INSTRUCTIONS FOR USE FOR: English
|
|
|
- Archibald West
- 5 years ago
- Views:
Transcription
1 INSTRUCTIONS FOR USE FOR: en English
2 INSTRUCTIONS FOR USE FOR GORE VIABAHN Endoprosthesis Carefully read all instructions prior to use. Observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. DESCRIPTION The GORE VIABAHN Endoprosthesis is a flexible, self-expanding endoluminal endoprosthesis consisting of an expanded polytetrafluoroethylene (eptfe) lining with an external nitinol (NiTi = Nickel:Titanium) support extending along its entire length (Figure 1). The endoprosthesis is compressed and attached to a dual lumen delivery catheter (Figure 2). The larger central catheter lumen is used for flushing and guidewire introduction. The smaller lumen contains elements of the deployment mechanism. The delivery catheter hub assembly has one port for the deployment system and one port for flushing and guidewire insertion. To facilitate accurate endoprosthesis placement, two radiopaque metallic bands are attached to the catheter shaft, marking the ends of the compressed endoprosthesis. The GORE VIABAHN Endoprosthesis is supplied STERILE. The GORE VIABAHN Endoprosthesis should not be resterilized. FIGURE 1: GORE VIABAHN ENDOPROSTHESIS External Nitinol Stent eptfe Lining FIGURE 2: GORE VIABAHN ENDOPROSTHESIS DELIVERY SYSTEM Deployment Knob Catheter Shaft Radiopaque Markers Hub Assembly Guidewire / Flushing Port Constrained Endoprosthesis INTENDED USE / INDICATIONS The GORE VIABAHN Endoprosthesis is a flexible, self-expanding endoluminal prosthesis for endovascular grafting of peripheral arteries. The GORE VIABAHN Endoprosthesis is also indicated for improving blood flow in symptomatic obstructions of peripheral veins. CONTRAINDICATIONS Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. TABLE 1: SIZING TABLE Device Sizing Labeled Device Recommended Vessel Diameter (mm) Diameter1 (mm) Introducer Sheath Size (Fr) Available Device Lengths2 (cm) Guidewire Diameter Recommended Balloon Diameter for Device Touch-up (mm)3 Deployment Direction , 5, 10, (0.889 mm) 5.0 Tip to hub , 5, 10, (0.889 mm) 6.0 Tip to hub , 5, 10, (0.889 mm) 7.0 Tip to hub , 5, 10, (0.889 mm) 8.0 Tip to hub , 10, (0.889 mm) 9.0 Tip to hub , 10, (0.889 mm) 10.0 Tip to hub , (0.889 mm) 12.0 Tip to hub , (0.889 mm) 14.0 Tip to hub 1 Recommended endoprosthesis compression within the vessel is approximately 5 20%. 2 Labeled device lengths are nominal. 3 For the 11 and 13 mm diameter devices, balloon inflation pressure should not exceed 8 atm. 4 The 10 mm diameter device is compatible with the following 10 Fr introducer sheaths: Cordis AVANTI Sheath Introducer, Boston Scientific SUPER SHEATH Introducer Sheath, B. Braun INTRADYN Tear-Away Introducer Sheath. PACKAGE HANDLING Store in a cool dry place. This product has an expiration date and should be used before the labeled use by (expiration) date marked on the box. METHOD Preparation of patients receiving the GORE VIABAHN Endoprosthesis should include initiation of an appropriate dosage of oral antiplatelet medication prior to and following the procedure. Effective anticoagulation therapy should be maintained throughout the procedure and continued into the postoperative period, as deemed appropriate by the treating physician. Prior to implantation of the GORE VIABAHN Endoprosthesis, the physician should refer to the Sizing Table (Table 1) and read the Directions for Use. When used in the treatment of stenotic or occlusive lesions, placement of the GORE VIABAHN Endoprosthesis should immediately follow successful transluminal balloon angioplasty confirmed by angiography. The endoprosthesis must be sized in accordance with the Sizing Table (Table 1) using accurate measurement techniques. Proper placement of the endoprosthesis should be monitored and confirmed using fluoroscopy. Sterile precautions should be the same as for any device implant procedure. To ensure an optimal result, the endoprosthesis must be dilated after deployment with an appropriately sized balloon (Table 1). Do not extend balloon dilatation beyond the ends of the device and into healthy vessel. 1
3 WARNINGS W. L. Gore & Associates has insufficient clinical and experimental data upon which to base any conclusions regarding the effectiveness of the GORE VIABAHN Endoprosthesis in applications other than the endovascular grafting of peripheral arteries and improving blood flow in symptomatic obstructions of peripheral veins. W. L. Gore & Associates has insufficient clinical and experimental data upon which to base any conclusions regarding the effectiveness of the GORE VIABAHN Endoprosthesis in applications where the device is deployed within stents or stent grafts other than the GORE VIABAHN Endoprosthesis. Other devices may interfere with the deployment of the GORE VIABAHN Endoprosthesis resulting in deployment failure or other device malfunction. The GORE VIABAHN Endoprosthesis is not indicated for use in the central circulatory system; such as, pulmonary arteries, aorta, coronary arteries, coronary bypass grafts, coronary sinus, carotid arteries, vertebral arteries, brachiocephalic (innominate) arteries, vena cavae or pulmonary veins. W. L. Gore & Associates has insufficient clinical and experimental data upon which to base any conclusions regarding the effectiveness of the GORE VIABAHN Endoprosthesis in applications where the endoprosthesis may experience repeated and extreme flexion, such as across the popliteal fossa and the anticubital fossa. Clinical conditions such as excessive bending, tortuosity, and / or repeated and extreme flexion may result in compromised performance or failure of the endoprosthesis. Do not use the GORE VIABAHN Endoprosthesis for the treatment of lesions that would not allow an operative salvage bypass procedure. Do not use the GORE VIABAHN Endoprosthesis for the treatment of ostial lesions or lesions involving a major side branch that may be covered by the endoprosthesis. Do not use in patients with less than one distal run-off vessel which has continuous patency to the ankle. Do not use in patients with a history of intolerance or adverse reaction to antiplatelet and / or anticoagulation therapies, bleeding diathesis, severe hypertension or renal failure. Special care should be taken to ensure that the appropriate size endoprosthesis, compatible sheath and guidewire are selected prior to introduction. Native vessel dimensions must be accurately measured, not estimated. Do not cannulate or puncture the GORE VIABAHN Endoprosthesis. Cannulating or puncturing the endoprosthesis may result in damage to the eptfe lining and / or the external nitinol support, resulting in compromised performance or failure of the endoprosthesis. Do not cut the endoprosthesis. The endoprosthesis should only be placed and deployed using the supplied catheter system. Do not use a kinked introducer sheath. A kinked introducer sheath may increase the force necessary to deploy the endoprosthesis and may cause a deployment failure or catheter breakage on removal. Do not attempt to deploy the endoprosthesis or manipulate the delivery system without an appropriately sized guidewire (Table 1) or fluoroscopic guidance. Do not withdraw the GORE VIABAHN Endoprosthesis back into the introducer sheath once the endoprosthesis is fully introduced. Withdrawing the GORE VIABAHN Endoprosthesis back into the sheath can cause damage to the endoprosthesis, premature deployment, deployment failure, and / or catheter separation. If removal prior to deployment is necessary, withdraw the GORE VIABAHN Endoprosthesis to a position close to but not into the introducer sheath. Both the GORE VIABAHN Endoprosthesis and introducer sheath can then be removed in tandem. After removal, do not reuse the GORE VIABAHN Endoprosthesis or introducer sheath. Inadvertent, partial, or failed deployment or migration of the endoprosthesis may require surgical intervention. PRECAUTIONS The GORE Medical Device is designed for single use only; do not reuse device. Gore does not have data regarding reuse of this device. Reuse may cause device failure or procedural complications including device damage, compromised device biocompatibility, and device contamination. Reuse may result in infection, serious injury, or patient death. W. L. Gore & Associates has insufficient technical information to recommend reuse or reprocessing of the GORE VIABAHN Endoprosthesis. However, reasonably foreseeable clinical complications that may result from inappropriate reuse or reprocessing include, but are not limited to: deployment failure, catheter failure, infection, irritation, vessel damage or loss of biocompatibility. Do not use the GORE VIABAHN Endoprosthesis if the sterile package is compromised or the GORE VIABAHN Endoprosthesis is damaged. Do not use the GORE VIABAHN Endoprosthesis after the labeled use by (expiration) date. Do not resterilize the GORE VIABAHN Endoprosthesis. The GORE VIABAHN Endoprosthesis should only be used by physicians trained in its use. Follow the Directions for Use supplied with all accessories used in conjunction with the GORE VIABAHN Endoprosthesis. Once deployment is started, repositioning the endoprosthesis should not be attempted. Do not dilate the endoprosthesis with a balloon longer than the labeled endoprosthesis length (Table 1). Refer to Sizing Table (Table 1) for selection of appropriate balloon diameter. Do not attempt to withdraw or reposition a balloon catheter within the lumen of the deployed endoprosthesis unless the balloon is completely deflated. Antiplatelet medication should be initiated prior to placement of the GORE VIABAHN Endoprosthesis. Effective anticoagulation therapy should be maintained at a dosage deemed appropriate by the physician. No clinical events related to heating effects of GORE VIABAHN Endoprostheses in the MRI environment have been reported. The effect of heating in the MRI environment for devices with fractured stent struts is not known. MRI Safety and Compatibility MR Conditional Non-clinical testing has demonstrated that the GORE VIABAHN Endoprosthesis is MR Conditional. It can be scanned safely under the following conditions: Static magnetic field of 1.5 or 3.0 Tesla Spatial gradient field of 720 Gauss/cm Maximum scanner displayed whole-body-averaged specific absorption rate (SAR) of 3.0W/kg for 15 minutes of scanning. 