Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system
|
|
|
- Kristian Stevens
- 6 years ago
- Views:
Transcription
1 Morphological characterization of Cryptosporidium parvum life-cycle stages in an in vitro model system 13 H. BOROWSKI 1,R.C.A.THOMPSON 1 *, T. ARMSTRONG 1 and P. L. CLODE 2 1 WHO Collaborating Centre for the Molecular Epidemiology of Parasitic Infections, Veterinary and Biomedical Sciences, Murdoch University, South Street, Murdoch, WA 6150, Australia 2 Centre for Microscopy, Characterisation and Analysis, The University of Western Australia, 35 Stirling Hwy, Crawley, WA 6009, Australia (Received 30 January 2009; revised 25 May, 15 June and 19 June 2009; accepted 22 June 2009; first published online 20 August 2009) SUMMARY Cryptosporidium parvum is a zoonotic protozoan parasite that mainly affects the ileum of humans and livestock, with the potential to cause severe enteric disease. We describe the complete life cycle of C. parvum in an in vitro system. Infected cultures of the human ileocecal epithelial cell line (HCT-8) were observed over time using electron microscopy. Additional data are presented on the morphology, development and behavioural characteristics of the different life-cycle stages as well as determining their time of occurrence after inoculation. Numerous stages of C. parvum and their behaviour have been visualized and morphologically characterized for the first time using scanning electron microscopy. Further, parasite-host interactions and the effect of C. parvum on host cells were also visualized. An improved understanding of the parasite s biology, proliferation and interactions with host cells will aid in the development of treatments for the disease. Key words: Cryptosporidium parvum, morphology, host cell interaction, phylogenetic affinity, gregarines, electron microscopy. INTRODUCTION Cryptosporidium is a protozoan enteric parasite of humans and other vertebrates (Fayer et al. 1997). Numerous Cryptosporidium species have been described (Smith et al. 2005) most of which are specific to their vertebrate host. The species C. parvum is of medical and economic relevance as it affects both humans and cattle with its primary site of infection being the gastrointestinal tract. It affects the epithelial lining of the ileum, resulting in self-limiting diarrhoea in immunocompetent individuals or in life-threatening diarrhoeal diseases in immunocompromised individuals. Belonging to the phylum of apicomplexan parasites, Cryptosporidium shares common life-cycle features and morphological characteristics with other members of this phylum (Tetley et al. 1998). Initially, Cryptosporidium was categorized as a coccidian parasite (Levine, 1988). However, more recent studies show that Cryptosporidium lacks key morphological characteristics of coccidians and is insensitive to anti-coccidial agents (O Donoghue, 1995; Fayer et al. 1997; Carreno et al. 1999). Further * Corresponding author: WHO Collaborating Centre for the Molecular Epidemiology of Parasitic Infections, Veterinary and Biomedical Sciences, Murdoch University, South Street, Murdoch, WA 6150, Australia. Tel: +(08) Fax: +(08) a.thompson@ murdoch.edu.au phylogenomic analysis has since revealed that Cryptosporidium is most closely related to gregarines (Barta and Thompson, 2006). Cryptosporidium shares many features in common with gregarines, including an extracytoplasmic location and connection to the host cell via a myzocytosis-like feeding mechanism (Barta and Thompson, 2006). The primary difference between these two groups is that Cryptosporidium induces the host cell to overlay it with the host cell apical membrane (Barta and Thompson, 2006; Butaeva et al. 2006). The parasite appears on the surface of cells, residing in a parasitophorous vacuole (PV) between the cytoplasmic membrane and the apical membrane (Huang et al. 2004). Critically, the mechanisms of Cryptosporidium pathogenesis are not fully understood, but both parasite stimuli as well as host immune responses are thought to play critical roles (Barta and Thompson, 2006). The life cycle and the mechanisms of infection by Cryptosporidium have recently been reviewed in detail by Smith et al. (2005) and Borowski et al. (2008). Importantly, previous studies by Hijjawi et al. (2001, 2004) described the life cycle of C. parvum in vitro, using light microscopy while more recent studies by Valigurova et al. (2008) described the morphology of various life-cycle stages of 2 different Cryptosporidium species from mice and toads in vivo using electron microscopy. The aim of this study was to expand on this earlier work and to gain a better understanding of the biology and relationship with host cells of the Parasitology (2010), 137, f Cambridge University Press 2009 doi: /s Printed in the United Kingdom
2 H. Borowski and others 14 economically and medically important species, C. parvum. In this study the human ileocecal epithelial cell line HCT-8 was used as an in vitro model to monitor the developmental process of C. parvum and to study the effects upon target cells. Cryptosporidium was observed to proliferate in our culture system for 5 days. Hence, infected cells were monitored for this period, with data obtained using scanning (SEM) and transmission (TEM) electron microscopy. From this, a more complete life cycle of C. parvum has been visualized and the behaviour and morphological characteristics of numerous lifecycle stages described for the first time with the aid of SEM. MATERIALS AND METHODS Cell culture The C. parvum cattle isolate used during this study was originally obtained from the Institute of Parasitology, University of Zurich. Oocysts were subsequently passaged through, and purified from, infected ARC/Swiss mice as described by Meloni and Thompson (1996). For routine passaging, HCT-8 cells were cultured in RPMI medium with 2gL x1 sodium bicarbonate, 0. 3g L x1 L-glutamine, g L x1 HEPES buffer (15 mmol L x1 ) and 10% fetal calf serum (FCS) at ph 7. 4, 37 xc with 5% CO 2. Pre-treatment of oocysts C. parvum oocysts were bleached with 200 ml of household bleach in 10 ml of water for 30 min at room temperature (RT). Sterilized oocysts were inoculated into excystation medium (0. 5% trypsin, ph of 2. 5) for 30 min at 37 xc. Excysted oocysts were resuspended in maintenance medium consisting of the RPMI medium described above plus 3 g L x1 sodium bicarbonate, 0. 2gL x1 bovine salt, 1 g L x1 glucose, 250 mg L x1 folic acid, 1 mg L x1 4 amino benzoic acid, 500 mg L x1 calcium pentothenate, mg L x1 ascorbic acid and 1% FCS. Cell line infection and cell-free culture Twenty-four h prior to an infection of cells with C. parvum, HCT-8 cells were plated onto thermonox cover slips in 24-well plates. Each cell was then infected with C. parvum pre-treated oocysts ( per cm 2 ) in 1 ml of maintenance medium and maintained at 37 xc with 5% CO 2. Infected cultures were sampled and processed for microscopy at 6 h, 7 h, 24 h, 48 h, 72 h, 96 h and 120 h post-inoculation. Sample preparation for electron microscopy Cover-slips with adherent cells were fixed in 2. 5% glutaraldehyde in 1rPBS. Additionally, to investigate extracellular C. parvum stages present within the supernatant of infected cells, medium from cells was aspirated and added to an equal volume of 5% glutaraldehyde in 2rPBS. Cellular material within this supernatant was subsequently attached to poly-l-lysine coated glass cover-slips for SEM investigation by applying several drops of concentrated supernatant and incubating for 20 min. All samples were post-fixed in 1% OsO 4 in PBS, and then dehydrated in a graded series of ethanols using a Pelco Biowave Microwave Processor. Samples destined for SEM were then critical-point dried, mounted on stubs with carbon tabs, and coated with 3 nm platinum for high-resolution imaging. Samples destined for TEM were infiltrated and embedded in Spurr s Resin. Thermonox cover-slips were removed under liquid nitrogen, and samples re-embedded. Sections, approximately 100 nm thick, were cut on a diamond knife and mounted on copper grids. Imaging SEM images were acquired at 3 kv using the in lens secondary electron detector, on a Zeiss 1555VP field emission SEM. TEM sections were viewed unstained at 120 kv using a JEOL 2100 TEM. Images were digitally acquired with a Gatan SC1000 ORIUS digital camera. RESULTS AND DISCUSSION All recognized C. parvum life-cycle stages (Table 1) were observed on the surface of epithelial cells or in the supernatant. Small trophozoites (<1 mm) were observed as early as 6 h post-inoculation with welldistinguishable meronts I and free merozoites type I being observed after 24 h. This implies that oocyst excystation and sporozoite invasion must have occurred immediately post-inoculation. Consistent with this, previous studies by Forney et al. (1999), observed sporozoites invading host cells as early as 5 min post-inoculation at the point of sporozoite emergence from the oocyst. Subsequent infection increased over the next 2 3 days as a result of ongoing oocyst excystation, and the ability of C. parvum to replicate asexually. After 2 days post-infection, only inoculated oocysts, sporozoites, trophozoites, meronts I and merozoites type I were observed in culture. After 3 days, meronts II as well as merozoites type II were seen, while gametocytes were not observed until 4 days post-inoculation. This timeline of Cryptosporidium development correlates with previous light microscopic data from in vitro cultures of C. parvum (Hijjawi et al. 2001, 2002, 2004), as well as electron microscopy studies of Cryptosporidium sp. toad and C. muris from experimentally infected toads and mice respectively
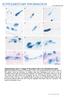
3 Morphological characterization of C. parvum in vitro 15 Table 1. Distinguishing characteristics of Cryptosporidium life-cycle stages Time (h) Stage Size Morphological Feature Fig. 0 Oocyst 5r7 mm Ovular, smooth surface with cleft for 1A sporozoite release >24 Excysted oocyst 5r7 mm Perforated surface 1B >3 Sporozoite 5r0. 5 mm Rough surface, pointed apical region 1B D (elongated when in proximity to host cells), rounded posterior region >6 Early trophozoite <1 mm Smooth surface formed by the host cell 1E apical membrane, hood like shape >24 Trophozoite 2. 5 mm Epicellular, smooth surface, electron dense 2A G band, feeder-organelle, PV, cytoplasmic granulation, hood like shape >24 Trophozoite Merging of apical membranes 2D G clusters engulfing individual parasites >24 Meronts I 1. 5 mm Epicellular, smooth surface 5A,B >24 Merozoites 0. 4r1 mm Rod like shape, pointed 5E H Type I apical region, rough surface >72 Meronts II 3. 5 mm Epicellular, smooth thick membrane 6A >72 Merozoites mm Round, rough surface 6A C Type II >96 Microgamont 2r2 mm Extracellular, densely packed with 8A C microgametes >96 Microgamete 0. 1 mm Spherical, rough surface 8A C >96 Macrogamont 4r5 mm Extracellular, ovular, rough surface 7A C 48 Extracellular 2 mm Forms within parent stage, rough surface 9A trophozoite 96 Extracellular meront <2 mm Round, extracellular accumulation of trophozoites 9B (Valigurova et al. 2008). This consistency with an in vivo system confirms the validity of our in vitro model system of C. parvum infection. In addition to the widely accepted life-cycle stages, we observed stages that are not commonly reported or known to date (Table 1). Such stages include trophozoites that formed within parent stages without host cell invasion, and extracellular accumulations of trophozoites. Oocyst excystation and sporozoite host cell invasion Following inoculation, intact C. parvum oocysts were only ever found in the supernatant and these were ovular, 5 mmr7 mm in size and possessed a smooth surface (Fig. 1A). This correlates with previous findings of Hijjawi et al. (2001). On the surface of these oocysts a cleft was visible and presumably it is along this cleft that the oocyst opens to release sporozoites during the excystation process. Excysted oocysts were observed adhered to cells after 24 h but not before. It is known that oocysts possess surface lectins that are thought to hinder their adherence to host tissue in vivo until the target tissue is reached where they then mediate attachment to host cells (Kuznar and Elimelech, 2006). In the present study, oocysts were directly applied to target cells but did not attach immediately. Perhaps without passage through the gastrointestinal tract, oocysts do not begin to express the surface lectins needed for host cell adherence until after extended exposure to host cells or simply until a certain progression of time after excystation stimuli are initiated. The outer membrane of excysted oocysts appeared rough (Fig. 1B). This is probably due to membrane perforation during the excystation processes. Sporozoites observed in this study measured about 5 mmr0. 5 mm and showed well-defined apical ends (Fig. 1B D). The hatching sporozoite shown in Fig. 1B as well as the sporozoite on the surface of the host cell in Fig. 1D, both showed an enlarged posterior region and a well-defined apical region, which appeared thin and elongated. Sporozoite apical organelle discharge is known to mediate host cell contact and according to previous findings already occurs during excystation (Snelling et al. 2007). As the sporozoites shown in Fig. 1B and D appear to be making host cell contact, apical organelle discharge of molecules, which are discharged in the presence of host cells, might account for the occurrence of this thin and elongated apical region. In contrast, sporozoites isolated from supernatant did not display this apical elongation and their shape remained largely regular along their entire length (Fig. 1C). Sporozoites were only observed up to 48 h post-inoculation. As sporozoites are known to invade host cells within 5 min after excystation (Forney et al. 1999) it can be assumed that after
