: 1. What affect does the selection of the outgroup have
Caecilians (Gymnophiona)
 Caecilians (Gymnophiona) David J. Gower* and Mark Wilkinson Department of Zoology, The Natural History Museum, London SW7 5BD, UK *To whom correspondence should be addressed (d.gower@nhm. ac.uk) Abstract
Caecilians (Gymnophiona) David J. Gower* and Mark Wilkinson Department of Zoology, The Natural History Museum, London SW7 5BD, UK *To whom correspondence should be addressed (d.gower@nhm. ac.uk) Abstract
Phylogenetic analysis of Ehrlichia canis and Rhipicephalus spp. genes and subsequent primer and probe design.
 Phylogenetic analysis of Ehrlichia canis and Rhipicephalus spp. genes and subsequent primer and probe design. Name: V.H. de Visser (3051684) Supervisor: prof. dr. F. Jongejan Division: Utrecht Centre for
Phylogenetic analysis of Ehrlichia canis and Rhipicephalus spp. genes and subsequent primer and probe design. Name: V.H. de Visser (3051684) Supervisor: prof. dr. F. Jongejan Division: Utrecht Centre for
INQUIRY & INVESTIGATION
 INQUIRY & INVESTIGTION Phylogenies & Tree-Thinking D VID. UM SUSN OFFNER character a trait or feature that varies among a set of taxa (e.g., hair color) character-state a variant of a character that occurs
INQUIRY & INVESTIGTION Phylogenies & Tree-Thinking D VID. UM SUSN OFFNER character a trait or feature that varies among a set of taxa (e.g., hair color) character-state a variant of a character that occurs
The Making of the Fittest: LESSON STUDENT MATERIALS USING DNA TO EXPLORE LIZARD PHYLOGENY
 The Making of the Fittest: Natural The The Making Origin Selection of the of Species and Fittest: Adaptation Natural Lizards Selection in an Evolutionary and Adaptation Tree INTRODUCTION USING DNA TO EXPLORE
The Making of the Fittest: Natural The The Making Origin Selection of the of Species and Fittest: Adaptation Natural Lizards Selection in an Evolutionary and Adaptation Tree INTRODUCTION USING DNA TO EXPLORE
Evolutionary patterns in snake mitochondrial genomes
 Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2006 Evolutionary patterns in snake mitochondrial genomes Zhijie Jiang Louisiana State University and Agricultural
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2006 Evolutionary patterns in snake mitochondrial genomes Zhijie Jiang Louisiana State University and Agricultural
Postilla PEABODY MUSEUM OF NATURAL HISTORY YALE UNIVERSITY NEW HAVEN, CONNECTICUT, U.S.A.
 Postilla PEABODY MUSEUM OF NATURAL HISTORY YALE UNIVERSITY NEW HAVEN, CONNECTICUT, U.S.A. Number 117 18 March 1968 A 7DIAPSID (REPTILIA) PARIETAL FROM THE LOWER PERMIAN OF OKLAHOMA ROBERT L. CARROLL REDPATH
Postilla PEABODY MUSEUM OF NATURAL HISTORY YALE UNIVERSITY NEW HAVEN, CONNECTICUT, U.S.A. Number 117 18 March 1968 A 7DIAPSID (REPTILIA) PARIETAL FROM THE LOWER PERMIAN OF OKLAHOMA ROBERT L. CARROLL REDPATH
Phylogeny Reconstruction
 Phylogeny Reconstruction Trees, Methods and Characters Reading: Gregory, 2008. Understanding Evolutionary Trees (Polly, 2006) Lab tomorrow Meet in Geology GY522 Bring computers if you have them (they will
Phylogeny Reconstruction Trees, Methods and Characters Reading: Gregory, 2008. Understanding Evolutionary Trees (Polly, 2006) Lab tomorrow Meet in Geology GY522 Bring computers if you have them (they will
The melanocortin 1 receptor (mc1r) is a gene that has been implicated in the wide
 Introduction The melanocortin 1 receptor (mc1r) is a gene that has been implicated in the wide variety of colors that exist in nature. It is responsible for hair and skin color in humans and the various
Introduction The melanocortin 1 receptor (mc1r) is a gene that has been implicated in the wide variety of colors that exist in nature. It is responsible for hair and skin color in humans and the various
Prof. Neil. J.L. Heideman
 Prof. Neil. J.L. Heideman Position Office Mailing address E-mail : Vice-dean (Professor of Zoology) : No. 10, Biology Building : P.O. Box 339 (Internal Box 44), Bloemfontein 9300, South Africa : heidemannj.sci@mail.uovs.ac.za
Prof. Neil. J.L. Heideman Position Office Mailing address E-mail : Vice-dean (Professor of Zoology) : No. 10, Biology Building : P.O. Box 339 (Internal Box 44), Bloemfontein 9300, South Africa : heidemannj.sci@mail.uovs.ac.za
COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST
 COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST In this laboratory investigation, you will use BLAST to compare several genes, and then use the information to construct a cladogram.
COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST In this laboratory investigation, you will use BLAST to compare several genes, and then use the information to construct a cladogram.
PARTIAL REPORT. Juvenile hybrid turtles along the Brazilian coast RIO GRANDE FEDERAL UNIVERSITY
 RIO GRANDE FEDERAL UNIVERSITY OCEANOGRAPHY INSTITUTE MARINE MOLECULAR ECOLOGY LABORATORY PARTIAL REPORT Juvenile hybrid turtles along the Brazilian coast PROJECT LEADER: MAIRA PROIETTI PROFESSOR, OCEANOGRAPHY
RIO GRANDE FEDERAL UNIVERSITY OCEANOGRAPHY INSTITUTE MARINE MOLECULAR ECOLOGY LABORATORY PARTIAL REPORT Juvenile hybrid turtles along the Brazilian coast PROJECT LEADER: MAIRA PROIETTI PROFESSOR, OCEANOGRAPHY
Comparing DNA Sequences Cladogram Practice
 Name Period Assignment # See lecture questions 75, 122-123, 127, 137 Comparing DNA Sequences Cladogram Practice BACKGROUND Between 1990 2003, scientists working on an international research project known
Name Period Assignment # See lecture questions 75, 122-123, 127, 137 Comparing DNA Sequences Cladogram Practice BACKGROUND Between 1990 2003, scientists working on an international research project known
17.2 Classification Based on Evolutionary Relationships Organization of all that speciation!
 Organization of all that speciation! Patterns of evolution.. Taxonomy gets an over haul! Using more than morphology! 3 domains, 6 kingdoms KEY CONCEPT Modern classification is based on evolutionary relationships.