3.0 Tesla Temperature Rise: In non-clinical testing, the GORE VIABAHN Endoprosthesis produced a temperature rise of 2.5ºC at an MR system reported maximum whole bodied averaged specific absorption rate (SAR) of 3.0W/kg for 15 minutes of MR scanning in a 3.0 Tesla, Excite, General Electric active-shield, horizontal field MR scanner using G B Software and placed in a worst-case location in a phantom designed to simulate human tissue. The SAR calculated using calorimetry was 2.8 W/kg. 1.5 Tesla Temperature Rise: In non-clinical testing, the GORE VIABAHN Endoprosthesis produced a temperature rise of 2.4ºC at an MR system reported maximum whole bodied averaged specific absorption rate (SAR) of 2.8W/kg for 15 minutes of MR scanning in a 1.5 Tesla, Magnetom, Siemens Medical Solutions, active-shield, horizontal field MR scanner using Numaris/4 Software and placed in a worst-case location in a phantom designed to simulate human tissue. The SAR calculated using calorimetry was 1.5 W/kg. Image Artifact: The image artifact extends approximately 2 4 mm from the device, both inside and outside the device lumen when scanned in nonclinical testing using sequence: T1 weighted, spin echo and gradient echo pulse sequences in a 3.0 Tesla, Excite, General Electric activeshield, horizontal field MR system with a send-receive RF body coil. 2
4 For each vascular device and assembly, the artifacts that appeared on the MR images were shown as localized signal voids (i.e., signal loss) that were minor in size relative to the size and shape of these implants. The gradient echo pulse sequence produced larger artifacts than the T1 weighted, spin echo pulse sequence for the GORE VIABAHN Endoprosthesis. MR image quality may be compromised if the area of interest is in the exact same area or relatively close to the position of the GORE VIABAHN Endoprosthesis. Therefore, it may be necessary to optimize the MR imaging parameters to compensate for the presence of this implant. HAZARDS AND ADVERSE EVENTS Procedure Related: As with all procedures that utilize techniques for introducing a catheter into a vessel, complications may be expected. These complications include, but are not limited to: access site infection; entry site bleeding and / or hematoma; vessel thrombosis, occlusion, pseudoaneurysm, and trauma to the vessel wall (including rupture or dissection); distal embolization; arteriovenous fistula formation; transient or permanent contrast induced renal failure; renal toxicity; sepsis; shock; radiation injury; myocardial infarction; fever; pain; malposition; malapposition; inflammation; and / or death. Device Related: Complications and adverse events can occur when using any endovascular device. These complications include, but are not limited to: hematoma; stenosis, thrombosis or occlusion; distal embolism; side branch occlusion; vessel wall trauma and / or rupture; false aneurysm; infection; inflammation; fever and / or pain in the absence of infection; deployment failure; migration; and device failure. MATERIALS REQUIRED FOR IMPLANTATION GORE VIABAHN Endoprosthesis Marker guidewire or catheter (for calibrated measurement reference) Syringe filled with heparinized saline Introducer sheath of appropriate size (Table 1) 0.035" (0.889 mm) diameter stiff guidewire Guidewire length should be at least twice the length of the GORE VIABAHN Endoprosthesis delivery catheter Appropriate angioplasty balloon catheters and accessories (Table 1) Appropriate diagnostic catheters and accessories DIRECTIONS FOR USE Treatment of Vessel Obstruction A. Access 1. Using appropriate local anesthesia, access is achieved using the appropriate vessel. When possible, a percutaneous Seldinger technique is preferred. A cutdown may be performed when indicated. 2. Using standard technique, insert the appropriately sized angiographic vascular introducer sheath into the vessel. B. Imaging and Measurement 1. To achieve accurate measurement and ensure precise sizing and placement of the endoprosthesis, use image-centered, magnifiedview contrast angiography, including a marker guidewire or catheter. C. Percutaneous Transluminal Angioplasty (PTA) (if treating stenotic or occlusive lesions) 1. Refer to manufacturer s Directions for Use. 2. Inflate the angioplasty balloon to its nominal pressure according to manufacturer s Directions for Use. Ensure full expansion of the balloon within the lesion. Note: Carefully mark the margins of the angioplasty treatment segment in order to ensure complete coverage with the endoprosthesis. 3. Following deflation of the angioplasty balloon, evaluate the results angiographically. For reference, measure the native vessel diameter, lesion length, and residual percent stenosis. D. Sizing and Selection of the GORE VIABAHN Endoprosthesis 1. Prior to opening the sterile package, check that the diameter and length of the endoprosthesis as well as the delivery catheter length are correct before removing from the packaging. a. In selecting the appropriate size endoprosthesis, a careful assessment of the vessel is necessary. In general, to assure adequate anchoring, the diameter of the endoprosthesis should be approximately 5 20% larger than the healthy vessel diameter immediately proximal and distal to the lesion (Table 1). b. The endoprosthesis lengths of the GORE VIABAHN Endoprosthesis listed in Table 1 are nominal. It is, therefore, important that the endoprosthesis overlap the native vessel at least 1 cm beyond the proximal and distal margins of the lesion when treating stenotic or occlusive lesions and preferably at least 2 cm beyond the proximal and distal margins of the lesion when treating aneurysmal lesions. When treating an arteriovenous graft outflow lesion that starts 30 mm or less from the venous anastomosis, the endoprosthesis should overlap the prosthetic graft by at least 1 cm. c. Verify that there is sufficient catheter length to access the treatment site. 2. When overlapping (telescoping) multiple devices, the following are suggested: a. Balloon touch-up (post-dilatation) should be performed on the first device prior to placing the second device. b. To ensure proper seating, at least 1 cm of overlap between devices is suggested. c. If unequal device diameters are used, the smaller device should be placed first and then the larger device should be placed inside of the smaller device. d. Overlapping devices should not differ by more than 1 mm in diameter (with the exception that the 13 mm diameter device may be overlapped into the 11 mm diameter device). e. When overlapping inside aneurysmal lesions, at least 2 cm of overlap between devices is suggested. E. Preparation of the GORE VIABAHN Endoprosthesis 1. Opening the Sterile Package. Carefully inspect the packaging for damage to the sterile barrier. Do not use the GORE VIABAHN Endoprosthesis after the use by (expiration) date. Peel back the outer pouch and remove the sterile inner pouch and coil containing the GORE VIABAHN Endoprosthesis. Beginning at one corner, peel back the edge of the inner pouch and gently remove the GORE VIABAHN Endoprosthesis. 2. Inspection Prior to Use. a. Prior to using the GORE VIABAHN Endoprosthesis, all materials and equipment to be used for the procedure should be carefully examined for bends, kinks, or other damage. b. Do not use any defective equipment. c. Do not use the GORE VIABAHN Endoprosthesis if the sterile package is compromised or the GORE VIABAHN Endoprosthesis is damaged. 3. Preparation of the GORE VIABAHN Endoprosthesis delivery catheter. a. Flush the delivery catheter by attaching a syringe of sterile saline to the flushing port on the catheter adapter (Figure 2). Continue flushing until a steady stream of fluid exits the tip of the catheter and the deployment lumen at the proximal end of the device. After flushing the catheter, remove the syringe. F. Introduction and Positioning of the GORE VIABAHN Endoprosthesis 1. Select the compatible size introducer sheath from Table Ensure the 0.035" (0.889 mm) diameter stiff guidewire has a length at least twice that of the delivery catheter. 3