4 H. Borowski and others 16 Fig. 1. Oocyst excystation and sporozoite host cell invasion. (A) Intact oocyst from supernatant, 3 h post-inoculation. (B) Oocyst excystation in vitro, 48 h post inoculation. (C) Free sporozoite isolated from supernatant 3 h post-inoculation. (D) Free sporozoite on host cell, 7 h post-inoculation. Arrows indicate the apical regions of sporozoites. (E) Encapsulated by the host cell apical membrane, invading sporozoites transform into the trophozoite stage epicellularly, at 6 h and 24 h respectively. Scale bars: (A) 4 mm; (B) 2 mm; (C,D,E) 1 mm. 48 h all oocysts have excysted and that no new sporozoites are produced. Invasion of host cells by sporozoites is shown in Fig. 1E. Invading sporozoites eventually become completely encapsulated by the host cell apical membrane, which initially appeared as a hood-like structure at this early invasion stage (Fig. 1E). A similar process has also been described in the in vivo toad system by Valigurova et al. (2008). As part of the infection process, sporozoites transform into the trophozoite stage and undergo merogony, which leads to trophozoite growth. This initial trophozoite stage measured <1 mm in diameter (Fig. 1E) and was observed as early as 6 h post-inoculation, showing that host cell infection followed by parasite development occurs rapidly after parasite-host tissue contact. Trophozoites on the host cell surface 48 h post-inoculation During the invasion process, C. parvum sporozoites always remain epicellular and appear to transform into the trophozoite stage extracytosolic. Thus, stages that result from host cell invasion show a similar cellular location as gregarines, with the only difference being that Cryptosporidium becomes encapsulated by a host cell apical membrane (Fig. 2A G; Barta and Thompson, 2006). Trophozoites shown in
5 Morphological characterization of C. parvum in vitro 17 Fig. 2. Trophozoites on the host cell surface 48 h post-inoculation. (A) Cross-section through an early trophozoite showing the feeder-organelle (arrow) attachment to the host cell cytosol. (B) Mature trophozoite. (C) Cross-section through a mature trophozoite revealing the electron-dense band (arrow) that separates the parasite from the host cell. (D,E,F,G) Accumulations of trophozoites on the host cell surface. Scale bars: (A) 0. 5 mm; (B,C,G) 1 mm; (D,E,F) 2 mm. Fig. 2A G may have developed from either sporozoites or from merozoites type I after host cell invasion. Trophozoites varied in size depending upon their developmental stage. Early trophozoites observed from 6 h post-inoculation onwards, were f1 mm in size (Fig. 1E), whereas well-developed trophozoites observed after 24 h post-inoculation, were up to 2. 5 mm (Fig. 2B). These mature trophozoites appeared attached to the surface of cells, but were separated from the host cell via an electrondense band (Fig. 2C). The formation of a feeder organelle structure is hypothesized for all C. parvum stages and is shown here in Fig. 2A. Previous morphological studies by Huang et al. (2004) have already described this extracytosolic location of the parasite and its feeder organelle attachment. Our data show sporozoites becoming engulfed by the host cell apical membrane. This phenomenon was proposed (Perkins et al. 1999; Elliott and Clark, 2000; Pollok et al. 2003; Chen et al. 2004a, b, 2005; Hashim et al. 2006) and recently confirmed by Valigurova et al. (2008). It has been suggested that the parasite induces the host cell to encapsulate itself with the host cell apical membrane (Borowski et al. 2008) to escape the hosts defence mechanisms. Trophozoites were always found to reside within a PV and to be engulfed by the host cell apical membrane, which displayed a smooth surface. In later stages of trophozoite development, the basal membrane developed a hood-like structure and cytoplasmic granulation occurred leading to merozoite development within the trophozoite (Fig. 2A and B). Single trophozoites were regularly seen attached to infected cells (Fig. 2B), but accumulations of 2 or more trophozoites were more frequently observed (Fig. 2D G). The 4 trophozoites shown in Fig. 2D are likely to have developed from sporozoites that simultaneously excysted from a single oocyst. However, the 6 trophozoites observed in Fig. 2E are more likely to have resulted from merozoite type I host cell invasion, as one meront I is believed to contain 6 or 8 merozoites (Hijjawi et al. 2001). A merging of membranes engulfing 2 or more parasites was also often observed (Fig. 2D G). This leads to the assumption that clusters of 2 or more trophozoites result from invasion of infective zoites (sporozoites or merozoites) in closest proximity. Hijjawi et al. (2004) made the observation that zoite stages commonly accumulate in cell-free cultures. Possibly, there is a weak unspecific binding between infective
6 H. Borowski and others 18 Fig. 3. The parasite s effect on host cell microvilli. (A) Gliding trail composed of elongated microvilli between an excysted oocyst and trophozoites 3 days post-inoculation. (B,C) Abnormal microvilli clusters surround trophozoites 48 h post-inoculation. Scale bars: 2 mm. zoites (Fig. 6A C). Chen et al. (2000) reported that zoite stages express surface lectins that have adhesive properties. It is possible therefore, that these lectins also facilitate the attachment of infective zoites to each other. Taken together, these data suggest that excysted sporozoites and merozoites can be adherent to each other during host cell attachment and invasion of cells (Fig. 6C). This close proximity of invading stages results in the merging of membranes between individuals, forming clusters of rapidly growing trophozoites. The effect on host cell microvilli Protozoan parasites possess gliding motility. Gliding motility of sporozoites has been observed in previous studies (Barnes et al. 1998; Riggs et al. 1999; Wetzel et al. 2005) leading to the suggestion that gliding along the host cell surface is a prerequisite to host cell invasion. C. parvum gliding trails along host cell surfaces were observed in this study (Fig. 3A). These gliding trails were evident as trails of elongated microvilli between an excysted oocyst and newly formed trophozoites; extending up to 15 mm. As C. parvum is known to utilize microvilli material to establish itself in its niche (Bonnin et al. 1995), it can be suggested that gliding sporozoites use microvilli material for their gliding motion and thus cause this abnormality seen in gliding trails. As gliding trails were not observed in all cases of oocyst excystation, it appears that not all sporozoites glide along the host membrane surface until they are ready to invade a cell at a particular area. Some sporozoites are thought to invade directly at their origin of excystation (Fig. 2F), exhibiting gliding movements in a small area only, whereas other sporozoites might even travel through the culture medium (Fig. 1C) before establishing host cell contact and invading cells some distance from excystation. Microvillus abnormality has not only been observed in gliding trails, but also around developing trophozoites (Fig. 3B and C). Abnormally abundant microvilli were frequently found to surround trophozoites (Fig. 3B) and in some cases were significantly elongated (Fig. 3C). As the expression of microvilli around trophozoite clusters was found to be higher than in non-infected areas, C. parvum might even induce the production of microvilli to satisfy its need for microvillar components. Microvilli of infected cells were also seen to appear to
7 Morphological characterization of C. parvum in vitro 19 C Fig. 4. Cryptosporidium parvum binary fission and syzygy. (A,B) Binary fission of C. parvum stages 4 days post-inoculation. (C,D) C. parvum syzygy 5 days post-inoculation. Basal discs are indicated by arrows. Scale bars: (A,B,C) 2 mm; (D) 1 mm. become incorporated into the membrane engulfing the parasites and to aid in securing the parasite to the host cell surface (Fig. 2B and G). This phenomenon has not been described before, but microvillar material has been identified in the membrane components engulfing the parasite using molecular techniques (Bonnin et al. 1995). Binary fission and syzygy in the C. parvum life cycle Additional C. parvum stages were also found that were characteristically different from those described above. Figure 4A and B raise the possibility that C. parvum may undergo binary fission, i.e. the splitting of a parent cell into 2 daughter cells, other than during the course of merogony and shizogony. In Fig. 4A, two microgamonts that seem to be in the process of binary fission, are engulfed by a single membrane that may have initially encapsulated the parent trophozoite on the host cell surface. The finding of 2 parasites that appear to be dividing from 1 parent and seem to be the same life-cycle stage (Fig. 4A), demonstrates that C. parvum possibly undergoes asexual reproduction other than in the course of merogony. As explained below, as C. parvum progresses through its life cycle, it appears to become more extracellular. This is evident in stages that appear to have little or no attachment to host cells (Fig. 4A C). All of these stages are likely to be microgamonts, one of which is clearly visible in Fig. 4A. Microgamonts occur later in the life cycle once sexual reproduction has been initiated. Thus, these stages show a more extracellular location. Our observations raise the question whether C. parvum stages with more epicellular location are still attached to and encapsulated by the host cell apical membrane, or have broken contact with host cells, but still retain the surrounding outer host cell membrane. Our data also indicate that C. parvum might employ syzygy (Fig. 4C). Syzygy is defined as the association of gamonts (pre-gametes) end-to-end or in lateral pairing prior to the formation of gametes that may be employed to ensure genetic diversity and has been described in gregarines (Landers, 2001; Lacombe et al. 2002; Barta and Thompson, 2006; Toso and Omoto, 2007). Thus, it is reasonable that syzygy may occur in closely related Cryptosporidium species. Connecting discs between adjacent parasites are visible (Fig. 4C and D) and these appear to be the same as the basal discs already described in Cryptosporidium by Valigurova et al. (2008). The C. parvum stages involved in syzygy shown in