Organization of all that speciation! Patterns of evolution.. Taxonomy gets an over haul! Using more than morphology! 3 domains, 6 kingdoms KEY CONCEPT Modern classification is based on evolutionary relationships.
Research Note. A novel method for sexing day-old chicks using endoscope system
 Research Note A novel method for sexing day-old chicks using endoscope system Makoto Otsuka,,1 Osamu Miyashita,,1 Mitsuru Shibata,,1 Fujiyuki Sato,,1 and Mitsuru Naito,2,3 NARO Institute of Livestock and
Research Note A novel method for sexing day-old chicks using endoscope system Makoto Otsuka,,1 Osamu Miyashita,,1 Mitsuru Shibata,,1 Fujiyuki Sato,,1 and Mitsuru Naito,2,3 NARO Institute of Livestock and
COOPERATIVE BREEDING IN THE TROPICAL MOCKINGBIRD (MIMUS GILVUS) IN THE PANAMA CANAL ZONE
 SHORT COMMUNICATIONS ORNITOLOGIA NEOTROPICAL 15: 417 421, 2004 The Neotropical Ornithological Society COOPERATIVE BREEDING IN THE TROPICAL MOCKINGBIRD (MIMUS GILVUS) IN THE PANAMA CANAL ZONE Eugene S.
SHORT COMMUNICATIONS ORNITOLOGIA NEOTROPICAL 15: 417 421, 2004 The Neotropical Ornithological Society COOPERATIVE BREEDING IN THE TROPICAL MOCKINGBIRD (MIMUS GILVUS) IN THE PANAMA CANAL ZONE Eugene S.
Species: Panthera pardus Genus: Panthera Family: Felidae Order: Carnivora Class: Mammalia Phylum: Chordata
 CHAPTER 6: PHYLOGENY AND THE TREE OF LIFE AP Biology 3 PHYLOGENY AND SYSTEMATICS Phylogeny - evolutionary history of a species or group of related species Systematics - analytical approach to understanding
CHAPTER 6: PHYLOGENY AND THE TREE OF LIFE AP Biology 3 PHYLOGENY AND SYSTEMATICS Phylogeny - evolutionary history of a species or group of related species Systematics - analytical approach to understanding
Final Report for Research Work Order 167 entitled:
 Final Report for Research Work Order 167 entitled: Population Genetic Structure of Marine Turtles, Eretmochelys imbricata and Caretta caretta, in the Southeastern United States and adjacent Caribbean region
Final Report for Research Work Order 167 entitled: Population Genetic Structure of Marine Turtles, Eretmochelys imbricata and Caretta caretta, in the Southeastern United States and adjacent Caribbean region
Based on the DNA sequences, most of the trnas could be folded as cloverleaf
 Putative secondary structures of trnas Based on the DNA sequences, most of the trnas could be folded as cloverleaf secondary structures. A few of them possessed nonwatsoncrick matches, aberrant loops,
Putative secondary structures of trnas Based on the DNA sequences, most of the trnas could be folded as cloverleaf secondary structures. A few of them possessed nonwatsoncrick matches, aberrant loops,
Ciccaba virgata (Mottled Owl)
 Ciccaba virgata (Mottled Owl) Family: Strigidae (Typical Owls) Order: Strigiformes (Owls) Class: Aves (Birds) Fig. 1. Mottled owl, Ciccaba virgata. [http://www.owling.com/mottled13.htm, downloaded 12 November
Ciccaba virgata (Mottled Owl) Family: Strigidae (Typical Owls) Order: Strigiformes (Owls) Class: Aves (Birds) Fig. 1. Mottled owl, Ciccaba virgata. [http://www.owling.com/mottled13.htm, downloaded 12 November
PHYLOGENY OF THE RATTLESNAKES (CROTALUS AND SISTRURUS) INFERRED FROM SEQUENCES OF FIVE MITOCHONDRIAL DNA GENES
 PHYLOGENY OF THE RATTLESNAKES (CROTALUS AND SISTRURUS) INFERRED FROM SEQUENCES OF FIVE MITOCHONDRIAL DNA GENES ROBERT W. MURPHY 1, JINZHONG FU 1,2, AMY LATHROP 1, JOSHUA V. FELTHAM 1,3, AND VIERA KOVAC
PHYLOGENY OF THE RATTLESNAKES (CROTALUS AND SISTRURUS) INFERRED FROM SEQUENCES OF FIVE MITOCHONDRIAL DNA GENES ROBERT W. MURPHY 1, JINZHONG FU 1,2, AMY LATHROP 1, JOSHUA V. FELTHAM 1,3, AND VIERA KOVAC
Incidence, Antimicrobial Susceptibility, and Toxin Genes Possession Screening of Staphylococcus aureus in Retail Chicken Livers and Gizzards
 Foods 2015, 4, 115-129; doi:10.3390/foods4020115 Article OPEN ACCESS foods ISSN 2304-8158 www.mdpi.com/journal/foods Incidence, Antimicrobial Susceptibility, and Toxin Genes Possession Screening of Staphylococcus
Foods 2015, 4, 115-129; doi:10.3390/foods4020115 Article OPEN ACCESS foods ISSN 2304-8158 www.mdpi.com/journal/foods Incidence, Antimicrobial Susceptibility, and Toxin Genes Possession Screening of Staphylococcus
Bio 1B Lecture Outline (please print and bring along) Fall, 2006
 Bio 1B Lecture Outline (please print and bring along) Fall, 2006 B.D. Mishler, Dept. of Integrative Biology 2-6810, bmishler@berkeley.edu Evolution lecture #4 -- Phylogenetic Analysis (Cladistics) -- Oct.