5 3. Be sure to remove the balloon catheter while maintaining the position of the guidewire beyond the target lesion. 4. With the delivery catheter as straight as possible, insert the guidewire into the tip of the delivery catheter while supporting the delivery catheter and the compressed endoprosthesis. Carefully advance the endoprosthesis in small increments (approximately 0.5 cm) over the guidewire, through the hemostasis valve and introducer sheath, and into the access vessel. Note: If excessive resistance is felt as the GORE VIABAHN Endoprosthesis is introduced through the hemostasis valve, remove and inspect the delivery system for damage. Do not reuse the GORE VIABAHN Endoprosthesis if damaged. Ensure a compatible introducer sheath size (Table 1), and that the introducer sheath is free of kinks. 5. Using fluoroscopic guidance, advance the delivery catheter over the guidewire via the angiographic sheath. Advance cautiously, especially if resistance is felt. If excessive resistance is felt, remove the delivery catheter and angiographic sheath together as described in step F Position the GORE VIABAHN Endoprosthesis across the target lesion using the radiopaque hub and tip markers on the catheter. These markers identify the proximal and distal ends of the endoprosthesis, respectively. Note: If PTA is performed, the endoprosthesis length should cover the entire vessel segment treated with balloon angioplasty. For treatment of stenotic or occlusive lesions, the endoprosthesis should extend at least 1 cm proximal and distal to the margins of the lesion, and at least 2 cm beyond the proximal and distal margins of the lesion when treating aneurysmal lesions. For a lesion that starts within 30 mm of the venous anastomosis of an arteriovenous graft, the endoprosthesis should overlap the prosthetic graft by at least 1 cm. 7. Once the optimal position is verified fluoroscopically, the endoprosthesis is ready to be deployed. Note: Should it become necessary to remove the GORE VIABAHN Endoprosthesis from the vessel prior to deployment, do not withdraw the GORE VIABAHN Endoprosthesis back into the introducer sheath after the endoprosthesis is fully introduced. To remove the GORE VIABAHN Endoprosthesis prior to deployment, the GORE VIABAHN Endoprosthesis can be withdrawn to a position close to but not into the introducer sheath. Both the GORE VIABAHN Endoprosthesis and introducer sheath can then be removed in tandem. After removal, neither the GORE VIABAHN Endoprosthesis nor the introducer sheath should be reused. G. Deployment of the GORE VIABAHN Endoprosthesis 1. Stabilize the delivery catheter at the hemostasis valve of the introducer sheath. It is also important to stabilize the delivery catheter and introducer sheath relative to the patient. This will minimize catheter movement during deployment and ensure accurate endoprosthesis positioning. 2. Untwist the screw-connector at the base of the deployment knob. While keeping the extracorporeal segment of the catheter as straight as possible, slowly pull the deployment knob away from the adapter. Deployment of the endoprosthesis will occur from the tip of the delivery catheter toward the hub. If deployed as instructed, the endoprosthesis should not appreciably shorten. Note: Once deployment has started, repositioning of the endoprosthesis should not be attempted. 3. While maintaining the position of the guidewire across the treated lesion, carefully withdraw the delivery catheter through the lumen of the endoprosthesis and remove it via the introducer sheath. Moderate resistance may be felt when the distal tip exit through the hemostasis valve of the introducer sheath. Note: If, during catheter removal, the tip olive catches on the leading edge of the endoprosthesis, a slight back and forth motion of the catheter may aid in release. Excessive or abrupt force during catheter removal may damage the endoprosthesis or cause separation at the catheter tip. 4. After deployment, the endoprosthesis must be smoothed and seated against the vessel wall by inflating an angioplasty balloon within it. Touch-up balloon diameter should be selected according to Table 1. It should be inflated to the desired diameter along the entire length of the endoprosthesis. If the endoprosthesis length exceeds that of the balloon, multiple inflations may be needed. After the balloon is inflated throughout the endoprosthesis, attention is required to ensure complete deflation of the balloon prior to cautious removal of the balloon catheter to prevent endoprosthesis displacement. Do not extend balloon dilatation beyond the ends of the device and into healthy vessel. 5. Using contrast angiography, evaluate the treated segment prior to completing the procedure. Further balloon inflations may be necessary if residual endoprosthesis folds or invaginations are visualized angiographically. A final angiographic run to evaluate vessel patency to the foot is recommended. 6. When clinically appropriate, remove the introducer sheath and achieve hemostasis of the puncture site. DEFINITIONS Use By Caution Consult Instructions for Use 2 STERILIZE Do Not Resterilize Do Not Reuse Catalogue Number Batch Code Serial Number Authorised Representative in the European Community MR Conditional CAUTION: USA Federal Law restricts the sale, distribution,or use of this device to, by, or on the order of a physician. Sterile Sterilized using Ethylene Oxide Do Not Use if Package is Damaged Keep Dry Store in a Cool Place Diameter Catheter Length Device Deploys from Tip to Hub Guidewire Compatibility Vessel Diameter 4
6 Manufacturer W. L. Gore & Associates, Inc North Fourth Street Flagstaff, Arizona United States Order Information: Tel.: Tel.: Technical Information: Tel.: Tel.: For international contacts and additional product information, visit GORE, VIABAHN, and designs are trademarks of W. L. Gore & Associates. AVANTI is a trademark of Johnson & Johnson Gateway, LLC. SUPER SHEATH is a trademark of Boston Scientific Corporation. INTRADYN is a trademark of B. Braun Medical, Inc. 2007, 2009, 2011, 2014 W. L. Gore & Associates, Inc. OCTOBER 2014 Printed on recyclable paper
INSTRUCTIONS FOR USE FOR:
 INSTRUCTIONS FOR USE FOR: NON-REMOVABLE THE GORE VIABIL SHORT WIRE BILIARY ENDOPROSTHESIS IS INTENDED FOR PALLIATION OF MALIGNANT STRICTURES IN THE BILIARY TREE English The GORE VIABIL Short Wire Biliary
INSTRUCTIONS FOR USE FOR: NON-REMOVABLE THE GORE VIABIL SHORT WIRE BILIARY ENDOPROSTHESIS IS INTENDED FOR PALLIATION OF MALIGNANT STRICTURES IN THE BILIARY TREE English The GORE VIABIL Short Wire Biliary
The only device incorporating infrarenal anchors for active fixation with proven migration prevention through 5 years of follow-up.
 The Endograft Active Fixation The only device incorporating infrarenal anchors for active fixation with proven migration prevention through 5 years of follow-up.* Flexibility The deployment catheter is
The Endograft Active Fixation The only device incorporating infrarenal anchors for active fixation with proven migration prevention through 5 years of follow-up.* Flexibility The deployment catheter is
No Sacrifice of Pushability or Torquability = More Control
 No Sacrifice of Pushability or Torquability = More Control Design enables the operator to utilize hydrophilic performance and preserve the desired pushability and torquability attributes Tapered tip enhances
No Sacrifice of Pushability or Torquability = More Control Design enables the operator to utilize hydrophilic performance and preserve the desired pushability and torquability attributes Tapered tip enhances
No Sacrifice of Pushability or Torquability = More Control
 No Sacrifice of Pushability or Torquability = More Control Design enables the operator to utilize hydrophilic performance and preserve the desired pushability and torquability attributes Tapered tip enhances
No Sacrifice of Pushability or Torquability = More Control Design enables the operator to utilize hydrophilic performance and preserve the desired pushability and torquability attributes Tapered tip enhances
VALUE ANALYSIS and PRODUCT INFORMATION KIT
 VALUE ANALYSIS and PRODUCT INFORMATION KIT New SHORT WIRE Delivery System SHORT W IRE BILIARY ENDOPROSTHESIS Table of contents PRODUCT OVERVIEW Key considerations................. 1.1 Features and benefits...............
VALUE ANALYSIS and PRODUCT INFORMATION KIT New SHORT WIRE Delivery System SHORT W IRE BILIARY ENDOPROSTHESIS Table of contents PRODUCT OVERVIEW Key considerations................. 1.1 Features and benefits...............
Features & Benefits WITHOUT VENT PLUG/ RED CAP WITH VENTED WITH VENT PLUG. 20 Fr 33cm A2016 A2016V 24 cm B2016 B2016V
 REINFORCED, STRAIGHT TIP RING REINFORCED, STRAIGHT TIP RING ARTERIAL CANNULAE Andocor arterial cannulae are intended for perfusion of the ascending aorta during cardiopulmonary bypass. Connection site
REINFORCED, STRAIGHT TIP RING REINFORCED, STRAIGHT TIP RING ARTERIAL CANNULAE Andocor arterial cannulae are intended for perfusion of the ascending aorta during cardiopulmonary bypass. Connection site
EC-AH-011v1 January 2018 Page 1 of 5. Standard Operating Procedure Equine Center Clemson University
 EC-AH-011v1 January 2018 Page 1 of 5 Standard Operating Procedure Equine Center Clemson University SOP ID: EC-AH-011v1 January 2018 Title: Injection Techniques Author(s): Julia Tagher, CU Equine Center
EC-AH-011v1 January 2018 Page 1 of 5 Standard Operating Procedure Equine Center Clemson University SOP ID: EC-AH-011v1 January 2018 Title: Injection Techniques Author(s): Julia Tagher, CU Equine Center
St George/Sutherland Hospitals And Health Services (SGSHHS)
 VASCATH INSTILLATION OF ANTICOAGULATION / ANTIBIOTIC LOCK Cross references (including NSW Health/ SESIAHS policy directives) NSW Health Policy for Medication Handling in NSW Public Hospitals PD207_007
VASCATH INSTILLATION OF ANTICOAGULATION / ANTIBIOTIC LOCK Cross references (including NSW Health/ SESIAHS policy directives) NSW Health Policy for Medication Handling in NSW Public Hospitals PD207_007
SOP: Blood Collection in Swine
 SOP: Blood Collection in Swine These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol
SOP: Blood Collection in Swine These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol
Cardiac MRI Morphology 2004
 Cardiac MRI Morphology 2004 1 2 Disclaimers The information in this presentation is strictly educational and is not intended to be used for instruction as to the practice of medicine. Healthcare practitioners
Cardiac MRI Morphology 2004 1 2 Disclaimers The information in this presentation is strictly educational and is not intended to be used for instruction as to the practice of medicine. Healthcare practitioners
Codman Neurovascular Product Portfolio
 Codman Neurovascular Product Portfolio EMBOLIC COILS FRAME Spherical PRESIDIO MICROCOIL Cerecyte 10 System, Frame PC410041230 4 mm 11.5 cm PC410051730 5 mm 17 cm PC410062630 6 mm 26 cm PC410073030 7 mm
Codman Neurovascular Product Portfolio EMBOLIC COILS FRAME Spherical PRESIDIO MICROCOIL Cerecyte 10 System, Frame PC410041230 4 mm 11.5 cm PC410051730 5 mm 17 cm PC410062630 6 mm 26 cm PC410073030 7 mm
GASTRO-INTESTINAL (ENTERAL)
 GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 95 A D Gastrostomy Feeding Tubes & Accessories Tri-Funnel Replacement Gastrostomy Tube Green inflator cuff, sterile. ard 12 French, 5 cc balloon.
GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 95 A D Gastrostomy Feeding Tubes & Accessories Tri-Funnel Replacement Gastrostomy Tube Green inflator cuff, sterile. ard 12 French, 5 cc balloon.
GASTRO-INTESTINAL (ENTERAL)
 GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 97 D Gastrostomy Feeding Tubes & Accessories Feeding Tube 18 French, 7-10 ml balloon. 058436 Each Tri-Funnel Replacement Gastrostomy Tube Green inflator
GASTRO-INTESTINAL (ENTERAL) GASTRO-INTESTINAL (ENTERAL) 97 D Gastrostomy Feeding Tubes & Accessories Feeding Tube 18 French, 7-10 ml balloon. 058436 Each Tri-Funnel Replacement Gastrostomy Tube Green inflator
RADIAL APPROACH ACCESSORIES Rad Board Radial Arm Board 3 Rad Board Accessories 3-4 Rad Board Xtra 3 Rad Trac 4 Rad Rest 4
 RADIAL APPROACH ORIES Rad Board Radial Arm Board 3 Rad Board Accessories 3-4 Rad Board Xtra 3 Rad Trac 4 Rad Rest 4 NEEDLES & SCALPELS Merit Advance Angiographic Needles 5 SecureLoc Safety Introducer Needles
RADIAL APPROACH ORIES Rad Board Radial Arm Board 3 Rad Board Accessories 3-4 Rad Board Xtra 3 Rad Trac 4 Rad Rest 4 NEEDLES & SCALPELS Merit Advance Angiographic Needles 5 SecureLoc Safety Introducer Needles
SOP: Blood Collection in the Horse
 SOP: Blood Collection in the Horse These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
SOP: Blood Collection in the Horse These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
Blood Collection Healthcare
 Blood Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms
Blood Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control Forms
all access Neurovascular guide
 all access Neurovascular guide Introduction... 3 Guiding Catheters Ordering Information... 4 Guiding Catheters 5F... 6 Guiding Catheters 6-8F... 7 Guidewires Ordering Information... 8 Guidewires -10...
all access Neurovascular guide Introduction... 3 Guiding Catheters Ordering Information... 4 Guiding Catheters 5F... 6 Guiding Catheters 6-8F... 7 Guidewires Ordering Information... 8 Guidewires -10...
Antimicrobial Selection and Therapy for Equine Musculoskeletal Trauma
 Antimicrobial Selection and Therapy for Equine Musculoskeletal Trauma Lucio Petrizzi DVM DECVS Università degli Studi di Teramo Surgical site infections (SSI) Microbial contamination unavoidable Infection
Antimicrobial Selection and Therapy for Equine Musculoskeletal Trauma Lucio Petrizzi DVM DECVS Università degli Studi di Teramo Surgical site infections (SSI) Microbial contamination unavoidable Infection
Product Name: Uricult Moderately Complex Item Number: Intuition: Title: Title: Discontinued By
 Moderately Complex Item Number: 1000 Intuition: Prepared By: Date: Title: Accepted By: Date: Title: Accepted By: Date: Discontinued By Date: SECTION 1 - TEST NAME Uricult SECTION 2 - INTENDED USAGE For
Moderately Complex Item Number: 1000 Intuition: Prepared By: Date: Title: Accepted By: Date: Title: Accepted By: Date: Discontinued By Date: SECTION 1 - TEST NAME Uricult SECTION 2 - INTENDED USAGE For
Advancing Lives and the Delivery of Health Care
 Advancing Lives and the Delivery of Health Care TERMS AND CONDITIONS OF SALE The following terms and conditions of sale constitute an integral part of the Price List for C. R. Bard, Inc. and its affiliates,
Advancing Lives and the Delivery of Health Care TERMS AND CONDITIONS OF SALE The following terms and conditions of sale constitute an integral part of the Price List for C. R. Bard, Inc. and its affiliates,
Withdrawal period: 93 days Milk: Not authorised for use in animals producing milk for human consumption.
 A. LABELLING PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE CARTON BOX AND LABELS OF 100 ml and 250 ml 1. NAME OF THE VETERINARY MEDICINAL PRODUCT TILKOMAY 300 mg/ml + 90 mg/ml solution
A. LABELLING PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE CARTON BOX AND LABELS OF 100 ml and 250 ml 1. NAME OF THE VETERINARY MEDICINAL PRODUCT TILKOMAY 300 mg/ml + 90 mg/ml solution
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE
 APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
APPLICATION FOR LIVE ANIMAL USE IN TEACHING AT FAULKNER STATE COMMUNITY COLLEGE MARK WITH AN X IN THE BOX FOR ONE OF THE FOLLOWING AND TYPE YOUR CURRENT PROTOCOL NUMBER IF NEEDED: X New application Amendment
MEDICATION ADMINSITRATION: ANTIBIOTIC LOCK THERAPY GUIDELINE
 MEDICATION ADMINSITRATION: ANTIBIOTIC LOCK THERAPY GUIDELINE I. PURPOSE Central venous catheters are an integral part in medical management for patients requiring long-term total parenteral nutrition,
MEDICATION ADMINSITRATION: ANTIBIOTIC LOCK THERAPY GUIDELINE I. PURPOSE Central venous catheters are an integral part in medical management for patients requiring long-term total parenteral nutrition,
Regional and Local Anesthesia of the Wrist and Hand Aided by a Forearm Sterile Elastic Exsanguination Tourniquet - A Review
 H E M A C L E A R P R E S S A u g u s t 2 0 1 2 P a g e 1 Regional and Local Anesthesia of the Wrist and Hand Aided by a Forearm Sterile Elastic Exsanguination Tourniquet - A Review Noam Gavriely, MD,
H E M A C L E A R P R E S S A u g u s t 2 0 1 2 P a g e 1 Regional and Local Anesthesia of the Wrist and Hand Aided by a Forearm Sterile Elastic Exsanguination Tourniquet - A Review Noam Gavriely, MD,
The Choice. V e r s a t i l i t y. S t r e n g t h. F l e x i b i l i t y. of surgeons for half a century
 The Choice of surgeons for half a century V e r s a t i l i t y S t r e n g t h F l e x i b i l i t y S t o p b l e e d i n g f a s t w i t h t h r e e p r o v e n p e r f o r m e r s SURGICEL Absorbable
The Choice of surgeons for half a century V e r s a t i l i t y S t r e n g t h F l e x i b i l i t y S t o p b l e e d i n g f a s t w i t h t h r e e p r o v e n p e r f o r m e r s SURGICEL Absorbable
ONE collar. flea larvae. REPELS and kills fleas. REPELS and kills ticks. cat convenient, easy-to-apply collar. 8month protection
 top view lid ONE collar REPELS and kills fleas REPELS and kills ticks flea larvae convenient, easy-to-apply collar 8month protection against fleas & ticks Odorless 3 visibility reflectors included For
top view lid ONE collar REPELS and kills fleas REPELS and kills ticks flea larvae convenient, easy-to-apply collar 8month protection against fleas & ticks Odorless 3 visibility reflectors included For
BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT. Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin
 BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin 2. STATEMENT OF ACTIVE AND OTHER SUBSTANCES Each ml contains: Tilmicosin 300 mg;
BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Hymatil 300 mg/ml solution for injection for cattle and sheep Tilmicosin 2. STATEMENT OF ACTIVE AND OTHER SUBSTANCES Each ml contains: Tilmicosin 300 mg;
Standard Angiographic Catheters
 Standard 5 Fr Standard Catheters for Peripheral and Cerebral Angiography with high torque control and maximum flow rate through a one-piece braided structure. Flow, smoothness and superior safety thanks
Standard 5 Fr Standard Catheters for Peripheral and Cerebral Angiography with high torque control and maximum flow rate through a one-piece braided structure. Flow, smoothness and superior safety thanks
300 Yard Trainer GDT Product Manual
 300 Yard Trainer GDT00-16298 Product Manual Have questions about your Remote Trainer or need training tips for your pet? Our Customer Care representatives are here to help you. Call our USA-based Customer
300 Yard Trainer GDT00-16298 Product Manual Have questions about your Remote Trainer or need training tips for your pet? Our Customer Care representatives are here to help you. Call our USA-based Customer
IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee. Blood Collection Guidelines
 IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee Blood Collection Guidelines Purpose To provide Iowa State University (ISU) Institutional Animal Care and Use Committee (IACUC) guidelines