8 H. Borowski and others 20 Fig. 5. Meronts I and merozoites type I. (A,B) Developing meronts I with internal merozoites type I. (C,D) Mature meronts I at the stage of merozoite I excystation. (E,F,G) Free merozoites type I showing well-defined apical regions (arrow) at 6 h of culture. (H) Merozoite type I host cell invasion 6 h post-inoculation. Scale bars: (A,B,C,E,F) 1 mm; (D) 2 mm; (G,H) 0. 5 mm. Fig. 4C and D are probably microgamonts. As syzygy was first observed when sexual life-cycle stages occurred in culture, it can be suggested that predominantly gamont stages (here microgamonts) employ syzygy. This correlates with findings on gregarines and further supports the affinity of Cryptosporidium with these apicomplexans (Landers, 2001; Toso and Omoto, 2007). Meronts and merozoites Trophozoites that result from sporozoite host cell invasion develop into meronts I. Meronts I with visible internal merozoites type I, were observed as early as 24 h post-inoculation (Fig. 5A and B). Like trophozoites, meronts I were found to be attached to host cells and engulfed by the host cell apical membrane (Fig. 5A and B). Developing meronts measured approximately 1. 5 mm in diameter (Fig. 5A and B), whereas mature meronts at the stage of merozoite release were larger, measuring 2. 5 mm (Fig. 5C and D). From this, it appears that trophozoites developing into meronts I undergo merogony and begin to form internal merozoites, before they have reached their full size. Merozoites seemed to be aligned in a parallel orientation within intact meronts I (Fig. 5A and B) which is consistent with observations by Hijjawi et al. (2001). Once excysted, the merozoites type I in a meront numbered 6 or 8, which also correlates with previous findings by Hijjawi et al. (2001). Excysting merozoites type I showed a rod-like shape with a pointed apical region. In contrast to excysting oocysts, the membranes of meronts I appear not to become perforated to
9 Morphological characterization of C. parvum in vitro 21 Fig. 6. Meront II and merozoites type II. (A) Excysting meront II with internal merozoites type II (arrow) 3 days post-inoculation. (B) Merozoite type II host cell invasion. (C) Pairing of a merozoite type II with a merozoite type I 9 h post-inoculation. Scale bars: (A) 1 mm; (B,C) 0. 5 mm. facilitate infective zoite release. During merozoite release, the membranes engulfing the parasite still appeared smooth (Fig. 5C and D) and seemed to either open up (Fig. 5 D) or rupture (Fig. 5C). When merozoites type I are released from the meront a residual body is left behind (Fig. 5C). Similar observations have been made on the Cryptosporidium sp. toad model (Valigurova et al. 2008). Free merozoites type I were seen in cell culture from 24 h post-inoculation onwards (Fig. 5E H). Merozoites, which previously were probably incorrectly referred to as microgametes (Thompson et al. 2005) type I were 0. 4 mmr1 mm, with both a rounded and a pointed end (Fig. 5E H). The pointed end appeared similar to that of sporozoites, which houses the apical organelles for host cell invasion (Tetley et al. 1998). These free merozoites must be adhered to the host cells in some manner (Fig. 5E 5H), otherwise they would have been removed during sample preparation. Surface lectins which have been detected on infective zoite stages (Chen et al. 2000) might mediate this attachment to host tissue. Merozoites appear initially to adhere to host cells along their full body length, but become stouter as cellular invasion occurs (Fig. 5H). Thus it can be hypothesized that after initial host cell attachment involving surface lectins, a re-orientation of merozoite organelles occurs, bringing the apical complex into host cell contact to initiate cellular invasion. Once merozoites type I are present, the parasite employs 2 ways to replicate in its host which both occur concurrently: (i) it progresses via asexual reproduction by the formation of meronts I and (ii) it progresses via sexual reproduction by formation of meronts II which then further develop into microand macrogamonts (Borowski et al. 2008). Meronts II as well as merozoites type II were observed in culture from 72 h post-inoculation. Meronts II were found to be bigger than meronts I measuring 3. 5 mm in diameter (Fig. 6). Meronts II appeared to possess a thicker outer membrane than
10 H. Borowski and others 22 Fig. 7. Macrogamonts and their attachment zones. (A) Macrogamont with feeder organelle (arrow) 4 days post-inoculation. (B) Developing macrogamont with feeder organelle (arrow). (C) Cryptosporidium parvum attachment zones 5 days post-inoculation. Scale bars: (A,C) 2 mm; (B) 1 mm. meronts I (Fig. 6A). Merozoites type II were round and measured between 0. 5 mm and 1 mm in diameter (Fig. 6A C). Similar to meronts I, the membrane engulfing the merozoites appeared smooth and not perforated for their release (Fig. 6A) suggesting the host cell origin of this membrane. Similar to the adhesion of sporozoites to each other via surface lectins, merozoites type I and type II can also adhere to each other and co-invade cells (Fig. 6B and C). Microgamonts and macrogamonts Merozoites type II that are released in culture, invade cells to transform into either micro- or macrogamonts. Four days post-inoculation microand macrogamonts were observed for the first time (Figs 7 and 8). Both gamont stages appeared to have less contact with host cells than trophozoites or meronts. This supports our suggestion that the further the parasite progresses in its life cycle, the more extracellular it appears to become. Macrogamonts were ovular and measured about 4 mmr5 mm (Fig. 7A). Feeder organelle attachment of a macrogamont is visible in Fig. 7A. The surface of this macrogamont appeared rough, suggesting that it has already broken host cell contact leaving the host cell-derived membrane that once surrounded it behind. Such empty attachment-zones often show a ring-like structure (Fig. 7C), presumably where feeding organelles were attached. These findings correlate with observations by Valigurova et al. (2008) who described a similar extracellular location of macrogamonts, their detachment from host cells, the granular structure of their feeder organelles, as well as detachment zones in vivo, in their Cryptosporidium sp. toad model. Microgamonts were rounder and smaller than macrogamonts, measuring about 2 mmr2 mm (Fig. 8A C). They contained a large number of microgametes (Fig. 8A and C), which are released to fertilize macrogamonts. This observation is not consistent with findings by Hijjawi et al. (2001), who identified only 16 microgametes in 1 microgamont with light microscopy. This difference is likely to be due to the difficulty resolving such structures with light microscopy. Microgametes measure about 0. 1 mm in diameter and are spherical. Hijjawi et al. (2001) believed that they were non-flagellated which is confirmed by our SEM study. The microgamonts shown in Fig. 8A seem to be surrounded by presumably host cell-derived membrane. Fig. 8C shows
11 Morphological characterization of C. parvum in vitro 23 Fig. 8. Microgamonts. (A,B) Microgamonts without stalk. The arrows in A indicate a cleft along which the macrogamonts open. (C,D) Microgamonts with stalk (arrow). Scale bars: (A,B) 1 mm; (C,D) 2 mm. a feeder organelle attachment of the same origin as that of the macrogamont in Fig. 7A. Approximately half of all microgamonts seen in culture possessed a stalk-like structure that either appeared as if the stalk had broken (Fig. 8D) or seemed to attach the parasite to the host cell (Fig. 8C). Again, a similar finding has also been reported by Valigurova et al. (2008). This stalk on microgamonts might result from the parasite being released from the attachment zones described above in a similar manner to macrogamonts. The microgamonts that possess a stalk (Fig. 8C and D) appeared to have less host cell contact compared to those which did not show such a structure (Fig. 8A and B). Additionally, their membrane appeared perforated (Fig. 8C) in contrast to microgamonts without this stalk. Like trophozoites, microgamonts were also observed to occur in clusters of 2 or more. The accumulation of microgamonts (Fig. 8D) probably resulted from microgamonts that had released from their attachment zone and adhered to each other, whereas the 2 adjacent microgamonts in Fig. 8B are more likely to be a result of merozoite type II host cell invasion in close proximity. Development of C. parvum in host cell culture without invasion of host cells The present study is consistent with the intracellular life cycle of C. parvum described by Hijjawi et al. (2001). The stages described above all resulted from host cell invasion, yet, complete cell-free development of C. parvum has also been documented at the light microscope level (Hijjawi et al. 2004) in vitro. From our own observations, we hypothesize that extracellular life-cycle stages might be part of the C. parvum life cycle, or co-exist resembling rudimentary stages of an ancestral life cycle. Our data show that C. parvum can develop extracellularly in the presence of host cells in an in vitro model, without invasion. We have observed stages of C. parvum that have been described before at the light microscopic level but are not as yet considered as part of the life cycle. Life-cycle stages of C. parvum that developed extracellularly showed a rough surface like that of previously described free parasite stages (Fig. 5E H and Fig. 6A C), and they did not appear to be engulfed by the host cell apical membrane.
12 H. Borowski and others 24 undergoing merogony and transforming into a trophozoite stage, without invasion of host cells (Fig. 9A). This suggestion is supported by our findings of a proposed extracellular meront 4 days post-inoculation (Fig. 9B). The meront measured approximately 8 mm in diameter and was round in shape. The zoite stages observed within the meront, measured up to 2 mm and appeared to be densely packed. It is likely that this extracellular meront has formed through the clumping of infective zoite stages in culture, of which each single one has undergone merogony to form a trophozoite. In support of this, accumulations of merozoites and/or trophozoites, as well as the formations of meronts in cellfree culture, have already been described (Hijjawi et al. 2004). Critically, how extracellular trophozoites and meronts progress in their life cycle without cellular invasion is still not understood. Each extracellular trophozoite might progress in a manner typical of an intracellular trophozoite, or each single trophozoite might transform into the next life-cycle stage. The observation that C. parvum also develops extracellularly, despite the presence of host cells, further supports the proposal that Cryptosporidium has a close affinity with gregarines (Carreno et al. 1999; Barta and Thompson, 2006; Valigurova et al. 2007). CONCLUSIONS Fig. 9. Development of Cryptosporidium parvum in host cell culture without the invasion of host cells. (A) Trophozoite development within an inoculated oocyst after 2 days. (B) Possible extracellular meront after 4 days of culture. Scale bars: (A) 2 mm; (B) 1 mm. After 48 h inoculation, we observed what we believe to be trophozoite development directly within an oocyst (Fig. 9A). The membrane of the oocyst releasing the trophozoites appeared rough and perforated, a characteristic already observed during the release of sporozoites (Fig. 1B). Similar to these so-called intracellular trophozoites, trophozoites that formed directly within an oocyst measured approximately 2 mm. As these stages were observed as early as 48 h post-inoculation (Fig. 9A) it can be hypothesized that the trophozoites developed within an inoculated oocyst, and were eventually released into culture. From our observations it can be suggested that each sporozoite and each merozoite is capable of For the first time, our study reveals the morphology of each stage in the currently accepted life cycle of C. parvum. The development of life-cycle stages described here extends previous reports that were based on light microscopic findings (Hijjawi et al. 2001, 2002, 2004). Data from our in vitro model system are further supported by similar morphological observations from in vivo studies on C. muris and Cryptosporidium sp. toad (Valigurova et al. 2008). Surprisingly, the species C. parvum observed in our study shows more similarities to the species Cryptosporidium sp. toad than to C. muris (Valigurova et al. 2008). Apart from revealing the morphology of accepted life-cycle stages in C. parvum our study describes previously unreported features of C. parvum lifecycle stages, which include their morphological structure and interaction with host cells. Further, extracellular stages of C. parvum are reported and characterized for the first time in an in vitro model of cultured host cells at an electron microscopic level. It is not clear whether extracellular stages of C. parvum occur in vivo, and whether they are part of the parasite s life cycle or represent rudimentary stages of an ancestral life cycle. Future studies are needed to identify the existence of these stages in vivo and clarify their nature.