Bio 1B Lecture Outline (please print and bring along) Fall, 2006 B.D. Mishler, Dept. of Integrative Biology 2-6810, bmishler@berkeley.edu Evolution lecture #4 -- Phylogenetic Analysis (Cladistics) -- Oct.
Phylogenetic Relationships and Biogeography of Xantusiid Lizards, Inferred from Mitochondria1 DNA Sequences '
 Phylogenetic Relationships and Biogeography of Xantusiid Lizards, Inferred from Mitochondria1 DNA Sequences ' S. Blair Hedges, * Robert L. Bezy,? and Linda R. Maxson * *Department of Biology and Institute
Phylogenetic Relationships and Biogeography of Xantusiid Lizards, Inferred from Mitochondria1 DNA Sequences ' S. Blair Hedges, * Robert L. Bezy,? and Linda R. Maxson * *Department of Biology and Institute
LABORATORY EXERCISE 6: CLADISTICS I
 Biology 4415/5415 Evolution LABORATORY EXERCISE 6: CLADISTICS I Take a group of organisms. Let s use five: a lungfish, a frog, a crocodile, a flamingo, and a human. How to reconstruct their relationships?
Biology 4415/5415 Evolution LABORATORY EXERCISE 6: CLADISTICS I Take a group of organisms. Let s use five: a lungfish, a frog, a crocodile, a flamingo, and a human. How to reconstruct their relationships?
HERPETOLOGY BIO 404 COURSE SYLLABUS, SPRING SEMESTER, 2001
 HERPETOLOGY BIO 404 COURSE SYLLABUS, SPRING SEMESTER, 2001 Lecture: Mon., Wed., Fri., 1:00 1:50 p. m., NS 523 Laboratory: Mon., 2:00-4:50 p.m., NS 522 and Field Trips PROFESSOR: RICHARD D. DURTSCHE OFFICE:
HERPETOLOGY BIO 404 COURSE SYLLABUS, SPRING SEMESTER, 2001 Lecture: Mon., Wed., Fri., 1:00 1:50 p. m., NS 523 Laboratory: Mon., 2:00-4:50 p.m., NS 522 and Field Trips PROFESSOR: RICHARD D. DURTSCHE OFFICE:
Testing Phylogenetic Hypotheses with Molecular Data 1
 Testing Phylogenetic Hypotheses with Molecular Data 1 How does an evolutionary biologist quantify the timing and pathways for diversification (speciation)? If we observe diversification today, the processes
Testing Phylogenetic Hypotheses with Molecular Data 1 How does an evolutionary biologist quantify the timing and pathways for diversification (speciation)? If we observe diversification today, the processes
muscles (enhancing biting strength). Possible states: none, one, or two.
 Reconstructing Evolutionary Relationships S-1 Practice Exercise: Phylogeny of Terrestrial Vertebrates In this example we will construct a phylogenetic hypothesis of the relationships between seven taxa
Reconstructing Evolutionary Relationships S-1 Practice Exercise: Phylogeny of Terrestrial Vertebrates In this example we will construct a phylogenetic hypothesis of the relationships between seven taxa
Cladistics (reading and making of cladograms)
 Cladistics (reading and making of cladograms) Definitions Systematics The branch of biological sciences concerned with classifying organisms Taxon (pl: taxa) Any unit of biological diversity (eg. Animalia,
Cladistics (reading and making of cladograms) Definitions Systematics The branch of biological sciences concerned with classifying organisms Taxon (pl: taxa) Any unit of biological diversity (eg. Animalia,
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/20908 holds various files of this Leiden University dissertation. Author: Kok, Philippe Jacques Robert Title: Islands in the sky : species diversity, evolutionary
Cover Page The handle http://hdl.handle.net/1887/20908 holds various files of this Leiden University dissertation. Author: Kok, Philippe Jacques Robert Title: Islands in the sky : species diversity, evolutionary
COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST
 Big Idea 1 Evolution INVESTIGATION 3 COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST How can bioinformatics be used as a tool to determine evolutionary relationships and to
Big Idea 1 Evolution INVESTIGATION 3 COMPARING DNA SEQUENCES TO UNDERSTAND EVOLUTIONARY RELATIONSHIPS WITH BLAST How can bioinformatics be used as a tool to determine evolutionary relationships and to
Characterization of the Multidrug-Resistant Acinetobacter
 Ann Clin Microbiol Vol. 7, No. 2, June, 20 http://dx.doi.org/0.55/acm.20.7.2.29 pissn 2288-0585 eissn 2288-6850 Characterization of the Multidrug-Resistant Acinetobacter species Causing a Nosocomial Outbreak
Ann Clin Microbiol Vol. 7, No. 2, June, 20 http://dx.doi.org/0.55/acm.20.7.2.29 pissn 2288-0585 eissn 2288-6850 Characterization of the Multidrug-Resistant Acinetobacter species Causing a Nosocomial Outbreak
Staphylococcus aureus is More Prevalent in Retail Beef Livers than in Pork and other Beef Cuts
 Pathogens 2015, 4, 182-198; doi:10.3390/pathogens4020182 Article OPEN ACCESS pathogens ISSN 2076-0817 www.mdpi.com/journal/pathogens Staphylococcus aureus is More Prevalent in Retail Beef Livers than in
Pathogens 2015, 4, 182-198; doi:10.3390/pathogens4020182 Article OPEN ACCESS pathogens ISSN 2076-0817 www.mdpi.com/journal/pathogens Staphylococcus aureus is More Prevalent in Retail Beef Livers than in
Tropical Screech Owl - Megascops choliba
 Tropical Screech Owl - Megascops choliba Formerly Otus choliba Description: A relatively small screech owl with short ear tufts that are raised mostly during daytime. There are grey-brown, brown and rufous
Tropical Screech Owl - Megascops choliba Formerly Otus choliba Description: A relatively small screech owl with short ear tufts that are raised mostly during daytime. There are grey-brown, brown and rufous
You have 254 Neanderthal variants.