IOWA STATE UNIVERSITY Institutional Animal Care and Use Committee Blood Collection Guidelines Purpose To provide Iowa State University (ISU) Institutional Animal Care and Use Committee (IACUC) guidelines
Treatment of septic peritonitis
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Treatment of septic peritonitis Author : Andrew Linklater Categories : Companion animal, Vets Date : November 2, 2016 Septic
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Treatment of septic peritonitis Author : Andrew Linklater Categories : Companion animal, Vets Date : November 2, 2016 Septic
Administering wormers (anthelmintics) effectively
 COWS www.cattleparasites.org.uk Administering wormers (anthelmintics) effectively COWS is an industry initiative promoting sustainable control strategies for parasites in cattle Wormer administration Dec
COWS www.cattleparasites.org.uk Administering wormers (anthelmintics) effectively COWS is an industry initiative promoting sustainable control strategies for parasites in cattle Wormer administration Dec
USA Product Label
 BAYER HEALTHCARE LLC Animal Health Division USA Product Label http://www.vetdepot.com P.O. BOX 390, SHAWNEE MISSION, KS, 66201 0390 Customer Service Tel.: 800 633 3796 Customer Service Fax: 800 344 4219
BAYER HEALTHCARE LLC Animal Health Division USA Product Label http://www.vetdepot.com P.O. BOX 390, SHAWNEE MISSION, KS, 66201 0390 Customer Service Tel.: 800 633 3796 Customer Service Fax: 800 344 4219
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Medicinal product no longer authorised
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Zubrin 50 mg oral lyophilisates for dogs Zubrin 100 mg oral lyophilisates for dogs Zubrin 200 mg oral lyophilisates
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Zubrin 50 mg oral lyophilisates for dogs Zubrin 100 mg oral lyophilisates for dogs Zubrin 200 mg oral lyophilisates
Feline blood transfusions: preliminary considerations
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk Feline blood transfusions: preliminary considerations Author : Andrea Harvey Categories : RVNs Date : September 1, 2011 ABSTRACT
Vet Times The website for the veterinary profession https://www.vettimes.co.uk Feline blood transfusions: preliminary considerations Author : Andrea Harvey Categories : RVNs Date : September 1, 2011 ABSTRACT
Page 1 of 6 INVENTION TITLE. Electromagnetic frequencies as a means to treat internal parasites in animals.
 Page 1 of 6 INVENTION TITLE Electromagnetic frequencies as a means to treat internal parasites in animals. DESCRIPTION The present invention relates to a process using electrical frequencies to treat internal
Page 1 of 6 INVENTION TITLE Electromagnetic frequencies as a means to treat internal parasites in animals. DESCRIPTION The present invention relates to a process using electrical frequencies to treat internal
CLINICAL ESSENTIAL HUDDLE CARD. All associates must comply with their state practice acts.
 CLINICAL ESSENTIAL HUDDLE CARD All associates must comply with their state practice acts. QUESTIONS FOR DISCUSSION Where can you find information about your state practice acts? If you are unclear of what
CLINICAL ESSENTIAL HUDDLE CARD All associates must comply with their state practice acts. QUESTIONS FOR DISCUSSION Where can you find information about your state practice acts? If you are unclear of what
400 Yard Trainer GDT Product Manual
 400 Yard Trainer GDT00-16301 Product Manual Remote Training Collar Have questions about your Remote Trainer or need training tips for your pet? Our Customer Care representatives are here to help you. Call
400 Yard Trainer GDT00-16301 Product Manual Remote Training Collar Have questions about your Remote Trainer or need training tips for your pet? Our Customer Care representatives are here to help you. Call
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site:
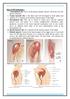 Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
Sites of IM injections : 1. Ventrogluteal site: site is in the gluteus medius muscle, which lies over the gluteus minimus. 2. Vastus lateralis site: is the thick and well developed in both adults and children.
HEARTWORM DISEASE AND THE DAMAGE DONE
 HEARTWORM DISEASE AND THE DAMAGE DONE Stephen Jones, DVM There are now more months of the year where environmental conditions favor mosquito survival and reproduction. Warmer temperatures Indoor environments
HEARTWORM DISEASE AND THE DAMAGE DONE Stephen Jones, DVM There are now more months of the year where environmental conditions favor mosquito survival and reproduction. Warmer temperatures Indoor environments
B. PACKAGE LEAFLET 1
 B. PACKAGE LEAFLET 1 PACKAGE LEAFLET FOR: Cadorex 300 mg/ml solution for injection for cattle, sheep and pigs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION
B. PACKAGE LEAFLET 1 PACKAGE LEAFLET FOR: Cadorex 300 mg/ml solution for injection for cattle, sheep and pigs 1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION
Discovery. DIFFERENTIAL DIAGNOSES Septic joint or tendon sheath Abscess Vascular damage Fracture Tendon or ligament damage
 Discovery Applied Research for Today s Equine Athlete March 2012 Volume 3 Case File: Contrast-Enhanced Computed Tomography (CT) SIGNALMENT AND HISTORY 1-year-old Morgan colt January 1, 2011, Trooper was
Discovery Applied Research for Today s Equine Athlete March 2012 Volume 3 Case File: Contrast-Enhanced Computed Tomography (CT) SIGNALMENT AND HISTORY 1-year-old Morgan colt January 1, 2011, Trooper was
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT NOSEDORM 5 mg/ml Solution for injection for dogs and cats [DE, ES, FR, PT] 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each
ANNEX III LABELLING AND PACKAGE LEAFLET
 ANNEX III LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE Card box and package leaflet for brown glass bottle (Type 1) 1. NAME OF THE
ANNEX III LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE AND THE IMMEDIATE PACKAGE Card box and package leaflet for brown glass bottle (Type 1) 1. NAME OF THE
Synopsis. Takeda Pharmaceutical Company Limited Name of the finished product UNISIA Combination Tablets LD, UNISIA Combination Tablets
 Synopsis Name of the sponsor Takeda Pharmaceutical Company Limited Name of the finished product UNISIA Combination Tablets LD, UNISIA Combination Tablets Name of active ingredient Title of the study Study
Synopsis Name of the sponsor Takeda Pharmaceutical Company Limited Name of the finished product UNISIA Combination Tablets LD, UNISIA Combination Tablets Name of active ingredient Title of the study Study
Antibiotic Line Lock Guideline
 Antibiotic Line Lock Guideline Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target audience, state if Trust wide): Guideline for the management of long-term catheterrelated
Antibiotic Line Lock Guideline Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target audience, state if Trust wide): Guideline for the management of long-term catheterrelated
large dog lbs REPELS AND kills ticks, fleas and mosquitoes
 DO NOT USE ON CATS 81356823 108 x 34 x 120 Topical Prevention and Treatment of Ticks, Fleas, Mosquitoes, Biting Flies and Lice for Monthly Use Only on Dogs and Puppies 7 Weeks of Age and Older and Weighing
DO NOT USE ON CATS 81356823 108 x 34 x 120 Topical Prevention and Treatment of Ticks, Fleas, Mosquitoes, Biting Flies and Lice for Monthly Use Only on Dogs and Puppies 7 Weeks of Age and Older and Weighing
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CLYNAV solution for injection for Atlantic salmon 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 0.05 ml dose
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE VETERINARY MEDICINAL PRODUCT CLYNAV solution for injection for Atlantic salmon 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 0.05 ml dose
SINGLE ANNUAL IMPLANT
 Manage pet ferret adrenal cortical disease with a SINGLE ANNUAL IMPLANT NOT APPROVED BY FDA Legally marketed as an FDA Indexed Product under MIF 900-013. FOR USE IN FERRETS ONLY. Extra-label use is prohibited.