13 Morphological characterization of C. parvum in vitro 25 REFERENCES Barnes, D. A., Bonnin, A., Huang, J. X., Gousset, L., Wu, J., Gut, J., Doyle, P., Dubremetz, J. F., Ward, H. and Petersen, C. (1998). A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Molecular and Biochemical Parasitology 96, Barta, J. R. and Thompson, R. C. A. (2006). What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends in Parasitology 22, Bonnin, A., Gut, J., Dubremetz, J. F., Nelson, R. G. and Camerlynck, P. (1995). Monoclonal antibodies identify a subset of dense granules in Cryptosporidium parvum zoites and gamonts. The Journal of Eukaryotic Microbiology 42, Borowski, H., Clode, P. L. and Thompson, R. C. A. (2008). Active invasion and/or encapsulation? A reappraisal of host-cell parasitism by Cryptosporidium. Trends in Parasitology 24, Butaeva, F., Paskerova, G. and Entzeroth, R. (2006). Ditrypanocystis sp. (Apicomplexa, Gregarinia, Selenidiidae): the mode of survival in the gut of Enchytraeus albidus (Annelida, Oligochaeta, Enchytraeidae) is close to that of the coccidian genus Cryptosporidium. Tsitologiia 48, Carreno, R. A., Martin, D. S. and Barta, J. R. (1999). Cryptosporidium is more closely related to the gregarines than to coccidia as shown by phylogenetic analysis of apicomplexan parasites inferred using small-subunit ribosomal RNA gene sequences. Parasitology Research 85, Chen, X. M., Huang, B. Q., Splinter, P. L., Orth, J. D., Billadeau, D. D., McNiven, M. A. and LaRusso, N. F. (2004a). Cdc42 and the actin-related protein/neural Wiskott-Aldrich syndrome protein network mediate cellular invasion by Cryptosporidium parvum. Infection and Immunity 72, Chen, X. M. and LaRusso, N. F. (2000). Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology 118, Chen, X. M., O Hara, S. P., Huang, B. Q., Splinter, P. L., Nelson, J. B. and LaRusso, N. F. (2005). Localized glucose and water influx facilitates Cryptosporidium parvum cellular invasion by means of modulation of host-cell membrane protrusion. Proceedings of the National Academy of Sciences, USA 102, Chen, X. M., Splinter, P. L., Tietz, P. S., Huang, B. Q., Billadeau, D. D. and LaRusso, N. F. (2004b). Phosphatidylinositol 3-kinase and frabin mediate Cryptosporidium parvum cellular invasion via activation of Cdc42. The Journal of Biological Chemistry 279, Elliott, D. A. and Clark, D. P. (2000). Cryptosporidium parvum induces host cell actin accumulation at the host-parasite interface. Infection and Immunity 68, Fayer, R., Speer, C. A. and Dubey, J. P. (1997). The general biology of Cryptosporidium. In Cryptosporidium and Cryptosporidiosis (ed. Fayer, R.), pp CRC Press LLC, New York, USA. Forney, J. R., DeWald, D. B., Yang, S., Speer, C. A. and Healey, M. C. (1999). A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infection and Immunity 67, Hashim, A., Mulcahy, G., Bourke, B. and Clyne, M. (2006). Interaction of Cryptosporidium hominis and Cryptosporidium parvum with primary human and bovine intestinal cells. Infection and Immunity 74, Hijjawi, N. S., Meloni, B. P., Morgan, U. M. and Thompson, R. C. A. (2001). Complete development and long-term maintenance of Cryptosporidium parvum human and cattle genotypes in cell culture. International Journal for Parasitology 31, Hijjawi, N. S., Meloni, B. P., Ryan, U. M., Olson, M. E. and Thompson, R. C. A. (2002). Successful in vitro cultivation of Cryptosporidium andersoni: evidence for the existence of novel extracellular stages in the life cycle and implications for the classification of Cryptosporidium. International Journal for Parasitology 32, Hijjawi, N. S., Meloni, B. P., Ng anzo, M., Ryan, U. M., Olson, M. E., Cox, P. T., Monis, P. T. and Thompson, R. C. A. (2004). Complete development of Cryptosporidium parvum in host cell-free culture. International Journal for Parasitology 34, Huang, B. Q., Chen, X. M. and LaRusso, N. F. (2004). Cryptosporidium parvum attachment to and internalization by human biliary epithelia in vitro: a morphologic study. The Journal of Parasitology 90, Kuznar, Z. A. and Elimelech, M. (2006). Cryptosporidium oocyst surface macromolecules significantly hinder oocyst attachment. Environmental Science and Technology 40, Lacombe, D., Jakowska, S. and Silva, E. (2002). Gregarine Cephaloidophora communis mawrodiadi, 1908 in the barnacle Euraphia rhyzophorae, Oliveira, 1940 from Brazil. Memórias do Instituto Oswaldo Cruz 97, Landers, S. C. (2001). Pterospora floridiensis, a new species of acephaline gregarine (Apicomplexa) from the maldanid polychaete Axiothella mucosa in St. Andrew Bay, Florida. Systematic Parasitology 48, Levine, N. D. (1988) The Protozoan Phylum Apicomplexa, Vols 1 and 2. CRC, Boca Raton, FL, USA. Meloni, B. P. and Thompson, R. C. A. (1996). Simplified methods for obtaining purified oocysts from mice and for growing Cryptosporidium parvum in vitro. The Journal of Parasitology 82, O Donoghue, P. J. (1995). Cryptosporidium and cryptosporidiosis in man and animals. International Journal for Parasitology 25, Perkins, M. E., Riojas, Y. A., Wu, T. W. and Le Blancq, S. M. (1999). CpABC, a Cryptosporidium parvum ATP-binding cassette protein at the host-parasite boundary in intracellular stages. Proceedings of the National Academy of Sciences, USA 96,
14 H. Borowski and others 26 Pollok, R. C., McDonald, V., Kelly, P. and Farthing, M. J. (2003). The role of Cryptosporidium parvum-derived phospholipase in intestinal epithelial cell invasion. Parasitology Research 90, Riggs, M. W., McNeil, M. R., Perryman, L. E., Stone, A. L., Scherman, M. S. and O Connor, R. M. (1999). Cryptosporidium parvum sporozoite pellicle antigen recognized by a neutralizing monoclonal antibody is a beta-mannosylated glycolipid. Infection and Immunity 67, Smith, H. V., Nichols, R. A. and Grimason, A. M. (2005). Cryptosporidium excystation and invasion: getting to the guts of the matter. Trends in Parasitology 21, Snelling, W. J., Lin, Q., Moore, J. E., Millar, B. C., Tosini, F., Pozio, E., Dooley, J. S. and Lowery, C. J. (2007). Proteomics analysis and protein expression during sporozoite excystation of Cryptosporidium parvum (Coccidia, Apicomplexa). Molecular and Cellular Proteomics: MCP 6, Tetley, L., Brown, S. M., McDonald, V. and Coombs, G. H. (1998). Ultrastructural analysis of the sporozoite of Cryptosporidium parvum. Microbiology 144, Thompson, R. C. A., Olson, M. E., Zhu, G., Enomoto, S., Abrahamsen, M. S. and Hijjawi, N. S. (2005). Cryptosporidium and cryptosporidiosis. Advances in Parasitology 59, Toso, M. A. and Omoto, C. K. (2007). Ultrastructure of the Gregarina niphandrodes nucleus through stages from unassociated trophozoites to gamonts in syzygy and the syzygy junction. The Journal of Parasitology 93, Valigurova, A., Hofmannova, L., Koudela, B. and Vavra, J. (2007). An ultrastructural comparison of the attachment sites between Gregarina steini and Cryptosporidium muris. The Journal of Eukaryotic Microbiology 54, Valigurova, A., Jirku, M., Koudela, B., Gelnar, M., Modry, D. and Slapeta, J. (2008). Cryptosporidia: epicellular parasites embraced by the host cell membrane. International Journal for Parasitology 38, Wetzel, D. M., Schmidt, J., Kuhlenschmidt, M. S., Dubey, J. P. and Sibley, L. D. (2005). Gliding motility leads to active cellular invasion by Cryptosporidium parvum sporozoites. Infection and Immunity 73,
Phylum:Apicomplexa Class:Sporozoa
 Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Phylum:Apicomplexa Class:Sporozoa The most characteristic features of sporozoa are 1-unique appearance of most protozoa makes it possible for knowledge able person to identifiy them to level of genus and
Apicomplexans Apicomplexa Intro
 Apicomplexans Apicomplexa Intro Cryptosporidium Apicomplexan Select Characteristics Gliding motility Apical Complex organelle for invasion of host cell Life cycle alternates b/w sexual and asexual phases
Apicomplexans Apicomplexa Intro Cryptosporidium Apicomplexan Select Characteristics Gliding motility Apical Complex organelle for invasion of host cell Life cycle alternates b/w sexual and asexual phases
Protozoa. Apicomplexa Sarcomastigophora Ciliophora. Gregarinea Coccidia Piroplasma
 Protozoa Apicomplexa Sarcomastigophora Ciliophora Gregarinea Coccidia Piroplasma Coccidia characterized by thick-walled oocysts excreted in feces In Humans Cryptosporidium Isospora Cyclospora Sarcocystis
Protozoa Apicomplexa Sarcomastigophora Ciliophora Gregarinea Coccidia Piroplasma Coccidia characterized by thick-walled oocysts excreted in feces In Humans Cryptosporidium Isospora Cyclospora Sarcocystis
1) Most common, infectious, pathogenic animal (zoonotic) parasite of humans; estimated that 13% of humans are infected
 XX Phylum Apicomplexa (Chapter 8) 2005 A. Characteristics 1. All are parasitic 2. APICAL COMPLEX a. Group of organelles used to invade host cells b. Visible only with electron microscopy Picture Slide
XX Phylum Apicomplexa (Chapter 8) 2005 A. Characteristics 1. All are parasitic 2. APICAL COMPLEX a. Group of organelles used to invade host cells b. Visible only with electron microscopy Picture Slide
Coccidia. Nimit Morakote, Ph.D.