 1 of 5 1/3/2018 1:21 PM Joseph Roberts Neanderthal Ancestry Neanderthal Ancestry Neanderthals were ancient humans who interbred with modern humans before becoming extinct 40,000 years ago. This report
1 of 5 1/3/2018 1:21 PM Joseph Roberts Neanderthal Ancestry Neanderthal Ancestry Neanderthals were ancient humans who interbred with modern humans before becoming extinct 40,000 years ago. This report
Lineage Classification of Canine Title Disorders Using Mitochondrial DNA 宮原, 和郎, 鈴木, 三義. Journal of Veterinary Medical Sci Citation
 ' ' Lineage Classification of Canine Title Disorders Using Mitochondrial DNA TAKAHASI, Shoko, MIYAHARA, Kazuro Author(s) Hirosi, ISHIGURO, Naotaka, SUZUKI 宮原, 和郎, 鈴木, 三義 Journal of Veterinary Medical Sci
' ' Lineage Classification of Canine Title Disorders Using Mitochondrial DNA TAKAHASI, Shoko, MIYAHARA, Kazuro Author(s) Hirosi, ISHIGURO, Naotaka, SUZUKI 宮原, 和郎, 鈴木, 三義 Journal of Veterinary Medical Sci
Screening and deciphering antibiotic resistance in Acinetobacter baumannii: a state of the art
 For reprint orders, please contact reprints@expert-reviews.com Screening and deciphering antibiotic resistance in Acinetobacter baumannii: a state of the art Expert Rev. Anti Infect. Ther. 11(6), 571 583
For reprint orders, please contact reprints@expert-reviews.com Screening and deciphering antibiotic resistance in Acinetobacter baumannii: a state of the art Expert Rev. Anti Infect. Ther. 11(6), 571 583
Supplementary Figure S WebLogo WebLogo WebLogo 3.0
 A B Normalized Count Density Density -10 CC A T A T C A T C A T C T AA 5' Fragment End A T C CT AA TC AC CTA T -5 0 CC AT TAC AC T T Supplementary Figure S1 A TA C C TCT TC TC CA C A AAAT TC CT TAA 5 10
A B Normalized Count Density Density -10 CC A T A T C A T C A T C T AA 5' Fragment End A T C CT AA TC AC CTA T -5 0 CC AT TAC AC T T Supplementary Figure S1 A TA C C TCT TC TC CA C A AAAT TC CT TAA 5 10
A Field Guide to the Herpetofauna on Dominica, W.I. by Brandi Quick Wildlife and Fisheries Science Texas A&M University.
 A Field Guide to the Herpetofauna on Dominica, W.I. by Brandi Quick Wildlife and Fisheries Science Texas A&M University June 11, 2001 Study Abroad Dominica 2001 Dr. Thomas Lacher Dr. Bob Wharton ABSTRACT
A Field Guide to the Herpetofauna on Dominica, W.I. by Brandi Quick Wildlife and Fisheries Science Texas A&M University June 11, 2001 Study Abroad Dominica 2001 Dr. Thomas Lacher Dr. Bob Wharton ABSTRACT
Dipsas trinitatis (Trinidad Snail-eating Snake)
 Dipsas trinitatis (Trinidad Snail-eating Snake) Family: Dipsadidae (Rear-fanged Snakes) Order: Squamata (Lizards and Snakes) Class: Reptilia (Reptiles) Fig. 1. Trinidad snail-eating snake, Dipsas trinitatis.
Dipsas trinitatis (Trinidad Snail-eating Snake) Family: Dipsadidae (Rear-fanged Snakes) Order: Squamata (Lizards and Snakes) Class: Reptilia (Reptiles) Fig. 1. Trinidad snail-eating snake, Dipsas trinitatis.
MULTI-DRUG RESISTANT GRAM-NEGATIVE ENTERIC BACTERIA ISOLATED FROM FLIES AT CHENGDU AIRPORT, CHINA
 MULTI-DRUG RESISTANT GRAM-NEGATIVE ENTERIC BACTERIA ISOLATED FROM FLIES AT CHENGDU AIRPORT, CHINA Yang Liu 1, 2, Yu Yang 2, Feng Zhao 2, Xuejun Fan 2, Wei Zhong 2, Dairong Qiao 1 and Yi Cao 1 1 College
MULTI-DRUG RESISTANT GRAM-NEGATIVE ENTERIC BACTERIA ISOLATED FROM FLIES AT CHENGDU AIRPORT, CHINA Yang Liu 1, 2, Yu Yang 2, Feng Zhao 2, Xuejun Fan 2, Wei Zhong 2, Dairong Qiao 1 and Yi Cao 1 1 College
LABORATORY EXERCISE 7: CLADISTICS I
 Biology 4415/5415 Evolution LABORATORY EXERCISE 7: CLADISTICS I Take a group of organisms. Let s use five: a lungfish, a frog, a crocodile, a flamingo, and a human. How to reconstruct their relationships?
Biology 4415/5415 Evolution LABORATORY EXERCISE 7: CLADISTICS I Take a group of organisms. Let s use five: a lungfish, a frog, a crocodile, a flamingo, and a human. How to reconstruct their relationships?
A Theileria sp. was detected by PCR in blood samples collected from dogs in the
 Chapter 6: Detection of Theileria sp. infections in dogs in South Africa. 6.1. Abstract A Theileria sp. was detected by PCR in blood samples collected from dogs in the Pietermaritzburg area and also found
Chapter 6: Detection of Theileria sp. infections in dogs in South Africa. 6.1. Abstract A Theileria sp. was detected by PCR in blood samples collected from dogs in the Pietermaritzburg area and also found
A Comparison of morphological differences between Gymnophthalmus spp. in Dominica, West Indies
 209 A Comparison of morphological differences between Gymnophthalmus spp. in Dominica, West Indies Marie Perez June 2015 Texas A&M University Dr. Thomas Lacher and Dr. Jim Woolley Department of Wildlife
209 A Comparison of morphological differences between Gymnophthalmus spp. in Dominica, West Indies Marie Perez June 2015 Texas A&M University Dr. Thomas Lacher and Dr. Jim Woolley Department of Wildlife
Selection, Recombination and History in a Parasitic Flatworm (Echinococcus) Inferred from Nucleotide Sequences
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 93(5): 695-702, Sep./Oct. 1998 Selection, Recombination and History in a Parasitic Flatworm (Echinococcus) Inferred from Nucleotide Sequences KL Haag, AM Araújo,
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 93(5): 695-702, Sep./Oct. 1998 Selection, Recombination and History in a Parasitic Flatworm (Echinococcus) Inferred from Nucleotide Sequences KL Haag, AM Araújo,
Interpreting Evolutionary Trees Honors Integrated Science 4 Name Per.