Manage pet ferret adrenal cortical disease with a SINGLE ANNUAL IMPLANT NOT APPROVED BY FDA Legally marketed as an FDA Indexed Product under MIF 900-013. FOR USE IN FERRETS ONLY. Extra-label use is prohibited.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Oncept IL-2 lyophilisate and solvent for suspension for injection for cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1/18 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Oncept IL-2 lyophilisate and solvent for suspension for injection for cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Starts working through contact
 DO NOT USE ON CATS 81356777 108 x 34 x 120 Once-A-Month Topical Treatment for Fleas and Lice For Use Only on Dogs and Puppies 7 Weeks and Older and Weighing 11 20 lbs. READ THE ENTIRE LABEL BEFORE EACH
DO NOT USE ON CATS 81356777 108 x 34 x 120 Once-A-Month Topical Treatment for Fleas and Lice For Use Only on Dogs and Puppies 7 Weeks and Older and Weighing 11 20 lbs. READ THE ENTIRE LABEL BEFORE EACH
adult fleas flea eggs flea larvae adult ticks tick nymphs tick larvae KILLS & REPELS: mosquitoes KEEP OUT OF REACH OF CHILDREN CAUTION
 For Use Only on Medium 15-30 lbs. Dogs/Puppies & 12 weeks of age or older 3 MONTH SUPPLY Flea & Tick Spot On For DOGS Includes Applicator KILLS: adult fleas flea eggs flea larvae adult ticks Applicator
For Use Only on Medium 15-30 lbs. Dogs/Puppies & 12 weeks of age or older 3 MONTH SUPPLY Flea & Tick Spot On For DOGS Includes Applicator KILLS: adult fleas flea eggs flea larvae adult ticks Applicator
They're not all the same: Why FDA approval of animal drugs matters
 They're not all the same: Why FDA approval of animal drugs matters Elizabeth Luddy, DVM Deputy Director, Office of New Animal Drug Evaluation Center for Veterinary Medicine US Food and Drug Administration
They're not all the same: Why FDA approval of animal drugs matters Elizabeth Luddy, DVM Deputy Director, Office of New Animal Drug Evaluation Center for Veterinary Medicine US Food and Drug Administration
Topical prevention and treatment of ticks, fleas, mosquitoes, biting flies and lice for monthly use on dogs and puppies 7 weeks of age and older
 BAYER HEALTHCARE LLC Animal Health Division P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390 Customer Service Tel.: 800-633-3796 Customer Service Fax: 800-344-4219 Website: www.bayer-ah.com Every effort has
BAYER HEALTHCARE LLC Animal Health Division P.O. BOX 390, SHAWNEE MISSION, KS, 66201-0390 Customer Service Tel.: 800-633-3796 Customer Service Fax: 800-344-4219 Website: www.bayer-ah.com Every effort has
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Acecare 2mg/ml Solution for Injection for Dogs and Cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution contains
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Acecare 2mg/ml Solution for Injection for Dogs and Cats 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of solution contains
EPA Est. No IL-001. Assurity and Elanco. EPA Reg. No
 Monthly application is recommended to prevent and treat flea infestations. Assurity starts working in 30 minutes, kills adult fleas before they can lay eggs and continues killing fleas for a full month
Monthly application is recommended to prevent and treat flea infestations. Assurity starts working in 30 minutes, kills adult fleas before they can lay eggs and continues killing fleas for a full month
Kristy Broaddus. Bite Wounds: Why are they so hard to manage? Bite Wounds 2/9/2016
 Kristy Broaddus Bite Wounds: Why are they so hard to manage? Kristy Broaddus, DVM, MS, DACVS VESC Richmond VA Michigan State DVM Auburn University internship and surgery residency Oklahoma State University
Kristy Broaddus Bite Wounds: Why are they so hard to manage? Kristy Broaddus, DVM, MS, DACVS VESC Richmond VA Michigan State DVM Auburn University internship and surgery residency Oklahoma State University
ADVANTAGE FOR DOGS
 ADVANTAGE FOR DOGS For use on dogs only. Do not use on cats or rabbits. For use on puppies from 8 weeks of age INDICATION A spot-on insecticide for flea control in dogs and cats. Indicated for use in dogs
ADVANTAGE FOR DOGS For use on dogs only. Do not use on cats or rabbits. For use on puppies from 8 weeks of age INDICATION A spot-on insecticide for flea control in dogs and cats. Indicated for use in dogs
Pigeon Spike Plastic Narrow Bird Spikes Includes specs for: Pigeon Spike Plastic Narrow Bird Spike and Surface Cleaning Systems.
 Three-Part Specifications - Copyright 2016 - Nixalite of America Inc Pigeon Spike Plastic Narrow Bird Spikes Includes specs for: Pigeon Spike Plastic Narrow Bird Spike and Surface Cleaning Systems. Nixalite
Three-Part Specifications - Copyright 2016 - Nixalite of America Inc Pigeon Spike Plastic Narrow Bird Spikes Includes specs for: Pigeon Spike Plastic Narrow Bird Spike and Surface Cleaning Systems. Nixalite
Anesthesia Check-off Form
 Anesthesia Check-off Form 5231 SW 91st Drive Gainesville, FL 32608 (352) 377-6003 The doctors and staff at Haile Plantation Animal Clinic would like to offer the most advanced medical care and services
Anesthesia Check-off Form 5231 SW 91st Drive Gainesville, FL 32608 (352) 377-6003 The doctors and staff at Haile Plantation Animal Clinic would like to offer the most advanced medical care and services
Pectus Excavatum (Funnel Chest) Dr Hasan Nugud Consultant Paediatric Surgeon
 Pectus Excavatum (Funnel Chest) Dr Hasan Nugud Consultant Paediatric Surgeon Pectus excavatum Pectus excavatum (PE) is an abnormal development of the rib cage where the breastbone (sternum) caves in,
Pectus Excavatum (Funnel Chest) Dr Hasan Nugud Consultant Paediatric Surgeon Pectus excavatum Pectus excavatum (PE) is an abnormal development of the rib cage where the breastbone (sternum) caves in,
RESEARCH AND TEACHING SURGERY GUIDELINES FOR MSU-OWNED ANIMALS
 RESEARCH AND TEACHING SURGERY GUIDELINES FOR MSU-OWNED ANIMALS I. Purpose/Scope These guidelines apply to all surgical procedures performed on animals at Mississippi State University in which the animals
RESEARCH AND TEACHING SURGERY GUIDELINES FOR MSU-OWNED ANIMALS I. Purpose/Scope These guidelines apply to all surgical procedures performed on animals at Mississippi State University in which the animals
Gastric Dilatation-Volvulus
 Gastric Dilatation-Volvulus The term "ACVS Diplomate" refers to a veterinarian who has been board certified in veterinary surgery. Only veterinarians who have successfully completed the certification requirements
Gastric Dilatation-Volvulus The term "ACVS Diplomate" refers to a veterinarian who has been board certified in veterinary surgery. Only veterinarians who have successfully completed the certification requirements
1. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER AND OF THE MANUFACTURING AUTHORISATION HOLDER RESPONSIBLE FOR BATCH RELEASE, IF DIFFERENT
 PACKAGE LEAFLET FOR: Dormilan solution for injection for dogs and cats [FR] Dormilan 1 mg/ml solution for injection for dogs and cats [DE, PT, UK] Reanest 1 mg/ml solution for injection for dogs and cats
PACKAGE LEAFLET FOR: Dormilan solution for injection for dogs and cats [FR] Dormilan 1 mg/ml solution for injection for dogs and cats [DE, PT, UK] Reanest 1 mg/ml solution for injection for dogs and cats
UiTM CARE APPLICATION FORM
 UiTM CARE APPLICATION FORM (Committee on Animal Research and Ethics) FOR UiTM CARE OFFICE USE ONLY Proposal No.:... Date of hard copy receipt:... INFORMATION FOR PRINICIPAL INVESTIGATOR Submit the duly
UiTM CARE APPLICATION FORM (Committee on Animal Research and Ethics) FOR UiTM CARE OFFICE USE ONLY Proposal No.:... Date of hard copy receipt:... INFORMATION FOR PRINICIPAL INVESTIGATOR Submit the duly
Ensure you have the right acute CVC for any treatment and every patient.
 Ensure you have the right acute CVC for any treatment and every patient. Cook Spectrum ANTIMICROBIAL TECHNOLOGY 2, 3, and 5 lumens WITH HIGH FLOW RATES CENTRAL VENOUS CATHETERS POWER-INJECTABLE Maximal
Ensure you have the right acute CVC for any treatment and every patient. Cook Spectrum ANTIMICROBIAL TECHNOLOGY 2, 3, and 5 lumens WITH HIGH FLOW RATES CENTRAL VENOUS CATHETERS POWER-INJECTABLE Maximal
LABELLING AND PACKAGE LEAFLET
 LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE CARTON BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MILTEFORAN 20 mg/ml oral solution for dogs miltefosine 2.
LABELLING AND PACKAGE LEAFLET 1 A. LABELLING 2 PARTICULARS TO APPEAR ON THE OUTER PACKAGE CARTON BOX 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MILTEFORAN 20 mg/ml oral solution for dogs miltefosine 2.
for For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs. for CATS
 Effectively breaks the flea life cycle For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs. For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs ACTIVE INGREDIENTS: Imidacloprid 9.10%
Effectively breaks the flea life cycle For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs. For use ONLY on cats 8 weeks and Older and Weighing 5 to 9 lbs ACTIVE INGREDIENTS: Imidacloprid 9.10%
Critically Appraised Topics in the Radiodiagnosis Curriculum
 Critically Appraised Topics in the Radiodiagnosis Curriculum What is a Critically Appraised Topic? There are different ways to interpret the term Critically Appraised Topic. Within the RANZCR Radiodiagnosis
Critically Appraised Topics in the Radiodiagnosis Curriculum What is a Critically Appraised Topic? There are different ways to interpret the term Critically Appraised Topic. Within the RANZCR Radiodiagnosis
large dog 5-way protection against: fleas/ticks/biting flies/mosquitoes/lice WARNING pack flea & tick protection KEEP OUT OF REACH OF CHILDREN
 from the makers of 5-way protection against: fleas/ticks/biting flies/mosquitoes/lice Topical prevention and treatment of fleas, ticks, mosquitoes, biting flies, and lice for monthly use only on dogs and
from the makers of 5-way protection against: fleas/ticks/biting flies/mosquitoes/lice Topical prevention and treatment of fleas, ticks, mosquitoes, biting flies, and lice for monthly use only on dogs and
Operating Guide. PBC Rechargeable Bark Control Collar. Please read this entire guide before beginning.