 Coccidia Nimit Morakote, Ph.D. 1 Learning objectives After class, students will be able to: Describe morphology, life cycle, signs and symptoms, prevention and control, laboratory diagnosis and treatment
Coccidia Nimit Morakote, Ph.D. 1 Learning objectives After class, students will be able to: Describe morphology, life cycle, signs and symptoms, prevention and control, laboratory diagnosis and treatment
Diagnosis, treatment and control: dealing with coccidiosis in cattle
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Diagnosis, treatment and control: dealing with coccidiosis in cattle Author : Adam Martin Categories : Vets Date : January
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Diagnosis, treatment and control: dealing with coccidiosis in cattle Author : Adam Martin Categories : Vets Date : January
Apicomplexa of Intestinal Pathology
 LECTURES #4, #5 & #6: APICOMPLEXA 1 Apicomplexa of Intestinal Pathology Cryptosporidium, Eimeria, Cystoisospora General Characteristics of Apicomplexa A. Morphology by stage Zoite o Tear-shaped (cylindrical
LECTURES #4, #5 & #6: APICOMPLEXA 1 Apicomplexa of Intestinal Pathology Cryptosporidium, Eimeria, Cystoisospora General Characteristics of Apicomplexa A. Morphology by stage Zoite o Tear-shaped (cylindrical
PLASMODIUM MODULE 39.1 INTRODUCTION OBJECTIVES 39.2 MALARIAL PARASITE. Notes
 Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Plasmodium MODULE 39 PLASMODIUM 39.1 INTRODUCTION Malaria is characterized by intermittent fever associated with chills and rigors in the patient. There may be enlargement of the liver and spleen in the
Key words: Coccidia, Choleoeimeria rochalimai, fine structure, gall bladder epithelium, Hemidactylus mabouia, Brazil
 FOLIA PARASITOLOGICA 47: 91-96, 2000 Ultrastructural study of meronts and gamonts of Choleoeimeria rochalimai (Apicomplexa: Eimeriidae) developing in the gall bladder of the gecko Hemidactylus mabouia
FOLIA PARASITOLOGICA 47: 91-96, 2000 Ultrastructural study of meronts and gamonts of Choleoeimeria rochalimai (Apicomplexa: Eimeriidae) developing in the gall bladder of the gecko Hemidactylus mabouia
HISTOPATHOLOGY. Introduction:
 Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
Introduction: HISTOPATHOLOGY Goats and sheep are the major domestic animal species in India. Much of the economy of the country has been depend upon the domestication of these animals. Especially economy
SCANNING electron - microscopy has
 Characteristics of the Absorptive Surface of the Small Intestine of the Chicken from 1 Day to 14 Weeks of Age 1 R. C. BAYER, C. B. CHAWAN, F. H. BIRD AND S. D. MUSGRAVE Department of Animal and Veterinary
Characteristics of the Absorptive Surface of the Small Intestine of the Chicken from 1 Day to 14 Weeks of Age 1 R. C. BAYER, C. B. CHAWAN, F. H. BIRD AND S. D. MUSGRAVE Department of Animal and Veterinary
for presence of cryptosporidia by microscopy using aniline-carbol-methyl violet staining, and Cryptosporidium
 doi: http://folia.paru.cas.cz Research Article Cryptosporidium testudinis sp. n., Cryptosporidium ducismarci Traversa, 2010 and Cryptosporidium tortoise genotype III (Apicomplexa: Cryptosporidiidae) in
doi: http://folia.paru.cas.cz Research Article Cryptosporidium testudinis sp. n., Cryptosporidium ducismarci Traversa, 2010 and Cryptosporidium tortoise genotype III (Apicomplexa: Cryptosporidiidae) in
Giardia and Apicomplexa. G. A. Lozano UNBC
 Giardia and Apicomplexa G. A. Lozano UNBC NINE Protozoan diseases/parasites Ciliphora, Ichthyophthirius, Ick Sarcomastigophora, Giardia, giardiasis Apicomplexa: Eimeria, Toxoplasma, Sarcocystis, Cryptosporidium.
Giardia and Apicomplexa G. A. Lozano UNBC NINE Protozoan diseases/parasites Ciliphora, Ichthyophthirius, Ick Sarcomastigophora, Giardia, giardiasis Apicomplexa: Eimeria, Toxoplasma, Sarcocystis, Cryptosporidium.
Cryptosporidium: Cryptosporidium: Director, UK Cryptosporidium Reference Unit the global challenge in monit toring
 Cryptosporidium: still cryptic after all these years Dr Rachel Chalmers Cryptosporidium: Director, UK Cryptosporidium Reference Unit the global challenge in monit toring urtesy of the Francis A. Countway
Cryptosporidium: still cryptic after all these years Dr Rachel Chalmers Cryptosporidium: Director, UK Cryptosporidium Reference Unit the global challenge in monit toring urtesy of the Francis A. Countway
Sarcocystis heydorni, n. sp. (Apicomplexa: Protozoa) with cattle (Bos taurus) and human
 1 Sarcocystis heydorni, n. sp. (Apicomplexa: Protozoa) with cattle (Bos taurus) and human (Homo sapiens) cycle Jitender P. Dubey 1, Erna van Wilpe 2, Rafael Calero-Bernal 1, Shiv Kumar Verma 1, Ronald
1 Sarcocystis heydorni, n. sp. (Apicomplexa: Protozoa) with cattle (Bos taurus) and human (Homo sapiens) cycle Jitender P. Dubey 1, Erna van Wilpe 2, Rafael Calero-Bernal 1, Shiv Kumar Verma 1, Ronald
Key words: Plasmodium, Kentropyx calcarata, Brazil, merogony, gametocytes, ultrastructure
 FOLIA PARASITOLOGICA 49: 2-8, 2002 Fine structure of erythrocytic stages of a Plasmodium tropiduri-like malaria parasite found in the lizard Kentropyx calcarata (Teiidae) from north Brazil Ilan Paperna
FOLIA PARASITOLOGICA 49: 2-8, 2002 Fine structure of erythrocytic stages of a Plasmodium tropiduri-like malaria parasite found in the lizard Kentropyx calcarata (Teiidae) from north Brazil Ilan Paperna
A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S.
 VI. Malaria A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S. because they were resistant to malaria & other diseases 3. Many
VI. Malaria A. Effect upon human culture 1. Control of malaria has contributed to world=s population explosion 2. Africans brought to U.S. because they were resistant to malaria & other diseases 3. Many
BIO Parasitology Spring 2009
 BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 10 Malaria-Life Cycle a. Micro and macrogametocytes in mosquito stomach. b. Ookinete
BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 10 Malaria-Life Cycle a. Micro and macrogametocytes in mosquito stomach. b. Ookinete
Cryptosporidium spp. Oocysts
 Sampling and Source Tracking of Cryptosporidium spp. Oocysts June 28, 2005 Kristen L. Jellison, Ph.D. Department of Civil & Environmental Engineering Lehigh University Bethlehem, Pennsylvania Ultimate
Sampling and Source Tracking of Cryptosporidium spp. Oocysts June 28, 2005 Kristen L. Jellison, Ph.D. Department of Civil & Environmental Engineering Lehigh University Bethlehem, Pennsylvania Ultimate
Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis
 Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis A. Reagents: 1. DMEM or RPMI DMEM (4.5g/L glucose) RPMI 1640 Cellgro #MT-10-017-CM Cellgro #MT-10-040-CM 2. Giemsa
Infecting Anopheles stephensi With Rodent Malaria Parasites Alida Coppi & Photini Sinnis A. Reagents: 1. DMEM or RPMI DMEM (4.5g/L glucose) RPMI 1640 Cellgro #MT-10-017-CM Cellgro #MT-10-040-CM 2. Giemsa
Ultrastructure of Endogenous Stages of Eimeria ninakohlyakimovae Yakimoff & Rastegaieff, 1930 Emend. Levine, 1961 in Experimentally Infected Goat
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 92(4): 533-538, Jul./Aug. 1997 Ultrastructure of Endogenous Stages of Eimeria ninakohlyakimovae Yakimoff & Rastegaieff, 1930 Emend. Levine, 1961 in Experimentally
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 92(4): 533-538, Jul./Aug. 1997 Ultrastructure of Endogenous Stages of Eimeria ninakohlyakimovae Yakimoff & Rastegaieff, 1930 Emend. Levine, 1961 in Experimentally
Systemic Apicomplexans. Toxoplasma
 Systemic Apicomplexans Toxoplasma Protozoan Groups Historically, protozoa have been grouped by mode of motility. Flagellates Hemoflagellates Trypanosoma cruzi Leishmania infantum Mucoflagellates Tritrichomonas
Systemic Apicomplexans Toxoplasma Protozoan Groups Historically, protozoa have been grouped by mode of motility. Flagellates Hemoflagellates Trypanosoma cruzi Leishmania infantum Mucoflagellates Tritrichomonas
The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 95(1): 43-47, Jan./Feb. 2000 The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 95(1): 43-47, Jan./Feb. 2000 The Fine Structure of the Endogenous Stages of Isospora hemidactyli Carini, 1936 in the Gecko Hemidactylus mabouia from North Brazil
Protozoan Parasites of Veterinary importance 2017
 Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Protozoan Parasites of Veterinary importance 2017 VPM-122 Laboratory 4 Spencer J. Greenwood PhD, DVM Dept. of Biomedical Sciences Room 2332N AVC North Annex sgreenwood@upei.ca Office phone # 566-6002 To
Biology of toxoplasmosis
 1 Biology of toxoplasmosis E. Petersen 1 and J. P. Dubey 2 1 Statens Seruminstitut, Copenhagen, Denmark 2 U.S. Department of Agriculture, Beltsville, USA History Toxoplasma gondii is a coccidium, with
1 Biology of toxoplasmosis E. Petersen 1 and J. P. Dubey 2 1 Statens Seruminstitut, Copenhagen, Denmark 2 U.S. Department of Agriculture, Beltsville, USA History Toxoplasma gondii is a coccidium, with
Parasitology Departement Medical Faculty of USU
 Malaria Mechanism of infection Parasitology Departement Medical Faculty of USU Introduction Malaria parasites Phylum Order Suborder Family Genus Species : : Apicomplexa : Eucoccidiida : Haemosporida :
Malaria Mechanism of infection Parasitology Departement Medical Faculty of USU Introduction Malaria parasites Phylum Order Suborder Family Genus Species : : Apicomplexa : Eucoccidiida : Haemosporida :
Gliding Motility Leads to Active Cellular Invasion by Cryptosporidium parvum Sporozoites
 INFECTION AND IMMUNITY, Sept. 2005, p. 5379 5387 Vol. 73, No. 9 0019-9567/05/$08.00 0 doi:10.1128/iai.73.9.5379 5387.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Gliding
INFECTION AND IMMUNITY, Sept. 2005, p. 5379 5387 Vol. 73, No. 9 0019-9567/05/$08.00 0 doi:10.1128/iai.73.9.5379 5387.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Gliding
Revajová, Viera, Loószová, Adrian. The Journal of Protozoology Resea Citation RightsNational Research Center for Prot
 ' ' Morphological study of partridge Title development in the foreign host - (Gallus gallus) Revajová, Viera, Loószová, Adrian Author(s) Maria, Zibrín, Martin, Herich, Ro Mikulas The Journal of Protozoology
' ' Morphological study of partridge Title development in the foreign host - (Gallus gallus) Revajová, Viera, Loószová, Adrian Author(s) Maria, Zibrín, Martin, Herich, Ro Mikulas The Journal of Protozoology
BLOOD PARASITES MORPHOTYPES OF ROCK LIZARDS OF ARMENIA
 PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY C h e m i s t r y a n d B i o l o g y 2015, 2, p. 45 49 B i o l o g y BLOOD PARASITES MORPHOTYPES OF ROCK LIZARDS OF ARMENIA T. K. HARUTYUNYAN, F. D. DANIELYAN,
PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY C h e m i s t r y a n d B i o l o g y 2015, 2, p. 45 49 B i o l o g y BLOOD PARASITES MORPHOTYPES OF ROCK LIZARDS OF ARMENIA T. K. HARUTYUNYAN, F. D. DANIELYAN,
NEUTRALIZATION OF CRYPTOSPORIDIUM MURIS SPOROZOITES BY RABBIT ANTI-C. MURIS SERUM
 Jpn. J. Trop. Med. Hyg., Vol. 23, No. 4, 1995, pp. 233-238 233 NEUTRALIZATION OF CRYPTOSPORIDIUM MURIS SPOROZOITES BY RABBIT ANTI-C. MURIS SERUM SHIGEHIKO UNI1, SHINJI HAYASHI1, AKIHIRO FUKUNAGA2, KENICHI
Jpn. J. Trop. Med. Hyg., Vol. 23, No. 4, 1995, pp. 233-238 233 NEUTRALIZATION OF CRYPTOSPORIDIUM MURIS SPOROZOITES BY RABBIT ANTI-C. MURIS SERUM SHIGEHIKO UNI1, SHINJI HAYASHI1, AKIHIRO FUKUNAGA2, KENICHI
Solar Radiation Induces Non-Nuclear Perturbations and a False Start to Regulated Exocytosis in Cryptosporidium parvum
 Solar Radiation Induces Non-Nuclear Perturbations and a False Start to Regulated Exocytosis in Cryptosporidium parvum Brendon J. King 1 *, Daniel Hoefel 1, Pao Ee Wong 2, Paul T. Monis 1 1 South Australian
Solar Radiation Induces Non-Nuclear Perturbations and a False Start to Regulated Exocytosis in Cryptosporidium parvum Brendon J. King 1 *, Daniel Hoefel 1, Pao Ee Wong 2, Paul T. Monis 1 1 South Australian
Coccidia. Toxoplasma gondii, Sarcocystis spp., Isospora belli, Cryptosporidium spp., Cyclospora cayetanenesis. Nimit Morakote, Ph.D.