 Interpreting Evolutionary Trees Honors Integrated Science 4 Name Per. Introduction Imagine a single diagram representing the evolutionary relationships between everything that has ever lived. If life evolved
Interpreting Evolutionary Trees Honors Integrated Science 4 Name Per. Introduction Imagine a single diagram representing the evolutionary relationships between everything that has ever lived. If life evolved
Name: Date: Hour: Fill out the following character matrix. Mark an X if an organism has the trait.
 Name: Date: Hour: CLADOGRAM ANALYSIS What is a cladogram? It is a diagram that depicts evolutionary relationships among groups. It is based on PHYLOGENY, which is the study of evolutionary relationships.
Name: Date: Hour: CLADOGRAM ANALYSIS What is a cladogram? It is a diagram that depicts evolutionary relationships among groups. It is based on PHYLOGENY, which is the study of evolutionary relationships.
Volume 2 Number 1, July 2012 ISSN:
 Volume 2 Number 1, July 2012 ISSN: 229-9769 Published by Faculty of Resource Science and Technology Borneo J. Resour. Sci. Tech. (2012) 2: 20-27 Molecular Phylogeny of Sarawak Green Sea Turtle (Chelonia
Volume 2 Number 1, July 2012 ISSN: 229-9769 Published by Faculty of Resource Science and Technology Borneo J. Resour. Sci. Tech. (2012) 2: 20-27 Molecular Phylogeny of Sarawak Green Sea Turtle (Chelonia
ON COLOMBIAN REPTILES AND AMPHIBIANS COLLECTED BY DR. R. E. SCHULTES. By BENJAMIN SHREVE Museum of Comparative Zoology, cambridge, U. S. A.
 HERPETOLOGIA ON COLOMBIAN REPTILES AND AMPHIBIANS COLLECTED BY DR. R. E. SCHULTES By BENJAMIN SHREVE Museum of Comparative Zoology, cambridge, U. S. A. From Dr. Richard Evans Schultes, who has been engaged
HERPETOLOGIA ON COLOMBIAN REPTILES AND AMPHIBIANS COLLECTED BY DR. R. E. SCHULTES By BENJAMIN SHREVE Museum of Comparative Zoology, cambridge, U. S. A. From Dr. Richard Evans Schultes, who has been engaged
UNIT III A. Descent with Modification(Ch19) B. Phylogeny (Ch20) C. Evolution of Populations (Ch21) D. Origin of Species or Speciation (Ch22)
 UNIT III A. Descent with Modification(Ch9) B. Phylogeny (Ch2) C. Evolution of Populations (Ch2) D. Origin of Species or Speciation (Ch22) Classification in broad term simply means putting things in classes
UNIT III A. Descent with Modification(Ch9) B. Phylogeny (Ch2) C. Evolution of Populations (Ch2) D. Origin of Species or Speciation (Ch22) Classification in broad term simply means putting things in classes
New Records of Cladocera (Crustacea) for Trinidad, West Indies
 New Records of Cladocera (Crustacea) for Trinidad, West Indies Azad Mohammed Mohammed, A. 2004. A New Records of Cladocera (Crustacea) for Trinidad, West Indies. Living World, Journal of The Trinidad and
New Records of Cladocera (Crustacea) for Trinidad, West Indies Azad Mohammed Mohammed, A. 2004. A New Records of Cladocera (Crustacea) for Trinidad, West Indies. Living World, Journal of The Trinidad and
What are taxonomy, classification, and systematics?
 Topic 2: Comparative Method o Taxonomy, classification, systematics o Importance of phylogenies o A closer look at systematics o Some key concepts o Parts of a cladogram o Groups and characters o Homology
Topic 2: Comparative Method o Taxonomy, classification, systematics o Importance of phylogenies o A closer look at systematics o Some key concepts o Parts of a cladogram o Groups and characters o Homology
The Divergence of the Marine Iguana: Amblyrhyncus cristatus. from its earlier land ancestor (what is now the Land Iguana). While both the land and
 Chris Lang Course Paper Sophomore College October 9, 2008 Abstract--- The Divergence of the Marine Iguana: Amblyrhyncus cristatus In this course paper, I address the divergence of the Galapagos Marine
Chris Lang Course Paper Sophomore College October 9, 2008 Abstract--- The Divergence of the Marine Iguana: Amblyrhyncus cristatus In this course paper, I address the divergence of the Galapagos Marine
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore
 Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore Activitydevelop EXPLO RING VERTEBRATE CL ASSIFICATIO N What criteria
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore Activitydevelop EXPLO RING VERTEBRATE CL ASSIFICATIO N What criteria
Molecular Phylogeny of the Chipmunk Genus Tamias Based on the Mitochondrial Cytochrome Oxidase Subunit II Gene
 Journal of Mammalian Evolution, Vol. 7, No. 3, 2000 Molecular Phylogeny of the Chipmunk Genus Tamias Based on the Mitochondrial Cytochrome Oxidase Subunit II Gene Antoinette J. Piaggio 1 and Greg S. Spicer
Journal of Mammalian Evolution, Vol. 7, No. 3, 2000 Molecular Phylogeny of the Chipmunk Genus Tamias Based on the Mitochondrial Cytochrome Oxidase Subunit II Gene Antoinette J. Piaggio 1 and Greg S. Spicer
J.K. McCoy CURRICULUM VITAE. J. Kelly McCoy. Department of Biology Angelo State University San Angelo, TX
 CURRICULUM VITAE J. Kelly McCoy Department of Biology Angelo State University San Angelo, TX 76909 325-486-6646 Kelly.McCoy@angelo.edu Education: B.S. 1990 Zoology Oklahoma State University Ph.D. 1995
CURRICULUM VITAE J. Kelly McCoy Department of Biology Angelo State University San Angelo, TX 76909 325-486-6646 Kelly.McCoy@angelo.edu Education: B.S. 1990 Zoology Oklahoma State University Ph.D. 1995
Modern taxonomy. Building family trees 10/10/2011. Knowing a lot about lots of creatures. Tom Hartman. Systematics includes: 1.