 Operating Guide PBC00-15999 Rechargeable Bark Control Collar Please read this entire guide before beginning. Welcome You and your pet were made for each other. Our aim is to help you have the best companionship
Operating Guide PBC00-15999 Rechargeable Bark Control Collar Please read this entire guide before beginning. Welcome You and your pet were made for each other. Our aim is to help you have the best companionship
extra large dog over 55 lbs kills flea eggs
 Seite 1: Layout aussen Seite 2: Layout innen Seite 3: Lack und Prägung DO NOT USE ON CATS 81356831 108 x 34 x 120 after handling and before eating, drinking, chewing gum, using tobacco or using the toilet.
Seite 1: Layout aussen Seite 2: Layout innen Seite 3: Lack und Prägung DO NOT USE ON CATS 81356831 108 x 34 x 120 after handling and before eating, drinking, chewing gum, using tobacco or using the toilet.
and older weighing Puppies 7 weeks on Dogs and For use ONLY FOR DOGS For use ONLY on Dogs and Puppies 7 weeks and older weighing lbs
 113072 MONTH 4 113072 4 MONTH WATERPROOF KILLS FLEAS, FLEA EGGS, LARVAE, PUPAE PREVENTS REINFESTATIONS Convenient, easy-to-apply and fragrance free monthly topical solution. NET CONTENTS: 0.336 fl. oz
113072 MONTH 4 113072 4 MONTH WATERPROOF KILLS FLEAS, FLEA EGGS, LARVAE, PUPAE PREVENTS REINFESTATIONS Convenient, easy-to-apply and fragrance free monthly topical solution. NET CONTENTS: 0.336 fl. oz
In this guide: Technology Overview. Proven Technology
 Fenestra VC User Guide with GE Imaging Hardware Imaging of Vascular Anatomy in SD Rats: Visualization of Normal Vascular Anatomy Using a GE explore Locus (GE Healthcare) Scanner Proven Technology Powerful
Fenestra VC User Guide with GE Imaging Hardware Imaging of Vascular Anatomy in SD Rats: Visualization of Normal Vascular Anatomy Using a GE explore Locus (GE Healthcare) Scanner Proven Technology Powerful
ASSEMBLY & INSTRUCTION MANUAL
 ASSEMBLY & INSTRUCTION MANUAL Congratulations on the purchase of your Ocean Treasures Collection aquarium. Each aquarium has been fabricated to enable a beautiful design, and optimal functionality. We
ASSEMBLY & INSTRUCTION MANUAL Congratulations on the purchase of your Ocean Treasures Collection aquarium. Each aquarium has been fabricated to enable a beautiful design, and optimal functionality. We
4MONTHS FORDOGS MEDIUM DOG WARNING MEDIUM DOG LBS REPELS AND KILLS TICKS, FLEAS, & MOSOUITOS
 FOR USE ONLY ON DOGS AND PUPPIES 7 WEEKS OF AGE AND OLDER WEIGHING MONTHS 4 4MONTHS MONTHS 4 CONTAINS IMIDACLOPRID, PERMETHRIN & PYRIPROXYFEN 4MONTHS REPELS AND KILLS TICKS, FLEAS, & MOSOUITOS FOR USE
FOR USE ONLY ON DOGS AND PUPPIES 7 WEEKS OF AGE AND OLDER WEIGHING MONTHS 4 4MONTHS MONTHS 4 CONTAINS IMIDACLOPRID, PERMETHRIN & PYRIPROXYFEN 4MONTHS REPELS AND KILLS TICKS, FLEAS, & MOSOUITOS FOR USE
S Fault Indicators. S.T.A.R. Type CR Faulted Circuit Indicator Installation Instructions. Contents PRODUCT INFORMATION
 Fault Indicators S.T.A.R. Type CR Faulted Circuit Indicator Installation Instructions Service Information S320-75-1 Contents Product Information..........................1 Safety Information............................2
Fault Indicators S.T.A.R. Type CR Faulted Circuit Indicator Installation Instructions Service Information S320-75-1 Contents Product Information..........................1 Safety Information............................2
AGILITY OBSTACLE GUIDELINES
 FEDERATION CYNOLOGIQUE INTERNATIONALE (AISBL) Place Albert 1 er, 13 B 6530 Thuin, tel : +32.71.59.12.38, fax : +32.71.59.22.29, internet : http://www.fci.be AGILITY OBSTACLE GUIDELINES January 1 2018 TABLE
FEDERATION CYNOLOGIQUE INTERNATIONALE (AISBL) Place Albert 1 er, 13 B 6530 Thuin, tel : +32.71.59.12.38, fax : +32.71.59.22.29, internet : http://www.fci.be AGILITY OBSTACLE GUIDELINES January 1 2018 TABLE
Composite Sterile Aneroid Sphygmomanometer And Rubber Bandage Tourniquet: Indications, Techniques and Results
 ISPUB.COM The Internet Journal of Orthopedic Surgery Volume 5 Number 2 Composite Sterile Aneroid Sphygmomanometer And Rubber Bandage Tourniquet: Indications, Techniques and Results A Ogbemudia Citation
ISPUB.COM The Internet Journal of Orthopedic Surgery Volume 5 Number 2 Composite Sterile Aneroid Sphygmomanometer And Rubber Bandage Tourniquet: Indications, Techniques and Results A Ogbemudia Citation
x 3 *Combiva II for Cats is not manufactured or distributed by Bayer. Advantage is a registered trademark of Bayer.
 5-9 lbs Contains the same active ingredients as in Advantage II for Cats* 5-9 lbs ACTIVE INGREDIENTS: Imidacloprid... 9.10 % Pyriproxyfen... 0.46 % OTHER INGREDIENTS:... 90.44 % TOTAL... 100.00 % Net Contents:
5-9 lbs Contains the same active ingredients as in Advantage II for Cats* 5-9 lbs ACTIVE INGREDIENTS: Imidacloprid... 9.10 % Pyriproxyfen... 0.46 % OTHER INGREDIENTS:... 90.44 % TOTAL... 100.00 % Net Contents:
VCH PHC SURGICAL PROPHYLAXIS RECOMMENDATIONS
 VCH PHC SURGICAL PROPHYLAXIS RECOMMENDATIONS CARDIAC Staphylococcus aureus, S. epidermidis, except for For patients with known MRSA colonization, recommend decolonization with Antimicrobial Photodynamic
VCH PHC SURGICAL PROPHYLAXIS RECOMMENDATIONS CARDIAC Staphylococcus aureus, S. epidermidis, except for For patients with known MRSA colonization, recommend decolonization with Antimicrobial Photodynamic
Cefuroxime 1.5gm IV and Metronidazole 500mg IV. Metronidazole 500mg IV/Ampicillin-sulbactam e 3g/Ceftriaxone 2gm. +Metronidazole 500mg/Ertapenem 1gm
 SURGICAL ANTIBIOTIC PROPHYLAXIS GENERAL SURGERY* PROCEDURE RECOMMENDED AGENTS a,b Clean None None ALTERNATIVE AGENTS (If allergic to penicillin or colonized/infected with MRSA at any site) Clean with potential
SURGICAL ANTIBIOTIC PROPHYLAXIS GENERAL SURGERY* PROCEDURE RECOMMENDED AGENTS a,b Clean None None ALTERNATIVE AGENTS (If allergic to penicillin or colonized/infected with MRSA at any site) Clean with potential
(12) United States Patent
 USOO7785.364B2 (12) United States Patent Styrc (54) KIT TO BE IMPLANTED IN A BLOOD CIRCULATION CONDUIT (75) Inventor: Mykolaj Styrc, Kopstal (LU) (73) Assignee: Laboratoires Perouse, Ivry le Temple (FR)
USOO7785.364B2 (12) United States Patent Styrc (54) KIT TO BE IMPLANTED IN A BLOOD CIRCULATION CONDUIT (75) Inventor: Mykolaj Styrc, Kopstal (LU) (73) Assignee: Laboratoires Perouse, Ivry le Temple (FR)
Giving IV Medication by Balloon System (EIS)
 Giving IV Medication by Balloon System (EIS) Medication: Click here to enter text. What do I do when I get the medication from HomeMed? Always check the prescription and label for final instructions. o
Giving IV Medication by Balloon System (EIS) Medication: Click here to enter text. What do I do when I get the medication from HomeMed? Always check the prescription and label for final instructions. o
Lameness Exams. Evaluating the Lame Horse
 Lameness Exams Evaluating the Lame Horse Stress, strain, or injury can take a toll on any horse, even one with no obvious conformation defects. When lameness occurs, you should contact your veterinarian
Lameness Exams Evaluating the Lame Horse Stress, strain, or injury can take a toll on any horse, even one with no obvious conformation defects. When lameness occurs, you should contact your veterinarian
IEEE Std 592 Test Program using Current Cable Accessories and Installation Practices