 Coccidia Toxoplasma gondii, Sarcocystis spp., Isospora belli, Cryptosporidium spp., Cyclospora cayetanenesis Nimit Morakote, Ph.D. 1 เอกสารประกอบการบรรยายน จ ดทาสาหร บกระบวนว ชา 317331, ภาค เร ยนท 2 ป
Coccidia Toxoplasma gondii, Sarcocystis spp., Isospora belli, Cryptosporidium spp., Cyclospora cayetanenesis Nimit Morakote, Ph.D. 1 เอกสารประกอบการบรรยายน จ ดทาสาหร บกระบวนว ชา 317331, ภาค เร ยนท 2 ป
A Scanning Electron Microscopic Study of Eggshell Surface Topography of Leidynema portentosae and L. appendiculatum (Nematoda: Oxyuroidea)
 The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 88, Issue 5 (December, 1988) 1988-12 A Scanning Electron Microscopic
The Ohio State University Knowledge Bank kb.osu.edu Ohio Journal of Science (Ohio Academy of Science) Ohio Journal of Science: Volume 88, Issue 5 (December, 1988) 1988-12 A Scanning Electron Microscopic
Malaria. This sheet is from both sections recording and includes all slides and diagrams.
 Malaria This sheet is from both sections recording and includes all slides and diagrams. Malaria is caused by protozoa family called plasmodium (Genus) mainly affect blood system specially RBCs and each
Malaria This sheet is from both sections recording and includes all slides and diagrams. Malaria is caused by protozoa family called plasmodium (Genus) mainly affect blood system specially RBCs and each
The specimens of Ameiva ameiva (Linn) were
 Article available at http://www.parasite-journal.org or http://dx.doi.org/10.1051/parasite/1999064359 FINE STRUCTURE OF THE EPICYTOPLASMIC EIMERID COCCIDIUM ACROEIMERIA PINTOI LAINSON & PAPERNA, 1999,
Article available at http://www.parasite-journal.org or http://dx.doi.org/10.1051/parasite/1999064359 FINE STRUCTURE OF THE EPICYTOPLASMIC EIMERID COCCIDIUM ACROEIMERIA PINTOI LAINSON & PAPERNA, 1999,
Parasitology Amoebas. Sarcodina. Mastigophora
 Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Parasitology Amoebas Sarcodina Entamoeba hisolytica (histo = tissue, lytica = lyse or break) (pathogenic form) o Trophozoite is the feeding form o Life Cycle: personfeces cyst with 4 nuclei with thicker
Development of the Intestinal Villi Associated
 Development of the Intestinal Villi Associated with the Increased Epithelial Cell Mitosis in Chickens Koh-en YAMAUCHI, Eiji NAKAMURA and Yutaka ISSHIKI Laboratory of Animal Science, Faculty of Agriculture,
Development of the Intestinal Villi Associated with the Increased Epithelial Cell Mitosis in Chickens Koh-en YAMAUCHI, Eiji NAKAMURA and Yutaka ISSHIKI Laboratory of Animal Science, Faculty of Agriculture,
Blood protozoan: Plasmodium
 Blood protozoan: Plasmodium Dr. Hala Al Daghistani The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans: four species are associated The Plasmodium spp.
Blood protozoan: Plasmodium Dr. Hala Al Daghistani The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans: four species are associated The Plasmodium spp.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
doi:10.1038/nature11046 Supplementary Figure 1: Images of PB-positive cells in the subepidermal region (a-i) Representative images of PB positive cells in the subepidermis of the upper beak of the pigeon.
DISEASES OF AQUATIC ORGANISMS Vol. 62: , 2004 Published November 23 Dis Aquat Org
 DISEASES OF AQUATIC ORGANISMS Vol. 62: 133 145, 2004 Published November 23 Dis Aquat Org Cryptosporidium scophthalmi n. sp. (Apicomplexa: Cryptosporidiidae) from cultured turbot Scophthalmus maximus. Light
DISEASES OF AQUATIC ORGANISMS Vol. 62: 133 145, 2004 Published November 23 Dis Aquat Org Cryptosporidium scophthalmi n. sp. (Apicomplexa: Cryptosporidiidae) from cultured turbot Scophthalmus maximus. Light
FACULTY OF VETERINARY MEDICINE
 FACULTY OF VETERINARY MEDICINE DEPARTMENT OF VETERINARY PARASITOLOGY AND ENTOMOLOGY M.Sc. AND Ph.D. DEGREE PROGRAMMES The postgraduate programmes of the Department of Veterinary Parasitology and Entomology
FACULTY OF VETERINARY MEDICINE DEPARTMENT OF VETERINARY PARASITOLOGY AND ENTOMOLOGY M.Sc. AND Ph.D. DEGREE PROGRAMMES The postgraduate programmes of the Department of Veterinary Parasitology and Entomology
Coccidiosis in macropods and other species
 Coccidiosis in macropods and other species Author: Derek Spielman Wildlife Assistance and Information Foundation; Sydney School of Veterinary Science, the University of Sydney Abstract This presentation
Coccidiosis in macropods and other species Author: Derek Spielman Wildlife Assistance and Information Foundation; Sydney School of Veterinary Science, the University of Sydney Abstract This presentation
LABORATORY. The Protozoa. At the Bench
 LABORATORY Laboratory 8, Page 1 8 The Protozoa Introduction: The protozoa are unicellular animals that are classified on the basis of the organelles used for locomotion (flagella, pseudopodia, cilia or
LABORATORY Laboratory 8, Page 1 8 The Protozoa Introduction: The protozoa are unicellular animals that are classified on the basis of the organelles used for locomotion (flagella, pseudopodia, cilia or
Joerg Kinne, Mansoor Ali*, Ulrich Wernery, and J. P. Dubey
 J. Parasitol., 88(3), 2002, pp. 548 552 American Society of Parasitologists 2002 CLINICAL LARGE INTESTINAL COCCIDIOSIS IN CAMELS (CAMELUS DROMEDARIUS) IN THE UNITED ARAB EMIRATES: DESCRIPTION OF LESIONS,
J. Parasitol., 88(3), 2002, pp. 548 552 American Society of Parasitologists 2002 CLINICAL LARGE INTESTINAL COCCIDIOSIS IN CAMELS (CAMELUS DROMEDARIUS) IN THE UNITED ARAB EMIRATES: DESCRIPTION OF LESIONS,
Understanding Epidemics Section 3: Malaria & Modelling
 Understanding Epidemics Section 3: Malaria & Modelling PART B: Biology Contents: Vector and parasite Biology of the malaria parasite Biology of the anopheles mosquito life cycle Vector and parasite Malaria
Understanding Epidemics Section 3: Malaria & Modelling PART B: Biology Contents: Vector and parasite Biology of the malaria parasite Biology of the anopheles mosquito life cycle Vector and parasite Malaria
Blood protozoan: Plasmodium
 Blood protozoan: Plasmodium The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans:four species are associated The Plasmodium spp. life cycle can be divided
Blood protozoan: Plasmodium The causative agent of including Plasmodium vivax P. falciparum P. malariae P. ovale. malaria in humans:four species are associated The Plasmodium spp. life cycle can be divided
9 Parasitology 9 EXERCISE EQA. Objectives EXERCISE
 0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
0696T_c09_81-90.qxd 07/01/2004 23:19 Page 81 EXERCISE 9 Parasitology Exercise Pre-Test Attempt to answer the following questions before starting this exercise. They will serve as a guide to important concepts.
The South American opossum, Didelphis marsupialis, from Brazil as another definitive host for Sarcocystis speeri Dubey and Lindsay, 1999
 The South American opossum, Didelphis marsupialis, from Brazil as another definitive host for Sarcocystis speeri Dubey and Lindsay, 1999 589 J. P. DUBEY *, C. E. KERBER, D. S. LINDSAY, N. KASAI and H.
The South American opossum, Didelphis marsupialis, from Brazil as another definitive host for Sarcocystis speeri Dubey and Lindsay, 1999 589 J. P. DUBEY *, C. E. KERBER, D. S. LINDSAY, N. KASAI and H.
Cryptosporidiosis in Cattle
 Cryptosporidiosis in Cattle The Moredun Foundation News Sheet Vol. 6, No. 1, February 2014 Beth Wells BSc, PhD Sarah Thomson BSc, MRes Moredun Research Institute Key points Cryptosporidiosis is the disease
Cryptosporidiosis in Cattle The Moredun Foundation News Sheet Vol. 6, No. 1, February 2014 Beth Wells BSc, PhD Sarah Thomson BSc, MRes Moredun Research Institute Key points Cryptosporidiosis is the disease
A Study of Coccidiosis in Livestock in the Island of Dominica. Joshua Santelises. Study Abroad Texas A&M University. Dr.