 Modern taxonomy Building family trees Tom Hartman www.tuatara9.co.uk Classification has moved away from the simple grouping of organisms according to their similarities (phenetics) and has become the study
Modern taxonomy Building family trees Tom Hartman www.tuatara9.co.uk Classification has moved away from the simple grouping of organisms according to their similarities (phenetics) and has become the study
Colonisation, diversificationand extinctionof birds in Macaronesia
 Colonisation, diversificationand extinctionof birds in Macaronesia Juan Carlos Illera Research Unit of Biodiversity (UO-PA-CSIC) http://www.juancarlosillera.es / http://www.unioviedo.es/umib/ MACARONESIA
Colonisation, diversificationand extinctionof birds in Macaronesia Juan Carlos Illera Research Unit of Biodiversity (UO-PA-CSIC) http://www.juancarlosillera.es / http://www.unioviedo.es/umib/ MACARONESIA
Geo 302D: Age of Dinosaurs LAB 4: Systematics Part 1
 Geo 302D: Age of Dinosaurs LAB 4: Systematics Part 1 Systematics is the comparative study of biological diversity with the intent of determining the relationships between organisms. Humankind has always
Geo 302D: Age of Dinosaurs LAB 4: Systematics Part 1 Systematics is the comparative study of biological diversity with the intent of determining the relationships between organisms. Humankind has always
Evolution of Birds. Summary:
 Oregon State Standards OR Science 7.1, 7.2, 7.3, 7.3S.1, 7.3S.2 8.1, 8.2, 8.2L.1, 8.3, 8.3S.1, 8.3S.2 H.1, H.2, H.2L.4, H.2L.5, H.3, H.3S.1, H.3S.2, H.3S.3 Summary: Students create phylogenetic trees to
Oregon State Standards OR Science 7.1, 7.2, 7.3, 7.3S.1, 7.3S.2 8.1, 8.2, 8.2L.1, 8.3, 8.3S.1, 8.3S.2 H.1, H.2, H.2L.4, H.2L.5, H.3, H.3S.1, H.3S.2, H.3S.3 Summary: Students create phylogenetic trees to
Genotypes of Cornel Dorset and Dorset Crosses Compared with Romneys for Melatonin Receptor 1a
 Genotypes of Cornell Dorset and Dorset Crosses Compared with Romneys for Melatonin Receptor 1a By Christian Posbergh Cornell Undergraduate Honor Student, Dept. Animal Science Abstract: Sheep are known
Genotypes of Cornell Dorset and Dorset Crosses Compared with Romneys for Melatonin Receptor 1a By Christian Posbergh Cornell Undergraduate Honor Student, Dept. Animal Science Abstract: Sheep are known
Fig Phylogeny & Systematics
 Fig. 26- Phylogeny & Systematics Tree of Life phylogenetic relationship for 3 clades (http://evolution.berkeley.edu Fig. 26-2 Phylogenetic tree Figure 26.3 Taxonomy Taxon Carolus Linnaeus Species: Panthera
Fig. 26- Phylogeny & Systematics Tree of Life phylogenetic relationship for 3 clades (http://evolution.berkeley.edu Fig. 26-2 Phylogenetic tree Figure 26.3 Taxonomy Taxon Carolus Linnaeus Species: Panthera
May 10, SWBAT analyze and evaluate the scientific evidence provided by the fossil record.
 May 10, 2017 Aims: SWBAT analyze and evaluate the scientific evidence provided by the fossil record. Agenda 1. Do Now 2. Class Notes 3. Guided Practice 4. Independent Practice 5. Practicing our AIMS: E.3-Examining
May 10, 2017 Aims: SWBAT analyze and evaluate the scientific evidence provided by the fossil record. Agenda 1. Do Now 2. Class Notes 3. Guided Practice 4. Independent Practice 5. Practicing our AIMS: E.3-Examining
ASSESSMENT OF GENETIC VARIATION WITHIN AND AMONG NATURAL AND CAPTIVE POPULATIONS OF ALLIGATOR SNAPPING TURTLES (MACROCHELYS TEMMINCKII)
 ASSESSMENT OF GENETIC VARIATION WITHIN AND AMONG NATURAL AND CAPTIVE POPULATIONS OF ALLIGATOR SNAPPING TURTLES (MACROCHELYS TEMMINCKII) By JOSEPH C. HACKLER Bachelor of Science Oklahoma State University
ASSESSMENT OF GENETIC VARIATION WITHIN AND AMONG NATURAL AND CAPTIVE POPULATIONS OF ALLIGATOR SNAPPING TURTLES (MACROCHELYS TEMMINCKII) By JOSEPH C. HACKLER Bachelor of Science Oklahoma State University
Do the traits of organisms provide evidence for evolution?
 PhyloStrat Tutorial Do the traits of organisms provide evidence for evolution? Consider two hypotheses about where Earth s organisms came from. The first hypothesis is from John Ray, an influential British
PhyloStrat Tutorial Do the traits of organisms provide evidence for evolution? Consider two hypotheses about where Earth s organisms came from. The first hypothesis is from John Ray, an influential British
AP Lab Three: Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST
 AP Biology Name AP Lab Three: Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST In the 1990 s when scientists began to compile a list of genes and DNA sequences in the human genome
AP Biology Name AP Lab Three: Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST In the 1990 s when scientists began to compile a list of genes and DNA sequences in the human genome
DOWNLOAD OR READ : PRELIMINARY AMPHIBIAN AND REPTILE SURVEY OF THE SIOUX DISTRICT OF THE CUSTER NATIONAL FOREST PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : PRELIMINARY AMPHIBIAN AND REPTILE SURVEY OF THE SIOUX DISTRICT OF THE CUSTER NATIONAL FOREST PDF EBOOK EPUB MOBI Page 1 Page 2 preliminary amphibian and reptile survey of the sioux district
DOWNLOAD OR READ : PRELIMINARY AMPHIBIAN AND REPTILE SURVEY OF THE SIOUX DISTRICT OF THE CUSTER NATIONAL FOREST PDF EBOOK EPUB MOBI Page 1 Page 2 preliminary amphibian and reptile survey of the sioux district
These small issues are easily addressed by small changes in wording, and should in no way delay publication of this first- rate paper.