 IEEE Std 592 Test Program using Current Cable Accessories and Installation Practices Thomas J. Parker GTRC 1 Notice a. The material contained herein is, to our knowledge, accurate and reliable at the date
IEEE Std 592 Test Program using Current Cable Accessories and Installation Practices Thomas J. Parker GTRC 1 Notice a. The material contained herein is, to our knowledge, accurate and reliable at the date
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 18 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Cardisure flavoured 10 mg Tablets For dogs (Austria, Belgium, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands,,
SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 18 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Cardisure flavoured 10 mg Tablets For dogs (Austria, Belgium, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands,,
extra large dog 5-way protection 3 pack extra large dog WARNING extra large dog flea & tick protection over 55 lbs KEEP OUT OF REACH OF CHILDREN pack
 Seite 1: Layout aussen Seite 2: Layout innen Seite 3: Lack und Prägung against: fleas/ticks/biting flies/mosquitoes/lice flea & tick protection DO NOT USE ON CATS pack 3 81946760 108 x 34 x 120 11556-134_DefenseCare
Seite 1: Layout aussen Seite 2: Layout innen Seite 3: Lack und Prägung against: fleas/ticks/biting flies/mosquitoes/lice flea & tick protection DO NOT USE ON CATS pack 3 81946760 108 x 34 x 120 11556-134_DefenseCare
Published with the permission of LAVC Close window to return to IVIS pág 65 The Latin American Veterinary Conference TLAVC 2006
 pág 65 COMMON EMERGENCIES IN REPTILE PATIENTS Douglas R. Mader, MS, DVM, ABVP Marathon Veterinary Hospital Marathon, Florida, USA Reptiles take a very long time to get sick. Likewise, amphibians tend to
pág 65 COMMON EMERGENCIES IN REPTILE PATIENTS Douglas R. Mader, MS, DVM, ABVP Marathon Veterinary Hospital Marathon, Florida, USA Reptiles take a very long time to get sick. Likewise, amphibians tend to
DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS
 DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS 1. TITLE SLIDE: DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS. Hands are one of the most common sources of the spread of pathogenic microorganisms. Hand
DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS 1. TITLE SLIDE: DISCUSS HAND HYGIENE AND PERFORM HAND ANTISEPSIS. Hands are one of the most common sources of the spread of pathogenic microorganisms. Hand
LATARJET Open Surgical technique
 1 LATARJET Open Surgical technique Steps A. Exposure B. Preparation of coracoid holes C. Cutting the coracoid D. Fixing the Double Cannula to the coracoid E. Exposure of both sides of Subscapularis F.
1 LATARJET Open Surgical technique Steps A. Exposure B. Preparation of coracoid holes C. Cutting the coracoid D. Fixing the Double Cannula to the coracoid E. Exposure of both sides of Subscapularis F.
USA Product Label PARASTAR PLUS (45-88 LBS.) Novartis. (fipronil/cyphenothrin) 3 EASY-TO-USE APPLICATIONS. For dogs lbs.
 USA Product Label http://www.vetdepot.com NOVARTIS ANIMAL HEALTH US, INC. 3200 NORTHLINE AVE. SUITE 300, GREENSBORO, NC, 27408 Customer Service: 800-332-2761 Professional Services: 800-637-0281 Fax: 336-387-1168
USA Product Label http://www.vetdepot.com NOVARTIS ANIMAL HEALTH US, INC. 3200 NORTHLINE AVE. SUITE 300, GREENSBORO, NC, 27408 Customer Service: 800-332-2761 Professional Services: 800-637-0281 Fax: 336-387-1168
IQ Range. Electrical Data 3-Phase Power Supplies. Keeping the World Flowing
 IQ Range Electrical Data 3-Phase Power Supplies Keeping the World Flowing Contents Section Page Introduction 3 50 Hz 380 V 5 0 V 6 415 V 7 4 V 8 500 V 9 6 V 60 Hz 8 V 11 2 V 0 V 13 4 V 14 460 V 15 480
IQ Range Electrical Data 3-Phase Power Supplies Keeping the World Flowing Contents Section Page Introduction 3 50 Hz 380 V 5 0 V 6 415 V 7 4 V 8 500 V 9 6 V 60 Hz 8 V 11 2 V 0 V 13 4 V 14 460 V 15 480
PET DOOR. Deluxe Aluminium IMPORTANT! READ AND FOLLOW THESE INSTRUCTIONS CAREFULLY AND KEEP FOR FUTURE REFERENCE. Product Codes: #1168, #1169, #1170
 Deluxe Aluminium PET DOOR Product Codes: #1168, #1169, #1170 IMPORTANT! READ AND FOLLOW THESE INSTRUCTIONS CAREFULLY AND KEEP FOR FUTURE REFERENCE. Installation video available at: www.hakunapets.com/deluxe-aluminium-pet-door
Deluxe Aluminium PET DOOR Product Codes: #1168, #1169, #1170 IMPORTANT! READ AND FOLLOW THESE INSTRUCTIONS CAREFULLY AND KEEP FOR FUTURE REFERENCE. Installation video available at: www.hakunapets.com/deluxe-aluminium-pet-door
x 3 CrossBlock II CrossBlock II 3-10 lbs 3-10 lbs For Dogs and Puppies For Dogs and Puppies 7 weeks or older 7 weeks or older
 x 3 Waterproof Kills Fleas Kills Flea Larvae Kills Flea Eggs CrossBlock II ACTIVE INGREDIENTS : Imidacloprid... 9.10% Pyriproxyfen... 0.46% OTHER INGREDIENTS:... 90.44% TOTAL... 100.00% EPA Est. No. 74720-DEU-01
x 3 Waterproof Kills Fleas Kills Flea Larvae Kills Flea Eggs CrossBlock II ACTIVE INGREDIENTS : Imidacloprid... 9.10% Pyriproxyfen... 0.46% OTHER INGREDIENTS:... 90.44% TOTAL... 100.00% EPA Est. No. 74720-DEU-01
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS Issued March 2017 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats Recicort vet 1.77 mg/ml + 17.7 mg/ml
SUMMARY OF PRODUCT CHARACTERISTICS Issued March 2017 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats Recicort vet 1.77 mg/ml + 17.7 mg/ml
Management of CRBSI Leilani Paitoonpong MD MSc Chusana Suankratay MD PhD Division of Infectious Diseases Chulalongkorn University
 Management of CRBSI Leilani Paitoonpong MD MSc Chusana Suankratay MD PhD Division of Infectious Diseases Chulalongkorn University A 60-year-old man was admitted for CABG surgery due to triple-vessel disease.
Management of CRBSI Leilani Paitoonpong MD MSc Chusana Suankratay MD PhD Division of Infectious Diseases Chulalongkorn University A 60-year-old man was admitted for CABG surgery due to triple-vessel disease.
Package leaflet: Information for the user. GENTAMICIN VISION 3 mg/ml eye drops, solution Gentamicin
 Package leaflet: Information for the user GENTAMICIN VISION 3 mg/ml eye drops, solution Gentamicin Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the user GENTAMICIN VISION 3 mg/ml eye drops, solution Gentamicin Read all of this leaflet carefully before you start taking this medicine because it contains important
Pigeon Spike Composite Bird Spikes Includes specifications for: Pigeon Spike Composite Bird Spike and Surface Cleaning Systems.
 Three-Part Specifications - Copyright 2014 - Nixalite of America Inc Pigeon Spike Composite Bird Spikes Includes specifications for: Pigeon Spike Composite Bird Spike and Surface Cleaning Systems. Nixalite
Three-Part Specifications - Copyright 2014 - Nixalite of America Inc Pigeon Spike Composite Bird Spikes Includes specifications for: Pigeon Spike Composite Bird Spike and Surface Cleaning Systems. Nixalite
DREXEL UNIVERSITY COLLEGE OF MEDICINE ANIMAL CARE AND USE COMMITTEE POLICY FOR PREOPERATIVE AND POSTOPERATIVE CARE FOR NON-RODENT MAMMALS
 DREXEL UNIVERSITY COLLEGE OF MEDICINE ANIMAL CARE AND USE COMMITTEE POLICY FOR PREOPERATIVE AND POSTOPERATIVE CARE FOR NON-RODENT MAMMALS OBJECTIVE: This policy is to ensure that appropriate provisions
DREXEL UNIVERSITY COLLEGE OF MEDICINE ANIMAL CARE AND USE COMMITTEE POLICY FOR PREOPERATIVE AND POSTOPERATIVE CARE FOR NON-RODENT MAMMALS OBJECTIVE: This policy is to ensure that appropriate provisions