 A Study of Coccidiosis in Livestock in the Island of Dominica Joshua Santelises Study Abroad 2012 Texas A&M University Dr. Thomas Lacher Dr. Jim Woolley Abstract The following experiment was done to investigate
A Study of Coccidiosis in Livestock in the Island of Dominica Joshua Santelises Study Abroad 2012 Texas A&M University Dr. Thomas Lacher Dr. Jim Woolley Abstract The following experiment was done to investigate
Eukaryotic Organisms
 Eukaryotic Organisms A Pictoral Guide of Supportive Illustrations to accompany Select Topics on Eukaryotic Oranisms Bacteria (Not Shown) Agent of Disease Reservoir Vector By Noel Ways Favorable Environmental
Eukaryotic Organisms A Pictoral Guide of Supportive Illustrations to accompany Select Topics on Eukaryotic Oranisms Bacteria (Not Shown) Agent of Disease Reservoir Vector By Noel Ways Favorable Environmental
Ectoparasites Myobia musculi Radfordia affinis Radfordia ensifera
 Ectoparasites Fleas, ticks, and lice are uncommon in modern laboratory facilities, but may be seen on wild or feral rodents. Most ectoparasite infestations seen in rats and mice used for research are various
Ectoparasites Fleas, ticks, and lice are uncommon in modern laboratory facilities, but may be seen on wild or feral rodents. Most ectoparasite infestations seen in rats and mice used for research are various
Cryptosporidium Taxonomy: Recent Advances and Implications for Public Health
 CLINICAL MICROBIOLOGY REVIEWS, Jan. 2004, p. 72 97 Vol. 17, No. 1 0893-8512/04/$08.00 0 DOI: 10.1128/CMR.17.1.72 97.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Cryptosporidium
CLINICAL MICROBIOLOGY REVIEWS, Jan. 2004, p. 72 97 Vol. 17, No. 1 0893-8512/04/$08.00 0 DOI: 10.1128/CMR.17.1.72 97.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Cryptosporidium
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign
 A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
A:Malaria (Plasmodium species) Plasmodium falciparum causes malignant tertian malaria P. malariae: causes Quartan malaria P. vivax: causes benign tertian malaria P. ovale: causes benign tertian malaria
EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS WORK-PROGRAMME PROPOSAL Version 2 VISAVET. Universidad Complutense de Madrid
 EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL Directorate D Animal Health and Welfare Unit D1- Animal health and Standing Committees EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS
EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL Directorate D Animal Health and Welfare Unit D1- Animal health and Standing Committees EUROPEAN REFERENCE LABORATORY (EU-RL) FOR BOVINE TUBERCULOSIS
Developmental Biology of Sporozoite-Host. Malaria: Implications for Vaccine Design. Javier E. Garcia, Alvaro Puentes and Manuel E.
 Developmental Biology of Sporozoite-Host Interactions in Plasmodium falciparum Malaria: Implications for Vaccine Design Javier E. Garcia, Alvaro Puentes and Manuel E. Patarroyo Clin. Microbiol. Rev. 2006,
Developmental Biology of Sporozoite-Host Interactions in Plasmodium falciparum Malaria: Implications for Vaccine Design Javier E. Garcia, Alvaro Puentes and Manuel E. Patarroyo Clin. Microbiol. Rev. 2006,
cyst&' appeared to be of two kinds-one smaller and Smnith "is inclined to regard these epithelial cell parasites as
 COCCIDIA IN SUBEPITHELIAL INFECTIONS OF THE INTESTINES OF BIRDS PHILIP B. HADLEY From the Agricultural Experiment Station of the Rhode Island State College' Received for publication, July 10, 1916 In an
COCCIDIA IN SUBEPITHELIAL INFECTIONS OF THE INTESTINES OF BIRDS PHILIP B. HADLEY From the Agricultural Experiment Station of the Rhode Island State College' Received for publication, July 10, 1916 In an
Protozoan Parasites: Lecture 20 Apicomplexans II Coccidia Part II & Cryptosporidium Pages 28-36
 Protozoan Parasites: Lecture 20 Apicomplexans II Coccidia Part II & Cryptosporidium Pages 28-36 Coccidia: Life cycle & treatment/control effectiveness? Asexual stages Sexual stages Prophylactic drugs Current
Protozoan Parasites: Lecture 20 Apicomplexans II Coccidia Part II & Cryptosporidium Pages 28-36 Coccidia: Life cycle & treatment/control effectiveness? Asexual stages Sexual stages Prophylactic drugs Current
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12 1. NAME OF THE VETERINARY MEDICINAL PRODUCT HALOCUR 0.5 mg/ml oral solution for calves 2. Qualitative and quantitative composition Active substance Halofuginone
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/12 1. NAME OF THE VETERINARY MEDICINAL PRODUCT HALOCUR 0.5 mg/ml oral solution for calves 2. Qualitative and quantitative composition Active substance Halofuginone
Evaluation of the hair growth and retention activity of two solutions on human hair explants
 activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
activity of two solutions on human hair explants Study Directed by Dr E. Lati of Laboratoire Bio-EC, Centre de Recherches Biologiques et d Experimentations Cutanees, on behalf of Pangaea Laboratories Ltd.
Gliding Motility Assay for P. berghei Sporozoites
 Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Gliding Motility Assay for P. berghei Sporozoites Important Notes: 1. For all dilutions (including antibodies and sporozoites), always make slightly more than needed. For instance, if you need 200 µl sporozoites
Intestinal and Luminal protozoa
 Intestinal and Luminal protozoa Bushehr University of Medical Sciences Department: Microbiology and Parasitology Module: Medical Parasitology Instructor: Mohammad Rayani, PhD 1 Flagellates: Giardia lamblia
Intestinal and Luminal protozoa Bushehr University of Medical Sciences Department: Microbiology and Parasitology Module: Medical Parasitology Instructor: Mohammad Rayani, PhD 1 Flagellates: Giardia lamblia
Malaria in the Mosquito Dr. Peter Billingsley
 Malaria in the Mosquito Senior Director Quality Systems and Entomology Research Sanaria Inc. Rockville MD. 1 Malaria: one of the world s foremost killers Every year 1 million children die of malaria 250
Malaria in the Mosquito Senior Director Quality Systems and Entomology Research Sanaria Inc. Rockville MD. 1 Malaria: one of the world s foremost killers Every year 1 million children die of malaria 250
Mesosomes are a definite event in antibiotic-treated Staphylococcus aureus ATCC 25923
 Tropical Biomedicine 24(1): 105 109 (2007) Mesosomes are a definite event in antibiotic-treated Staphylococcus aureus ATCC 25923 Santhana Raj, L. 1*, Hing, H.L. 2, Baharudin Omar 2, Teh Hamidah, Z. 1,
Tropical Biomedicine 24(1): 105 109 (2007) Mesosomes are a definite event in antibiotic-treated Staphylococcus aureus ATCC 25923 Santhana Raj, L. 1*, Hing, H.L. 2, Baharudin Omar 2, Teh Hamidah, Z. 1,
Electron Microscopic Observations on Ciliated Epithelium of Tracheal Organ Cultures Infected with Bordetella bronchiseptica
 Microbiol. Immunol. Vol. 33 (2), 111-121, 1989 Electron Microscopic Observations on Ciliated Epithelium of Tracheal Organ Cultures Infected with Bordetella bronchiseptica Kachiko SEKIYA,*,1 Yutaka FUTAESAKU,2
Microbiol. Immunol. Vol. 33 (2), 111-121, 1989 Electron Microscopic Observations on Ciliated Epithelium of Tracheal Organ Cultures Infected with Bordetella bronchiseptica Kachiko SEKIYA,*,1 Yutaka FUTAESAKU,2
PRINCIPAL INVESTIGATOR: Dr. Jetsumon (Sattabongkot) Prachumsri
 AD (Leave blank) Award Number: W81XWH-07-2-0090 TITLE: Proteomic Study of Human Malaria Parasite Plasmodium Vivax Liver Stages for Development of Vaccines and Drugs PRINCIPAL INVESTIGATOR: Dr. Jetsumon
AD (Leave blank) Award Number: W81XWH-07-2-0090 TITLE: Proteomic Study of Human Malaria Parasite Plasmodium Vivax Liver Stages for Development of Vaccines and Drugs PRINCIPAL INVESTIGATOR: Dr. Jetsumon
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii. Yates, Lauren A.
 A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
A comparison of placental tissue in the skinks Eulamprus tympanum and E. quoyii Yates, Lauren A. Abstract: The species Eulamprus tympanum and Eulamprus quoyii are viviparous skinks that are said to have
Fungal Disease. What is a fungus?
 Fungal Disease What is a fungus? A fungus is a living organism. It goes through a complicated life cycle and is able to spread in the environment by producing large numbers of spores that are easily dispersed
Fungal Disease What is a fungus? A fungus is a living organism. It goes through a complicated life cycle and is able to spread in the environment by producing large numbers of spores that are easily dispersed
Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220
 Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220 Introduction Enzootic Bovine Leukosis is a transmissible disease caused by the Enzootic Bovine Leukosis Virus (BLV)
Enzootic Bovine Leukosis: Milk Screening and Verification ELISA: VF-P02210 & VF-P02220 Introduction Enzootic Bovine Leukosis is a transmissible disease caused by the Enzootic Bovine Leukosis Virus (BLV)
Ahead of print online version
 Folia Parasitologica 60 [3]: 232 236, 2013 ISSN 0015-5683 (print), ISSN 1803-6465 (online) Institute of Parasitology, Biology Centre ASCR http://folia.paru.cas.cz/ A new species of Choleoeimeria (Apicomplexa:
Folia Parasitologica 60 [3]: 232 236, 2013 ISSN 0015-5683 (print), ISSN 1803-6465 (online) Institute of Parasitology, Biology Centre ASCR http://folia.paru.cas.cz/ A new species of Choleoeimeria (Apicomplexa:
DLS Sample Preparation Guide
 DLS Sample Preparation Guide The Leica TCS SP8 DLS is an innovative concept to integrate the Light Sheet Microscopy technology into the confocal microscope. Due to its unique optical architecture samples
DLS Sample Preparation Guide The Leica TCS SP8 DLS is an innovative concept to integrate the Light Sheet Microscopy technology into the confocal microscope. Due to its unique optical architecture samples
Avian coccidiosis, a disease of major economic
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction
Overview. There are commonly found arrangements of bacteria based on their division. Spheres, Rods, Spirals
 Bacteria Overview Bacteria live almost everywhere. Most are microscopic ranging from 0.5 5 m in size, and unicellular. They have a variety of shapes when viewed under a microscope, most commonly: Spheres,
Bacteria Overview Bacteria live almost everywhere. Most are microscopic ranging from 0.5 5 m in size, and unicellular. They have a variety of shapes when viewed under a microscope, most commonly: Spheres,
Seasonal Variations of yeso sika Deer Skin and its Vegetable Tanned Leather
 Seasonal Variations of yeso sika Deer Skin and its Vegetable Tanned Leather Shigeharu Fukunaga, Akihiko Yoshie, Ikuo Yamakawa, Fumio Nakamura Laboratory of Animal By-product Science, Graduate School of
Seasonal Variations of yeso sika Deer Skin and its Vegetable Tanned Leather Shigeharu Fukunaga, Akihiko Yoshie, Ikuo Yamakawa, Fumio Nakamura Laboratory of Animal By-product Science, Graduate School of
DOWNLOAD OR READ : VETERINARY CLINICAL PARASITOLOGY PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : VETERINARY CLINICAL PARASITOLOGY PDF EBOOK EPUB MOBI Page 1 Page 2 veterinary clinical parasitology veterinary clinical parasitology pdf veterinary clinical parasitology Use these links
DOWNLOAD OR READ : VETERINARY CLINICAL PARASITOLOGY PDF EBOOK EPUB MOBI Page 1 Page 2 veterinary clinical parasitology veterinary clinical parasitology pdf veterinary clinical parasitology Use these links
Arrested oocyst maturation in Plasmodium parasites. lacking type II NADH:ubiquinone dehydrogenase
 Supplemental Information for: Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase Katja E. Boysen and Kai Matuschewski Contents: - Supplemental Movies 1 and
Supplemental Information for: Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase Katja E. Boysen and Kai Matuschewski Contents: - Supplemental Movies 1 and
Anti-protozoan study of a medicinal herb, Bidens pilosa
 1 2017 JITMM Anti-protozoan study of a medicinal herb, Bidens pilosa Meng-Ting Yang, Tien-Fen Kuo, Yueh-Chen Wu, Cicero L.T. Chang and Wen-Chin Yang Taiwan International Graduate Program Molecular and
1 2017 JITMM Anti-protozoan study of a medicinal herb, Bidens pilosa Meng-Ting Yang, Tien-Fen Kuo, Yueh-Chen Wu, Cicero L.T. Chang and Wen-Chin Yang Taiwan International Graduate Program Molecular and
VETERINARY BIOMEDICAL SCIENCES (VBSC)
 Veterinary Biomedical Sciences (VBSC) 1 VETERINARY BIOMEDICAL SCIENCES (VBSC) VBSC 5000 Master s Research and Thesis Prerequisites: Graduate standing. Description: Research problem for meeting requirements
Veterinary Biomedical Sciences (VBSC) 1 VETERINARY BIOMEDICAL SCIENCES (VBSC) VBSC 5000 Master s Research and Thesis Prerequisites: Graduate standing. Description: Research problem for meeting requirements
PARASITOLOGICAL EXAMINATIONS CATALOGUE OF SERVICES AND PRICE LIST
 INSTITUTE OF PARASITOLOGY Biomedical Research Center Seltersberg Justus Liebig University Giessen Schubertstrasse 81 35392 Giessen Germany Office: +49 (0) 641 99 38461 Fax: +49 (0) 641 99 38469 Coprological
INSTITUTE OF PARASITOLOGY Biomedical Research Center Seltersberg Justus Liebig University Giessen Schubertstrasse 81 35392 Giessen Germany Office: +49 (0) 641 99 38461 Fax: +49 (0) 641 99 38469 Coprological
Consequences of Antimicrobial Resistant Bacteria. Antimicrobial Resistance. Molecular Genetics of Antimicrobial Resistance. Topics to be Covered
 Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
MID 23. Antimicrobial Resistance. Consequences of Antimicrobial Resistant Bacteria. Molecular Genetics of Antimicrobial Resistance
 Antimicrobial Resistance Molecular Genetics of Antimicrobial Resistance Micro evolutionary change - point mutations Beta-lactamase mutation extends spectrum of the enzyme rpob gene (RNA polymerase) mutation
Antimicrobial Resistance Molecular Genetics of Antimicrobial Resistance Micro evolutionary change - point mutations Beta-lactamase mutation extends spectrum of the enzyme rpob gene (RNA polymerase) mutation
Molecular characterisation of selected. enteric pathogens and antimicrobial resistance. of Salmonella in rangeland goats in Western.