 Reviewers' comments: Reviewer #1 (Remarks to the Author): This paper reports on a highly significant discovery and associated analysis that are likely to be of broad interest to the scientific community.
Reviewers' comments: Reviewer #1 (Remarks to the Author): This paper reports on a highly significant discovery and associated analysis that are likely to be of broad interest to the scientific community.
Sample Questions: EXAMINATION I Form A Mammalogy -EEOB 625. Name Composite of previous Examinations
 Sample Questions: EXAMINATION I Form A Mammalogy -EEOB 625 Name Composite of previous Examinations Part I. Define or describe only 5 of the following 6 words - 15 points (3 each). If you define all 6,
Sample Questions: EXAMINATION I Form A Mammalogy -EEOB 625 Name Composite of previous Examinations Part I. Define or describe only 5 of the following 6 words - 15 points (3 each). If you define all 6,
Higher-Level Snake Phylogeny Inferred from Mitochondrial DNA Sequences of 12s rrna and 16s rrna Genes
 Higher-Level Snake Phylogeny Inferred from Mitochondrial DNA Sequences of 12s rrna and 16s rrna Genes Philip J. Heise, * Linda R. Maxson, * Herndon G. Dowling,? and S. Blair Hedges* *Department o f Biology
Higher-Level Snake Phylogeny Inferred from Mitochondrial DNA Sequences of 12s rrna and 16s rrna Genes Philip J. Heise, * Linda R. Maxson, * Herndon G. Dowling,? and S. Blair Hedges* *Department o f Biology
Ch 1.2 Determining How Species Are Related.notebook February 06, 2018
 Name 3 "Big Ideas" from our last notebook lecture: * * * 1 WDYR? Of the following organisms, which is the closest relative of the "Snowy Owl" (Bubo scandiacus)? a) barn owl (Tyto alba) b) saw whet owl
Name 3 "Big Ideas" from our last notebook lecture: * * * 1 WDYR? Of the following organisms, which is the closest relative of the "Snowy Owl" (Bubo scandiacus)? a) barn owl (Tyto alba) b) saw whet owl
Veterinary Parasitology
 Veterinary Parasitology 172 (2010) 311 316 Contents lists available at ScienceDirect Veterinary Parasitology journal homepage: www.elsevier.com/locate/vetpar Identification and genetic characterization
Veterinary Parasitology 172 (2010) 311 316 Contents lists available at ScienceDirect Veterinary Parasitology journal homepage: www.elsevier.com/locate/vetpar Identification and genetic characterization
Evolution in dogs. Megan Elmore CS374 11/16/2010. (thanks to Dan Newburger for many slides' content)
 Evolution in dogs Megan Elmore CS374 11/16/2010 (thanks to Dan Newburger for many slides' content) Papers for today Vonholdt BM et al (2010). Genome-wide SNP and haplotype analyses reveal a rich history
Evolution in dogs Megan Elmore CS374 11/16/2010 (thanks to Dan Newburger for many slides' content) Papers for today Vonholdt BM et al (2010). Genome-wide SNP and haplotype analyses reveal a rich history
Obituary A Monument to Natural History Henry S. Fitch ( )
 Phyllomedusa 8(2):75-79, 2009 2009 Departamento de Ciências Biológicas - ESALQ - USP ISSN 1519-1397 Obituary A Monument to Natural History Henry S. Fitch (1909-2009) William E. Duellman Biodiversity Institute,
Phyllomedusa 8(2):75-79, 2009 2009 Departamento de Ciências Biológicas - ESALQ - USP ISSN 1519-1397 Obituary A Monument to Natural History Henry S. Fitch (1909-2009) William E. Duellman Biodiversity Institute,
Comparing DNA Sequence to Understand
 Comparing DNA Sequence to Understand Evolutionary Relationships with BLAST Name: Big Idea 1: Evolution Pre-Reading In order to understand the purposes and learning objectives of this investigation, you
Comparing DNA Sequence to Understand Evolutionary Relationships with BLAST Name: Big Idea 1: Evolution Pre-Reading In order to understand the purposes and learning objectives of this investigation, you
NAME: DATE: SECTION:
 NAME: DATE: SECTION: MCAS PREP PACKET EVOLUTION AND BIODIVERSITY 1. Which of the following observations best supports the conclusion that dolphins and sharks do not have a recent common ancestor? A. Dolphins
NAME: DATE: SECTION: MCAS PREP PACKET EVOLUTION AND BIODIVERSITY 1. Which of the following observations best supports the conclusion that dolphins and sharks do not have a recent common ancestor? A. Dolphins
Sergio, A NEW GENUS OF GHOST SHRIMP FROM THE AMERICAS (CRUSTACEA: DECAPODA: CALLIANASSIDAE)
 NAUPLIUS, Rio Grande, 1: 39-43, 1991!* ^ Sergio, A NEW GENUS OF GHOST SHRIMP FROM THE AMERICAS (CRUSTACEA: DECAPODA: CALLIANASSIDAE) R. B. MANNING & R. LEMAITRE Department of Invertebrate Zoology National
NAUPLIUS, Rio Grande, 1: 39-43, 1991!* ^ Sergio, A NEW GENUS OF GHOST SHRIMP FROM THE AMERICAS (CRUSTACEA: DECAPODA: CALLIANASSIDAE) R. B. MANNING & R. LEMAITRE Department of Invertebrate Zoology National
FIRST RECORD OF Platemys platycephala melanonota ERNST,
 FIRST RECORD OF Platemys platycephala melanonota ERNST, 1984 (REPTILIA, TESTUDINES, CHELIDAE) FOR THE BRAZILIAN AMAZON Telêmaco Jason Mendes-Pinto 1,2 Sergio Marques de Souza 2 Richard Carl Vogt 2 Rafael
FIRST RECORD OF Platemys platycephala melanonota ERNST, 1984 (REPTILIA, TESTUDINES, CHELIDAE) FOR THE BRAZILIAN AMAZON Telêmaco Jason Mendes-Pinto 1,2 Sergio Marques de Souza 2 Richard Carl Vogt 2 Rafael
Evolution as Fact. The figure below shows transitional fossils in the whale lineage.