 Molecular characterisation of selected enteric pathogens and antimicrobial resistance of Salmonella in rangeland goats in Western Australia Thesis presented by Khalid Rasheed Saif Al-Habsi BSc, MScAnSc
Molecular characterisation of selected enteric pathogens and antimicrobial resistance of Salmonella in rangeland goats in Western Australia Thesis presented by Khalid Rasheed Saif Al-Habsi BSc, MScAnSc
Alveolar proteins stabilize cortical microtubules in Toxoplasma gondii
 Alveolar proteins stabilize cortical microtubules in Toxoplasma gondii Clare R. Harding 1,*, Matthew Gow 2, Joon Ho Kang 3,, Emily Shortt 1, Scott R. Manalis,5,6, Markus Meissner 2,7, and Sebastian Lourido
Alveolar proteins stabilize cortical microtubules in Toxoplasma gondii Clare R. Harding 1,*, Matthew Gow 2, Joon Ho Kang 3,, Emily Shortt 1, Scott R. Manalis,5,6, Markus Meissner 2,7, and Sebastian Lourido
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and
 Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere
THE MICROSCOPE PATHOGEN IDENTIFICATION
 CONTENTS 5 ABOUT THE AUTHOR 5 ACKNOWLEDGEMENTS 6 OVERVIEW 6 What is the Purpose of this Book? 6 What are the Limitations of Light Microscopy as a Diagnostic Tool? 7 When Should I Contact a Veterinarian?
CONTENTS 5 ABOUT THE AUTHOR 5 ACKNOWLEDGEMENTS 6 OVERVIEW 6 What is the Purpose of this Book? 6 What are the Limitations of Light Microscopy as a Diagnostic Tool? 7 When Should I Contact a Veterinarian?
ECHINOCOCCOSIS. By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine).
 ECHINOCOCCOSIS By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine). INTRODUCTION Species under genus Echinococcus are small tapeworms of carnivores with larval stages known as hydatids proliferating
ECHINOCOCCOSIS By Dr. Ameer kadhim Hussein. M.B.Ch.B. FICMS (Community Medicine). INTRODUCTION Species under genus Echinococcus are small tapeworms of carnivores with larval stages known as hydatids proliferating
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5870/1679/dc1 Supporting Online Material for Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes Chung-hui Yang, Priyanka
www.sciencemag.org/cgi/content/full/319/5870/1679/dc1 Supporting Online Material for Drosophila Egg-Laying Site Selection as a System to Study Simple Decision-Making Processes Chung-hui Yang, Priyanka
Bioinformatics: Investigating Molecular/Biochemical Evidence for Evolution
 Bioinformatics: Investigating Molecular/Biochemical Evidence for Evolution Background How does an evolutionary biologist decide how closely related two different species are? The simplest way is to compare
Bioinformatics: Investigating Molecular/Biochemical Evidence for Evolution Background How does an evolutionary biologist decide how closely related two different species are? The simplest way is to compare
TOPIC CLADISTICS
 TOPIC 5.4 - CLADISTICS 5.4 A Clades & Cladograms https://upload.wikimedia.org/wikipedia/commons/thumb/4/46/clade-grade_ii.svg IB BIO 5.4 3 U1: A clade is a group of organisms that have evolved from a common
TOPIC 5.4 - CLADISTICS 5.4 A Clades & Cladograms https://upload.wikimedia.org/wikipedia/commons/thumb/4/46/clade-grade_ii.svg IB BIO 5.4 3 U1: A clade is a group of organisms that have evolved from a common
Title. CitationJapanese Journal of Veterinary Research, 52(2): 101- Issue Date Doc URL. Type. File Information
 Title INFORMATION: Thesis for the Doctor of Veterinary Med CitationJapanese Journal of Veterinary Research, 52(2): 101- Issue Date 2004-08 Doc URL http://hdl.handle.net/2115/10515 Type bulletin File Information
Title INFORMATION: Thesis for the Doctor of Veterinary Med CitationJapanese Journal of Veterinary Research, 52(2): 101- Issue Date 2004-08 Doc URL http://hdl.handle.net/2115/10515 Type bulletin File Information
National Research Center
 National Research Center Update of immunodiagnosis of cystic echinococcosis cysts Global distribution of zoonotic strains of Echinococcus granulosus (Adapted from Eckert and Deplazes, 2004) Echinococcus
National Research Center Update of immunodiagnosis of cystic echinococcosis cysts Global distribution of zoonotic strains of Echinococcus granulosus (Adapted from Eckert and Deplazes, 2004) Echinococcus
Vaccines for Cats. 2. Feline viral rhinotracheitis, FVR caused by FVR virus, also known as herpes virus type 1, FHV-1
 Vaccines for Cats Recent advances in veterinary medical science have resulted in an increase in the number and type of vaccines that are available for use in cats, and improvements are continuously being
Vaccines for Cats Recent advances in veterinary medical science have resulted in an increase in the number and type of vaccines that are available for use in cats, and improvements are continuously being
Avian Reproductive System Female
 extension Avian Reproductive System Female articles.extension.org/pages/65372/avian-reproductive-systemfemale Written by: Dr. Jacquie Jacob, University of Kentucky For anyone interested in raising chickens
extension Avian Reproductive System Female articles.extension.org/pages/65372/avian-reproductive-systemfemale Written by: Dr. Jacquie Jacob, University of Kentucky For anyone interested in raising chickens
Chapter 1 COPYRIGHTED MATERIAL. Introduction to Veterinary Pathology. What is pathology? Who does pathology?
 What is pathology? Who does pathology? Chapter 1 Introduction to Veterinary Pathology Anatomic pathology Clinical pathology Microbiology Parasitology Immunology Toxicology Veterinary forensic pathology
What is pathology? Who does pathology? Chapter 1 Introduction to Veterinary Pathology Anatomic pathology Clinical pathology Microbiology Parasitology Immunology Toxicology Veterinary forensic pathology
VETERINARY BACTERIOLOGY FROM THE DARK AGES TO THE PRESENT DAY
 VETERINARY BACTERIOLOGY FROM THE DARK AGES TO THE PRESENT DAY D.J.TAYLOR MA PhD VetMB DipECPHM DipECVPH MRCVS EMERITUS PROFESSOR OF VETERINARY BACTERIOLOGY AND PUBLIC HEALTH UNIVERSITY OF GLASGOW INTRODUCTION
VETERINARY BACTERIOLOGY FROM THE DARK AGES TO THE PRESENT DAY D.J.TAYLOR MA PhD VetMB DipECPHM DipECVPH MRCVS EMERITUS PROFESSOR OF VETERINARY BACTERIOLOGY AND PUBLIC HEALTH UNIVERSITY OF GLASGOW INTRODUCTION
Protozoan Parasites: Flagellates, Amoebae, Ciliates & Apicomplexans
 Protozoan Parasites: Flagellates, Amoebae, Ciliates & Apicomplexans Spencer Greenwood BSc, MSc, PhD, DVM Dept. of Biomedical Sciences Office: 2332N AVC-North Annex Phone: 566-6002 Home: 892-4686 E-mail:
Protozoan Parasites: Flagellates, Amoebae, Ciliates & Apicomplexans Spencer Greenwood BSc, MSc, PhD, DVM Dept. of Biomedical Sciences Office: 2332N AVC-North Annex Phone: 566-6002 Home: 892-4686 E-mail:
Feline Tritrichomonas foetus infection
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Feline Tritrichomonas foetus infection Author : ANDREW SPARKES Categories : Vets Date : November 24, 2008 ANDREW SPARKES discusses
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Feline Tritrichomonas foetus infection Author : ANDREW SPARKES Categories : Vets Date : November 24, 2008 ANDREW SPARKES discusses
Biology of Isospora spp. from Humans, Nonhuman Primates, and Domestic Animals
 CLINICAL MICROBIOLOGY REVIEWS, Jan. 1997, p. 19 34 Vol. 10, No. 1 0893-8512/97/$04.00 0 Copyright 1997, American Society for Microbiology Biology of Isospora spp. from Humans, Nonhuman Primates, and Domestic
CLINICAL MICROBIOLOGY REVIEWS, Jan. 1997, p. 19 34 Vol. 10, No. 1 0893-8512/97/$04.00 0 Copyright 1997, American Society for Microbiology Biology of Isospora spp. from Humans, Nonhuman Primates, and Domestic
Antimicrobial Resistance
 Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Consequences of Antimicrobial Resistant Bacteria Change in the approach to the administration of empiric antimicrobial therapy Increased number of hospitalizations Increased length
Antimicrobial Resistance Acquisition of Foreign DNA
 Antimicrobial Resistance Acquisition of Foreign DNA Levy, Scientific American Horizontal gene transfer is common, even between Gram positive and negative bacteria Plasmid - transfer of single or multiple
Antimicrobial Resistance Acquisition of Foreign DNA Levy, Scientific American Horizontal gene transfer is common, even between Gram positive and negative bacteria Plasmid - transfer of single or multiple