 Evolution as Fact Evolution is a fact. Organisms descend from others with modification. Phylogeny, the lineage of ancestors and descendants, is the scientific term to Darwin's phrase "descent with modification."
Evolution as Fact Evolution is a fact. Organisms descend from others with modification. Phylogeny, the lineage of ancestors and descendants, is the scientific term to Darwin's phrase "descent with modification."
Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST
 Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST INVESTIGATION 3 BIG IDEA 1 Lab Investigation 3: BLAST Pre-Lab Essential Question: How can bioinformatics be used as a tool to
Comparing DNA Sequences to Understand Evolutionary Relationships with BLAST INVESTIGATION 3 BIG IDEA 1 Lab Investigation 3: BLAST Pre-Lab Essential Question: How can bioinformatics be used as a tool to
Comparisons of mitochondrial DNA (mtdna) sequences. (16S rrna gene) between oviparous and viviparous strains of Lacerta vivipara: a preliminary study
 Molecular Ecology (1999) 8, 1627 1631 Comparisons of mitochondrial DNA (mtdna) sequences Blackwell Science, Ltd (16S rrna gene) between oviparous and viviparous strains of Lacerta vivipara: a preliminary
Molecular Ecology (1999) 8, 1627 1631 Comparisons of mitochondrial DNA (mtdna) sequences Blackwell Science, Ltd (16S rrna gene) between oviparous and viviparous strains of Lacerta vivipara: a preliminary
Evidence for Evolution by Natural Selection. Hunting for evolution clues Elementary, my dear, Darwin!
 Evidence for Evolution by Natural Selection Hunting for evolution clues Elementary, my dear, Darwin! 2006-2007 Evidence supporting evolution Fossil record shows change over time Anatomical record comparing
Evidence for Evolution by Natural Selection Hunting for evolution clues Elementary, my dear, Darwin! 2006-2007 Evidence supporting evolution Fossil record shows change over time Anatomical record comparing
Who Cares? The Evolution of Parental Care in Squamate Reptiles. Ben Halliwell Geoffrey While, Tobias Uller
 Who Cares? The Evolution of Parental Care in Squamate Reptiles Ben Halliwell Geoffrey While, Tobias Uller 1 Parental Care any instance of parental investment that increases the fitness of offspring 2 Parental
Who Cares? The Evolution of Parental Care in Squamate Reptiles Ben Halliwell Geoffrey While, Tobias Uller 1 Parental Care any instance of parental investment that increases the fitness of offspring 2 Parental
LABORATORY EXERCISE: CLADISTICS III. In fact, cladistics is becoming increasingly applied in a wide range of fields. Here s a sampling:
 Biology 4415 Evolution LABORATORY EXERCISE: CLADISTICS III The last lab and the accompanying lectures should have given you an in-depth introduction to cladistics: what a cladogram means, how to draw one
Biology 4415 Evolution LABORATORY EXERCISE: CLADISTICS III The last lab and the accompanying lectures should have given you an in-depth introduction to cladistics: what a cladogram means, how to draw one
Whit I e y G. P The fishes of Michaelmas Cay, North Queensland. Ree. Austrar. Mus., v \V hit I e y G. P Studies in ichthyology no
 Whit I e y G. P. 1927. The fishes of Michaelmas Cay, North Queensland. Ree. Austrar. Mus., v. 16. 1 \V hit I e y G. P. 1932. Studies in ichthyology no 6.- Ree. Austral. Mus., v. 18. Whit 1 e y G. P. 1933.
Whit I e y G. P. 1927. The fishes of Michaelmas Cay, North Queensland. Ree. Austrar. Mus., v. 16. 1 \V hit I e y G. P. 1932. Studies in ichthyology no 6.- Ree. Austral. Mus., v. 18. Whit 1 e y G. P. 1933.
W. R. Heyer, 1 R. O. de Sá, 2 and A. Rettig 2. Herpetologia Petropolitana, Ananjeva N. and Tsinenko O. (eds.), pp
 Herpetologia Petropolitana, Ananjeva N. and Tsinenko O. (eds.), pp. 35 39 35 SIBLING SPECIES, ADVERTISEMENT CALLS, AND REPRODUCTIVE ISOLATION IN FROGS OF THE Leptodactylus pentadactylus SPECIES CLUSTER
Herpetologia Petropolitana, Ananjeva N. and Tsinenko O. (eds.), pp. 35 39 35 SIBLING SPECIES, ADVERTISEMENT CALLS, AND REPRODUCTIVE ISOLATION IN FROGS OF THE Leptodactylus pentadactylus SPECIES CLUSTER
Status of the Six-lined Racerunner (Aspidoscelis sexlineata) in Michigan
 Status of the Six-lined Racerunner (Aspidoscelis sexlineata) in Michigan Teresa A. Yoder, Ghada Sharif, Ann Sturtevant & Ernest Szuch University of Michigan-Flint Throughout its range, Aspidoscelis sexlineata:
Status of the Six-lined Racerunner (Aspidoscelis sexlineata) in Michigan Teresa A. Yoder, Ghada Sharif, Ann Sturtevant & Ernest Szuch University of Michigan-Flint Throughout its range, Aspidoscelis sexlineata:
Main Points. 2) The Great American Interchange -- dispersal versus vicariance -- example: recent range expansion of nine-banded armadillos
 Main Points 1) Mammalian Characteristics: Diversity, Phylogeny, and Systematics: -- Infraclass Eutheria -- Orders Scandentia through Cetacea 2) The Great American Interchange -- dispersal versus vicariance
Main Points 1) Mammalian Characteristics: Diversity, Phylogeny, and Systematics: -- Infraclass Eutheria -- Orders Scandentia through Cetacea 2) The Great American Interchange -- dispersal versus vicariance
